Abstract
Background
Mild expiratory flow limitation may not be recognized using traditional spirometric criteria based on the ratio of FEV1/FVC.
Research Question
Does slow vital capacity (SVC) instead of FVC increase the sensitivity of spirometry to identify patients with early or mild obstructive lung disease?
Study Design and Methods
We included 854 current and former smokers from the Subpopulations and Intermediate Outcome Measures in COPD Study cohort with a postbronchodilator FEV1/FVC ≥ 0.7 and FEV1 % predicted of ≥ 80% at enrollment. We compared baseline characteristics, chest CT scan features, exacerbations, and progression to COPD (postbronchodilator FEV1/FVC, < 0.7) during the follow-up period between 734 participants with postbronchodilator FEV1/SVC of ≥ 0.7 and 120 with postbronchodilator FEV1/SVC < 0.7 at the enrollment. We performed multivariate linear and logistic regression models and negative binomial and interval-censored proportion hazards regression models adjusted for demographics and smoking exposure to examine the association of FEV1/SVC < 0.7 with those characteristics and outcomes.
Results
Participants with FEV1/SVC < 0.7 were older and had lower FEV1 and more emphysema than those with FEV1/SVC ≥ 0.7. In adjusted analysis, individuals with postbronchodilator FEV1/SVC < 0.7 showed a greater percentage of emphysema by 0.45% (95% CI, 0.09%-0.82%), percentage of gas trapping by 2.52% (95% CI, 0.59%-4.44%), and percentage of functional small airways disease based on parametric response mapping by 2.78% (95% CI, 0.72%-4.83%) at baseline than those with FEV1/SVC ≥ 0.7. During a median follow-up time of 1,500 days, an FEV1/SVC < 0.7 was not associated with total exacerbations (incident rate ratio [IRR], 1.61; 95% CI, 0.97-2.64), but was associated with severe exacerbations (IRR, 2.60; 95% CI, 1.04-4.89). An FEV1/SVC < 0.7 was associated with progression to COPD during a 3-year follow-up even after adjustment for demographics and smoking exposure (hazard ratio, 3.93; 95% CI, 2.71-5.72). We found similar results when we examined the association of prebronchodilator FEV1/SVC < 0.7 or FEV1/SVC less than the lower limit of normal with chest CT scan features and progression to COPD.
Interpretation
Low FEV1 to SVC in current and former smokers with normal spirometry results can identify individuals with CT scan features of COPD who are at risk for severe exacerbations and is associated with progression to COPD in the future.
Trial Registry
ClinicalTrials.gov; No.: NCT01969344T4; URL: www.clinicaltrials.gov
Key Words: COPD, pulmonary, pulmonary function test, slow vital capacity, SVC
Abbreviations: CAT, COPD Assessment Test; LLN, lower limit of normal; mMRC, Modified Medical Research Council dyspnea; SVC, slow vital capacity; SPIROMICS, Subpopulations and Intermediate Outcome Measures in COPD Study; VC, vital capacity
FOR EDITORIAL COMMENT, SEE PAGE 7
The diagnosis of COPD is based on the presence of airflow obstruction defined by the ratio of FEV1/FVC of less than the lower limit of normal or 0.7.1 About one-quarter of smokers with normal FEV1 and FEV1/FVC show visual emphysema on chest CT scanning.2 Smokers with preserved lung function and COPD Assessment Test (CAT) score of > 10 experience more respiratory exacerbations than those with CAT score of < 10.3 Many high-risk individuals with evidence of COPD features are not diagnosed formally with COPD based on current diagnostic criteria.2, 3, 4
In comparison with FVC, slow vital capacity (SVC) may reflect better the true vital capacity (VC) in obstructive lung disease because of possible underestimation of FVC that results from dynamic compression of the airways during the forced expiratory maneuver and reduced exhalation time.5 In these settings, SVC may be more appropriate to calculate the FEV1/VC according to the American Thoracic Society guidelines.6 In a single-center study that included people referred for pulmonary function test and who showed a FEV1/FVC and total lung capacity of more than the lower limit of normal (LLN), 20.4% of the participants demonstrated a FEV1/SVC less than the LLN.7 The frequency of obstructive lung disease diagnosis by health care provider was higher in participants with FEV1/SVC less than the LLN compared with those with FEV1/SVC equal to the LLN or more based on chart review of a randomly selected subgroup. The association of low FEV1/SVC with objective features of obstructive lung disease like radiographic emphysema and progression to spirometric obstruction in the future among those at risk for COPD with normal FEV1/FVC is unknown. We hypothesized that, in smokers with normal spirometry results according to the current standards, an FEV1/SVC < 0.7 is associated with respiratory symptoms, chest CT scan emphysema, and increased likelihood of COPD developing. To investigate our hypothesis, we analyzed data from current and former smokers with normal spirometry results, defined as postbronchodilator FEV1/FVC of ≥ 0.7 and FEV1 % predicted of ≥ 80%, who were enrolled in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). We compared clinical, functional, and chest CT scan features between individuals with an FEV1/SVC < 0.7 and individuals with an FEV1/SVC ≥ 0.7.
Methods
This is a retrospective analysis of data from participants in SPIROMICS, a prospective observational cohort study conducted at multiple clinical centers across the United States (https://www.spiromics.org/spiromics/). The institutional review boards at each participating center approved the study protocol, and written informed consent was obtained from all participants (e-Appendix 1). Details of the study protocol have been published previously.8 Briefly, participants 40 to 80 years of age who were either current or former smokers with ≥ 20 pack-years of smoking were enrolled in the study. An obstructive lung disease diagnosis other than asthma and COPD, BMI of > 40 kg/m2 at baseline, and unstable cardiovascular disease were exclusion criteria. Of 2,770 current and former smokers with at least a 20-pack-years history of smoking enrolled in SPIROMICS, we included 924 participants with normal baseline spirometry results, defined as a postbronchodilator FEV1/FVC of ≥ 0.7 and FEV1 % predicted of ≥ 80% at enrollment. We used the reference spirometric values from the third National Health and Nutrition Examination Survey.9 We excluded 70 participants with no available SVC data. The remained 854 participants entered the analysis. Participants underwent a baseline visit and up to three annual in-person follow-up visits. At baseline, participants answered questionnaires, including the modified Medical Research Council dyspnea (mMRC) questionnaire,10 CAT,11 and St. George’s Respiratory Questionnaire.12 At baseline and at each follow-up visit, participants underwent prebronchodilator and postbronchodilator spirometry assessments. Prebronchodilator spirometry and expiratory SVC maneuvers were performed according to American Thoracic Society/European Respiratory Society guidelines.13 After four inhalations each of albuterol 90 μg/inhalation and ipratropium 18 μg/inhalation, spirometry and expiratory SVC maneuvers were repeated. Information regarding medical history, respiratory exposures, and current medications were collected. Six-minute walk distance in meters was tested and recorded as per SPIROMICS protocol.8 Chest CT scans were performed at baseline according to study protocols.14 Participants also received quarterly follow-up calls to assess health status and whether they had experienced an exacerbation.
Imaging
Baseline visit included high-resolution chest CT scans at maximum inspiration (total lung capacity) and maximal expiration (residual volume). We evaluated emphysema on high-resolution CT imaging by VIDA software. Percent emphysema was defined by using the percentage of voxels at maximum inspiration (total lung capacity by CT scan) with attenuation less than –950 Hounsfield units and gas trapping was quantified as the percentage of voxels at maximum expiration (residual volume by CT scan) with attenuation values of less than –856 Hounsfield units.14 Parametric response mapping analysis was performed using the Imbio Lung Density Analysis software application (Imbio, LLC) to distinguish regions of emphysema from regions of nonemphysematous gas trapping, functional small airways disease.15,16
Definitions and Outcomes
In the main analysis, postbronchodilator FEV1/SVC was calculated as the ratio of postbronchodilator FEV1 to postbronchodilator SVC. Chronic bronchitis was defined based on the St. George’s Respiratory Questionnaire results at baseline.17 History of exacerbation was defined as self-report of respiratory exacerbation in the year before enrollment at the baseline visit. Exacerbation was defined as respiratory events for which the participant received antibiotics, steroids, or both or that were evaluated by a health care professional. Severe exacerbations were defined as exacerbations that required hospital admission or ED visit. Progression to COPD was defined as postbronchodilator FEV1/FVC < 0.7 at a follow-up visit. After a postbronchodilator FEV1/FVC < 0.7 at a follow-up visit, some individuals may have bounced back to postbronchodilator FEV1/FVC of ≥ 0.7. For that reason, we also examined persistent COPD defined as a postbronchodilator FEV1/FVC < 0.7 at a follow-up visit that did not bounce back to postbronchodilator FEV1/FVC of ≥ 0.7 at the next visits.
Statistical Analysis
We stratified participants at the enrollment visit into those with FEV1/SVC ≥ 0.7 and those with FEV1/SVC < 0.7. We compared the characteristics of participants at the enrollment visit between the two groups using the Wilcoxon rank-sum test for continuous variables and the χ 2 or Fisher exact test for categorical variables. To identify factors associated with FEV1/SVC < 0.7, we created parsimonious multivariate logistic regression models. Clinically relevant variables associated with P < .1 in the univariate analysis were considered for multivariate analysis. Variables were selected for the final model using a stepwise backward variable elimination process to minimize the Akaike information criterion.18 We assessed for variable multicollinearity using correlation matrices and variance inflation factors.19 We repeated the multivariate analysis after the multiple imputation by chained equations package (five datasets) to account for missing variables.20,21
We also created multivariate linear regression models with percent emphysema, gas trapping, and parametric response mapping functional small airways disease as the dependent variables (outcomes) and FEV1/SVC < 0.7 as the main independent variable (exposure). Age, sex, race, smoking status at enrollment, and pack-years smoked were included as covariates in the models. We created zero-inflated negative binomial models to assess exacerbation rates, which included adjustment for age, sex, race, smoking status at enrollment, pack-years smoked, and diabetes mellitus as risk factors for exacerbations.22 Follow-up time was included as an offset in the models as described previously.23,24 We compared the frequency of participants who progressed to COPD during the study period using the χ 2 test. Interval-censored proportion hazards regression analysis was used to examine the association of FEV1/SVC < 0.7 with progression to COPD during the follow-up period, with adjustment for age, sex, race, smoking status at enrollment, and pack-years smoked. We created a multivariate logistic regression model with persistent COPD as the dependent variable (outcome) and FEV1/SVC < 0.7 as the main independent variable (exposure). Age, sex, race, smoking status at enrollment, and pack-years smoked were included as covariates in the models.
Although postbronchodilator spirometry is considered the gold standard for COPD diagnosis,25 both prebronchodilator and postbronchodilator spirometry is associated with clinical, functional, and radiographic features of COPD.26 Moreover, postbronchodilator spirometry is not always performed.27 Therefore, we performed a sensitivity analysis that included 645 participants with a prebronchodilator FEV1/FVC of ≥ 0.7 and FEV1 % predicted of ≥ 80% at the enrollment. In the sensitivity analysis, we repeated the same approach as in the main analysis, but the FEV1/SVC was computed using the prebronchodilator FEV1 and SVC, and COPD was defined based on prebronchodilator FEV1/FVC < 0.7. We examined the association of prebronchodilator FEV1/SVC < 0.7 with clinical, functional, and radiographic features of COPD. An additional analysis that included 864 current or former smokers with a prebronchodilator FEV1/FVC of the LLN or more and FEV1 equal to the LLN or more at the enrollment9 was performed to examine the association of prebronchodilator FEV1/SVC less than the LLN with clinically relevant outcomes. All statistical analyses were conducted using R statistical software (R Foundation for Statistical Computing) except interval-censored proportion hazards regression analysis, which was conducted using SAS software (SAS Institute).
Results
Of 854 current and former smokers with normal spirometry results at enrollment, defined as postbronchodilator FEV1/FVC of ≥ 0.7 and FEV1 % predicted of ≥ 80%, 120 participants showed a postbronchodilator FEV1/SVC < 0.7 and 734 participants showed an FEV1/SVC ≥ 0.7. Table 1 shows the characteristics of the two groups. In a parsimonious multivariate analysis, we identified factors associated with postbronchodilator FEV1/SVC < 0.7. Older age with an OR of 1.91 for every 10 years (95% CI, 1.51-2.45), postbronchodilator FEV1 % predicted (OR, 0.96 for every 1%; 95% CI, 0.94-0.97), and greater emphysema (OR, 1.13 for every 1% emphysema; 95% CI, 1.03-1.25) were associated with postbronchodilator FEV1/SVC < 0.7 (Table 2). We repeated the multivariate analysis after multiple imputations to account for missing variables showing similar results (e-Table 1).
Table 1.
Baseline Characteristics in Smokers With Normal Spirometry Resultsa Stratified by Postbronchodilator FEV1/SVC < 0.7 (n = 854)
| Variable | Postbronchodilator FEV1/SVC ≥ 0.7 (n = 734) | Postbronchodilator FEV1/SVC < 0.7 (n = 120) | P Valueb |
|---|---|---|---|
| Age, y | 59.13 ± 9.59 | 65.35 ± 9.02 | < .001 |
| Female sex | 391 (53.3) | 51 (42.5) | .036 |
| White race | 489 (66.6) | 92 (76.7) | .037 |
| BMI, kg/m2 | 28.73 ± 5.05 | 29.12 ± 4.87 | .36 |
| Pack-years smoking | 40.75 ± 24.29 | 50.31 ± 22.93 | < .001 |
| Currently smoking | 387 (53.2) | 45 (38.1) | .003 |
| Asthma | 107 (14.6) | 19 (15.8) | .83 |
| Congestive heart failure | 10 (1.4) | 2 (1.7) | .68 |
| Diabetes mellitus | 77 (10.6) | 22 (18.5) | .019 |
| Hypertension | 296 (40.6) | 65 (54.6) | .006 |
| OSA | 130 (17.7) | 21 (17.5) | 1 |
| Stroke | 26 (3.6) | 4 (3.4) | 1 |
| History of exacerbation | 97 (13.3) | 14 (11.8) | .74 |
| Bronchodilators | 149 (20.3) | 32 (26.7) | .14 |
| Inhaled glucocorticoids | 76 (10.4) | 13 (10.8) | 1 |
| Chronic bronchitis | 259 (37.1) | 46 (41.8) | .40 |
| MMRC ≥ 2 | 93 (12.8) | 11 (9.2) | .34 |
| CAT score ≥ 10 | 332 (49.0) | 57 (49.6) | .99 |
| Prebronchodilator FEV1, L | 2.71 ± 0.68 | 2.56 ± 0.66 | .047 |
| Prebronchodilator FEV1 % predicted | 94.17 ± 12.90 | 89.17 ± 11.52 | < .001 |
| Prebronchodilator FVC, L | 3.61 ± 0.89 | 3.69 ± 0.97 | .34 |
| Prebronchodilator FVC % predicted | 96.97 ± 12.61 | 97.02 ± 12.01 | .76 |
| Postbronchodilator FEV1, L | 2.87 ± 0.70 | 2.70 ± 0.67 | .026 |
| Postbronchodilator FEV1 % predicted | 99.76 ± 12.22 | 94.29 ± 9.94 | < .001 |
| Postbronchodilator FVC, L | 3.67 ± 0.89 | 3.70 ± 0.94 | .67 |
| Postbronchodilator FVC % predicted | 98.45 ± 12.01 | 97.63 ± 10.56 | .44 |
| Prebronchodilator SVC, L | 3.61 ± 0.96 | 3.84 ± 1.05 | .016 |
| Postbronchodilator SVC, L | 3.68 ± 0.92 | 4.03 ± 0.99 | < .001 |
| Bronchodilator response | 86 (11.7) | 19 (15.8) | .26 |
| RVCT, L | 2.78 ± 0.72 | 3.15 ± 0.79 | < .001 |
| TLCCT, L | 5.38 ± 1.27 | 5.84 ± 1.33 | < .001 |
| RVCT to TLCCT ratio, % | 52.56 ± 11.96 | 54.94 ± 11.89 | .008 |
| 6-MWT distance, m | 440.52 ± 94.96 | 426.77 ± 95.93 | .30 |
| Emphysema, % | 1.53 ± 1.82 | 2.30 ± 2.25 | < .001 |
| Gas trapping, % | 7.58 ± 9.53 | 12.09 ± 12.08 | < .001 |
| PRMfSAD, % | 8.01 ± 9.74 | 12.73 ± 12.27 | < .001 |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. 6-MWT = 6-min walk test distance; CAT = COPD Assessment Test; MMRC= Modified Medical Research Council; PRMfSAD = parametric response mapping functional small airways disease; RVCT = residual volume by CT scan; SVC = slow vital capacity; TLCCT = total lung capacity by CT scan.
Normal spirometry results defined as postbronchodilator FEV1 of ≥ 80% and postbronchodilator FEV1/FVC ≥ 0.7.
Characteristics of participants between the two groups compared using a t test for continuous variable and the χ 2 or Fisher exact test for categorical variables.
Table 2.
Factors Associated With Abnormal Postbronchodilator FEV1/SVC Ratio of < 0.7 Among Smokers With Normal Spirometry Resultsa (n = 854)
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age, every 10 y | 1.91 (1.51-2.45) | < .001 |
| Pack-years, every 10 y | 1.06 (0.99-1.14) | .09 |
| Postbronchodilator FEV1 % predicted | 0.96 (0.94-0.97) | < .001 |
| % Emphysema | 1.13 (1.03-1.25) | .008 |
| Female sex | 0.68 (0.45-1.04) | .08 |
Variables tested but not retained for the final model include: residual volume to total lung capacity ratio, race, and current smoking. Data regarding percent emphysema, residual volume to total lung capacity ratio, and current smoking were missing in 7, 9, and 9 participants, respectively. We performed an additional analysis after multiple imputations accounting for missing values showing similar findings (e-Table 1). SVC = slow vital capacity.
Normal spirometry results defined as postbronchodilator FEV1 ≥ 80% and postbronchodilator FEV1/FVC ≥ 0.7.
Chest CT Scan Features
After adjusting for demographics, pack-years smoking, and current smoking status, participants with postbronchodilator FEV1/SVC < 0.7 showed greater percent emphysema by 0.45% (95% CI, 0.09-0.82), percent gas trapping by 2.52% (95% CI, 0.59-4.44), and percent parametric response mapping functional small airways disease by 2.78% (95% CI, 0.72-4.83) at baseline than those with FEV1/SVC ≥ 0.7 (Table 3).
Table 3.
Association of Postbronchodilator FEV1 to SVC Ratio of < 0.7 With Chest CT Scan Features in Smokers With Normal Spirometry Resultsa (n = 854)
| Variable | β (95% CI) | P Value |
|---|---|---|
| % Emphysema | 0.45 (0.09-0.82) | .014 |
| % Gas trapping | 2.52 (0.59-4.44) | .010 |
| % PRMfSAD | 2.78 (0.72-4.83) | .0081 |
Each row represents a model. All models included the following covariates: age, sex, race, smoking status, and smoking pack-years at the enrollment. Data regarding % emphysema, % gas trapping, % PRMfSAD, and current smoking were missing in 7, 4, 96, and 9 participants, respectively. PRMfSAD = parametric response mapping functional small airways disease; SVC = slow vital capacity.
Normal spirometry results defined as postbronchodilator FEV1 ≥ 80% and postbronchodilator FEV1/FVC ≥ 0.7.
Exacerbations
During a median follow-up time of 1,500 days (interquartile range, 1,062-1,954 days), data for exacerbations were available for 710 participants with FEV1/SVC ≥ 0.7 and for 120 participant with FEV1/SVC < 0.7. Of 710 participants with postbronchodilator FEV1/SVC ≥ 0.7, 170 (23.9%) experienced at least one exacerbation, with 62 (8.7%) experiencing at least one severe exacerbation. Among 120 individuals with postbronchodilator FEV1/SVC < 0.7, 42 (35%) experienced at least one exacerbation, with 17 (14.2%) experiencing at least one severe exacerbation. In multivariate analysis adjusted for demographics, pack-years smoking, current smoking status, and diabetes mellitus, postbronchodilator FEV1/SVC < 0.7 was not associated with total exacerbation (incident rate ratio, 1.60; 95% CI, 0.97-2.64), but was associated with severe exacerbations (incident rate ratio, 2.60; 95% CI, 1.04-4.89) (Table 4).
Table 4.
Association of Postbronchodilator FEV1 to SVC Ratio of < 0.7 With Exacerbations and Progression to COPD in Smokers With Normal Spirometry Resultsa
| Variable | IRR (95% CI) | P Value |
|---|---|---|
| Total exacerbations | 1.60 (0.97-2.64) | .07 |
| Severe exacerbations | 2.60 (1.04-4.89) | .040 |
| HR (95% CI) | ||
| Progression to COPD | 3.93 (2.71-5.72) | < .001 |
| OR (95% CI) | ||
| Persistent COPD | 5.08 (3.09-8.37) | < .001 |
For exacerbation analysis, data for 830 participants were available. Zero-inflated negative binomial regression models with postbronchodilator FEV1/SVC < 0.7 as the main independent variable (exposure) and total exacerbations and severe exacerbations as the dependent variables (outcome) were performed. Models included the following covariates: age, sex, race, current smoking status, smoking pack-years, and diabetes mellitus in the count negative binomial regression and an intercept-only model in the zero component. Follow-up time was included as an offset in the models. For progression to COPD analysis, data for 845 participants were available. Interval-censored proportion hazards regression model for progression to COPD included the following covariates: age, sex, race, smoking status, and smoking pack-years. For progression to persistent COPD analysis, a logistic regression model was created with the same covariates. HR = hazard ratio; IRR = incident rate ratio; SVC = slow vital capacity.
Normal spirometry results defined as postbronchodilator FEV1 ≥ 80% and postbronchodilator FEV1/FVC ≥ 0.7.
Progression to COPD and Persistent COPD
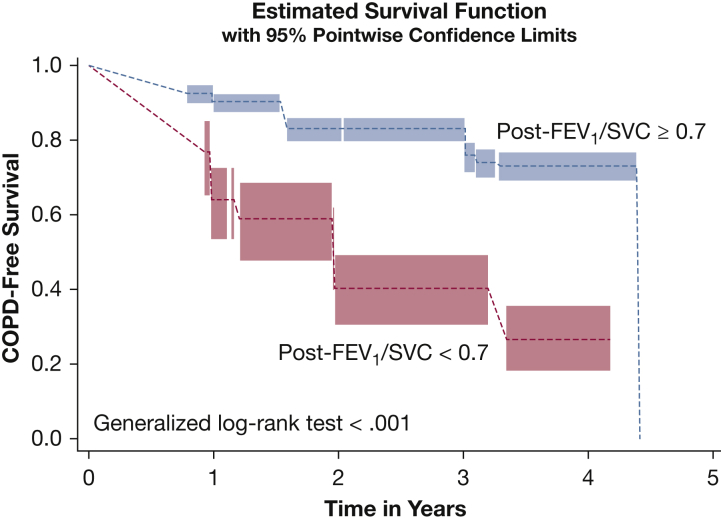
During a median follow-up time of 1,460 days (interquartile range, 1,024-1,946 days), spirometric follow-up data were available for 727 participants with FEV1/SVC ≥ 0.7 and for 118 participants with FEV1/SVC < 0.7. Of participants with FEV1/SVC ≥ 0.7, 12.7% demonstrated COPD, defined as postbronchodilator FEV1/FVC < 0.7, whereas 42.4% of those with FEV1/SVC < 0.7 demonstrated COPD during the follow-up period (P < .001). Figure 1 shows the COPD-free survival time in those with FEV1/SVC ≥ 0.7 and those with FEV1/SVC < 0.7. The association of postbronchodilator FEV1/SVC < 0.7 with progression to COPD during the follow-up period remained after adjusting for demographics, pack-years smoking, and current smoking status (hazard ratio, 3.93; 95% CI, 2.71-5.72) (Table 4). Postbronchodilator FEV1/SVC < 0.7 also was associated with persistent COPD after adjusting for demographics, pack-years smoking, and current smoking status (OR, 5.08; 95% CI, 3.09-8.37). e-Table 2 shows the characteristics in participants who demonstrated persistent COPD and those who did not.
Figure 1.
Line graph showing COPD-free survival in smokers with normal spirometry results (postbronchodilator FEV1 ≥ 80% and postbronchodilator FEV1/FVC ≥ 0.7; n = 845) stratified by postbronchodilator FEV1/SVC: postbronchodilator FEV1/SVC ≥ 0.7 (blue) and postbronchodilator FEV1/SVC < 0.7 (red). Interval-censored proportion hazards regression analysis was used to examine the association of FEV1/SVC < 0.7 with progression to COPD during the follow-up period. SVC = slow vital capacity.
Prebronchodilator Analysis
The prebronchodilator analysis included 645 participants with prebronchodilator FEV1/FVC of ≥ 0.7 and FEV1 % predicted of ≥ 80%, and it showed similar results to the postbronchodilator analysis, except that no association was found of prebronchodilator FEV1/SVC < 0.7 with percent gas trapping, small airway disease, and exacerbations, as opposed to the main analysis findings (e-Tables 3-7).
LLN Analysis
The LLN analysis included 864 participants with prebronchodilator FEV1/FVC = the LLN or more and FEV1 equal to the LLN or more, and it showed similar results to the postbronchodilator analysis, except that no association was found of prebronchodilator FEV1/SVC less than the LLN with small airway disease and exacerbations, as opposed to the main analysis findings (e-Tables 8, 9).
Discussion
Among current and former smokers who were not diagnosed with COPD based on normal postbronchodilator spirometry results,25 we found that participants with postbronchodilator FEV1/SVC < 0.7 experienced more emphysema, gas trapping, and severe exacerbations and that they were more likely to demonstrate COPD relative to those patients with postbronchodilator FEV1/SVC ≥ 0.7. We found similar results when we examined the association of prebronchodilator FEV1/SVC < 0.7 or FEV1/SVC less than the LLN with chest CT scan features and progression to COPD.
Vital capacity can be measured either at forced expiration (FVC), at slow expiration (SVC), or at inspiration (inspiratory VC).28 Although SVC and FVC in theory should be the same in a healthy population with normal lungs, SVC usually is larger than FVC, especially in obese people or those with airways disease.29,30 In individuals with no obstructive lung disease, the difference between SVC and FVC increases with increasing BMI and age.29,30 Current lung function test interpretation guidelines acknowledge that inspiratory or expiratory SVC may be a better estimate of VC than FVC, but they do not provide specific recommendations regarding whether SVC should be used to calculate the FEV1/VC.6 Nevertheless, professional organizations have proposed the FEV1 to VC instead of the FEV1/FVC as a diagnostic criterion for COPD at certain times.31 The FEV1/SVC may be more sensitive than the FEV1/FVC to diagnose COPD, likely because FVC often may underestimate the true VC. The FVC maneuver increases intrathoracic pressure, which may lead to the collapse of small airways before the end of expiration, an effect that also shortens exhalation time. In individuals with mild obstructive lung disease, this phenomenon may result in pseudonormalization of the FEV1/FVC, whereas in those with substantial obstructive lung disease, it may result in preserved ratio impaired spirometry results.32, 33, 34 Using the FEV1/SVC instead of the FEV1/FVC results in an increase in the reported prevalence of COPD35,36 and may lead to overdiagnosis, in particular among elderly individuals.7 Nevertheless, in the absence of a true gold standard for COPD diagnosis, the usefulness of a diagnostic test is related highly to its association with the clinical, functional, and radiographic features of a disease.37 The main purpose of this analysis was not to evaluate FEV1/SVC as a tool to diagnose the COPD. Instead, we decided to evaluate the usefulness of FEV1/SVC in individuals at risk for development, but without COPD according to current guidelines.1 Our goal was to use the information commonly obtained by routine spirometry further, which could be useful for identifying early airflow abnormalities predictive of the development of COPD.38
In our analysis, FEV1/SVC < 0.7 was not associated with high mMRC, CAT score, or chronic bronchitis, likely because the participants in the study had relatively preserved lung function and they were fairly asymptomatic. In a similar study by Saint-Pierre et al7 that included adults with a prebronchodilator FEV1/FVC of more than the LLN and total lung capacity of more than the LLN, the average of mMRC was only 1.5 in those FEV1/SVC less than the LLN and in those with FEV1/SVC equal to the LLN or more, the average of mMRC was 0.5. Our data contribute to the published literature by showing that, among smokers with normal postbronchodilator spirometry results, a postbronchodilator FEV1/SVC < 0.7 was associated with greater radiographic emphysema and gas trapping relative to those with postbronchodilator FEV1/SVC ≥ 0.7. The association of FEV1/SVC < 0.7 with emphysema is not surprising, because emphysema may result in expiratory airway collapse and increase in the difference between SVC and FVC.39 Thus, individuals with low to normal FEV1 who do not meet the criteria for COPD diagnosis using the FEV1/FVC often have an abnormal FEV1/SVC. Consistent with our findings, the study by Saint-Pierre et al7 showed that individuals with an FEV1/SVC less than the LLN demonstrated a higher airway resistance and residual volume than patients with FEV1/SVC equal to the LLN or more and were more likely to be diagnosed with obstructive lung diseases such as asthma and COPD relative to patients with FEV1/SVC equal to the LLN or more. We must note that in this cohort of current or formers smokers with normal spirometry results, approximately 15% of the participants self-reported asthma, which may be associated with different clinical features (ie, emphysema, exacerbations, and progression to airways obstruction) than those in individuals without history of asthma.
In contrast to several studies focused mainly on the association of low FEV1/SVC and the clinical characteristics of COPD,7 we also evaluated the outcomes over at least 3 years of follow-up, thus allowing for longitudinal evaluation of the significance of an abnormal FEV1/SVC. Our findings add to other recent reports demonstrating that other physiologic abnormalities may precede formal diagnosis of COPD.4,40,41 Air trapping based on radiographic lung volumes predicts accelerated spirometry decline and progression to COPD in smokers without obstruction.4,33,40,41 Based on the above, we conclude that FEV1/SVC can be a simple, routinely available spirometric index that can identify individuals who may benefit from early intervention such as smoking cessation. Confirmation of obstructive lung disease by an objective measurement like FEV1/SVC may motivate smokers to quit smoking,42,43 which is the only proven intervention that can modify disease progression. A study showed that pharmacotherapy in COPD patients with mild-to-moderate lung function impairment also may be beneficial.44 An ongoing multicenter randomized controlled trial aims to examine the effect of bronchodilators in symptomatic smokers with preserved spirometry.45
Apart from its retrospective nature, our study has some limitations. SPIROMICS was not a population-based study. We analyzed a cohort of heavy smokers older than 40 years; thus, any generalization warrants caution. We did not take into consideration other risk factors for obstructive lung disease like occupational exposure. Given the lack of widely accepted reference values for FEV1/SVC in the US population, we used the 0.7 as a cutoff for the FEV1/VC for the main analysis. Nevertheless, we observed similar findings when we examined the association of FEV1/SVC less than the LLN with radiographic features and progression to COPD in those with FEV1 equal to the LLN or more and FEV1/FVC equal to the LLN or more. In this regard, a study by Bhatt et al46 demonstrated that fixed ratio is actually superior to the LLN in the ability to predict hospitalization, mortality, or both.
In conclusion, an FEV1/SVC < 0.7 or LLN may be used as a metric of early obstruction and may be useful tool in identifying individuals at increased risk of COPD. An FEV1/SVC < 0.7 or LLN in current and former smokers with normal spirometry results can identify individuals with increased emphysema, gas trapping, and risk of progressing to COPD in the future. Further research should evaluate whether the FEV1/SVC should be used in addition to the current diagnostic criteria to identify individuals at high risk for COPD who potentially may benefit from early interventions like smoking cessation or pharmacotherapy.
Acknowledgments
Author contributions: S. F. and I. B. had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the analysis and contributed equally to the conception and design of the study. All authors contributed to data collection and drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. F. has received grants from the American Thoracic Society and Fisher &Paykel. I. B. has consulted with AstraZeneca, Boehringer Ingelheim, CSL Behring, Grifols, Verona Pharma, GE Healthcare, Mylan, Theravance, and GSK and has received research grants from AMGEN, Theravance, Mylan, and GE Healthcare. A. C. has consulted for GSK and VIDA Diagnostics. S. P. B. has served on advisory boards for Sunovion and GlaxoSmithKline. E. A. H. a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed. M. K. H. has consulted for GSK, Boehringer Ingelheim, and AstraZeneca and has received research support from Novartis and Sunovion. R. P. reports grants from NHLBI, grants from COPD Foundation, NHLBI and Department of Veterans Affairs, outside the submitted work. M. T. D. reports receiving grants from the NIH, the Department of Defense, and the American Heart Association; consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Yungjin, PneumRx/BTG, Pulmonx, Genentech, Boston Scientific, Quark Pharmaceuticals, and Mereo; and received grants from American Lung Association and NIH. R. G. Buhr received personal fees from GlaxoSmithKline and Mylan/Theravance Biopharma, grants from NIH/National Center for Advancing Translational Sciences, and grants from NIH/NHLBI outside the submitted work. R. G. Barr has received grants from the NIH, Foundation for the NIH, COPD Foundation, and Alpha-1 Foundation. M. B. D. reports grants from NIH-NHLBI, during the conduct of the study; personal fees from Boehringer-Ingelheim, personal fees from GlaxoSmithKline, personal fees from AstraZeneca, personal fees from Mylan-Theravance, grants from Department of Defense, personal fees from Novavax, personal fees from Parion, personal fees from Midmark, and personal fees from Philips, outside the submitted work. M. A. was supported by a research grant from the Flight Attendant Medical Research Institute (FAMRI). R. J. K. received grants and personal fees from Genentech, Boehringer Ingelheim, Medimmune/Astra Zeneca, and Gilead. V. K. has consulted for Boehringer Ingelheim, Gala Therapeutics, and AstraZeneca and has received personal fees from ABIM. J. L. C. is supported by Merit Review award I01 CX000911 from the Department of Veterans Affairs, and reports grants the Department of Defense, the National Institutes of Health, and personal fees from AstraZeneca. R. B. served on the advisory boards (GlaxoSmithKline, Boehringer Ingelheim, and Mylan Pharmaceuticals) and received research grants from GlaxoSmithKline and Boehringer Ingelheim. F. M. reports grants from NHLBI and the National Institutes of Health, personal fees from Continuing Education, personal fees from Forest Laboratories, Janssen, GlaxoSmithKline, Nycomed/Takeda, AstraZeneca, Boehringer Ingelheim, Bellerophon (formerly Ikaria), Genentech, Novartis, Pearl, Roche, Sunovion, Theravance, CME Incite, Annenberg Center for Health Sciences at Eisenhower, Integritas, InThought, National Association for Continuing Education, Paradigm Medical Communications, LLC, PeerVoice, UpToDate, Haymarket Communications, Western Society of Allergy and Immunology, Proterixbio (formerly Bioscale), Unity Biotechnology, ConCert Pharmaceuticals, Lucid, Methodist Hospital, Columbia University, Prime Healthcare, Ltd., WebMD, PeerView Network, California Society of Allergy and Immunology, Chiesi, and the Puerto Rico Thoracic Society outside the submitted work. W. W. L. reports nonfinancial support from Pulmonx and personal fees from Konica Minolta outside the submitted work. C. B. C. has consulted with PulmonX, has received research funding from Equinox Fitness Clubs and Amgen, and is employed part-time by the GlaxoSmithKline Global Respiratory Franchise. G. C. reports grants from Boehringer-Ingelheim, Novartis, AstraZeneca, Respironics, MedImmune, Actelion, Forest, Pearl, Ikaria, Aeris, PneumRx, and Pulmonx and other from HGE Health Care Solutions, Inc., Amirall, Boehringer-Ingelheim, and Holaira. N. N. H. reports grants from the National Institutes of Health, COPD Foundation, and Boehringer Ingelheim. P. W. reports receiving personal fees for consultancy from Theravance, AstraZeneca, Regeneron, Sanofi, Genentech, Roche, and mJanssen. Dr Barr reports receiving grants from the COPD Foundation, the Alpha1 Foundation, the USnEnvironmental Protection Agency (EPA), and the NIH. J. A. K. has received research grants from the NIH, the Patient Centered Outcomes Research Institute, ResMed and Inogen, and Sanofi outside of the submitted work. D. T. reports personal fees from Boehringer-Ingelheim, AstraZeneca, Sunovion, Theravance/Innoviva, and Mylan outside the submitted work. None declared (B. D., R. E. K., B. R., N. R. P. B., D. H., V. E. O., D. C.).
Subpopulations and Intermediate Outcome Measures in COPD Study Collaborators: Neil E. Alexis, PhD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Ronald G. Crystal, MD; Melissa Freeman, PhD; Annette T. Hastie, PhD; Eric C. Kleerup, MD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Deborah A. Meyers, PhD; John D. Newell Jr, MD; Elizabeth C. Oelsner, MD, MPH; Nirupama Putcha, MD, MHS; Mary Beth Scholand, MD; and Robert A. Wise, MD. The project officers from the Lung Division of the National Heart, Lung and Blood Institute were Lisa Postow, PhD, and Thomas Croxton, PhD, MD. More information about the study and how to access SPIROMICS data is at www.spiromics.org.
Data sharing statement: The datasets used or analyzed, or both, during the current study are available from the corresponding author on reasonable request.
Other contributions: The authors thank Miguel Quibrera, MS, for statistical assistance and the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Supported by the National Institutes of Health [Grants R01HL125432-01A1 (M. B. D.), T32HL007106-41 (R. M. B.), TL1TR001883-01 (R. G. Buhr), R01HL151421 (S. P. B.), R21EB027891 (S. P. B.), and K23HL133438 (S. P. B.)]. SPIROMICS was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health [Contracts HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C; Grants U01 HL137880 and U24 HL141762] and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer, Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici S.p.A., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Novartis Pharmaceuticals Corporation, Nycomed GmbH, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi; Sunovion, Takeda Pharmaceutical Company, and Theravance Biopharma and Mylan. The parametric response mapping analyses were supported by the National Heart, Lung, and Blood Institute, National Institutes of Health [Grants HL122438 and HL138188]. S. F. was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health, Veterans Rural Health Resource Center [Grant 14380] and the Health Services Research and Development Service through the Comprehensive Access and Delivery Research and Evaluation Center [Grant CIN 13-412].
Supplementary Data
Visual Abstract.
References
- 1.Singh D., Agusti A., Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 2.Regan E.A., Lynch D.A., Curran-Everett D. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff P.G., Barr R.G., Bleecker E. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe K.E., Regan E.A., Anzueto A. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019;6(5):384–399. doi: 10.15326/jcopdf.6.5.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chhabra S.K. Forced vital capacity, slow vital capacity, or inspiratory vital capacity: which is the best measure of vital capacity? J Asthma. 1998;35(4):361–365. doi: 10.3109/02770909809075669. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 7.Saint-Pierre M., Ladha J., Berton D.C. Is the slow vital capacity clinically useful to uncover airflow limitation in subjects with preserved FEV1/FVC? Chest. 2019;156(3):497–506. doi: 10.1016/j.chest.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Couper D., LaVange L.M., Han M. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 10.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones P.W., Harding G., Berry P., Wiklund I., Chen W.H., Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 12.Jones P.W., Quirk F.H., Baveystock C.M. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33-27. [DOI] [PubMed] [Google Scholar]
- 13.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Sieren J.P., Newell J.D., Jr., Barr R.G. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galban C.J., Han M.K., Boes J.L. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt S.P., Soler X., Wang X. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(2):178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim V., Crapo J., Zhao H. Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns R.J., Deschenes S.S., Schmitz N. Associations between depressive symptoms and social support in adults with diabetes: comparing directionality hypotheses with a longitudinal cohort. Ann Behav Med. 2016;50(3):348–357. doi: 10.1007/s12160-015-9760-x. [DOI] [PubMed] [Google Scholar]
- 19.Brecthel L., Gainey J., Penwell A., Nathaniel T.I. Predictors of thrombolysis in the telestroke and non telestroke settings for hypertensive acute ischemic stroke patients. BMC Neurol. 2018;18:215. doi: 10.1186/s12883-018-1204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortis S., O’Shea A.M.J., Beck Mae B.F. A simplified critical illness severity scoring system (CISSS): development and internal validation. J Crit Care. 2020;61:21–28. doi: 10.1016/j.jcrc.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):30. doi: 10.3978/j.issn.2305-5839.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueira Goncalves J.M., Garcia Bello M.A., Golpe R., Alonso Jerez J.L., Garcia-Talavera I. Impact of diabetes mellitus on the risk of severe exacerbation in patients with chronic obstructive pulmonary disease. Clin Respir J. 2020 doi: 10.1111/crj.13255. [DOI] [PubMed] [Google Scholar]
- 23.Han M.K., Quibrera P.M., Carretta E.E. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortis S., Comellas A., Make B.J. Combined forced expiratory volume in 1 second and forced vital capacity bronchodilator response, exacerbations, and mortality in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16(7):826–835. doi: 10.1513/AnnalsATS.201809-601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). https://goldcopd.org/gold-reports/. Accessed October 1, 2020.
- 26.Fortis S., Eberlein M., Georgopoulos D., Comellas A.P. Predictive value of prebronchodilator and postbronchodilator spirometry for COPD features and outcomes. BMJ Open Respir Res. 2017;4(1) doi: 10.1136/bmjresp-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han M.K., Kim M.G., Mardon R. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 28.Fortis S. Lost in interpretation: should the highest VC value be used to calculate the FEV1/VC? Int J Chron Obstruct Pulmon Dis. 2016;11:2167–2170. doi: 10.2147/COPD.S116214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortis S., Corazalla E.O., Wang Q., Kim H.J. The difference between slow and forced vital capacity increases with increasing body mass index: a paradoxical difference in low and normal body mass indices. Respir Care. 2015;60(1):113–118. doi: 10.4187/respcare.03403. [DOI] [PubMed] [Google Scholar]
- 30.Huprikar N.A., Skabelund A.J., Bedsole V.G. Comparison of forced and slow vital capacity maneuvers in defining airway obstruction. Respir Care. 2019;64(7):786–792. doi: 10.4187/respcare.06419. [DOI] [PubMed] [Google Scholar]
- 31.Swanney M.P., Ruppel G., Enright P.L. Using the lower limit of normal for the FEV1/FVC reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 32.Wan E.S., Fortis S., Regan E.A. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortis S., Comellas A., Kim V. Low FVC/TLC in preserved ratio impaired spirometry (PRISm) is associated with features of and progression to obstructive lung disease. Sci Rep. 2020;10(1):5169. doi: 10.1038/s41598-020-61932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz A., Arnold N., Skinner B. Preserved ratio impaired spirometry in a spirometry database. Respir Care. 2021;66(1):58–65. doi: 10.4187/respcare.07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toren K., Olin A.C., Lindberg A. Vital capacity and COPD: the Swedish CArdioPulmonary bioImage Study (SCAPIS) Int J Chron Obstruct Pulmon Dis. 2016;11:927–933. doi: 10.2147/COPD.S104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barros A.R., Pires M.B., Raposo N.M. Importance of slow vital capacity in the detection of airway obstruction. J Bras Pneumol. 2013;39(3):317–322. doi: 10.1590/S1806-37132013000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirozzi C.S., Gu T., Quibrera P.M. Heterogeneous burden of lung disease in smokers with borderline airflow obstruction. Respir Res. 2018;19(1):223. doi: 10.1186/s12931-018-0911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoesterey D., Das N., Janssens W. Spirometric indices of early airflow impairment in individuals at risk of developing COPD: spirometry beyond FEV1/FVC. Respir Med. 2019;156:58–68. doi: 10.1016/j.rmed.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topalovic M., Exadaktylos V., Peeters A. Computer quantification of airway collapse on forced expiration to predict the presence of emphysema. Respir Res. 2013;14:131. doi: 10.1186/1465-9921-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arjomandi M., Zeng S., Barjaktarevic I. Radiographic lung volumes predict progression to COPD in smokers with preserved spirometry in SPIROMICS. Eur Respir J. 2019;54(4):1802214. doi: 10.1183/13993003.02214-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng S., Tham A., Bos B., Jin J., Giang B., Arjomandi M. Lung volume indices predict morbidity in smokers with preserved spirometry. Thorax. 2019;74(2):114–124. doi: 10.1136/thoraxjnl-2018-211881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guirguis-Blake J.M., Senger C.A., Webber E.M., Mularski R.A., Whitlock E.P. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378–1393. doi: 10.1001/jama.2016.2654. [DOI] [PubMed] [Google Scholar]
- 43.Parkes G., Greenhalgh T., Griffin M., Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336(7644):598–600. doi: 10.1136/bmj.39503.582396.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y., Zhong N.S., Li X. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 45.National Institutes of Health Clinical Center . National Institutes of Health; Bethesda, MD: 2016. RETHINC: REdefining THerapy In Early COPD for the Pulmonary Trials Cooperative (RETHINC). ClinicalTrials.gov. [Google Scholar]
- 46.Bhatt S.P., Balte P.P., Schwartz J.E. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321(24):2438–2447. doi: 10.1001/jama.2019.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.