Abstract
Background
Pediatric pulmonary hypertension is a severe disease defined by sustained elevation of pulmonary artery pressures and pulmonary vascular resistance (PVR). Noninvasive diagnostic and prognostic markers that are more pulmonary vascular specific have been elusive because of disease heterogeneity and patient growth.
Research Question
Is soluble suppressor of tumorigenicity (ST2) associated with pulmonary hemodynamic and functional changes in pediatric pulmonary hypertension? Does ST2 improve mortality risk models in pediatric pulmonary hypertension?
Study Design and Methods
Two pediatric cohorts (age < 21 years) were assayed for ST2 and N-terminal prohormone B-natriuretic peptide: a cross-sectional cohort from the National Heart Lung and Blood Institute-funded National Biological Sample and Data Repository for PAH (PAHB) (N = 182), and a second longitudinal cohort from Children’s Hospital of Colorado (N = 61). Adjusted linear regression was used for association with clinical variables. Clinical mortality models (the Registry to Evaluate Early and Long-Term PAH Disease Management [REVEAL] score) with and without ST2 were used to predict worsening outcomes and compared. Pulmonary artery endothelial and smooth muscle cell ST2 expression and secretion were assayed in vitro.
Results
In an adjusted (age and sex) analysis in the PAHB, ST2 was significantly associated with shorter 6-min walk distance (P = .03) and increased PVR index (P = .02). In adjusted longitudinal regression in the Children’s Hospital of Colorado cohort, ST2 was significantly associated with higher PVR index (P < .001), shorter 6-min walk distance (P = .01), and higher mean pulmonary artery pressure (P < .001). Although the REVEAL Risk Score Calculator 2.0 was predictive of clinical worsening in the PAHB (hazard ratio, 1.88), addition of ST2 significantly improved the model (hazard ratio, 2.05). In cell culture, ST2 was produced and secreted predominately by endothelial cells as opposed to smooth muscle cells (P < .0001).
Interpretation
In two pediatric PAH cohorts, elevated ST2 was associated with unfavorable pulmonary hemodynamics and functional measures, clinical worsening, and significantly improved prediction of clinical worsening. Pulmonary artery endothelial cellular expression of ST2 suggests that ST2 is a more pulmonary vascular-specific marker for pulmonary hypertension.
Key Words: biomarkers, pediatric cardiology, pediatric pulmonary hypertension, pulmonary hypertension, ST2
Abbreviations: 6MWD, 6-min walk distance; APAH, associated pulmonary arterial hypertension; CHC, Children’s Hospital Colorado; IPAH, idiopathic pulmonary arterial hypertension; IQR, interquartile range; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal prohormone B-natriuretic peptide; NYHA FC, New York Heart Association functional class; PAH, pulmonary arterial hypertension; PAHB, National Biological Sample and Data Repository for PAH; PCWP, pulmonary capillary wedge pressure; PHBI, Pulmonary Hypertension Breakout Initiative; PVR, pulmonary vascular resistance; PVRi, pulmonary vascular resistance index; RAP, right atrial pressure; REVEAL, Registry to Evaluate Early and Long-Term PAH Disease Management; RHC, right heart catheterization; ST2, soluble suppressor of tumorigenicity; ST2-L, transmembrane ST2 receptor; WU, Woods unit
Pulmonary arterial hypertension (PAH) is a severe, progressive disease characterized by elevation of pulmonary artery pressures, with resulting right ventricular failure and death. Definitive diagnosis and accurate monitoring, via cardiac catheterization, are difficult in children because the procedure is invasive and requires sedation, increasing risk in infants and children. N-terminal prohormone B-natriuretic peptide (NT-proBNP) is difficult to interpret in pediatrics due to effects from age, sex, congenital heart disease, interindividual variability, dynamic day-to-day changes, and without age-specific normal values.1,2 Importantly, although PAH therapies are directed at the pulmonary vasculature, targeting vasodilation, NT-proBNP is exclusively expressed by cardiomyocytes in the setting of cardiac stretch.3 NT-proBNP may reflect cardiac stress due to PAH, a later finding, rather than being a vascular-specific marker of disease. In addition, NT-proBNP levels plateau, with further increases no longer indicating disease progression. A vascular-specific marker may add information about the pulmonary vasculature, independent from cardiac function, providing earlier and more specific information about PAH.
Clinical models for deleterious outcome are limited in pediatrics; the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) score is a multicomponent, weighted score, including NT-proBNP, that is well validated in adults and promising in pediatrics.4,5 The variables included are difficult to measure in pediatrics, including invasive hemodynamics, 6-min walk distance (6MWD), and New York Heart Association functional class (NYHA FC), or are not pediatric relevant (eg, age > 60 years). To bridge the gap, a noninvasive biomarker is needed that is stable across age groups, associated with progression of disease, and that can help guide therapy in pediatrics.
ST2, soluble suppresor of tumorgencity, is a member of the IL-1 receptor family, found as a transmembrane (ST2-L) and soluble form (hereafter called ST2), with IL-33 as the functional ligand.6 In response to inflammation, IL-33 is upregulated, and with transmembrane ST2-L, has a cardioprotective effect reducing fibrosis and hypertrophy.7,8 ST2, an isoform of the ST2-L receptor, scavenges IL-33, ablating its protective effect.9,10 ST2-L and ST2 have been found in cardiac and coronary endothelium but not in smooth muscle or cardiomyocytes.8,11 The pulmonary vasculature and lungs may be a primary source of ST2.12 In adults, circulating ST2 was associated with higher pulmonary artery pressures and mortality,13 and was upregulated both in the lungs and, systemically, in cardiogenic pulmonary edema.12 Carlomagno et al14 reported in adults that ST2 levels were associated with right ventricular dysfunction using MRI, whereas Zheng et al15 showed that ST2 was predictive of clinical worsening and shorter event-free survival. In a prior analysis of 59 children with idiopathic PAH (IPAH), ST2 was associated with mortality,16 whereas in a longitudinal study of infants, it was associated with worse disease over time.17
ST2 performance in a larger, more heterogeneous, multicenter group of pediatric PAH subjects and in a longitudinal analysis is unknown. We sought to evaluate ST2 as a potentially vascular-specific indicator of severity and prognostic marker of World Symposium on Pulmonary Hypertension Group 1 pediatric pulmonary hypertension using a multicenter cohort, with comparison vs the current clinical standard, NT-proBNP, and as an adjunct to a clinical model based on the REVEAL score. We also sought to evaluate expression and release of ST2 from human small pulmonary artery endothelial and smooth muscle cells.
Patients and Methods
Analytic Cohorts
Two cohorts of pediatric patients (age < 21 years) with PAH were studied: a cross-sectional cohort from the National Biological Sample and Data Repository for Pulmonary Arterial Hypertension (PAHB) and a longitudinal cohort from Children’s Hospital of Colorado (CHC). Each cohort was approved by the Institutional Review Boards, including those of Johns Hopkins University, CHC, Cincinnati Children’s Medical Center, and PAHB participating centers, with informed consent.
The PAHB is a resource of biological, genetic, and clinical data for the PAH research community funded by the National Heart, Lung, and Blood Institute (HL105333); it includes five pediatric enrolling centers (www.pahbiobank.org). De-identified biological samples and clinical data on enrollees (N = 182, < 21 years old) (Table 1) were available. Clinical severity parameters collected at enrollment included the World Symposium on Pulmonary Hypertension Group 1 subgroup, demographic characteristics and comorbidities, NYHA FC, 6MWD, drug therapy, and cardiac catheterization. 6MWD data were collected on subjects aged > 7 years. A subset of the PAHB had cardiac catheterizations within 12 months of enrollment (n = 66) and were used for hemodynamic analysis. Severity measures, including transplant and mortality, were collected prospectively up to 5 years and analyzed for the entire cohort.
Table 1.
Demographic and Clinical Characteristics of PAH Cohorts at Enrollment
| Characteristic | PAHB | Children’s Hospital Colorado |
|---|---|---|
| Demographics | ||
| Subjects, n | 182 | 61 |
| Age, y | 13 (8-17)a | 5 (3-8)a |
| Female sex, No. (%) | 108 (59) | 37 (60) |
| Weight, kg | 31.5 (15-51.5)a | 15.9 (11.7-26)a |
| Height, cm | 132 (102-156) | 104 (84-123) |
| BSA, m2 | 1 (0.6-1.4) | 0.69 (0.5-1) |
| NYHA FC, no. I/II/III/IV (% III/IV) | 24/55/49/12 (33) | 17/11/10/4 (33) |
| 6MWD, m (n) | 423 (90) | 427 (19) |
| REVEAL 2.0 score | 7 (6-8) | ... |
| REVEAL 2.0 score + ST2 | 8 (7-10) | ... |
| REVEAL variables available | 9 (8-10) | ... |
| Deaths, No. (%) | 6 (3) | 1 (1.6) |
| Events, No. (%) | 18 (9.8) | 11 (18) |
| Etiology, No. (%) | ||
| IPAH | 84 (46) | 23 (38) |
| FPAH | 11 (6) | 5 (8) |
| APAH | 84 (46) | 29 (48) |
| PVOD/PCH | 2 (1) | 1 (2) |
| Other | 1 (0.5) | 3 (4) |
| No. of visits | ... | 2 (1-3) |
| Time between visits, mo | ... | 16 (11-27) |
| Length of follow up, mo | ... | 29 (8-87) |
| Time from enrollment to censor, mo | 43 (27-56) | |
| Biomarker values | ||
| ST2, pg/mL | 3,061 (2,136-4,606) | 2,709 (1,939-5,120) |
| NT-proBNP, ng/mL | 218 (84-456) | 222 (118-537) |
| Hemodynamics | ||
| RAP, mm Hg | 7 (5-9) | 6 (5-8) |
| mPAP, mm Hg | 54 (39-63)a | 38 (28-52)a |
| PCWP, mm Hg | 9 (7-11) | 8 (6.5-9) |
| PVR, Wood units | 15.6 (8-20.4) | 11 (7-16) |
| PVRi, Wood units • m2 | 10.7 (6.99-27.4) | 7 (4.6-13) |
| Cardiac output, L/min | 3.3 (2.3-4.1) | 2.8 (1.8-3.6) |
| Cardiac index, L/min/m2 | 3.7 (2.9-5) | 3.6 (2.8-4.8) |
| Therapies, No. (%) | ||
| PDE5 inhibitor | 167 (92) | 43 (70) |
| ERA | 119 (65) | 20 (33) |
| IV/SC prostacyclin | 62 (34) | 17 (28) |
| CCB | 29 (16) | 4 (6.5) |
Data are expressed as median (interquartile range) unless otherwise specified.
6MWD = 6-min walk distance; APAH = associated pulmonary arterial hypertension; BSA = body surface area; CCB = calcium channel blocker; ERA = endothelin receptor antagonist; FPAH = familial pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; mPAP = mean pulmonary arterial pressure; NT-proBNP = N-terminal prohormone B-natriuretic peptide; NYHA FC = New York Heart Association Functional Class; PAH = pulmonary arterial hypertension; PAHB = National Biological Sample and Data Repository for PAH; PCWP = pulmonary capillary wedge pressure; PDE5 = phosphodiesterase-5; PVOD/PCH = pulmonary veno-occlusive disease; PVR = pulmonary vascular resistance; PVRi = pulmonary vascular resistance index; RAP = right atrial pressure; REVEAL = Registry to Evaluate Early and Long-Term PAH Disease Management; SC = subcutaneous; ST2 = soluble suppressor of tumorigenicity.
P < .05 between groups.
A longitudinal cohort of pediatric patients with PAH were enrolled at the CHC (N = 61) (Table 1). The patient data are maintained by CHC; inclusion and exclusion criteria, clinical assessments, therapy, and cardiac catheterizations have been previously published.18 A subset of the CHC subjects were enrolled in the PAHB, and thus the CHC cohort was used for longitudinal analysis to prevent any repeat analysis. Subjects had prospective collection of serum samples, concurrent with cardiac catheterization, functional data, and medical therapy, during clinically indicated evaluation of PAH from 2008 to 2013. A total of 151 samples were collected from 61 subjects. All subjects had precapillary PAH defined by a resting mean pulmonary arterial pressure (mPAP) > 25 mm Hg with a mean pulmonary capillary wedge pressure (PCWP) < 15 mm Hg.
Laboratory Analysis
Cell lines were obtained from the Cardiovascular Medical Research and Education Fund Pulmonary Hypertension Breakout Initiative (PHBI) Penn Cell Center and included small pulmonary artery smooth muscle and endothelial cells from patients with severe PAH who underwent transplant (n = 25) and control cell lines from nontransplanted donor lungs (n = 11). The median age of the control cell lines was 49 years, and the median age of the PAH cell lines was 40 years. Methods for cell culture and RNA-sequencing are detailed in e-Appendix 1 and have been described elsewhere.19 A multiplex electrochemiluminescent immunosorbent assay was used to measure ST2 and NT-proBNP, as previously described.19 Biomarker values below the limit of detection were imputed as one-half the lower limit of detection.
Statistical Analysis
Continuous variables are presented as means with SDs or medians with interquartile range (IQR) as appropriate. Comparisons were made between variables with Student t tests, Kruskal-Wallis tests, or Mann-Whitney U tests as appropriate. A subset of the PAHB that underwent a cardiac catheterization within 1 year of enrollment (n = 66) was used to evaluate hemodynamics. ST2 and NT-proBNP concentrations were log transformed for regression due to non-normality.
Associations between biomarker levels (independent variable) and each clinical outcome were analyzed by using a regression model adjusted for age and sex. Clinical outcomes included hemodynamic measures (cardiac output, mean right atrial pressure [RAP], mPAP, mean PCWP, pulmonary vascular resistance [PVR], and pulmonary vascular resistance index [PVRi]), NYHA FC, and 6MWD. Because 6MWD varies by age and height, it was calculated as a percent predicted 6MWD based on studies of healthy children.20,21 Subjects with missing outcomes were removed from analysis of the respective outcome; demographic characteristics of subjects with any missing outcomes are shown in e-Table 1. The longitudinal association of hemodynamic and functional variables with ST2 and NT-proBNP were determined by using mixed model analysis in the CHC cohort. Multiple visits nested within individual subjects were modeled as a random intercept for every individual and adjusted for age and sex.
Relationships between ST2 and NT-proBNP and time to clinical worsening defined as mortality, transplant, or palliative shunt (eg, Pott’s shunt, atrial septostomy) were studied in the PAHB with Cox proportional hazards models.
A REVEAL score was calculated by using the REVEAL Risk Score Calculator 2.0 algorithm,4,22 a multivariable mortality prediction score that is well validated in adults and includes adjustment for low and high NT-proBNP levels. REVEAL scores were divided into five REVEAL risk categories based on the original REVEAL algorithm.4 Points were added to the score for higher ST2 concentration, and scores (REVEAL score + ST2) were re-categorized based on the original algorithm into the five REVEAL risk categories + ST2. The baseline REVEAL score/category and the bivariate REVEAL score/category + ST2 were compared by using C-statistics and Cox proportional hazards models to assess discrimination of outcome of each model. Each model was compared vs the model with ST2 added by using the Akaike information criterion. Detailed methodology for calculation of the REVEAL score, addition of biomarkers, and division into REVEAL risk categories is found in e-Appendix 1. Due to crossover of subjects, the REVEAL score was only calculated in the PAHB.
A P value < .05 was considered statistically significant. Statistical analyses were conducted with Stata version 15.1 (StataCorp).
Results
There were 182 PAHB subjects and 61 CHC subjects available for analysis. All subjects were diagnosed with World Symposium on Pulmonary Hypertension Group 1 PAH, with serum samples collected at a single time point (enrollment) in the PAHB and at multiple time points in the CHC. Demographic characteristics at enrollment are summarized in Table 1. PAH subtypes were not significantly different between the cohorts, with 46% IPAH and 46% APAH in the PAHB compared with 38% IPAH and 48% congenital heart disease-associated APAH in the CHC. Biomarkers according to PAH subtype are provided in e-Table 2.
In the CHC, 59 of 61 subjects were evaluated at two consecutive visits with cardiac catheterization, 28 at three consecutive visits with cardiac catheterization, and 23 at five consecutive visits with cardiac catheterization. The median length of follow-up was 29 months (IQR, 8-87 months) (Table 1), whereas the median time between visit 1 and visit 2 was 16 months (IQR, 11-27 months). Data on ST2, NT-proBNP, mPAP, PVRi, and 6MWD over visits 1 to 3 are shown in e-Figure 1.
The 6MWD was recorded in subjects aged > 7 years and was not significantly different between cohorts. NYHA FC was available for 72% and 53% of the PAHB and CHC cohorts, respectively, and did not differ between the two groups. PVRi and mPAP were higher in the PAHB cohort, with a median PVRi of 10.7 Woods unit WU • m2 and mPAP of 54 mm Hg, vs a median PVRi of 7 WU • m2 and mPAP of 38 mm Hg in the CHC.
Serum ST2 and NT-proBNP
The lower limit of detection was 1.21 pg/mL for ST2 and 0.67 pg/mL for NT-proBNP. The interplate coefficients of variation were 3.06% and 4.67% for ST2 and NT-proBNP, respectively. The median ST2 and NT-proBNP concentrations did not differ between the two cohorts (Table 1). ST2 was not different between PAH types in either cohort, although NT-proBNP levels were high in the congenital heart disease-associated APAH cohort in the PAHB (e-Table 2).
Serum ST2 and NT-proBNP Association With Hemodynamic and Functional Measures
ST2, but not NT-proBNP, was associated with hemodynamic measures in the cross-sectional PAHB. In adjusted linear regression in the PAHB (Table 2), each log increase in ST2 was associated with 3.65 WU higher PVR, 3.23 WU • m2 higher PVRi, and nonsignificantly higher mPAP (5.63 mm Hg; P = .05). ST2 was not associated with cardiac output or mean PCWP. After adjustment, NT-proBNP was associated with nonsignificantly higher mPAP (2.99 mm Hg; P = .05) and PVR (1.46 WU; P = .05).
Table 2.
Age- and Sex-Adjusted Linear Regressions of Biomarkers and Each Continuous Clinical Variable in the PAHB (n = 66)
| Variable | ST2 | NT-proBNP |
|---|---|---|
| RAP, mm Hg (n = 65) | 0.05 (–1 to 1.1, .9) | 0.37 (–0.2 to 0.9, .2) |
| mPAP, mm Hg (n = 66) | 5.63 (-0.1 to 11, .05) | 2.99 (–0.03 to 6, .05) |
| PCWP, mm Hg (n = 65) | –0.5 (–1.4 to 0.3, .3) | –0.09 (–0.6 to 0.3, .7) |
| PVR, Wood units (n = 61) | 3.65 (0.9- 6.39, .01)a | 1.46 (–0.01 to 2.95, .05) |
| PVRi, Wood units • m2 (n = 61) | 3.23 (0.07 to 6.39, .04)a | 1.27 (–0.4 to 2.98, .14) |
| Cardiac output, L/min (n = 63) | –0.2 (–0.8 to 0.38, .5) | –0.12 (–0.4 to 0.19, .5) |
| Cardiac index, L/min • m2 (n = 63) | –0.45 (–0.96 to 0.05, .07) | –0.04 (–0.32 to 0.23, .8) |
| 6MWD, m (n = 37) | –161.9 (–306 to –17.2, .03)a | –66 (–121 to –11, .02)a |
| 6MWD % predicted, % (n = 37) | –26 (–48 to –4.4, .02)a | –10 (–18 to –2, .017)a |
| NYHA FC (n = 61) | 1.6 (0.85 to 2.5, < .001)a | 0.7 (0.3 to 1.1, < .001)a |
Each regression coefficient represents a separate model (nine total) of ST2 or NT-proBNP effect (independent variable) on the hemodynamic or functional outcome, with adjustment for age and sex. Data are expressed as regression coefficient (95% CI, P value). The PAHB was limited to subjects with biomarkers obtained within 12 months of clinical tests. The total number of subjects analyzed after accounting for missing values is presented with variable. 6MWD = 6-min walk distance; mPAP = mean pulmonary arterial pressure; NT-proBNP = N-terminal prohormone B-natriuretic peptide; NYHA FC = New York Heart Association functional class; PAHB = National Biological Sample and Data Repository for PAH; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; PVRi = pulmonary vascular resistance index; RAP = right atrial pressure; ST2 = soluble suppressor of tumorigenicity.
P < 0.05.
Adjusted mixed model linear regression was performed comparing serial ST2 and NT-proBNP with contemporaneous hemodynamic measurements in the CHC. Regression coefficients are shown in Table 3. Each log increase in ST2 was associated with 0.98 mm Hg higher RAP, 8.4 mm Hg higher mPAP, 5.54 WU higher PVR, and 3.78 WU • m2 higher PVRi. Conversely, in this linear model, a log decrease in ST2 would be associated with an equivalently lower RAP, mPAP, PVR, and PVRi. ST2 was not associated with cardiac output or mean PCWP. Each log increase in NT-proBNP resulted in 0.24 L/min lower cardiac output, 0.75 mm Hg higher RAP, 2.96 mm Hg higher mPAP, 2.85 WU higher PVR, 2.1 WU • m2 higher PVRi, and 0.38 mm Hg higher mean PCWP.
Table 3.
Age- and Sex-Adjusted (Mixed Model Longitudinal) Linear Regressions of Biomarkers and Continuous Clinical Variables in Children’s Hospital Colorado Over Time
| Variable | ST2 | NT-proBNP |
|---|---|---|
| RAP, mm Hg | 0.98 (0.34 to 1.6, .003)a | 0.75 (0.46 to 1.0, < .001)a |
| mPAP, mm Hg | 8.4 (5.1 to 11.7, < .001)a | 2.96 (1.2 to 4.7, .001)a |
| PCWP, mm Hg | 0.087 (–0.51 to 0.69, .8) | 0.38 (0.09 to 0.68, .01)a |
| PVR, Wood units | 5.54 (3.6 to 7.5, < .001)a | 2.85 (1.9 to 3.8, < .001)a |
| PVRi, Wood units • m2 | 3.78 (2.4 to 5.2, < .001)a | 2.1 (1.4, 2.8, < .001)a |
| Cardiac output, L/min | –0.19 (–0.45 to 0.07, .9) | –0.24 (–0.37 to –0.11, < .001)a |
| 6MWD, m | –40.9 (–72.6 to –9.1, .01)a | –15.69 (–32.4 to 1.0, .065) |
| 6MWD % predicted, % | –7% (–11.5 to 1.5, .01)a | –1.9% (–4.5 to 0.7, .16) |
| NYHA FC | 0.43 (0.24 to 0.62, < .001)a | 0.22 (0.12 to 0.31, < .001)a |
Each regression coefficient represents a separate model (eight total) of ST2 or NT-proBNP effect (independent variable) on the hemodynamic or functional outcome, with adjustment for age and sex.
Data are expressed as regression coefficient (95% CI, P value). Note that data are reported for change in outcome per 1 natural log unit increase in biomarker. Inverse relationship with the outcome is implied for 1 natural log unit decrease in biomarker. Children’s Hospital Colorado biomarker and clinical tests all concurrent, n = 61, 151 samples. 6MWD = 6-min walk distance; mPAP = mean pulmonary arterial pressure; NT-proBNP = N-terminal prohormone B-natriuretic peptide; NYHA FC = New York Heart Association Functional Class; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; PVRi = pulmonary vascular resistance index; RAP = right atrial pressure; ST2 = soluble suppressor of tumorigenicity.
P < .05.
In adjusted linear regressions in the PAHB, higher ST2 was associated with 161.9 m shorter 6MWD (Table 2), which corresponded with a 26% shorter predicted 6MWD. In the longitudinal analysis (Table 3), for every log higher ST2, 6MWD decreased by 40.9 m, a 7% decrease in predicted 6MWD. Higher NT-proBNP was associated with a 66 m decrease in 6MWD in the PAHB, a 10% shorter predicted 6MWD. NT-proBNP had no relationship with 6MWD in the CHC.
Associations With Clinical Worsening
Serum ST2 and NT-proBNP, as well as individual clinical variables, were assessed as predictors of clinical worsening, defined as death, lung transplant, or palliative shunt (Pott’s shunt or atrial septostomy) by Cox proportional hazards modeling in the PAHB (Table 4). Female sex protected against clinical worsening, with a hazard ratio of 0.23 (95% CI, 0.06-0.85; P = .02). Serum ST2 and NT-proBNP were associated with clinical worsening, with hazard ratios of 2.4 (95% CI, 1.5- 3.9; P < .0001) and 1.96 (95% CI, 1.43-2.67; P < .001), respectively, with similar risk when adjusted for age, sex, and pulmonary hypertension type.
Table 4.
Cox Multivariable Hazards Ratios for Clinical Worsening in the PAHB
| Association of Single Covariates | Hazard Ratio (95% CI, P Value) |
|---|---|
| Sex (female, n = 181) | 0.23 (0.06-0.85, .02) |
| Age (y; n = 181) | 1.02 (0.92-1.12, .7) |
| PAH type (n = 181) | 0.94 (0.8-1.2, .7) |
| NYHA FC (n = 132) | 1.68 (0.75-3.7, .2) |
| 6MWD (m; n = 82) | 0.99 (0.98-0.99, .03) |
| SBP (mm Hg; n = 165) | 0.99 (0.97-1.02, .8) |
| Heart rate (beats/min; n = 158) | 0.98 (0.95-1.01, .25) |
| RAP (mm Hg, n = 178) | 1.03 (0.97-1.08, .3) |
| mPAP (mm Hg; n = 181) | 1.05 (1.02-1.08, .002) |
| PVR (Woods unit; n = 168) | 1.03 (0.99-1.06, .17) |
| PVRi (Woods unit • m2; n = 165) | 1.05 (1.0-1.1, .04) |
| Cardiac index (L/min/m2; n = 176) | 1.1 (0.87-1.4, .4) |
| REVEAL score (n = 181) | 1.62 (1.2-2.18, .001) |
| REVEAL risk category (n = 181) | 1.88 (1.2-2.8, .003) |
| ST2 models (hazard ratio per log change in ST2; n = 181) | |
| ST2 Unadjusted | 2.4 (1.5-3.9, < .001) |
| ST2 adjusted for age and sex | 2.85 (1.5-5.1, < .001) |
| ST2 adjusted for age, sex, and PAH type | 2.9 (1.6-5.4, .001) |
| NT-proBNP models (hazard ratio per log change in NT-proBNP; n = 181) | |
| NT-proBNP unadjusted | 1.96 (1.43-2.67, < .001) |
| NT-proBNP adjusted for age and sex | 1.9 (1.4-2.6, < .001) |
| NT-proBNP adjusted for age, sex, and PAH type | 1.91 (1.4-2.6, < .001) |
Biomarkers natural log-transformed. 6MWD = 6-min walk distance; mPAP = mean pulmonary arterial pressure; NT-proBNP = N-terminal prohormone B-natriuretic peptide; NYHA FC = New York Heart Association Functional Class; PAH = pulmonary arterial hypertension; PAHB = National Biological Sample and Data Repository for PAH; PVR = pulmonary vascular resistance; PVRi = pulmonary vascular resistance index; RAP = right atrial pressure; REVEAL = Registry to Evaluate Early and Long-Term PAH Disease Management; SBP = systolic BP; ST2 = soluble suppressor of tumorigenicity.
Addition of ST2 to the REVEAL score, and recalculation of the REVEAL risk category, improved the predictive ability of the REVEAL score, reclassifying 14 of the 18 subjects with events into a higher risk category (e-Table 3). The hazard ratio in the REVEAL risk category was 1.88 (95% CI, 1.2-2.8; P = .003) compared with a hazard ratio of 2.05 (95% CI, 1.3-3.3; P = .003) for the REVEAL risk category + ST2 (Table 5). The REVEAL scores were compared vs the bivariate REVEAL + ST2 model using the Akaike information criterion; in all cases, the model with ST2 showed a better fit, with a lower Akaike information criterion compared with the baseline REVEAL score (e-Table 4). The best outcome discrimination was in the REVEAL score + ST2 and the REVEAL risk category + ST2, both with a C-statistic of 0.78.
Table 5.
Cox Multivariable Hazard Ratios for REVEAL 2.0 score, REVEAL Risk Category, Baseline and With ST2 in PAHB (N = 181)
| Model | Model Description | Hazard Ratio, P Value | C-Statistic (95% CI) |
|---|---|---|---|
| A | REVEAL score | 1.62 (1.2-2.18, .001) | 0.73 (0.62-0.85) |
| B | REVEAL risk category | 1.88 (1.2-2.8, .003) | 0.69 (0.56-0.83) |
| C | REVEAL score + ST2 | 1.5 (1.2-1.8, < .0001) | 0.78 (0.67-0.9) |
| D | REVEAL risk category + ST2 | 2.05 (1.3-3.3, .003) | 0.78 (0.65-0.89) |
PAHB = National Biological Sample and Data Repository for PAH; REVEAL = Registry to Evaluate Early and Long-Term PAH Disease Management; ST2 = soluble suppressor of tumorigenicity.
ST2 Is Secreted by Pulmonary Artery Endothelial Cells
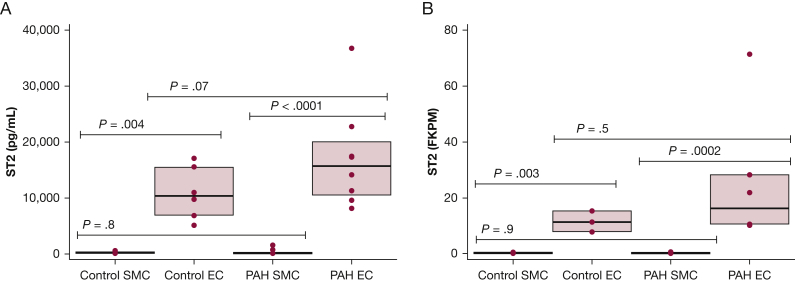
The pulmonary vascular source of ST2 was evaluated by using 25 PAH cell lines and 11 control cell lines from unique participants. As shown in Figure 1A, the cell-conditioned growth media ST2 concentration was significantly greater from endothelial cells compared with smooth muscle cells in both PAH (P = .004) and donor (P < .0001) cell lines. The PAH endothelial cell-conditioned growth media ST2 concentration (15,772 pg/mL) was 1.5-fold higher than the donor endothelial cells (10,321 pg/mL), although this difference was not statistically significant (endothelial cells, P = .07; smooth muscle cells, P = .8). RNA-sequencing confirmed findings using seven PAH endothelial cell lines, nine PAH smooth muscle cell lines, and four control endothelial and smooth muscle cell lines. The endothelial cells had significantly more ST2 messenger RNA compared with smooth muscle cells in both control (P = .003) and PAH (P = .0002) cell lines (Fig 1B). ST2 messenger RNA expression was 1.5-fold higher in the PAH endothelial cells (16.2 fragments per kilobase of transcript per million) compared with control cells (11.2 fragments per kilobase of transcript per million), although this difference was not statistically significant (endothelial cells, P = .5; smooth muscle cells, P = .9).
Figure 1.
ST2 concentration in PAH and control failed donor cells. SMC and EC are significantly different in both enzyme-linked immunosorbent assay and RNA-sequencing (P < .0001). A, ST2 concentration in cell-conditioned media from control and PAH SMC and EC. No significant difference between PAH and control SMC (P = .8) or between PAH and control EC (P = .07). B, ST2 expression (FKPM) by RNA-sequencing in PAH and control SMC and PAH and control EC. No significant difference was noted between PAH and control SMC (P = .9) or between PAH and control EC (P = .5). EC = endothelial cells; FKPM = fragments per kilobase of transcript per million; PAH = pulmonary arterial hypertension; SMC = smooth muscle cells; ST2 = soluble suppressor of tumorigenicity.
Discussion and Interpretation
In this multicenter and longitudinal study comparing ST2 and NT-proBNP, ST2 more strongly associated with pulmonary hemodynamics without an association with cardiac output, whereas NT-proBNP had a stronger association with cardiac output. ST2 was secreted in large amounts in unstimulated pulmonary artery endothelial cells, suggesting a pulmonary vascular role for ST2.
ST2 was consistently associated with the key hemodynamic findings of PAH (increased mPAP, PVR, and PVRi), with the long-term functional decline associated with the disease, and ultimately mortality or need for transplant/palliative shunt. The association of ST2 with abnormal pulmonary vascular hemodynamics is similar to that reported in prior adult studies.14,15,23 NT-proBNP, conversely, was associated with cardiac output and PCWP only in the CHC cohort; furthermore, the association of NT-proBNP with pulmonary hemodynamics was more modest, with less effect for the same proportional change in the biomarker. NT-proBNP may exhibit a secondary effect, reflecting cardiac function and right ventricular afterload rather than being a direct reflection of the pulmonary vasculature. Carlomagno et al14 noted elevated ST2 with right ventricular dysfunction and fibrosis. Although right ventricular function was not directly measured in this study, the majority of patients had a normal cardiac index, leading to the conclusion that elevated ST2 was related to the abnormal pulmonary vascular hemodynamics. It would be interesting to study whether ST2 increases further in the setting of worsening pulmonary pressures and subsequent right ventricular failure. The association with NT-proBNP and hemodynamics was only shown with concurrent hemodynamics and a serum sample, suggesting the known, dynamic variability in NT-proBNP. The closer association of ST2 with pulmonary hemodynamics in both groups, combined with the pulmonary artery endothelial secretion of ST2, suggests that ST2 could be used as a more stable vascular marker of clinical worsening/improvement in pediatric PAH and could be used as a noninvasive and reliable surrogate for traditional invasive diagnostic methods.
Although there are no well-validated clinical prognostic models for pediatric PAH, we show that the REVEAL 2.0 score performed moderately well in pediatric subjects using limited parameters. Adding ST2 to the score improved the predictive power of the score. This confirms findings from a large adult study in which ST2 improved REVEAL mortality prediction.13
ST2 is clearly expressed and secreted in large quantities by pulmonary artery endothelial cells, with a trend toward increased secretion in cells of subjects with PAH, shown in both cell-conditioned media and RNA-sequencing. The difference between ST2 expression/secretion between PAH and control cells is likely underpowered. In addition, the control cells in this case are from donated organs and may not reflect healthy control cells. The profibrotic mechanism of ST2, potentially produced in the local pulmonary artery environment, suggests a direct pathobiologic role in PAH, in which the IL-33/L-ST2 and ST2 pathway may have a potential mechanistic role in PAH beyond its known role in mediating cardiac fibrosis.
Limitations include a mix of incident and prevalent subjects and the relatively small size of each cohort for hemodynamic analysis, with 66 subjects in the PAHB cohort. The time (up to 12 months) between cardiac catheterization and sample in the PAHB, with potential changes in disease severity or therapy, may affect the association between biomarkers and clinical outcomes and contribute to the inconsistent performance of NT-proBNP. There was no significant effect of age on biomarkers in this study, although the study did not include infants. Prior studies have shown the utility of ST2 in infants.16,17 Shorter 6MWD may be limited by variability across age groups, although findings were consistent with changes in percent predicted walk distance. Although clinical worsening and mortality were significant outcomes in this study, the limited number of events makes it more difficult to build a predictive model for mortality. We were further unable to validate a time-to-event analysis in the CHC due to crossover between cohorts. Finally, the donors (both PAH and control subjects) used for cellular analysis were largely adult, and thus the cellular expression of ST2 may not reflect that of pediatric subjects.
ST2 and NT-proBNP are clinically useful biomarkers of pediatric PAH and heart failure. NT-proBNP, an excellent marker of cardiac function, is exclusively expressed by the myocardium3 and responds to increased afterload from the pulmonary vascular bed. Right ventricular function, reflected by NT-proBNP, is a known determinant of outcomes in PAH but is often evident in later disease, as the ventricle is able to adapt to increased afterload.24 ST2 seems to add prognostic value in PAH, with a tighter association with key hemodynamic measures of pulmonary vascular disease, and may be a more specific and earlier marker than NT-proBNP.
Further studies are needed to determine the temporal relationship of these markers in PAH. Based on these results, confirming prior studies of ST2 in pediatrics, ST2 could be added to NT-proBNP to inform clinical decision-making in pediatric PAH. Next steps should include development of a pediatric-specific risk model using noninvasive measures, including NT-proBNP and ST2. A noninvasive risk prediction tool could allow clinicians to follow up pediatric patients, intervening earlier to improve outcomes while reducing the risks posed by current clinical standards.
Take-home Point.
Study Question: Is ST2 a useful prognostic marker of hemodynamic worsening and adverse outcomes in pediatric pulmonary hypertension?
Results: In both cross-sectional and longitudinal cohorts, ST2 was associated with worse hemodynamics and shorter time to event. Addition of ST2 to the REVEAL score improved prediction of adverse outcomes. ST2 was produced by the pulmonary artery endothelium.
Interpretation: ST2 may be a pulmonary vascular-specific biomarker for worsening pediatric PAH.
Acknowledgments
Author contributions: M. G., J. Y., D. V., and A. D. E planned the project, analyzed the data, and wrote the manuscript; M. G., S. B., and J. Y. performed the experiments and interpreted the results; M. G., R. D., D. V., and J. Y. performed statistical analysis; and M. N., C. E. S., R. D., D. D. I., E. D. A, W. C. N., M. W. P., K. A. L., E. B. R., R. H., D. Y., and A. D. E. recruited subjects and performed research. All authors reviewed, revised, and approved the manuscript for submission.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: Serum/tissue samples were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI). Funding for the PHBI is provided under a National Heart, Lung, and Blood Institute R24 Grant [R24HL123767]. Samples and/or data from the PAHB, which receives government support under an investigator-initiated grant [R24 HL105333] awarded by the National Heart, Lung, and Blood Institute, were used in this study. The authors thank contributors, including the Pulmonary Hypertension Centers who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by National Institutes of Health/National Heart, Lung, and Blood Institute [R01HL135114 and R01 HL150070, A. D. E., M. N., J. Y., R. D., D. V., W. C. N., D. D. I., and E. D. A.; R24 HL105333, W. C. N. and M. W. P.; and F32 HL143835-01A1, C. E. S.]. M. G. was supported by the Pediatric Scientist Development Program, which is supported by K12-HD000850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. D. D. I. was supported by a National Institutes of Health/National Center for Advancing Translational Sciences Colorado CTSA Grant UL1 TR002535. M. N. was supported by a Matthew and Michael Wojciechowski Pulmonary Hypertension Pediatric Proof-of-Concept Grant [Dr. Robyn J. Barst Pediatric PH Research and Mentoring Fund Grant].
Supplementary Data
References
- 1.Ten Kate C.A., Tibboel D., Kraemer U.S. B-type natriuretic peptide as a parameter for pulmonary hypertension in children. A systematic review. Eur J Pediatr. 2015;174(10):1267–1275. doi: 10.1007/s00431-015-2619-0. [DOI] [PubMed] [Google Scholar]
- 2.Baggish A.L., van Kimmenade R.R., Januzzi J.L., Jr. Amino-terminal pro-B-type natriuretic peptide testing and prognosis in patients with acute dyspnea, including those with acute heart failure. Am J Cardiol. 2008;101(3a):49–55. doi: 10.1016/j.amjcard.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 3.GTex Portal. https://www.gtexportal.org/home/gene/NPPB. Accessed March 15, 2020.
- 4.Benza R.L., Gomberg-Maitland M., Elliott C.G. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL Risk Score Calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Ivy D., Rosenzweig E.B., Harrison C.E. Risk assessment in pediatric patients with pulmonary arterial hypertension (PAH): application of the REVEAL 2.0 Risk Calculator. Chest. 2019;156(4):A169–A171. [Google Scholar]
- 6.Pascual-Figal D.A., Garrido I.P., Blanco R. Soluble ST2 is a marker for acute cardiac allograft rejection. Ann Thorac Surg. 2011;92(6):2118–2124. doi: 10.1016/j.athoracsur.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Sanada S., Hakuno D., Higgins L.J., Schreiter E.R., McKenzie A.N., Lee R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117(6):1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demyanets S., Kaun C., Pentz R. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013;60:16–26. doi: 10.1016/j.yjmcc.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller A.M. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8(1):22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkar R., Lee R.T. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7(10):827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Wang W., Wang L. IL-33 initiates vascular remodelling in hypoxic pulmonary hypertension by up-regulating HIF-1alpha and VEGF expression in vascular endothelial cells. EBioMedicine. 2018;33:196–210. doi: 10.1016/j.ebiom.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayés-Genis A., González A., Lupón J. ST2 in heart failure. Circ Heart Fail. 2018;11(12) doi: 10.1161/CIRCHEARTFAILURE.118.005582. [DOI] [PubMed] [Google Scholar]
- 13.Simpson C.E., Damico R.L., Hassoun P.M. Noninvasive prognostic biomarkers for left-sided heart failure as predictors of survival in pulmonary arterial hypertension. Chest. 2020;157(6):1606–1616. doi: 10.1016/j.chest.2019.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlomagno G., Messalli G., Melillo R.M. Serum soluble ST2 and interleukin-33 levels in patients with pulmonary arterial hypertension. Int J Cardiol. 2013;168(2):1545–1547. doi: 10.1016/j.ijcard.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y.G., Yang T., He J.G. Plasma soluble ST2 levels correlate with disease severity and predict clinical worsening in patients with pulmonary arterial hypertension. Clin Cardiol. 2014;37(6):365–370. doi: 10.1002/clc.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chida A., Sato H., Shintani M. Soluble ST2 and N-terminal pro-brain natriuretic peptide combination. Circ J. 2014;78(2):436–442. doi: 10.1253/circj.cj-13-1033. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths M., Yang J., Everett A.D. Endostatin and ST2 are predictors of pulmonary hypertension disease course in infants. J Perinatol. 2020;40(11):1625–1633. doi: 10.1038/s41372-020-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkett D.A., Slorach C., Patel S.S. Impact of pulmonary hemodynamics and ventricular interdependence on left ventricular diastolic function in children with pulmonary hypertension. Circ Cardiovasc Imaging. 2016;9(9) doi: 10.1161/CIRCIMAGING.116.004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson C.E., Chen J.Y., Damico R.L. Cellular sources of interleukin-6 and associations with clinical phenotypes and outcomes in pulmonary a rterial hypertension. Eur Respir J. 2020;55(4):1901761. doi: 10.1183/13993003.01761-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A.M., Yin J., Au J.T. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med. 2007;176(2):174–180. doi: 10.1164/rccm.200607-883OC. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich S., Hildenbrand F.F., Treder U. Reference values for the 6-minute walk test in healthy children and adolescents in Switzerland. BMC Pulm Med. 2013;13:49. doi: 10.1186/1471-2466-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benza R.L., Gomberg-Maitland M., Miller D.P. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 23.Geenen L.W., Baggen V.J.M., Kauling R.M. The prognostic value of soluble ST2 in adults with pulmonary hypertension. J Clin Med. 2019;8(10):1517. doi: 10.3390/jcm8101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naeije R., Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev. 2014;23(134):476–487. doi: 10.1183/09059180.00007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.