Abstract
Addiction is best described as a disorder of maladaptive neuroplasticity involving the simultaneous strengthening of reward circuitry that drives compulsive drug seeking and weakening of circuits involved in executive control over harmful behaviors. Psychedelics have shown great promise for treating addiction, with many people attributing their therapeutic effects to insights gained while under the influence of the drug. However, psychedelics are also potent psychoplastogens—molecules capable of rapidly re-wiring the adult brain. The advent of non-hallucinogenic psychoplastogens with anti-addictive properties raises the intriguing possibility that hallucinations might not be necessary for all therapeutic effects of psychedelic-based medicines, so long as the underlying pathological neural circuitry can be remedied. One of these non-hallucinogenic psychoplastogens, tabernanthalog (TBG), appears to have long-lasting therapeutic effects in preclinical models relevant to alcohol and opioid addiction. Here, we discuss the implications of these results for the development of addiction treatments, as well as the next steps for advancing TBG and related non-hallucinogenic psychoplastogens as addiction therapeutics.
Keywords: Psychedelic, psychoplastogen, neuroplasticity, ibogaine, neuropsychiatric disorder, substance use disorder, addiction, alcohol use disorder, opioid use disorder, tabernanthalog, TBG
COMMENT ON: Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, Vargas MV, McCarroll MN, Taylor JC, Myers-Turnbull D, Liu T, Yaghoobi B, Laskowski LJ, Anderson EI, Zhang G, Viswanathan J, Brown BM, Tjia M, Dunlap LE, Rabow ZT, Fiehn O, Wulff H, McCorvy JD, Lein PJ, Kokel D, Ron D, Peters J, Zuo Y, Olson DE. A non-hallucinogenic psychedelic analog with therapeutic potential. Nature 2021;589:474–479.
Addiction, like many other neuropsychiatric disorders, is the product of pathological neuroplasticity—structural and functional changes in neural networks that result in altered information processing and ultimately manifest as maladaptive behaviors. However, the majority of currently available drugs for treating addiction are intended to curb withdrawal and/or reduce cravings (eg, methadone, naltrexone, acamprosate, etc.). None directly target the underlying pathological neural circuitry of addiction. Like addiction, post-traumatic stress disorder (PTSD) has been described as a disease of learning and memory, with addiction and PTSD involving maladaptive plasticity in circuits encoding drug reward or trauma, respectively. 1 These structural and functional changes support long-lasting drug-cue or trauma-related memories that drive continued drug seeking in the case of addiction and pervasive fear in PTSD. The goal of cognitive behavioral therapy is to overwrite these deleterious associative memories; however, the adult brain has a limited capacity for plasticity. As a result, pharmacological approaches to enhance neuroplasticity have been used to facilitate cognitive behavioral therapy. In principle, small molecules capable of selectively promoting plasticity in key circuits could be used to combat diseases of learning and memory like addiction and PTSD without requiring therapy. Of the many brain regions involved in addiction, the prefrontal cortex (PFC) is a particularly attractive target for selective modulation by small molecules.
The PFC plays a critical role in the pathophysiology of addiction due to its ability to regulate limbic reward circuitry, modulate attention, and exert top-down control over drug-seeking behavior. 2 The inability to perceive negative consequences of substance use is a defining characteristic of addiction that can lead to hazardous use, social or interpersonal problems related to use, and physical or psychological problems related to use—3 of the 11 criteria used to define substance use disorder (SUD) in the Diagnostic and Statistical Manual 5 (DSM-5). This “myopia for the future” has also been observed in patients with lesions to the ventromedial PFC. 3
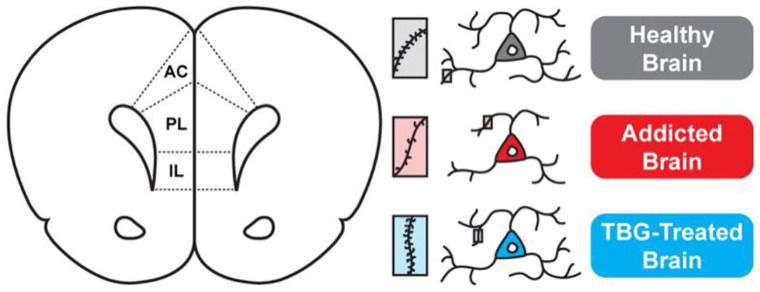
Numerous neuroimaging studies have observed abnormal PFC function in patients suffering from SUDs and alcohol use disorders (AUD). 2 Additionally, many drugs of abuse are known to impact the structure of cortical neurons, with reduced gray matter volume in the PFC being a hallmark of various SUDs including addiction to alcohol, heroin, methamphetamine, and cocaine. These structural and functional changes in the PFC are believed to worsen the disease by reducing executive control, exacerbating impulsivity, and leading to deficits in the extinction of drug-cue memories.1,4-6 Therefore, pharmacological strategies aimed at restoring PFC structure/function are hypothesized to have the potential for treating a variety of SUDs (Figure 1). Broadly speaking, the pathologic memories associated with the addicted brain are driven by overactive, bottom-up mesolimbic circuits, while therapeutic memories involve top-down corticolimbic circuits. Thus, treatments that enhance activity in corticolimbic circuits are potentially useful for treating addiction and related disorders involving cortical atrophy such as PTSD and depression.
Figure 1.
Hypothesized mechanism explaining the anti-addictive properties of TBG. The rodent medial PFC includes the infralimbic (IL), prelimbic (PL), and anterior cingulate (AC) cortex, which exert top-down control over a number of subcortical brain regions. Addiction is characterized by atrophy of neurons in the PFC (ie, the retraction of neurites and loss of dendritic spines). TBG-induced growth of cortical neurons is predicted to enhance the excitability of these neurons, thus re-establishing top-down corticolimbic control.
Perhaps the most straightforward approach for modulating such circuits is to change the structure and/or function of the atrophied cortical neurons in the addicted brain. The term “psychoplastogen” was introduced to describe drugs that produce such “mind-molding” effects. 7 Ketamine, an NMDA receptor antagonist, is perhaps the most well-known psychoplastogen. It induces rapid structural plasticity in the PFC and has shown promise for treating addiction in addition to other diseases involving cortical atrophy such as PTSD and depression. Classic psychedelics also produce psychoplastogenic effects including the growth of dendritic spines and the formation of new synapses. 8 Though many psychedelics are controlled substances, most are not addictive, and in fact, many have shown promise as anti-addictive therapeutics.9,10 While some researchers believe that the hallucinogenic properties of psychedelics play an essential role in their therapeutic mechanism of action, 11 the potent psychoplastogenic effects of psychedelics make it difficult to ascribe their therapeutic efficacy solely to psychological or neurobiological underpinnings. Hallucinogenic medicines are unlikely to become first-line treatments for mental illnesses because many patients, (1) are reluctant to take them, (2) are unable to take them due to contraindicated co-morbidities or genetic predispositions, or (3) are unable to afford taking them given the costs associated with medically supervised psychedelic sessions. If structural remodeling of the brain in the absence of hallucinations is sufficient to drive an anti-addictive phenotype, a larger number of patients could potentially benefit from non-hallucinogenic medicines inspired by psychedelic science. 12
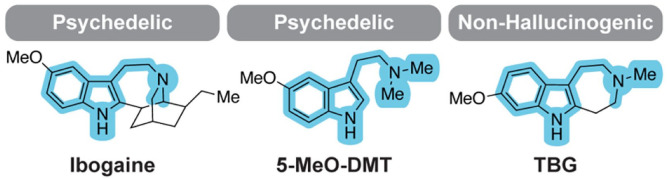
Recently, we described the design, synthesis, and evaluation of tabernanthalog (TBG)—a non-hallucinogenic psychoplastogen with structural similarities to ibogaine and 5-MeO-DMT (Figure 2). 13 Both ibogaine and 5-MeO-DMT have shown promise for treating addiction,14,15 but their hallucinogenic effects and cardiotoxicity have limited their therapeutic potential. The improved safety profile of TBG is encouraging given that it produces ibogaine-like efficacy in behavioral assays relevant to addiction. Moreover, TBG seems to work across addictive disorders, having demonstrated efficacy in assays relevant to treating both alcohol and heroin use disorders. Future research should determine if TBG’s anti-addictive properties extend to other substances such as nicotine, cocaine, and methamphetamine, as there are currently no FDA-approved drugs to treat cocaine or methamphetamine addiction. Therapeutic strategies capable of addressing multiple addictions simultaneously are rare, which emphasizes the uniqueness of a circuit-based approach to treating mental illness through restoration of PFC function. In fact, a single dose of TBG has recently been shown to rescue circuit-level deficits in the cortex induced by chronic unpredictable stress. 16
Figure 2.
Structural similarities of ibogaine, 5-MeO-DMT, and TBG. The common N,N-dimethyltryptamine core of all 3 molecules is highlighted.
The gold standard behavioral tests for measuring addiction in rodents are self-administration models, wherein animals self-regulate their own intake of drug by performing an operant response, typically a lever press, to obtain drug reward (ie, an intravenous infusion of heroin). Thus, we probed the therapeutic potential of TBG over multiple time points in a heroin self-administration model in rats. TBG potently inhibited heroin taking during self-administration, as well as heroin seeking when heroin was no longer available. Our initial studies found that the acute effects of TBG were not specific to heroin, as TBG also acutely inhibited lever pressing in animals trained to self-administer sucrose. Many researchers would tend to discount these “nonselective” effects on sucrose taking and seeking as performance deficits, but that might not be accurate given that TBG may be acting on circuits involved in both drug and nondrug rewards. 17 Moreover, TBG-treated animals did not exhibit any gross locomotor deficits while engaging in the operant task, so we were interested in performing additional experiments to determine if the ability of TBG to acutely reduce heroin taking and seeking was truly the result of a performance deficit.
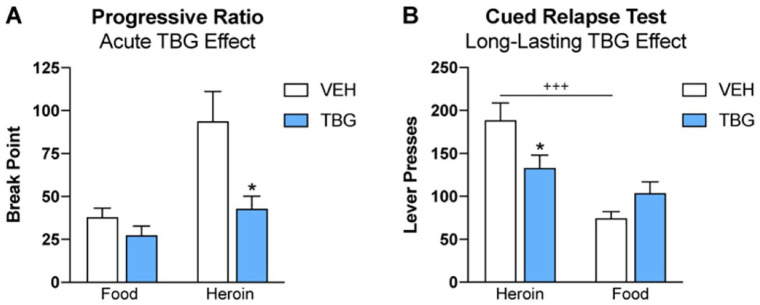
We trained animals to self-administer food or heroin by pressing distinct levers. Each reward was paired with a unique tone or light cue. After animals learned to self-administer both food and heroin, we subjected them to a progressive ratio test designed to assess their motivation to obtain each reward. The progressive ratio test has been used as a reliable measure of motivation because over the course of the test, the ratio requirement to obtain the reward is progressively increased on an exponential scale. Eventually, the animal reaches a break point, a price that the animal considers to be too high to obtain a single reward. When tested under these conditions, acute administration of TBG selectively reduced break points for heroin, but not food (Figure 3A). It is possible that the “nonselective” effects of TBG on sucrose taking that we reported previously may have been due, in part, to the low-price conditions of that test. When response requirements were raised, as in the progressive ratio test, a selective effect on motivation to seek heroin emerged.
Figure 3.
TBG specifically reduces motivation to seek heroin. (A) TBG (30 mg/kg, IP) or vehicle (VEH) was administered to male Wistar rats 30 minutes prior to a progressive ratio test of motivation to seek food or heroin (separate tests). TBG selectively reduced break points for heroin, but not food (planned comparison, 2-tailed t-test, *P < .05). Motivation to seek heroin is typically much greater than motivation to seek food. However, after administration of TBG, motivation to seek heroin and food were comparable. (B) Using male Wistar rats, we replicated the long-lasting effect of TBG on cued relapse for heroin (42.5 mg/kg cumulative dose, IP). This effect was specific to heroin relapse, and not food relapse (same test). Only VEH-treated rats relapsed at higher rates for heroin than for food (2-way RM ANOVA, Sidak’s post-hoc, *P < .05, +++P < .001).
In addition to acutely decreasing heroin seeking, a single administration of TBG protects against heroin relapse for at least 2 weeks. This long-lasting reduction in relapse rate was specific to heroin (ie, it was not observed in controls trained to self-administer sucrose) and is perhaps the most striking finding related to the anti-addictive properties of TBG. During self-administration, animals learn to associate the rewarding experience of drug administration with paired lights and tones. During extinction training, the rewarding drug is no longer available; animals learn that drug seeking is now futile, and drug seeking behavior is reduced. However, when exposed to drug cues (eg, lights and tones), pathological memories are activated that drive compulsive drug-seeking behavior leading to relapse. In such models, the PFC is a central regulator of relapse, perhaps in part due to the conflict that is created by extinction training.
Evidence suggests that extinction does not result in erasure of drug-cue memories that promote drug seeking, but rather creates a new, inhibitory memory trace that opposes this drive, resulting in active suppression of drug seeking. This notion is supported by findings demonstrating that deactivation of the infralimbic cortex—a subregion of the PFC—is sufficient to trigger relapse in these models.1,18 The infralimbic cortex serves a similar function in extinction of learned fear memories in animal models of PTSD, and brain-derived neurotrophic factor (BDNF) in the PFC is central to this type of therapeutic memory. 19 As mentioned above, the PFC is an important site of action for the antidepressant effects of psychoplastogens, and BDNF signaling is believed to play a key role in their effects on structural plasticity. 20 Thus, we hypothesize that structural plasticity in the infralimbic cortex might mediate the sustained anti-addictive effects of TBG (Figure 1). Using a photoactivatable Rac1, Liston and co-workers recently demonstrated that ketamine-induced spine growth in the PFC was responsible for its antidepressant effects in rodents. 21 A similar approach could be used to probe the importance of structural plasticity in the anti-addictive effects of TBG.
Drugs that produce long-lasting anti-addictive effects after a single administration are rare. Going forward, it will be important to determine whether added benefit is conferred by additional doses of TBG, and over what time frame. To mimic conditions that are likely to be used in clinical trials, we tested an increasing dose regimen of TBG in our preclinical heroin model—first with a dose of 2.5 mg/kg, then 10 mg/kg, and finally 30 mg/kg, with each administration spaced a few days apart. Thus, the cumulative dose was 42.5 mg/kg (compared to a single dose of 40 mg/kg used previously). Under these conditions, we again observed that TBG produced a long-lasting protective effect on heroin, but not food relapse (Figure 3B). These data provide the first internal replication of the long-lasting effect of TBG on heroin relapse and extend the finding to a cumulative dosing regimen. Though there are many additional steps toward translating these preclinical findings with TBG to the clinic, the data thus far are incredibly encouraging. Future studies should examine other dosing regimens, with multiple high doses of TBG, to enhance or extend the duration of TBG’s therapeutic effects on relapse. Additionally, mechanistic studies are needed to define both the receptors and circuits that mediate TBG’s anti-addictive properties. Finally, clinical studies in humans will be absolutely necessary for confirming and extending these preliminary findings.
We now know that through careful chemical design, it is possible to engineer non-hallucinogenic analogs of psychedelics capable of promoting structural neuroplasticity in cortical neurons. TBG is only one of several non-hallucinogenic psychoplastogens recently disclosed.22,23 It will be interesting to see if other non-hallucinogenic psychoplastogens like AAZ-A-154 (AAZ) produce similar anti-addictive effects, or if the therapeutic properties of TBG are unique to its ibogaine-like scaffold. We suspect that other psychoplastogens will also prove useful for treating addiction. Compounds that modulate neural circuits originating in the PFC that control drug-seeking behavior, motivation, and anxiety, have enormous potential for treating not only multiple SUDs, but also comorbid diseases such as depression and post-traumatic stress disorder (PTSD). Addiction is rarely an isolated disease, and compounds that simultaneously treat co-morbid conditions have a greater chance of achieving robust efficacy. While psychedelics have provided the blueprint for developing these types of circuit-modifying therapeutics, non-hallucinogenic psychoplastogens like TBG have distinct advantages that make them more likely to reach a larger patient population.
Footnotes
Declaration Of conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DEO is a co-founder of Delix Therapeutics, Inc. Delix Therapeutics has licensed technology from the University of California, Davis related to this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH R01DA045836 to JP; R01GM128997 to DEO.
Author Contributions: JP performed experiments and analyzed data; JP and DEO wrote the manuscript.
ORCID iD: David E Olson  https://orcid.org/0000-0002-4517-0543
https://orcid.org/0000-0002-4517-0543
References
- 1. Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458-1463. [DOI] [PubMed] [Google Scholar]
- 4. Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185-189. [DOI] [PubMed] [Google Scholar]
- 6. Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olson DE. Psychoplastogens: a promising class of plasticity-promoting neurotherapeutics. J Exp Neurosci. 2018;12:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ly C, Greb AC, Cameron LP, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dos Santos RG, Osório FL, Crippa JA, Riba J, Zuardi AW, Hallak JE. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol. 2016;6:193-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bogenschutz MP, Johnson MW. Classic hallucinogens in the treatment of addictions. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:250-258. [DOI] [PubMed] [Google Scholar]
- 11. Yaden DB, Griffiths RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cameron LP, Tombari RJ, Lu J, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 2021;589:474-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iyer RN, Favela D, Zhang G, Olson DE. The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs. Nat Prod Rep. 2021;38:307-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E. The epidemiology of 5-methoxy- N, N-dimethyltryptamine (5-MeO-DMT) use: benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J Psychopharmacol. 2018;32:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Ju, Tjia M, Mullen B, et al. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol Psychiatry. Published online May 25, 2021. doi: 10.1038/s41380-021-01159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology. 2019;44:465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peters J, Lalumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ly C, Greb AC, Vargas MV, et al. Transient stimulation with psychoplastogens is sufficient to initiate neuronal growth. ACS Pharmacol Transl Sci. 2021;4:452-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moda-Sava RN, Murdock MH, Parekh PK, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dunlap LE, Azinfar A, Ly C, et al. Identification of psychoplastogenic N,N-dimethylaminoisotryptamine (isoDMT) analogs through structure-activity relationship studies. J Med Chem. 2020;63:1142-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong C, Ly C, Dunlap LE, et al. Psychedelic-inspired drug discovery using an engineered biosensor. Cell. 2021;184:2779-2792.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]