Abstract
Background and Purpose
Stereotactic body radiation therapy delivered using MR-guided radiotherapy (MRgRT) and automatic breathold gating has shown to improve overall survival for locally advanced pancreatic cancer (LAPC) patients. The goal of our study was to evaluate feasibility of treating LAPC patients using abdominal compression (AC) and impact of potential intrafraction motion on planned dose on a 1.5T MR-linac.
Methods & Materials
Ten LAPC patients were treated with MRgRT to 50 Gy in 5 fractions with daily online plan adaptation and AC. Three orthogonal plane cine MRI were acquired to assess stability of AC pressure in minimizing tumor motion. Three sets of T2w MR scans, pre-treatment (MRIpre), verification (MRIver) and post-treatment (MRIpost) MRI, were acquired for every fraction. A total of 150 MRIs and doses were evaluated. Impact of intrafraction organ motion was evaluated by propagating pre-treatment plan and structures to MRIver and MRIpost, editing contours and recalculating doses. Gross tumor volume (GTV) coverage and organs-at-risk (OARs) doses were evaluated on MRIver and MRIpost.
Results
Median total treatment time was 75.5 (49–132) minutes. Median tumor motion in AC for all fractions was 1.7 (0.7–7), 2.1 (0.6–6.3) and 4.1 (1.4–10.0) mm in anterior-posterior, left–right and superior-inferior direction. Median GTV V50Gy was 78.7%. Median D5cm3 stomach_duodenum was 24.2 (18.4–29.3) Gy on MRIver and 24.2 (18.3–30.5) Gy on MRIpost. Median D5cm3 small bowel was 24.3 (18.2–32.8) Gy on MRIver and 24.4 (16.0–33.6) Gy on MRIpost.
Conclusion
Dose-volume constraints for OARs were exceeded for some fractions on MRIver and MRIpost. Longer follow up is needed to see the dosimetric impact of intrafraction motion on gastrointestinal toxicity.
Keywords: Ablative, SBRT, Pancreatic cancer, MR-linac, 1.5 Tesla
1. Introduction
Standard radiotherapy approaches, commonly delivering 40–60 Gy in 1.8–2.0 Gy per fraction add minimal to no survival benefit over chemotherapy alone for patients with unresectable locally advanced pancreatic cancer (LAPC) [1], [2], [3]. Ablative doses of greater than 100 Gy biological-effective dose (BED10) are likely needed to achieve local tumor control and improve overall survival [4]. For these patients, tumor doses significantly exceed the tolerance of the surrounding normal organs. Gastrointestinal (GI) toxicity related to radiosensitive organs such as stomach, duodenum and small bowel are dose-limiting for GI radiotherapy. Additional challenge to the safe delivery of such aggressive radiotherapeutic regimen is the respiratory motion management of the GI organs as well as day-to-day differences in luminal organ shape. Ablative doses to unresectable pancreatic tumors on a conventional linac are delivered in 15 or 25 fractions with respiratory gating, daily online image guidance and selective adaptive planning to address interfraction motion and limit the dose to surrounding luminal organs [4], [5]. These treatments have shown improved local tumor control and survival [6]. Ablative doses can also be delivered in a highly conformal manner using five fraction stereotactic body radiation therapy (SBRT) treatments and recent publication has shown superior outcomes with a SBRT dose of ≥40 Gy in five fractions [7].
MR-guided RT (MRgRT) systems such as hybrid MR-linacs allow delivery of SBRT techniques more effectively using daily adaptive planning and MR image guidance [8], [9], [10], [11], [12], [13]. Placidi et al. have shown the benefit of delivering 30–40 Gy in 5 fractions for LAPC patients using video-assisted inspiratory breath-hold on MR-guided radiotherapy (MRgRT) systems [12]. Bohoudi et al. reported on the benefit in target coverage and organ-at-risk (OAR) sparing of daily plan adaptation in LAPC patients treated with 40 Gy in five fractions [13] Patients treated with dose-escalated MRgRT are demonstrating improved overall survival [14]. Delivering 50 Gy in five fractions using mid-inspiration breath-hold on the MRgRT system has shown minimal severe treatment-related toxicity and encouraging early local control and overall survival [15], [16]. Abdominal compression is the method of choice on systems without automatic breath-hold, gating or other motion management options. Studies using abdominal compression for delivering such ablative prescriptions on MRgRT systems are lacking. In addition, the change in delivered doses to these GI OARs due to potential intrafraction motion has not been reported. The goal of our study was to report on the feasibility of delivering SBRT dose of 50 Gy in five fractions using a compression belt workflow and assess the impact of potential intrafraction motion on dose-volume histogram parameters on a 1.5 Tesla MRgRT system.
2. Materials and methods
2.1. Patient characteristics
Ten patients with LAPC were treated with a definitive intent between March and November 2020 on the Unity 1.5 T MR-linac system. The study was conducted under an IRB approved retrospective protocol number 21-129. Table 1 shows the detailed patient characteristics. Three tumors were located in the head of the pancreas and seven were in the body. Out of these, seven patients were considered inoperable per surgeon and six were node positive. One received RT preoperatively. Average GTV volume during simulation was 42.5 ± 24.8 cm3.
Table 1.
Patient characteristics.
| # of Patients | 10 |
|---|---|
| Age, Median (years, range) | 65.5 (60–78) |
| Gender, N (%) | |
| Male | 7 (70) |
| Female | 3 (30) |
| Pancreas Tumor Location | |
| Head | 3 (30) |
| Body | 7 (70) |
| Tail | 0 (0) |
| RT Indication | |
| Inoperable per Surgeon | 7 (70) |
| Pre-operative RT | 1 (10) |
| Post-operative recurrence | 1 (10) |
| Consolidation in setting of de novo metastatic to liver | 1 (10) |
| Node Positive | 6 (60) |
| Pre-RT Chemotherapy* | 9 (90) |
| Induction FOLFIRINOX | 7 (70) |
| Neoadjuvant FOLFIRINOX → gem/nab-paclitaxel | 1 (10) |
| Induction gem/nab-paclitaxel | 1 (10) |
| Tumor size on pre-RT CT (cm, range) | 3.2 (1.3–4.5) |
| Average compression belt pressure (mm Hg, range) | 32.5 (25–40) |
1 patient received adjuvant FOLFIRINOX 2 years prior at time of initial diagnosis but received no pre-RT chemotherapy at time of recurrence.
2.2. Compression belt simulation workflow
Each patient underwent MR fluoroscopy on the 3T Philips MR simulator (MR-sim) to assess the pressure the patient could tolerate on the compression belt (Aktina Medical, Congers, NY) while minimizing the GTV and nearby OAR motion up to 5 mm. Based on the patient’s size, a small, medium or large sized abdominal compression (AC) belt was chosen. The belt is a corset and has been modified in-house to make it MR safe. An air bladder under the belt is connected to a sphygmomanometer that ensures reproducible pressure is applied each time. The pressure was monitored and controlled from the console area.
MR fluoroscopy was performed using a balanced fast field echo (b-FFE) sequence with three orthogonal plane cine acquisition at 5 frames/sec and using the following parameters (TR/TE = 2.6/1.32 ms, slice thickness = 5 mm, FOV = 400(FH) × 424(RL) mm2, voxel size = 3 × 3 mm2). MR fluoroscopy was acquired at free breathing (FB) and then with compression belt after applying appropriate pressure. Patients were positioned arms up or arms perpendicular to the table depending on their convenience (Supplementary Fig. 1). 500 frames were acquired for each acquisition. Average AC pressure used in this study was 32.5 ± 4.8 mm Hg (Table 1). Additional MR sequences were acquired for contouring GTV. These sequences include a single-shot 2D T2w TSE (TR/TE = 1250/80 ms, slice thickness = 4 mm, voxel size = 1.3 × 1.6 mm2) and 3D radial T1w acquisition, also called 3DVANE (TR/TE = 4.1/1.68 ms, slice thickness = 4 mm, voxel size = 1.4 × 1.4 mm2). Both sequences were also acquired in FB to assess the impact of motion on image quality. MR fluoroscopy was followed by CT simulation (CT-sim) where the patient was immobilized in a Q-fix™ device along with an alphacradle™ or a Vac-lok™. This was followed by a dual phase-contrast CT with a 30 s injection delay (150 cm3 @ 5 cm3/s).
2.3. Planning details
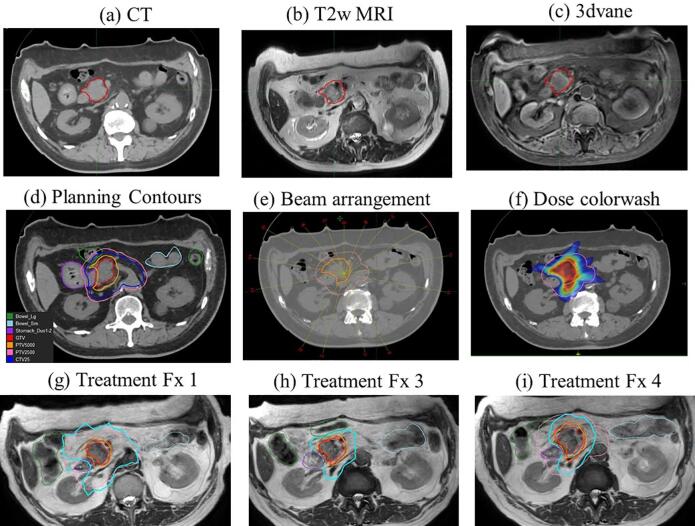
Each patient was prescribed a dose of 50 Gy in five fractions to the gross tumor plus margin along with a second dose level of 25 Gy in five fractions to the volume of risk harboring microscopic disease. Targets were delineated on the CT-sim scan with the help of 2D T2w TSE and 3DVANE MR-sim sequences. All the OARs were contoured on the CT-sim. Three GI organs at risk (OAR) were contoured – stomach with the first two segments of the duodenum (called stomach_duodenum), the rest of the small bowel and large bowel. Planning risk volume (PRV) margin on OARs was 1 mm and used in the planning optimization process to help achieve the desired dose constraints and dose distribution near the OARs. PTV50 was created using a 5 mm margin to the GTV. All OARs with an additional safety margin of 3 mm were excluded from PTV50. GTV was not cropped from any OARs. Low dose PTV, called PTV25, was created using a 5 mm set-up uncertainty margin on the CTV, which included a 1.0 cm expansion of the GTV as well as the celiac axis, and the superior mesenteric artery nodal basins [4]. Fig. 1 shows the MR-simulation images (top row a-c) along with the GTV contour and planning CT image with contours for GTV, PTV50, CTV25 and PTV25. Fifteen beams all around excluding gantry angles going through a specific high-density couch structure (~125°–145° and 215°–235°) and through the cryostat pipe (~10–20°) were used for generating a reference plan on CT using Monaco Monte Carlo based dose calculation system. A step-and-shoot intensity modulated radiation therapy (IMRT) plan using with a maximum of 100 segments per plan and dose calculation grid of 3 mm were used for plan generation. Plans were optimized to maximize target coverage until all OAR constraints were met. Plan normalization on the order of 1–2% was occasionally performed to achieve planning goals. Supplementary Table 1 shows our department dose volume constraints for 50 Gy in five fractions.
Fig. 1.
(a) CT simulation and (b-c) MR simulation images including T2wMRI and 3dvane (d) Planning contours for GTV (red), PTV50 (yellow), CTV25 (blue), PTV25 (pink), stomach (purple), small bowel (light blue) and large bowel (dark green) (e) Beam arrangement and (f) Dose color wash for 50 Gy (red) and 25 Gy (blue) (g-i) Online treatment plan performed on T2w 3D MRI for fraction 1, 3 and 4 with GTV and OAR contour as well as 33 Gy (cyan) and 50 Gy (red) isodose line displayed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Treatment workflow on unity
Each patient underwent five SBRT fractions with daily online plan adaptation using Elekta’s Adapt-to-Shape (ATS) workflow. Patients were asked to have nothing by mouth for 4 h prior to treatment. In the beginning of each treatment, 500 cine frames were acquired using 3 orthogonal planes to assess the stability of using the compression belt in minimizing the breathing motion. An attempt was made to keep the pressure consistent during the entire treatment. Patients were given a benzodiazepine (e.g., lorazepam) for each treatment to minimize any discomfort and anxiety associated with using the belt. During each treatment fraction, three sets of T2w 3D MRI (TR/TE = 1300/87 ms, voxel size = 1 × 1 × 2 mm3, FOV = 400 × 450 × 250 mm3) were acquired – pretreatment (MRIpre), verification (MRIver) and post-treatment (MRIpost). MRIpre was matched to the reference CT by first performing a rigid spine match and then adjusting the fusion to match the GTV and nearby soft-tissue anatomy or vessels. The remaining OARs were propagated using Monaco deformable image registration algorithm and edited by the physician and planners. The OAR contouring was done within a 2 cm ring around the low dose PTV (PTV25). A new adaptive plan was generated in Monaco for every fraction using fluence optimization and bulk electron density assignment derived from reference planning CT. During the first fraction, planning CT was used as a reference and average bulk electron density within each structure was propagated from CT to the MR. During subsequent fractions, the average bulk electron density is propagated from MR to MR. Once all the OAR constraints were met and right before beam-on, the structures and the optimized plan were overlaid on MRIver to visually assess GTV coverage and evaluated for any potential intrafraction motion that may have occurred during online contouring and planning. The contours were adjusted, and a new plan was generated on MRIver if significant volume of small bowel or stomach moved inside the 33 Gy isodose line. Otherwise, the treatment was delivered with cine MR monitoring. Three orthogonal MRs based on a bFFE scan were acquired at the centroid of the motion monitoring (MM) structure. MM structure was chosen to be either the GTV or an OAR (e.g., stomach) based on MDs discretion. Right before the completion of treatment, MRIpost was acquired. Once the treatment was completed, a post-treatment QA was performed using Arccheck QA phantom. In addition, the pre-treatment structures and plans were propagated to MRIver and MRIpost, the structures were adjusted accordingly, and doses recalculated. This was done to assess the impact on dose-volume histogram parameters due to potential intrafraction motion that may have occurred during online planning and delivery. Supplementary Fig. 2 shows our adaptive workflow on Unity.
2.5. Data analysis
2.5.1. Overall treatment time, patient setup and patient experience
Time taken for the ATS workflow broken down into online contouring, adaptive planning, physics QA check and beam-on time was recorded and analyzed. Overall treatment time, defined as the time from the beginning of the acquisition of the pre-treatment MRI to the end of the acquisition of the post-treatment MRI, was also logged. Physics QA check included independent monitor unit check and data integrity check between Monaco™ and Mosaiq™. Unity system does not have in-room lasers, hence day-to-day setup shifts were also analyzed to assess initial patient setup on the treatment table. Finally, patient questionnaire modified by McNair et al [17], [18] from MR-linac consortium was given to each patient and the cumulative statistics were generated.
2.5.2. Stability of abdominal compression belt for GTV motion using cine data
Cine images acquired during simulation and treatment were analyzed using an in-house developed software known as SequenceReg. A region of interest (ROI) representing the GTV was defined by the user in the first frame and subsequent frames were registered using rigid translation within that ROI based on minimizing the cost function defined by a normalized cross correlation coefficient. Cine frames from all three planes were analyzed separately. Orthogonal translational shifts for each frame with respect to the first frame were analyzed to calculate peak-to-peak amplitude by taking the height difference between sequential maximum and a minimum point. Supplementary Fig. 3 shows the method for selecting the ROI on the cine frame along with an example of tumor motion profile and the location of minimum and maximum points for calculating motion amplitude. The final output consists of the mean, median and standard deviation of all the amplitudes. The median amplitudes from the simulation scans were compared to the free breathing amplitude to judge the effectiveness of compression belt and its ability to limit breathing motion. The median (range) amplitude for each fraction was used to compare the stability and reproducibility of the compression belt during treatment. The left–right (LR), cranio-caudal (CC) and anterior-posterior (AP) motion was measured for each patient.
2.5.3. Dose-volume parameter coverage and post-treatment QA
Following dose volume histogram parameters were evaluated for daily online planning and post-treatment QA for target and OARs: Median (range) GTV coverage (V50Gy, D0.035cm3 and Dmean), Stomach_Duodenum (D0.035cm3, D2cm3 and D5cm3), Small bowel (D0.035cm3, D2cm3 and D5cm3). GTV coverage and organ doses using above parameters were also evaluated on MRIver and MRIpost to assess any potential intrafraction motion. After each treatment, GTV and OAR contours were transferred to MRIver and MRIpost and edited to match any differences in anatomy due to intrafraction motion. The treatment plan was then recalculated on each of these image sets and the dose volume parameters were extracted. Dose data from a total of 150 MR scans in 50 fractions was extracted.
3. Results
3.1. Overall treatment time, patient setup and patient experience
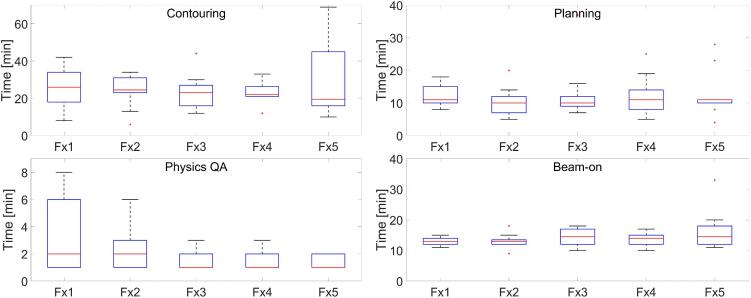
Median (range) total treatment time was 75.5 (49–132) mins. Overall adapt-to-shape time was divided into contouring, planning, physics QA and beam-on time as a function of fraction number and is displayed in Fig. 2. Combining all fractions, Median (range) contouring times was 23 (6–69) mins, planning time was 10 (4–37) mins, physics QA was 1.0 (1.0–8.0) mins and beam-on time was 14 (9–33) mins. Overall contouring time decreased with increased fractions as indicated by the median. The longer contouring time on a particular fraction was indicative of either machine issue, software crash or large intrafraction motion for a few patients that required recontouring on a new MR. Higher beam-on time was due to machine issue.
Fig. 2.
Overall Adapt-to-shape time as a function of fraction number divided into contouring, planning, physics QA and beam-on.
Average setup shifts performed based on tattoos for all the patients and all the fractions was −1.0 ± 9.0, −1.0 ± 11.0 and 0.0 ± 3.0 mm in LR, CC (in-out) and AP direction, indicating that therapists were comfortable setting up the patients without in-room lasers (Supplementary Fig. 4). In terms of patient comfort, on average patients reported acceptable tolerance to MR-linac treatments (Supplementary Fig. 5).
3.2. Stability of compression belt for GTV motion using cine data
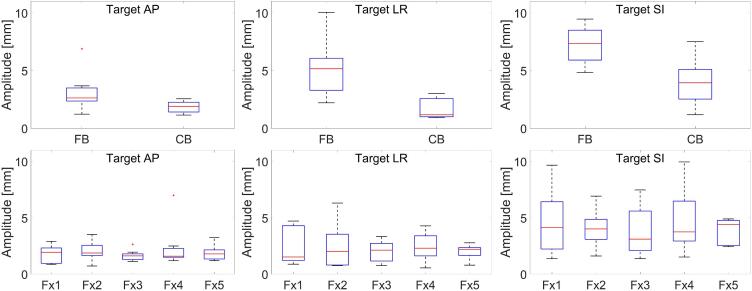
Median (range) AP, LR and SI motion in FB and with compression was 2.6 (1.2–6.9), 5.2 (2.2–10.1), 7.4 (4.8–9.5) and 1.9 (1.2–2.6), 1.2 (0.9–0.3), 4.0 (1.2–7.5) mm as shown in the boxplots in Fig. 3a. Average tumor motion in AP, LR and SI direction was calculated as a function of fraction number (Fig. 3b). Median (range) tumor motion in the belt for all fractions was 1.7 (0.7–7.0), 2.1 (0.6–6.3) and 4.1 (1.4–10.0) mm in AP, LR and SI direction.
Fig. 3.
(Top row) Target motion amplitude comparison in AP, LR and SI direction between FB cine and CB cine MRI scan measured on simulation MRI (Bottom row) Tumor motion amplitude variation as a function of treatment fraction in AP, LR and SI direction.
3.3. Dose-volume parameter coverage and post-treatment QA
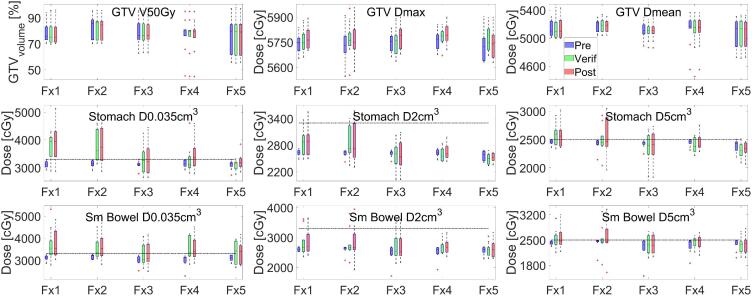
Median GTV V50Gy coverage was 78.7 (45.3–97) % for all fractions. Median GTV Dmax and GTV mean dose for all the fractions was 57.5 (55.4–59.1) Gy and 51.6 (45.5–53.7) Gy. Dose-volume criteria for stomach_duodenum and small bowel met our institutional constraints during daily planning on MRIpre. However, these parameters exceeded on MRIver and MRIpost due to intrafraction motion. Median D0.035cm3, D2cm3 and D5cm3 stomach dose was 32.6 (26.5–46.0), 26.5 (20.2–33.9) and 24.2 (18.4–29.3) Gy on MRIver and 33.5 (26.4–51.3), 26.1 (21.3–37.9) and 24.2 (18.3–30.5) Gy on MRIpost. Median D0.035cm3, D2cm3 and D5cm3 small bowel dose was 33.5 (26.4–53.2), 26.3 (21.3–37.9) and 24.3 (18.2–32.8) Gy on MRIver and 33.6 (26.4–51.7), 26.2 (19.1–39.5) and 24.4 (16.0–33.6) Gy on MRIpost. Stomach D0.035cm3, and D5cm3 dose exceeded our institutional constraints of 33 Gy and 25 Gy in 21/50 and 15/50 fractions on MRIver. The corresponding number of fractions exceeding stomach D0.035cm3 and D5cm3 constraint on MRIpost was 27/50 and 13/50. In terms of small bowel, D0.035cm3 and D5cm3 dose exceeded our institutional constraints of 33 Gy and 25 Gy in 26/50 and 15/50 fractions on MRIver. The corresponding number of fractions exceeding stomach D0.035cm3 and D5cm3 constraint on MRIpost was 26/50 and 19/50. Fig. 4 shows the dosimetric coverage for the online treatment on MRIpre, MRIver and MRIpost as a function of fraction number for GTV (top row), stomach_duodenum (middle row) and small bowel (bottom row).
Fig. 4.
Relevant dose volume parameter coverage as a function of treatment fraction for GTV V50Gy, Dmax and Dmean (top row), stomach_duodenum D0.035cm3, D2cm3, D5cm3 (middle row) and small bowel D0.035cm3, D2cm3, D5cm3 (bottom row) on MRIpre, MRIver and MRIpost. Dashed lines represent OAR planning constraints.
4. Discussion
In this study, we investigated the feasibility of delivering SBRT dose of 50 Gy in five fractions using a compression belt workflow on a MR-gRT system. Our analysis indicated that with the lack of automatic motion management techniques on Unity MR-Linac, the AC workflow and post-treatment QA enable delivery of ablative radiation doses for selected cases of LAPC patients. Our study also found that intrafraction motion of GI organs in some patients can result in violation of dose volume constraints on verification and post-treatment MRI indicating the importance of intrafraction motion management for these treatments.
Overall, the treatments with compression belt were well tolerated by our patient group. Our median treatment time per fraction from patient setup to patient leaving the room was 75 min with the exception of a few fractions exceeding this time due to software crash or intrafraction motion before beam-on that triggered another round of contouring and replanning. Out of 50 fractions, 8 fractions (6 patients) required second online adaptive replanning. Henke et al, in their early experience have reported the mean duration of 80 mins per fraction and that the adapted treatments were overall well tolerated [10]. To our knowledge, no studies have reported on the impact of intrafraction motion. This could be due to the fact that majority of these treatments were delivered with breathold where OAR motion will be minimal during the breathold period and automatic beam gating can allow any potential intrafraction/patient motion to be adjusted by a couch shift.
Median GTV motion in FB as well compression shows that compression belt was effective in minimizing patient breathing motion. The clinical objective of reducing the breathing motion of the tumor to a maximum excursion of 5 mm or less using compression belt was achieved in more than 75% of fractions implying compression belt to be a viable motion management option on MRgRT systems for this patient population. Thirteen fractions were greater than 5 mm motion as also evident from Fig. 3b with amplitude in SI directions. In addition, small standard deviation in the breathing amplitude for all the fractions implies that AC is also stable and reproducible in between treatment fractions. Currently, AC is used as a motion management technique for all GI MRgRT patients at our institution. With free breathing, even if the tumor does not move, nearby organ movement can cause blurring and impact daily soft tissue contouring of organs such as small bowel and duodenum. There is also a concern that compression can bring the organs closer. When we see this during simulation, those patients are deemed ineligible for MR-linac. One could also argue that there is still a possibility of organs getting closer to high dose target during treatment.
Patients with inoperable tumors mostly involving the neck and proximal body without direct abutment of the gastrointestinal tract were selected for MRgRT treatments. This patient cohort was chosen in order to investigate MRgRT ablative RT in the definitive setting. On T2w MRI, OARs are clearly visible. GTV and pancreas-duodenum interface is challenging to visualize but our physicians make use of near-by surrogates such as pancreatic duct or water in the duodenum. A median GTV V50Gy coverage of 78 (45.3–97.0)% was achieved, which compares favorably with patients treated with non-MRgRT ablative radiation using deep inspiratory breath hold and daily cone beam CT for image guidance [5], [19]. Prior analysis of the relationship between GTV coverage and treatment efficacy using a hypofractionated approach to deliver BED10 > 98 Gy for LAPC suggests that coverage as low as 70% does not result in a significant loss of efficacy [5]. Our median V45Gy and V40Gy GTV coverage was 90.5% (76.1–99.5) and 95% (84.3–100) respectively. Recent published study from Toesca et al has shown superior outcomes when patients received an SBRT dose of ≥40 Gy in 5 fractions [7] implying that maximizing the GTV coverage at 40 Gy is an important goal. Please note that GTV coverage did not change between pre-treatment and verification and post-treatment MRI because GTV contour had minimal motion and was not edited.
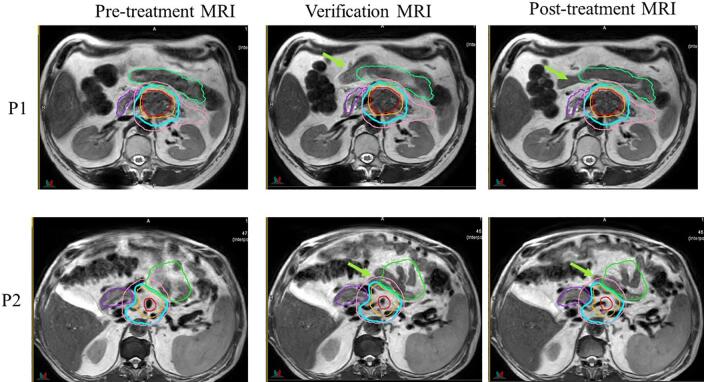
The OAR doses on verification and post-treatment changed because of intrafraction motion. The OAR motion was not consistent among all patients – some had large motion while others did not. Fig. 5 shows intrafraction motion between pretreatment, verification and post-treatment MRI for two example patients. For patient 1, small bowel movement was more consistent and lateral and stayed outside the 33 Gy isodose line whereas for patient 2, small bowel loop moved more posterior and into the 33 Gy isodose line. For some patients such as patient 1, the small bowel motion was also predictable from fraction to fraction whereas for patient 2 it was random every day. We are currently investigating patient related factors that can be correlated with intrafraction motion.
Fig. 5.
Intrafraction motion seen on verification and post-treatment MRI for two example patients. Contours for GTV (red), PTV50 (orange), PTV25 (pink) small bowel (green) and stomach_duodenum (purple) as well 33 Gy isodose line (cyan) color from pre-treatment plan is displayed on the verification and post-treatment MRI. Green arrows indicate the intrafraction motion of small bowel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To manage intrafraction motion in our current clinical workflow, with AC and no automatic beam gating, GI organ motion is assessed for first two fractions and conservative strategies are employed for later fractions, if found necessary. In this study, small bowel and stomach contours were adjusted and dose was recalculated on the verification and post-treatment MRI scans to assess dosimetric impact of intrafraction motion. This analysis was done before the subsequent fraction for every patient, giving the opportunity to discuss with the MDs before next fraction and modify the plan of action or strategy that we needed to employ if the dose has exceeded – i.e. making the PRV margin bigger to account for intrafraction motion or change the directive to keep stomach 0.035cm3 dose to <30 Gy. This is also evident from the OAR doses in later fractions in Fig. 4 where the OAR dose coverage on verification and post-treatment MRI was much closer for later fractions as compared to earlier fractions. Our group is very conservative to the D2cm3 dose to stomach_duodenum as stomach closer to high dose GTV will be less mobile compared to small bowel that can undergo larger variation in shape and size during treatment. In that respect, unintended over-irradiation maybe higher for stomach and duodenum due to limited range of motion in regions receiving the prescription dose. Although D2cm3 constraint is defined for stomach and small bowel PRV, physicians often prioritized the D2cm3 coverage on the structures itself as opposed to the PRV in order to determine safety while maintaining optimal GTV coverage.
Finally, we compared our GI OAR dose constraints with published constraints from MRgRT studies delivering ablative doses for LAPC patients. Applying GI constraints from Choung et al [20] showed that ~20% of our treatment fractions did not meet the maximum dose (V40Gy < 0.03 cc) constraints for stomach_duodenum and small bowel on verification and post-treatment MRI. Applying OAR constraints from Hassanzadeh et al [16] (V36Gy < 0.5 cc) and Bohoudi et al [11] (V33Gy < 1 cc) showed that ~10% of fractions did not meet the constraints on verification and post-treatment MRI scan. Supplementary Table 2 shows the detail analysis. Our department constraints for maximum dose of V33Gy < 0.035 cc for GI organs maybe more conservative than the published ones. We also believe our contouring is conservative due to volume averaging in the range of residual motion under compression. Motion due to respiration may spread the maximum dose point. Our followup is limited but so far we have not seen any grade II toxicity. None of the patients investigated in this study needed IV fluid or hospitalization. Intrafraction motion management is critical to our workflow and we are exploring options such as anti-peristalsis or medications that can slow down the bowel movements.
In conclusion, AC is a viable option for treating LAPC patients with ablative doses on MRgRT systems. However, intrafraction motion management is critical and can result GI OARs moving into high dose PTV area. Further work needs to be done in terms of intrafraction motion assessment and predictors, deformable image registration and dose accumulation to develop toxicity models and develop consensus on GI OAR dose volume constraints for ablative SBRT treatments of LAPC patient population.
5. Ethics approval and consent to participate
The study was conducted under MSKCC IRB approved retrospective protocol.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NT has received honorarium and travel support from Philips and Elekta healthcare. CHC and PBR have received travel support from Elekta healthcare.
Acknowledgement
This research was partially supported by the NIH/NCI Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2021.07.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Crane C.H. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res. 2016;57(Suppl 1):i53–i57. doi: 10.1093/jrr/rrw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammel P., Huguet F., van Laethem J.-L., Goldstein D., Glimelius B., Artru P. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 3.Loehrer P.J., Feng Y., Cardenes H., Wagner L., Brell J.M., Cella D. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyngold M., Parikh P., Crane C.H. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14:95. doi: 10.1186/s13014-019-1309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan S., Chadha A.S., Suh Y., Chen H.-C., Rao A., Das P. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyngold M., O’Reilly E.M., Varghese A.M., Fiasconaro M., Zinovoy M., Romesser P.B. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 2021;7:735–738. doi: 10.1001/jamaoncol.2021.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toesca D.A.S., Ahmed F., Kashyap M., Baclay J.R.M., von Eyben R., Pollom E.L. Intensified systemic therapy and stereotactic ablative radiotherapy dose for patients with unresectable pancreatic adenocarcinoma. Radiother Oncol. 2020;152:63–69. doi: 10.1016/j.radonc.2020.07.053. [DOI] [PubMed] [Google Scholar]
- 8.Boldrini L., Cusumano D., Cellini F., Azario L., Mattiucci G.C., Valentini V. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol. 2019;14:71. doi: 10.1186/s13014-019-1275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruynzeel A.M.E., Lagerwaard F.J. The role of biological dose-escalation for pancreatic cancer. Clin Transl Radiat Oncol. 2019;18:128–130. doi: 10.1016/j.ctro.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henke L., Kashani R., Robinson C., Curcuru A., DeWees T., Bradley J. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126:519–526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Bohoudi O., Bruynzeel A.M.E., Senan S., Cuijpers J.P., Slotman B.J., Lagerwaard F.J. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125:439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Placidi L., Romano A., Chiloiro G., Cusumano D., Boldrini L., Cellini F. On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: Clinical and dosimetric considerations. Tech Innov Patient Support Radiat Oncol. 2020;15:15–21. doi: 10.1016/j.tipsro.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohoudi O., Bruynzeel A.M.E., Meijerink M.R., Senan S., Slotman B.J., Palacios M.A. Identification of patients with locally advanced pancreatic cancer benefitting from plan adaptation in MR-guided radiation therapy. Radiother Oncol. 2019;132:16–22. doi: 10.1016/j.radonc.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Rudra S., Jiang N., Rosenberg S.A., Olsen J.R., Roach M.C., Wan L. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuong M.D., Bryant J., Mittauer K.E., Hall M., Kotecha R., Alvarez D. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2021;11:134–147. doi: 10.1016/j.prro.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Hassanzadeh C., Rudra S., Bommireddy A., Hawkins W.G., Wang-Gillam A., Fields R.C. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol. 2021;6:100506. doi: 10.1016/j.adro.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olausson K., Holst Hansson A., Zackrisson B., Edvardsson D., Östlund U., Nyholm T. Development and psychometric testing of an instrument to measure the patient's experience of external radiotherapy: The Radiotherapy Experience Questionnaire (RTEQ) Tech Innov Patient Support Radiat Oncol. 2017;3-4:7–12. doi: 10.1016/j.tipsro.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlander B.-M., Årestedt K., Engvall J., Maret E., Ericsson E. Development and validation of a questionnaire evaluating patient anxiety during Magnetic Resonance Imaging: the Magnetic Resonance Imaging-Anxiety Questionnaire (MRI-AQ) J Adv Nurs. 2016;72:1368–1380. doi: 10.1111/jan.12917. [DOI] [PubMed] [Google Scholar]
- 19.Reyngold M., O’Reilly E.M., Varghese A.M., Fiasconaro M., Zinovoy M., Romesser P.B. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 2021;7 doi: 10.1001/jamaoncol.2021.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuong M.D., Bryant J., Mittauer K.E., Hall M., Kotecha R., Alvarez D. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy (MRgRT) with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2020 doi: 10.1016/j.prro.2020.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.