Key Points
Question
Has the population-attributable risk fraction for cannabis use disorder in schizophrenia increased over time, as would be expected with increasing use and potency of cannabis?
Findings
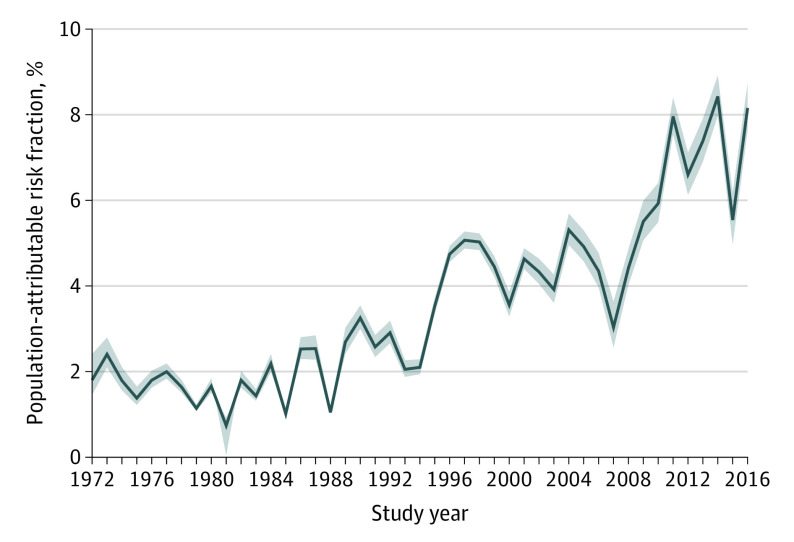
In this Danish nationwide, register-based cohort study, the population-attributable risk fraction for cannabis use disorder in schizophrenia increased from approximately 2% in the period to 1995 to approximately 6% to 8% since 2010.
Meaning
These findings may indicate that cannabis use disorders are associated with an increase in the proportion of cases of schizophrenia.
Abstract
Importance
Cannabis use and potency of cannabis have increased during the past 2 decades. If the association between cannabis use and schizophrenia is causal, this should be reflected in an increase in the proportion of cases of schizophrenia being attributable to cannabis, the population-attributable risk fraction (PARF).
Objective
To determine whether the PARF for cannabis use disorder in schizophrenia has increased over time.
Design, Setting, and Participants
This nationwide, register-based historical prospective cohort study included all people in Denmark born before December 31, 2000, who were alive and 16 years or older at some point from January 1, 1972, to December 31, 2016. Data analysis was performed from August 2020 to April 2021.
Exposure
Diagnosis of cannabis use disorder.
Main Outcomes and Measures
Diagnosis of schizophrenia, with estimated PARF of cannabis use disorder in schizophrenia from 1972 to 2016.
Results
A total of 7 186 834 individuals were included in the analysis, including 3 595 910 women (50.0%) and 3 590 924 men (50.0%). The adjusted hazard ratio for schizophrenia fluctuated at approximately 4 (with 95% CIs ranging from approximately 3 to 6) throughout most of the study period when people diagnosed with cannabis use disorder were compared with those without cannabis use disorder. The PARF of cannabis use disorder in schizophrenia also fluctuated, but with clear evidence of an increase from 1995 (when the PARF was relatively stable around 2.0%, with a 95% CI of approximately 0.3% to either side) until reaching some stability around 6.0% to 8.0% (with a 95% CI of approximately 0.5% to either side) since 2010.
Conclusions and Relevance
The results from these longitudinal analyses show the proportion of cases of schizophrenia associated with cannabis use disorder has increased 3- to 4-fold during the past 2 decades, which is expected given previously described increases in the use and potency of cannabis. This finding has important ramifications regarding legalization and control of use of cannabis.
This nationwide, register-based historical cohort study assesses whether the population-attributable risk fraction (PARF) for cannabis use disorder in schizophrenia has increased over time in Denmark.
Introduction
Several studies and meta-analyses1,2 have indicated that the risk of schizophrenia and related psychoses is increased for people who use cannabis. The association appears to be particularly driven by heavy and frequent cannabis use.2,3 This has led to a persisting hypothesis that cannabis may be a component cause of schizophrenia, at least in some people. This hypothesis is strengthened by several factors, such as strong attempts to control for reverse causation (eg, people self-medicating prodromal symptoms of schizophrenia). Similarly, cannabis-induced psychosis has been found to be associated with later onset of schizophrenia.4,5,6 Furthermore, experimental evidence strongly suggests that cannabis, even in relatively low doses, causes psychoticlike symptoms in healthy individuals.7,8,9
Despite this, some debate persists regarding whether the association between cannabis use and schizophrenia is truly causal. Epidemiological studies, although able to provide strong evidence, can never completely exclude the possibility of residual confounding. One argument against the causal hypothesis has been that, with increasing use and potency of cannabis in the population, one should have observed an increase in the incidence of schizophrenia, which has been postulated to be absent.10,11,12 A long-standing dogma states that the incidence of schizophrenia has remained stable over time, but Kühl et al13 recently showed that the incidence of schizophrenia has steadily increased in Denmark from 2000 to 2012, especially in younger groups. This study did not, however, make any claims that this should have been due to increasing use or potency of cannabis.13
Denmark has the benefit of having unselected nationwide Danish registers that allow for large-scale studies using the entire population. Using these registers, Nielson et al14 showed that cannabis use disorder, an indicator of regular use of cannabis, is associated with schizophrenia. Both cannabis use and cannabis use disorder have indeed been increasing over time in many countries, such as Denmark.12 The same is true for the potency of cannabis products available on the market.10,11 If all other risk factors for schizophrenia remained stable in the same period, this should then lead to an increase in the population-attributable risk fraction (PARF) of cannabis use disorder in schizophrenia. The PARF estimates the proportion of cases with schizophrenia that would have been prevented if nobody had cannabis use disorder. We thus aimed to investigate whether the PARF for cannabis use disorder in schizophrenia has increased over time in the Danish population.
Methods
We used the nationwide Danish registers, full linkage of which is made possible through the personal identification number issued to all people born in Denmark or obtaining legal residence in Denmark since 1968.15 We included all people born before December 31, 2000, who were alive and 16 years or older at some point from January 1, 1972, to December 31, 2016, ensuring that all people would be able to turn at least 16 years of age during follow-up. Purely register-based studies do not require ethics approval under Danish law. This study was approved by the Danish Data Protection Agency, and analyses were conducted on servers at Statistics Denmark using encrypted personal identification numbers, making identification of single individuals impossible. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Cannabis Use Disorder and Schizophrenia
Information on both cannabis use disorder and schizophrenia was obtained from the Psychiatric Central Research Register, in which all inpatient treatment at psychiatric departments has been registered since 1969, as well as outpatient treatment at psychiatric departments since 1995.16 In the case of cannabis use disorder, this was supplemented with data from the National Patient Register, which contains data on inpatient treatment at general (nonpsychiatric) departments since 1977 and outpatient treatment since 1995.17 Cannabis use disorder was defined as International Classification of Diseases, Eighth Revision (ICD-8) diagnostic code 304.5 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnostic code F12.X. Schizophrenia was defined as ICD-8 diagnostic code 295.X (except 295.7) and ICD-10 diagnostic code F20.X.
Potential Confounders
The following potential confounders were included in the analyses: alcohol use disorder (ICD-8 codes 291, 303, and 571.0; ICD-10 codes F10.x, E24.4, E52, G31.2, G62.1, G72.1, K29.2, K70, K86.0, O35.4, Y57.3, Z50.2, Z71.4, and Z72.1) and other substance use disorder (ICD-8 codes 304.x except 304.5; ICD-10 codes F1X.X except F10.X, F12.X, and F17.X), both as time-varying covariates identified in the Psychiatric Central Research Register and the National Patient Register; sex; and other psychiatric diagnoses except those pertaining to substance use disorder or schizophrenia (ICD-8 codes 290-315; ICD-10 codes FX, except those previously used) as time-varying covariates identified in the Psychiatric Central Research Register. We also included parental psychiatric history and parental history of alcohol, cannabis, and substance use disorder, using the same diagnostic codes as described previously. Analyses were further adjusted for sex, age at the beginning of each year, whether a person was born outside of Denmark, and the parents’ highest level of education. In cases of missing data, we included a unique value in the analyses indicating missingness.
Statistical Analysis
The data analysis was performed from August 2020 to April 2021. The PARF is calculated by the following formula: PARF = pd([RR − 1]/RR), where pd refers to the proportion of exposed participants among cases (ie, the proportion of those who develop schizophrenia who were diagnosed with cannabis use disorder), and RR is the rate ratio, which in this study was estimated using the hazard ratio following a Cox proportional hazards regression model with time-varying covariates. We applied this formula for the PARF because it is internally valid when confounding exists and adjusted rate ratios must be used.18 We used the Greenland method for estimating 95% CIs of the PARF.19
We estimated the PARF for each year from 1972 to 2016 by using delayed entry into the Cox proportional hazards regression model on January 1 of a given year, and censoring individuals on December 31 of the same year if they had not either developed incident schizophrenia or been censored owing to death or emigration. People were entered into the analyses no earlier than their 16th birthday. For each year under investigation, we considered a person positive for cannabis use disorder only if at least 1 diagnosis thereof had been given no more than 3 years before the start of that year. The proportion of individuals with diagnosed cannabis use disorder in a given year was evaluated using this time-varying covariate. Age of the individual was used as the underlying timescale, ensuring that analyses were adjusted for age.
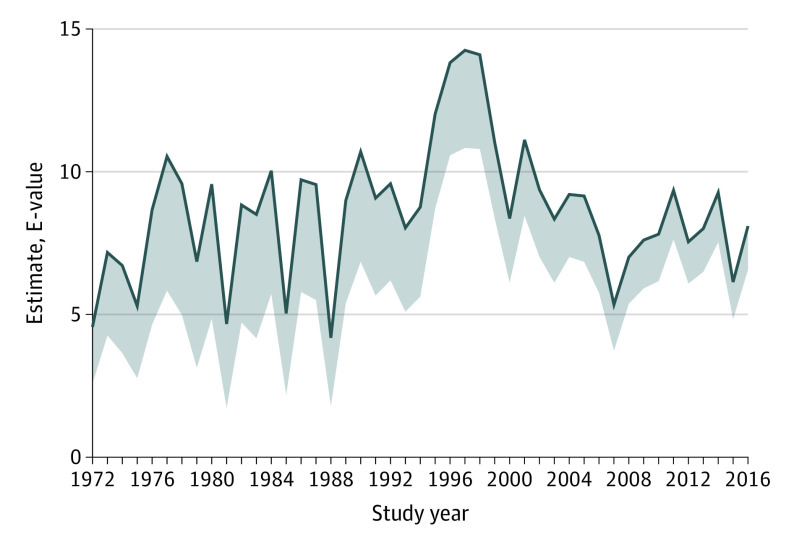
We also calculated the E-value for the estimate and the lower limit of the 95% CI each year in the same period. The E-value is a measure of the strength of unmeasured confounders on both cannabis use disorder and schizophrenia that would need to exist to completely explain the observed association.20
We conducted sensitivity analyses in which we censored individuals as they turned 50 years of age, to better reflect the population most at risk of both cannabis use disorder and incident schizophrenia. We conducted further sensitivity analyses, expanding the time frame for positive cannabis use disorder to 7 years and 1 sensitivity analysis without a time limit of exposure. In a final set of sensitivity analyses, we did not adjust for other psychiatric disorders, which may be a case of overadjustment, for instance because other psychiatric disorders might rather be considered mediators. All analyses were conducted in Stata/MP, version 16.1 (StataCorp LLC).
Results
We included 7 186 834 individuals in at least 1 of the yearly PARF analyses (3 595 910 women [50.0%] and 3 590 924 men [50.0%]). Additional characteristics of the study population are provided in the Table.
Table. Characteristics of Individuals Included in at Least 1 Analysis of Yearly PARF.
| Characteristic | No. (%) of participants (N = 7 186 834)a |
|---|---|
| Sex | |
| Male | 3 590 924 (50.0) |
| Female | 3 595 910 (50.0) |
| Decade of birth | |
| 1939 or earlier | 2 195 395 (30.5) |
| 1940s | 828 617 (11.5) |
| 1950s | 837 048 (11.6) |
| 1960s | 945 783 (13.2) |
| 1970s | 860 459 (12.0) |
| 1980s | 757 927 (10.5) |
| 1990s or 2000 | 761 605 (10.6) |
| Not born in Denmark | 656 739 (9.1) |
| Developed schizophrenia during follow-up | 42 611 (0.6) |
| Positive for cannabis use disorder in ≥1 analysis | 23 809 (0.3) |
| Positive for alcohol use disorder in ≥1 analysis | 169 571 (2.4) |
| Positive for other substance use disorder in ≥1 analysis | 135 066 (1.9) |
| Positive for other psychiatric disorders in ≥1 analysis | 726 991 (10.1) |
| Died during follow-upb | 2 075 920 (28.9) |
| Parental history | |
| Schizophrenia | 23 889 (0.3) |
| Alcohol or substance use disorder | 340 092 (4.7) |
| Other psychiatric disorder | 643 562 (9.0) |
| Highest level of parental education attainedc | |
| Primary/lower secondary | 762 319 (10.6) |
| Upper secondary (high school equivalent) | 1 357 350 (18.9) |
| Short-cycle tertiary | 147 817 (2.1) |
| Bachelor’s degree or equivalent | 590 162 (8.2) |
| Master’s degree or equivalent or higher | 257 325 (3.6) |
| Not available in registers | 4 071 861 (56.7) |
Percentages have been rounded and may not total 100.
Includes deaths occurring while at risk of schizophrenia. Consequently, deaths occurring after incident schizophrenia or after emigration are not counted here.
Uses International Standard Classification of Education definitions.
The association between cannabis use disorder and schizophrenia over time is depicted in Figure 1. Figure 1A and B show the hazard ratios, which, unadjusted, decreased from more than 100 to reaching some stability at approximately 50 or even 30. The adjusted hazard ratio fluctuated over time, but with no clear trend, and generally centered around an adjusted hazard ratio of approximately 4 (with 95% CIs ranging from approximately 3 to 6). The association remained statistically significant throughout the period, as indicated by the 95% CIs. The 95% CIs tended to narrow after 1995. This substantial decrease in the hazard ratios is likely a case of overadjustment, because most of the reduction occurred when adjusting for other psychiatric disorders. This would indicate that the fully adjusted hazard ratios may actually be artificially low. However, we opted to use these because conservative results, biased toward the null hypothesis, are preferable owing to our desire to avoid false-positive findings. Figure 1C and D show the development over the period of incident schizophrenia and of recently diagnosed (within 3 years) cannabis use disorder. The latter increased almost linearly throughout the period, whereas the incidence of schizophrenia showed a generally increasing tendency, albeit with some fluctuations. Parameter estimates for all variables are shown in the eTable in the Supplement for the years 1972, 2000, and 2016.
Figure 1. Association Between Cannabis Use Disorder and Schizophrenia Over Time.
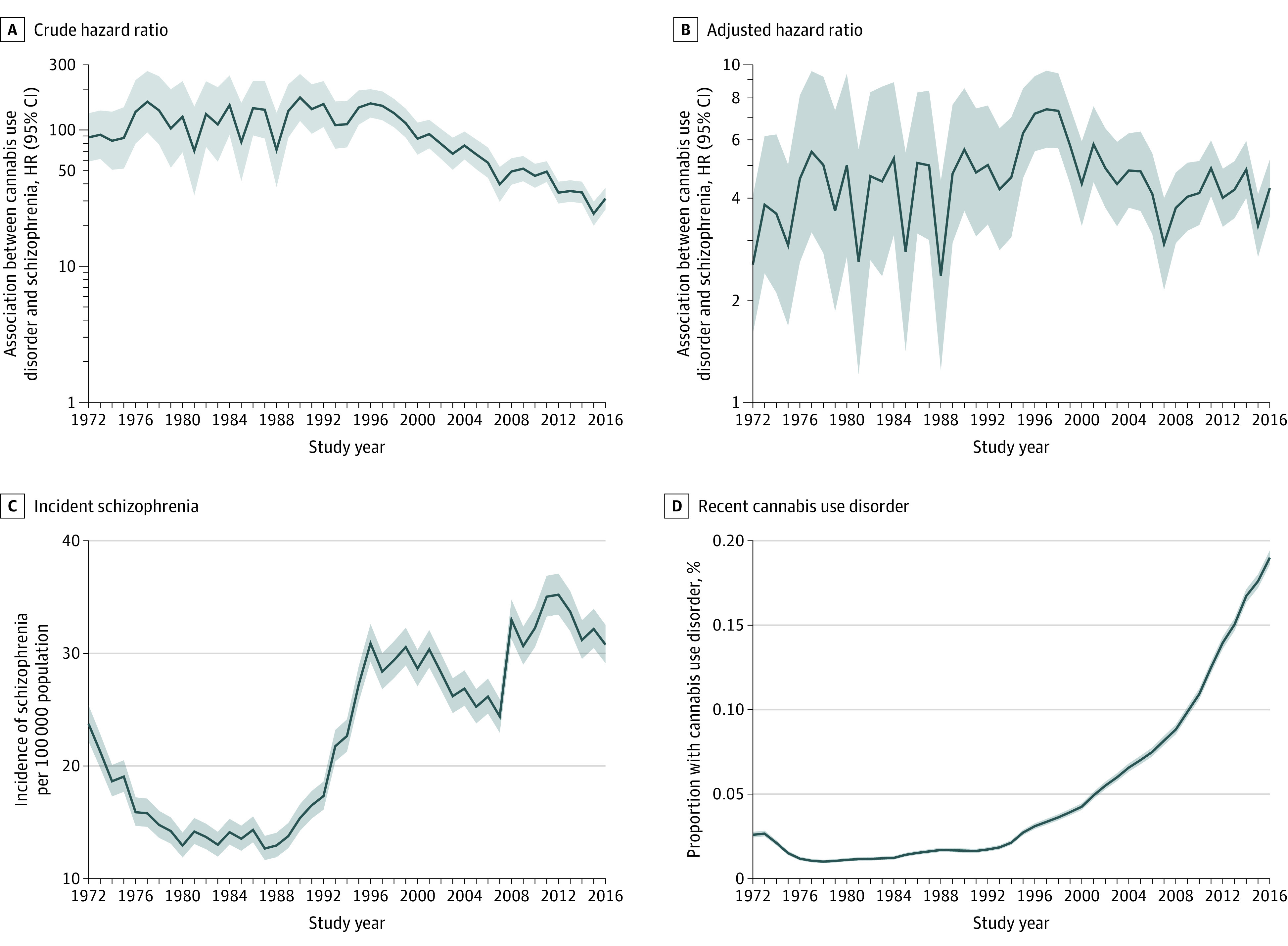
Development of the crude (A) (adjusted for age) and adjusted (B) (adjusted for alcohol use disorder, other psychiatric disorders, parental schizophrenia, parental other psychiatric disorder, parental alcohol or substance use disorder, parental educational attainment, sex, and age) hazard ratios (HRs) between cannabis use disorder and schizophrenia in Denmark and of the incidence of schizophrenia (C) and cannabis use disorder (D) in the same period. Shaded areas indicate 95% CIs.
The development of the PARF over time is depicted in Figure 2. Despite some fluctuation, a general increase over time is evident, starting first around 1995 (when the PARF was relatively stable at approximately 2.0% with a 95% CI of approximately 0.3% to either side) until reaching some stability around 6.0% to 8.0% (with a 95% CI of approximately 0.5% to either side) since 2010. Figure 3 shows the E-value over time, indicating that an unmeasured confounder would need to be associated with both cannabis use disorder and schizophrenia at a relative risk of well above 5, and sometimes even above 10, beyond the other variables already included in the adjusted analyses.
Figure 2. Development of the Population-Attributable Risk Fraction (PARF) of Cannabis Use Disorder in Schizophrenia in Denmark.
Shaded areas indicate 95% CIs.
Figure 3. Development of the E-Value for the Estimate Between Cannabis Use Disorder and Schizophrenia in Denmark.
The lower bound of the shaded area is the E-value for the lower bound of the 95% CI for the hazard ratio used to estimate the E-value.
Figure 4 shows results from the 4 sensitivity analyses. The results were largely the same, with the same apparent increase being evident, albeit with slightly higher PARFs.
Figure 4. Results From 4 Sensitivity Analyses of the Development of the Population-Attributable Risk Fraction of Cannabis Use Disorder in Schizophrenia in Denmark.
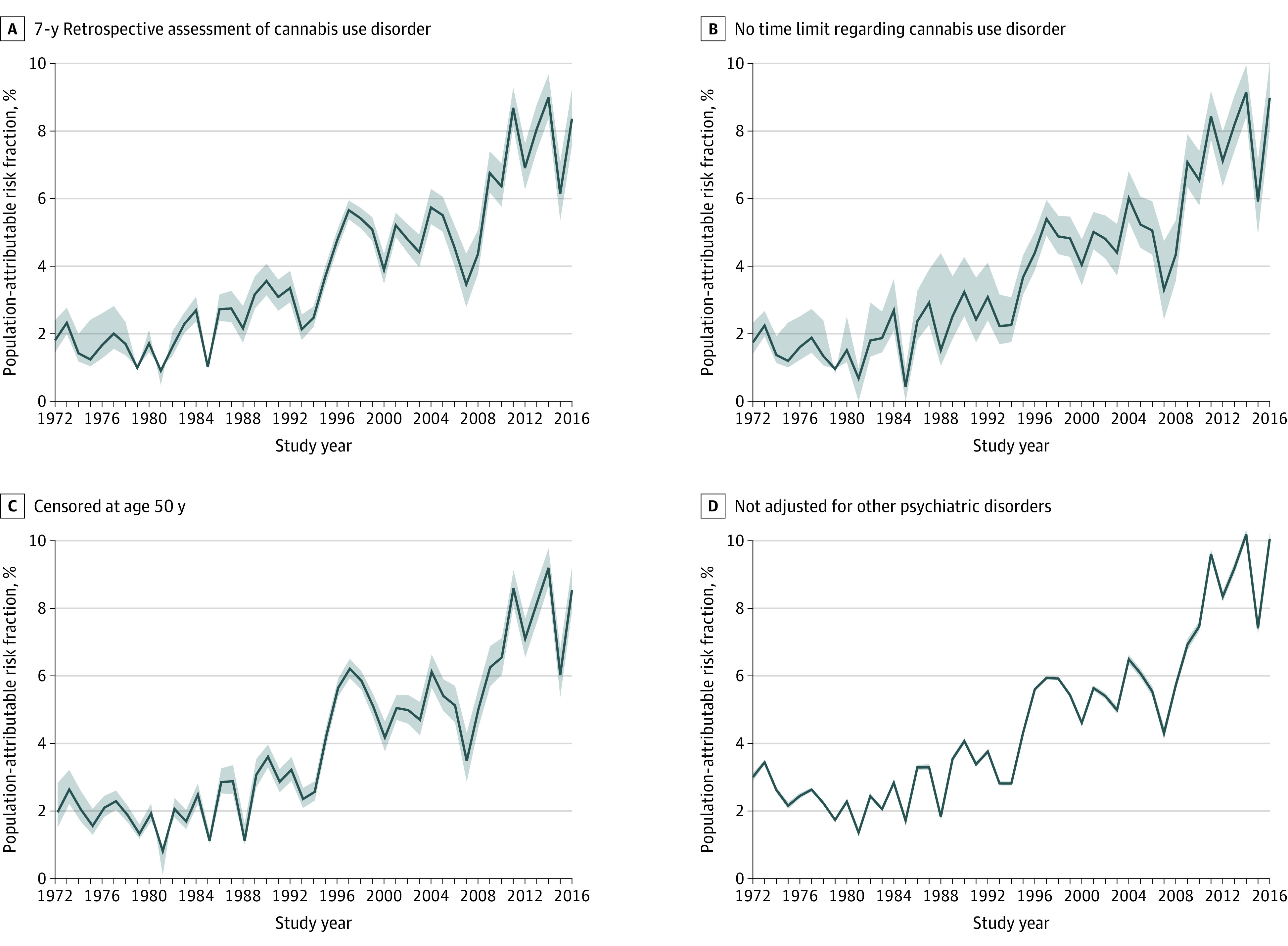
Shaded areas indicate 95% CIs.
Discussion
An increase in the PARF over time for cannabis use disorder in schizophrenia was clearly observed. The PARF increased from approximately 4.0% around the turn of the millennium to 8.0% since 2010, albeit with some fluctuations, especially in 2006, which appears to be an unexplained anomaly, because ignoring 2006 would yield a regular linear trend. A further increase was observed beginning already in the mid-1990s, until which point it had been relatively stable at around 2.0%. This was consistent with our hypothesis. Because the PARF was estimated under the assumption of potential causality, maintaining this assumption and increasing potency and frequency of use of cannabis use in recent years should yield a corresponding increase in PARF.10,11,12 Ours is, to our knowledge, the first study to demonstrate such an increase. Although not actual proof of a causal link, our results from longitudinal analyses of the associations over time between cannabis use disorder and schizophrenia provide evidence that some of this association may be causal.21 This evidence might also help explain the general increase in the incidence of schizophrenia that has been observed in recent years.13 In total, our study thus provides some support for a potential causal inference that the long-observed association between cannabis and schizophrenia is likely partially causal in nature.1,2
The PARF is an estimate of the proportion of cases of schizophrenia that would have been prevented if no individuals had been exposed (in this case to cannabis use disorder), under the assumption that the association between cannabis and schizophrenia may be causal. In light of this assumption, the PARF cannot confirm whether an association is truly causal. However, an increase in the PARF co-occurring alongside an increase in either the proportion using cannabis or in the potency of cannabis would be expected if the association was indeed causal. Previous data suggest an increase in the use and problematic use of cannabis in Denmark.12 Moreover, cannabis on the Danish market is noted to be among the most potent variants in Europe and has increased from approximately 13% Δ9-tetrahydrocannabinol in 2006 to nearly 30% in 2016.10 The fact that the hazard ratio did not increase over time was unexpected, because this would have been expected if the association between cannabis use disorder and schizophrenia was primarily driven by high-potency cannabis, as has previously been suggested.2 This could either indicate that potency of cannabis in Denmark has long been above the threshold for psychotogenic effects, or that cannabis use disorder is itself an indicator of severe exposure. Furthermore, we estimated the E-value over time, which is a measure of how strong an unmeasured confounding effect would be required to conclude that cannabis use disorder was not causally associated with schizophrenia. With some fluctuations, a requirement for such an unmeasured confounder would be association with both cannabis use disorder at a relative risk of above 5, often approximately 10, and with schizophrenia at the same magnitude of relative risk, after adjustment for all the covariates included in the present study (ie, alcohol or other substance use disorders, other psychiatric disorders, parental schizophrenia, parental other psychiatric disorders, parental alcohol or substance use disorder, parental educational attainment, sex, and age). Although some unmeasured confounding may exist, it is unlikely to have such strong associations as required by the E-value to explain the associations we observed in the present study. Use of tobacco has been suggested as such a potential risk factor for schizophrenia22,23,24; first, some evidence actually suggests that cannabis use is the confounder of this association and not the other way around23,24,25; and second, tobacco use is unlikely to yield relative risks from 5 to 10 with schizophrenia, although it may do so for cannabis use disorder.22 Another often-mentioned risk factor for schizophrenia is urbanicity24; we did not include urbanicity in our study because (1) urbanicity may be considered a mediator, in which people at risk of using cannabis and other substances gravitate toward urban centers, and (2) the variable was not available for the full study period. We did not have information on childhood trauma, but the associations between childhood trauma and either cannabis or schizophrenia are generally lower than our estimated E-value, and previous studies have indicated at least partial independence between the 2 risk factors.26,27,28 Other potential risk factors for schizophrenia, such as season of birth, vitamin D, and left-handedness, are likely not strongly associated with cannabis use disorder.24,29,30,31 Genetic liability has been suggested as a partial confounder (ie, that the association between cannabis use and schizophrenia is partly causal and partly confounded by genes; eg, by risk genes for schizophrenia increasing the risk of cannabis use).32,33
To our knowledge, ours is the first study to investigate the contribution of time to the PARF of cannabis in schizophrenia. However, the multisite EU-GEI (European Network of National Schizophrenia Networks Studying Gene-Environment Interactions) study recently found that sites with high rates of use of high-potency cannabis or high rates of daily use of cannabis were associated with the incidence of schizophrenia.34 In that study, researchers found that the theoretical removal of high-potency cannabis would prevent 12% of cases of schizophrenia, and with PARFs as high as 30% in London and 50% in Amsterdam.
Implications
Although not in itself proof of causality, our study provides evidence of the theory of cannabis being a component cause of schizophrenia. Furthermore, our study challenges the often-cited argument against causality that an expected increase in cases of schizophrenia attributable to cannabis use has not been observed. Our study is particularly important with the increasing legalization of cannabis for both medicinal and recreational uses seeming to lead to an increase in the perception of cannabis as relatively harmless and possibly in the uptake of cannabis use, especially among youth.35,36 Although psychosis is not the only outcome of interest in terms of cannabis use, our study clearly indicates that cannabis should not be considered harmless. Cannabis on the Danish market is among the strongest in the European market, which may compound the problem, because previous studies2,10,11 have shown that the cannabis-psychosis link may be most strongly relevant for high-potency cannabis. An important implication of our study may thus be that both use of cannabis and the potency of cannabis available should be reduced to achieve primary prevention of schizophrenia.
Strengths and Limitations
Our study holds both important strengths and limitations. Regarding strengths, we were able to use the unselected nationwide Danish registers to estimate annual associations between cannabis use disorder and schizophrenia, without the risk of selection biases related to, for instance, nonparticipation or dropouts. Furthermore, we were able to adjust for a range of potential confounders that may have changed their effect with the association between cannabis use disorder and schizophrenia over time. It is a further strength that we quantified the risk of unmeasured confounding through the use of the E-value. Finally, we conducted sensitivity analyses, revealing that our results were robust to choices regarding the analytical procedure.
A few limitations also warrant mention, however. Use of register-based information allowed us to assess only cannabis use disorder and not cannabis use per se. However, it has previously been suggested that the association between cannabis and schizophrenia is driven by high-potency cannabis and problematic use of cannabis.2 Consequently, this may not have a dramatic effect on our results. If anything, it would mean that some cases of schizophrenia were erroneously classified as unexposed to cannabis, making our results slightly conservative. If the probability of being erroneously classified as not having a cannabis use disorder has decreased over time, this might explain a small part of the increase in the PARF. A similar limitation exists in that we were only able to assess cannabis use disorder in patients who had contact with the treatment system. Again, this is likely to have yielded slightly conservative results, and without a clear effect on the development of the PARF. A third and related limitation is that we do not have information on the types or potency of cannabis use by the study population on an individual level.
Conclusions
In this Danish, register-based cohort study, an increase in the proportion of cases of schizophrenia that may be attributable to cannabis use disorder to a relatively stable level of 8.0% since 2010 was observed. This increase is in concordance with what would be expected, given observed increases in use and potency of cannabis. These results from longitudinal analyses of the associations over time between cannabis use disorder and schizophrenia lend further support to the hypothesis that cannabis may be involved in the etiology of schizophrenia.
eTable. All Parameter Estimates From the Regression Models for 1972, 2000, and 2016
References
- 1.Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328. doi: 10.1016/S0140-6736(07)61162-3 [DOI] [PubMed] [Google Scholar]
- 2.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull. 2016;42(5):1262-1269. doi: 10.1093/schbul/sbw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Forti M, Marconi A, Carra E, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2(3):233-238. doi: 10.1016/S2215-0366(14)00117-5 [DOI] [PubMed] [Google Scholar]
- 4.Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi: 10.1176/appi.ajp.2017.17020223 [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Prediction of onset of substance-induced psychotic disorder and its progression to schizophrenia in a Swedish national sample. Am J Psychiatry. 2019;176(9):711-719. doi: 10.1176/appi.ajp.2019.18101217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrie B, Lappin J, Large M, Sara G. Transition of substance-induced, brief, and atypical psychoses to schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2020;46(3):505-516. doi: 10.1093/schbul/sbz102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindley G, Beck K, Borgan F, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7(4):344-353. doi: 10.1016/S2215-0366(20)30074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjorthøj C, Posselt CM. Δ9-tetrahydrocannabinol: harmful even in low doses? Lancet Psychiatry. 2020;7(4):296-297. doi: 10.1016/S2215-0366(20)30093-6 [DOI] [PubMed] [Google Scholar]
- 9.Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39(10):1607-1616. doi: 10.1017/S0033291709005522 [DOI] [PubMed] [Google Scholar]
- 10.Freeman TP, Groshkova T, Cunningham A, Sedefov R, Griffiths P, Lynskey MT. Increasing potency and price of cannabis in Europe, 2006-16. Addiction. 2019;114(6):1015-1023. doi: 10.1111/add.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rømer Thomsen K, Lindholst C, Thylstrup B, et al. Changes in the composition of cannabis from 2000-2017 in Denmark: analysis of confiscated samples of cannabis resin. Exp Clin Psychopharmacol. 2019;27(4):402-411. doi: 10.1037/pha0000303 [DOI] [PubMed] [Google Scholar]
- 12.Sundhedsstyrelsen. The situation of narcotics in Denmark 2017 [in Danish]. November 2017. Accessed September 6, 2020. https://www.sst.dk/da/udgivelser/2017/narkotikasituationen-i-danmark-2017
- 13.Kühl JOG, Laursen TM, Thorup A, Nordentoft M. The incidence of schizophrenia and schizophrenia spectrum disorders in Denmark in the period 2000-2012: a register-based study. Schizophr Res. 2016;176(2-3):533-539. doi: 10.1016/j.schres.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen SM, Toftdahl NG, Nordentoft M, Hjorthøj C. Association between alcohol, cannabis, and other illicit substance abuse and risk of developing schizophrenia: a nationwide population based register study. Psychol Med. 2017;47(9):1668-1677. doi: 10.1017/S0033291717000162 [DOI] [PubMed] [Google Scholar]
- 15.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7)(suppl):22-25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 16.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7)(suppl):54-57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 17.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7)(suppl):30-33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 18.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15-19. doi: 10.2105/AJPH.88.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenland S. Re: “Confidence limits made easy: interval estimation using a substitution method”. Am J Epidemiol. 1999;149(9):884-884. doi: 10.1093/oxfordjournals.aje.a009905 [DOI] [PubMed] [Google Scholar]
- 20.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ. Can sophisticated study designs with regression analyses of observational data provide causal inferences? JAMA Psychiatry. 2021;78(3):244-246. doi: 10.1001/jamapsychiatry.2020.2588 [DOI] [PubMed] [Google Scholar]
- 22.Hunter A, Murray R, Asher L, Leonardi-Bee J. The effects of tobacco smoking, and prenatal tobacco smoke exposure, on risk of schizophrenia: a systematic review and meta-analysis. Nicotine Tob Res. 2020;22(1):3-10. doi: 10.1093/ntr/nty160 [DOI] [PubMed] [Google Scholar]
- 23.Scott JG, Matuschka L, Niemelä S, Miettunen J, Emmerson B, Mustonen A. Evidence of a causal relationship between smoking tobacco and schizophrenia spectrum disorders. Front Psychiatry. 2018;9:607. doi: 10.3389/fpsyt.2018.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belbasis L, Köhler CA, Stefanis N, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137(2):88-97. doi: 10.1111/acps.12847 [DOI] [PubMed] [Google Scholar]
- 25.Menezes P, Busatto G, McGuire P, Murray R, Scazufca M. Tobacco smoking and risk of first episode psychosis: a case-control study in Sao Paulo, Brazil. Schizophrenia. 2016;(suppl):T126. Published March 2016. Accessed June 14, 2021. https://www.nature.com/documents/npjschz20167.pdf [Google Scholar]
- 26.Popovic D, Schmitt A, Kaurani L, et al. Childhood trauma in schizophrenia: current findings and research perspectives. Front Neurosci. 2019;13:274. doi: 10.3389/fnins.2019.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheidell JD, Quinn K, McGorray SP, et al. Childhood traumatic experiences and the association with marijuana and cocaine use in adolescence through adulthood. Addiction. 2018;113(1):44-56. doi: 10.1111/add.13921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sideli L, Fisher HL, Murray RM, et al. Interaction between cannabis consumption and childhood abuse in psychotic disorders: preliminary findings on the role of different patterns of cannabis use. Early Interv Psychiatry. 2018;12(2):135-142. doi: 10.1111/eip.12285 [DOI] [PubMed] [Google Scholar]
- 29.Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29(3):587-593. doi: 10.1093/oxfordjournals.schbul.a007030 [DOI] [PubMed] [Google Scholar]
- 30.Hirnstein M, Hugdahl K. Excess of non–right-handedness in schizophrenia: meta-analysis of gender effects and potential biases in handedness assessment. Br J Psychiatry. 2014;205(4):260-267. doi: 10.1192/bjp.bp.113.137349 [DOI] [PubMed] [Google Scholar]
- 31.Ripke S, Neale BM, Corvin A, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillespie NA, Kendler KS. Use of genetically informed methods to clarify the nature of the association between cannabis use and risk for schizophrenia. JAMA Psychiatry. 2021;78(5):467-468. doi: 10.1001/jamapsychiatry.2020.3564 [DOI] [PubMed] [Google Scholar]
- 33.Pasman JA, Verweij KJH, Gerring Z, et al. ; 23andMe Research Team; Substance Use Disorders Working Group of the Psychiatric Genomics Consortium; International Cannabis Consortium . GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. 2018;21(9):1161-1170. doi: 10.1038/s41593-018-0206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Forti M, Quattrone D, Freeman TP, et al. ; EU-GEI WP2 Group . The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427-436. doi: 10.1016/S2215-0366(19)30048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung J, Chiu CYV, Stjepanović D, Hall W. Has the legalisation of medical and recreational cannabis use in the USA affected the prevalence of cannabis use and cannabis use disorders? Curr Addict Reports. 2018;5(4):403-417. doi: 10.1007/s40429-018-0224-9 [DOI] [Google Scholar]
- 36.Hall W, Stjepanović D, Caulkins J, et al. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet. 2019;394(10208):1580-1590. doi: 10.1016/S0140-6736(19)31789-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. All Parameter Estimates From the Regression Models for 1972, 2000, and 2016