Abstract
Immune checkpoint inhibitors (ICIs) have come to play an increasingly prominent role in the treatment of lung cancer, and some are recommended as a first-line treatment for late-stage non-small-cell lung cancer, either as a monotherapy or in combination with chemotherapy. Accordingly, the indications of Food and Drug Administration-approved ICIs have increased. In this background, China has implemented various policies to encourage and accelerate the marketing of domestic and imported innovative antitumor drugs. Eight ICIs have been approved in China. Among these, four imported programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors have received approval for six indications, and one domestic PD-1 inhibitor has received approval for one indication for lung cancer in 2018. Numerous clinical trials of ICIs for lung cancer are underway in China. This review aims to summarize the recent advances and future directions of ICIs, including PD-1 inhibitors, PD-L1 inhibitors, cytotoxic T lymphocyte-associated antigen-4 inhibitors, bi-specific antibodies, and a novel inhibitor of T-cell immune-receptor with Ig and immunoreceptor tyrosine-based inhibitory motif domains in immunotherapies for lung cancer in China.
Keywords: lung cancer, immunotherapy, progress, immune checkpoint inhibitors, China
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. The prognosis of lung cancer (non-small-cell lung cancer, NSCLC), with a 5-year overall survival (OS) rate of 21%, remains disappointing. 1 Immunotherapy targeting T-cell regulatory pathways is regarded a promising treatment for lung cancer and is expected to provide significant clinical benefits in cancer treatment. 2 Four programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors have demonstrated clinical activity and are approved by the National Comprehensive Cancer Network (NCCN) for the treatment of several indications in China. Nivolumab is recommended as a second-line monotherapy for advanced NSCLC. 3 Pembrolizumab is approved for first-line monotherapy of locally advanced or metastatic epidermal growth factor receptor (EGFR) mutation/anaplastic lymphoma kinase (ALK) mutation-negative NSCLC patients with a PD-1/PD-L1 tumor proportion score (TPS) of ⩾1%, first-line treatment with pemetrexed/platinum of metastatic non-squamous EGFR/ALK mutation-negative NSCLC, and first-line treatment with carboplatin and paclitaxel of metastatic squamous NSCLC. 4 Durvalumab is approved for the treatment of non-advanced stage III unresectable NSCLC after concurrent chemo-radiation. 5 Atezolizumab in combination with carboplatin and etoposide has been approved for the first-line treatment of extensive small-cell lung cancer (SCLC). 6 In this review, we summarize current research and ongoing trials of various immune checkpoint inhibitors (ICIs) and to examine the outcomes of treatments with these ICIs for the relevant indications in China with the aim to optimize treatment strategy. The presence of a small subset of long-term survivors suggests that inpatient ICI therapy is appropriate in certain cases. For this review, data were collected until February 2nd, 2021.
Data searches and study inclusion
PubMed, Embase, and Cochrane Library were systematically searched up to February 2nd, 2021, using the search terms “Carcinoma, lung cancer,” “immune checkpoint inhibitors,” “immune checkpoint blockade,” “Programmed death-1,” “Programmed death ligand-1”. The inclusion criteria were as follows: Original papers of human clinical trials that reported the outcomes of combination therapy using PD-1/PD-L1 inhibitors in Chinese patients with lung cancer. There were no restrictions on tumor stage, publication date, study population, language, or study design. The exclusion criteria were as follows: (i) studies from which data could not be extracted, (ii) duplicate reports (only the latest or the parent study was included), (iii) studies that reported only protocols, and (iv) abstracts for which the complete text was unavailable.
Approved ICIs
Nivolumab (Bristol Myers Squibb)
Nivolumab (Opdivo®) is a humanized anti-PD-1 IgG4 monoclonal antibody that exerts an antitumor effect by blocking the binding of PD-1 to PD-L1/PD-L2. 7 Nivolumab was approved by the Food and Drug Administration (FDA) for the second-line treatment of advanced squamous NSCLC. Based on the phase II CheckMate 063 trial 8 of nivolumab conducted in 2015, the optimal clinical dosage is 3 mg/kg. Two international, randomized, open-label, phase III trials, CheckMate 017 and CheckMate 057, compared the efficacy of nivolumab with those of docetaxel as a second-line treatment in patients with advanced squamous (CheckMate 017) 9 and non-squamous (CheckMate 057) NSCLCs. 10 These studies for the first time demonstrated the superiority of immunotherapy over chemotherapy as a second-line treatment for NSCLC and showed that nivolumab had improved efficacy and safety versus docetaxel in primarily North-American or European populations. The median OS in CheckMate017 was 9.2 months versus 6.0 months [hazard ratio (HR): 0.59, confidence interval (CI): 0.44–0.79, p < 0.001] whereas that in CheckMate057 was 12.2 months versus 9.4 months (HR: 0.73, CI: 0.62–0.88, p = 0.002), without a clinically significant difference in treatment safety. After a 3-year minimum follow-up, nivolumab achieved a higher OS rate at 18 months (39%) than docetaxel (23%), regardless of PD-L1 levels. 11 To evaluate the efficacy of nivolumab in the Chinese population and receive approval in China, a phase III study, CheckMate 078, 12 was registered. The study aimed to confirm the efficacy and safety of nivolumab as a second-line treatment in a predominantly Chinese population of patients with advanced or metastatic NSCLC. The CheckMate 078 results showed that nivolumab as a second-line monotherapy improved the median OS versus that of docetaxel (12.0 months versus 9.6 months, HR: 0.68, CI: 0.52–0.92, p < 0.001). Based on these results, nivolumab was approved for the second-line treatment of NSCLC in China in July 2018. This was the first anti-PD-1 monoclonal antibody to be approved in China. Long-term follow-up (minimum, 2 years) results showed that nivolumab maintained a superior OS over docetaxel (11.9 months versus 9.5 months, HR: 0.75, CI: 0.61–0.93, p < 0.001) in this predominantly Chinese patient population with previously treated NSCLC. 13 Nivolumab is currently used in nine clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China (Table 1).
Table 1.
Ongoing clinical studies of immune checkpoint inhibitors conducted for lung cancer in China.
| No. | Agents | Identifiers | Current status | Indications | Enrollment | Launched date |
|---|---|---|---|---|---|---|
| 1 | Nivolumab | CTR20200425 | Ongoing, not recruiting | Previously untreated locally advanced NSCLC | 888 | 4/3/2020 |
| CTR20180929 | Recruitment completed | NSCLC | 642 | 7/9/2018 | ||
| CTR20180914 | Recruiting | NSCLC | 420 | 7/9/2018 | ||
| CTR20171020 | Recruiting | NSCLC | 400 | 9/25/2017 | ||
| CTR20170694 | Recruitment completed | Extensive-stage SCLC | 907 | 7/24/2017 | ||
| CTR20170541 | Recruiting | Advanced or metastatic NSCLC (EGFR mutation+, T790M-, first-line TKI treatment failed) | 500 | 6/29/2017 | ||
| CTR20170304 | Recruiting | NSCLC | 721 | 4/13/2017 | ||
| CTR20160578 | Recruitment completed | Limited or extensive-stage NSCLC | 480 | 9/13/2016 | ||
| CTR20150767 | Recruitment completed | Advanced or metastatic NSCLC | 500 | 11/11/2015 | ||
| 2 | Pembrolizumab | CTR20191820 | Ongoing, not recruiting | Non-squamous NSCLC (EGFR-, ALK-) | 714 | 9/23/2019 |
| CTR20191591 | Recruiting | Neoadjuvant or adjuvant therapy of resectable | 570 | 9/16/2019 | ||
| Stage II B or III A NSCLC | ||||||
| CTR20191589 | Ongoing, not recruiting | First-line therapy of metastatic NSCLC with PD-L1+ | 620 | 8/12/2019 | ||
| CTR20181883 | Recruiting | Metastatic non-squamous NSCLC | 786 | 10/22/2018 | ||
| 3 | Durvalumab | CTR20200425 | Ongoing, not recruiting | Previously untreated locally advanced NSCLC | 888 | 4/3/2020 |
| CTR20190131 | Recruiting | Limited stage SCLC | 600 | 6/18/2019 | ||
| CTR20181576 | Recruiting | Locally advanced NSCLC | 360 | 4/8/2019 | ||
| CTR20170012 | Recruiting | Advanced NSCLC | 440 | 1/19/2017 | ||
| 4 | Atezolizumab | CTR20192714 | Ongoing, not recruiting | Extensive-stage SCLC | 200 | 2/17/2020 |
| CTR20190821 | Recruiting | Stage IV non-squamous NSCLC | 306 | 8/14/2019 | ||
| CTR20190668 | Recruiting | Locally advanced or metastatic NSCLC | 100 | 4/11/2019 | ||
| CTR20181628 | Ongoing, not recruiting | NSCLC | 302 | 11/7/2018 | ||
| CTR20180582 | Recruitment completed | Previously treated locally advanced or metastatic NSCLC | 621 | 7/2/2018 | ||
| CTR20171629 | Recruiting | Primary treatment of advanced or relapsed or metastatic NSCLC | 441 | 1/18/2018 | ||
| CTR20170064 | Recruitment completed | Previously untreated with chemotherapy stage IV non-squamous NSCLC | 578 | 4/6/2017 | ||
| CTR20160994 | Recruitment | Primary treatment of stage IV NSCLC with high expression of PD-L1 | 502 | 6/23/2017 | ||
| CTR20160988 | Recruitment completed | Stage III extensive-stage SCLC | 502 | 2/15/2017 | ||
| CTR20160510 | Recruiting | Completely resected stage IB-IIIA NSCLC | 760 | 1/11/2017 | ||
| 5 | Toripalimab | CTR20192525 | Ongoing, not recruiting | Recurrent and metastatic NSCLC | 124 | 12/19/2019 |
| CTR20192179 | Recruiting | Operative stage III NSCLC | 306 | 11/6/2019 | ||
| CTR20191139 | Recruiting | Extensive-stage SCLC | 406 | 4/11/2019 | ||
| CTR20190768 | Recruiting | Advanced NSCLC with positive EGFR sensitive mutation that failed TKI treatment | 350 | 4/19/2019 | ||
| CTR20190147 | Recruiting | Advanced NSCLC | 450 | 1/24/2019 | ||
| 6 | Sintilimab | CTR20192164 | Ongoing, not recruiting | Advanced NSCLC | 24 | 11/16/2019 |
| CTR20190972 | Recruiting | Non-squamous NSCLC | 480 | 6/14/2019 | ||
| CTR20190968 | Recruiting | Non-squamous NSCLC | 480 | 6/6/2019 | ||
| CTR20182559 | Active suspension | Non-squamous NSCLC | 480 | 1/22/2019 | ||
| CTR2018255 | Active suspension | Non-squamous NSCLC | 480 | 1/10/2019 | ||
| CTR20181437 | Recruitment completed | Advanced or metastatic NSCLC | 348 | 9/11/2018 | ||
| CTR20180975 | Recruitment | Non-squamous NSCLC | 378 | 7/23/2018 | ||
| completed | ||||||
| 7 | Camrelizumab | CTR20200638 | Ongoing, not recruiting | Relapse or metastasis PD-L1 positive NSCLC without systemic treatment | 762 | 4/13/2020 |
| CTR20200637 | Ongoing, not recruiting | Relapse or metastasis NSCLC without systemic treatment | 762 | 4/20/2020 | ||
| CTR20190113 | Recruiting | Stage IV non-squamous NSCLC with KRAS mutation | 230 | 2/13/2019 | ||
| CTR20181611 | Recruitment completed | Stage IV squamous NSCLC | 360 | 9/13/2018 | ||
| 8 | BGB-A317 | CTR20190511 | Recruiting | Extensive-stage SCLC | 364 | 5/17/2019 |
| CTR20181746 | Recruitment completed | Squamous NSCLC | 342 | 11/25/2019 | ||
| CTR20180292 | Recruitment completed | Squamous NSCLC | 342 | 7/26/2018 | ||
| CTR20180032 | Recruitment completed | Non-squamous NSCLC | 320 | 7/10/201 | ||
| CTR20171112 | Recruiting | NSCLC | 641 | 10/12/2017 | ||
| CTR20170361 | Recruitment completed | Lung cancer | 60 | 7/27/2017 | ||
| 9 | AK104 | CTR20191326 | Recruiting | Advanced solid tumor | 120 | 7/23/2019 |
| (NSCLC, melanoma, nasopharyngeal carcinoma, etc.) | ||||||
| 10 | KN046 | CTR20191219 | Recruiting | NSCLC | 50 | 6/25/2019 |
| CTR20190195 | Recruiting | NSCLC | 149 | 1/31/2019 |
ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1; SCLC, small cell lung cancer; TKI
Pembrolizumab (Merck)
Pembrolizumab (Keytruda®) is a selective human anti-PD-1 IgG4 monoclonal antibody that blocks PD-1 binding to PD-L1 and PD-L2. 14 Pembrolizumab has been approved for three indications for the first-line treatment of advanced NSCLC in China based on the results of three clinical studies, namely, KEYNOTE-042, 4 KEYNOTE-189, 15 and KEYNOTE-407. 16
KEYNOTE-042 was an open-label, randomized, controlled, large phase III clinical trial comparing pembrolizumab monotherapy with platinum-based chemotherapy as a first-line therapy for patients with locally advanced or metastatic NSCLC and a PD-L1 TPS of ⩾1%. The exploratory endpoints were OS and progression-free survival (PFS) in patients with a TPS of 1–49% and objective response among those with a TPS of ⩾50%, 1–49%, or ⩾1%. The KEYNOTE-042 results showed that pembrolizumab alone is effective in patients with a PD-L1 TPS of ⩾50%, with a median OS of 16.7 months, and in patients with a PD-L1 TPS of 1–49%. The final analysis results showed that pembrolizumab could significantly prolong total survival and was safer than chemotherapy, regardless of whether the PD-L1 TPS was ⩾50% (median OS: 20.0 months versus 12.2 months, HR 0.69, 0.56–0.85; p < 0.001), ⩾20% (median OS: 18.0 months versus 13.0 months, HR 0.77, 0.64–0.92; p = 0.002), or ⩾1% (median OS: 16.4 months versus 12.1 months, HR 0.81, 0.71–0.93; p = 0.002). A Chinese expansion cohort study (NCT03850444) of pembrolizumab was conducted parallel to the global KEYNOTE-042 study; 262 Chinese patients with PD-L1-positive (TPS ⩾1%) NSCLC were enrolled (global, n = 92; China extension, n = 170) and randomized to pembrolizumab (n = 128) or chemotherapy (n = 134). The results in terms of efficacy were consistent between the Chinese population cohort and the global cohort at the 2019 World Conference on Lung Cancer (WCLC). 17 Pembrolizumab provided a better survival benefit in all PD-L1 TPS groups (⩾50%, ⩾20%, and ⩾1%) of patients with untreated locally advanced or metastatic (no EGFR/ALK mutations) NSCLC than chemotherapy. Moreover, the median OS in the Chinese population was equivalent to that in the global cohort regardless of PD-L1 expression. No new adverse events (AEs) were observed in the Chinese expansion cohort study. Based on the results of KEYNOTE-042 and the expansion study, pembrolizumab was approved for the first-line monotherapy of locally advanced or metastatic EGFR/ALK-negative NSCLC with PD-L1 TPS of ⩾1% in China in October 2019.
To improve the objective response rate (ORR) further and maximize the benefits of immunotherapy, combinations of chemotherapy with an immune monoclonal antibody were extensively investigated. Based on the KEYNOTE-189 study, pembrolizumab was the first to receive approval for the first-line treatment of non-squamous cell carcinoma by immune-combined chemotherapy in China. KEYNOTE-189 was a large phase III, randomized, controlled study that included untreated, metastatic, wild-type EGFR/ALK, non-squamous NSCLC patients. The patients were randomly assigned (2:1) to pemetrexed and a platinum-based drug (cisplatin or carboplatin) plus pembrolizumab or placebo every 3 weeks for four cycles. After four cycles, the patients in the pembrolizumab group were maintained on pembrolizumab plus pemetrexed, whereas the patients in the control group were treated with placebo plus pemetrexed. After a median follow-up of 10.5 months, the estimated OS rate at 12 months was 69.2% (64.1–73.8%) in the pembrolizumab-combination group versus 49.4% (42.1–56.2%) in the placebo-combination group (HR: 0.49, CI: 0.38–0.64, p < 0.001). The median PFS was 8.8 months in the pembrolizumab-combination group and 4.9 months in the placebo-combination group (HR: 0.52, CI: 0.43–0.64, p < 0.001). Based on these results, pembrolizumab plus chemotherapy (cisplatin/carboplatin + pemetrexed) was recommended as a first-line treatment for non-squamous NSCLC in the NCCN guidelines 18 and was approved for this indication in China at the end of March 2019. According to the final results presented at the 2020 American Society of Clinical Oncology (ASCO) meeting, 19 the pembrolizumab plus chemotherapy group had significantly better outcomes than the control group, with a median OS of 22.0 months (19.5–24.5 months) versus 10.6 months (8.7–13.6 months; HR: 0.56, CI: 0.46–0.69, p < 0.001) and a median PFS of 9.0 months (8.1–10.4 months) versus 4.9 months (4.7–5.5 months; HR 0.49, CI: 0.41–0.59, p < 0.001). There were no significant differences in the incidence of AEs of any grade and the mortality rate between the immune and control groups. The 2-year OS rate was 45.7% versus 27.3%, the 2-year PFS rate was 22.0% versus 3.4%, and the ORR was 48.3% versus 19.9%. The addition of pembrolizumab to standard chemotherapy of pemetrexed and a platinum-based drug resulted in significantly improved efficacy and safety compared with those for chemotherapy alone.
KEYNOTE-407, a randomized, double-blind, phase III study in patients with squamous cell carcinoma, combined carboplatin and paclitaxel or albumin-bound paclitaxel, allowing patients in the control group to cross over to pembrolizumab after progression. With a median follow-up time of 7.8 months, the median OS was 15.9 months versus 11.3 months (HR: 0.64, CI: 0.49–0.85, p < 0.001), and the median PFS was 6.4 months versus 4.8 months (HR: 0.56, CI: 0.45–0.70, p < 0.001). Like for KEYNOTE-042, for approval for launch in China, a China expansion cohort study including 125 patients was carried out. 20 The chemotherapy regimen was paclitaxel plus carboplatin. The results showed that pembrolizumab combined with chemotherapy was significantly superior to chemotherapy alone, with a median OS of 17.3 months versus 12.6 months and a median PFS of 8.3 months versus 4.2 months. Interestingly, the HR value in the Chinese expansion cohort was lower than that in the global cohort (OS: HR: 0.44, CI: 0.24–0.81, p < 0.001; PFS: HR: 0.32, CI: 0.21–0.49, p < 0.001), suggesting that Chinese patients with squamous cell carcinoma may benefit more from the combined regimen. Based on these data, pembrolizumab combined with carboplatin and paclitaxel was approved as a first-line treatment for patients with metastatic squamous NSCLC in November 2019. Pembrolizumab is currently used in four clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China.
Durvalumab (AstraZeneca)
Durvalumab (Infinzi®), a fully human anti-PD-L1 IgG1 antibody, blocks the binding of PD-L1 and B7-1. The phase III PACIFIC trial compared durvalumab (10 mg/kg body weight, intravenously) or placebo consolidation every 2 weeks for up to 12 months in patients with unresectable stage III NSCLC. 21 Patients who had not progressed for at least two cycles after receiving concurrent radiotherapy and chemotherapy were randomly assigned (2:1) to durvalumab immune-monotherapy or a placebo. The median PFS was 16.8 months for durvalumab versus 5.6 months for the placebo (HR: 0.52, CI: 0.42–0.65; p < 0.001). Results of a prognosis analysis in the PACIFIC study demonstrating that low-grade pneumonitis should not deter the use of durvalumab was presented at the 2019 ASCO meeting. 22 Since then, durvalumab was established as a maintenance treatment for patients who did not progress after concurrent radiotherapy and chemotherapy and were eligible to receive immunotherapy. According to an update presented at the 2020 ASCO meeting, the median OS in the immunotherapy and placebo groups was 47.5 months and 29.1 months (HR: 0.71, CI: 0.57–0.88, p < 0.001), respectively. The 4-year OS rate was 49.6% versus 36.3%. Of 709 treated patients, 19% and 11% experienced immune-related (ir) AEs and non-pneumonia (np) irAEs of any grade, respectively; proportionally more patients had np irAEs with durvalumab (15%, 71/475) than with the placebo (2%, 5/234). Thyroid disorders (54/475; 11%), rash/dermatitis (9/475; 2%), and diarrhea/colitis (5/475; 1%) were the most common irAEs with durvalumab, and rash/dermatitis had the shortest time to onset. Among patients treated with durvalumab suffering irAEs, 11% had grade 3/4 irAEs, 41% of which were resolved, and none had fatal np irAEs. Durvalumab had a broadly manageable safety profile, irrespective of the occurrence of np irAEs. Thus, the safety profile was favorable, indicating that this ICI is well tolerated. Durvalumab is currently used in four clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China.
Atezolizumab (Roche)
Atezolizumab (Tecentriq®) is a selective, humanized, engineered anti-PD-L1 IgG1 isotype of PD-L1. IMpower133, a global, multicenter, phase III study, included patients with extensive-stage SCLC who were randomized (1:1) to receive carboplatin and etoposide with either atezolizumab or a placebo for four cycles with atezolizumab monotherapy or placebo maintenance therapy, according to a previous random assignment. 23 At a median follow-up of 13.9 months, the addition of atezolizumab significantly improved the OS. The median OS was 12.3 months in the atezolizumab group versus 10.3 months in the placebo group (HR: 0.70, CI: 0.54–0.91, p = 0.007), and the median PFS was also improved (5.2 months versus 4.3 months; HR: 0.77, CI: 0.62–0.96, p = 0.02). Therefore, atezolizumab in combination with carboplatin and etoposide was indicated as a first-line treatment for patients with extensive-stage SCLC in China in February, 2020, based on the breakthrough results of IMpower133 and after the European Society for Medical Oncology (ESMO) 2019 congress. 24 Updated results presented at the ESMO 2020 congress showed that the median follow-up time was 22.9 months, the median OS was 12.3 months versus 10.3 months (HR: 0.70, 0.54–0.91; p < 0.001), the median PFS was 5.2 months versus 4.3 months (HR: 0.77, 0.62–0.96, p = 0.017), and the median disease control rate (DCR) was 4.2 months versus 3.9 months (HR: 0.70, 0.53–0.92; p < 0.001). There was no significant difference in the incidence of AEs of any grade between the groups, and the overall safety was acceptable. Atezolizumab is currently used in 10 clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China.
ICIs in trials
Toripalimab (TopAlliance Biosciences)
Toripalimab, also known as JS-001, was the first humanized antibody against PD-1 to be approved for clinical trials by the China FDA. 25 JS-001 specifically binds to PD-1 antigen with an EC50 of 21 nmol/L, and effectively blocks the binding of PD-1 to PD-L1 and PD-L2 with IC50 values of 3.0 and 3.1 nmol/L, respectively. Kd values for the interaction between JS-001 and PD-1 antigens on CD8+ T cells of human and cynomolgus monkey were 2.1 nmol/L and 1.2 nmol/L, respectively. 25 Research data on toripalimab for lung cancer treatment have not been presented at any conference or published as an article. Five clinical studies of toripalimab in China registered at the Drug Evaluation Center of the State Drug Administration are presented in Table 1.
Sintilimab (Innovent)
All published data on sintilimab, an anti-PD-1 antibody also known as IBI308, in lung cancer are from phase I studies. One study investigated the efficacy of sintilimab combined with chemotherapy and sintilimab combined with an anti-angiogenesis drug for unresectable, late-stage NSCLC. Another study evaluated a combination of sintilimab with immune-adjuvant therapy in early-stage lung cancer.
The results of the phase I study on the combination of sintilimab and anlotinib (a multi-target tyrosine kinase inhibitor that inhibits tumor angiogenesis and proliferation) were presented at the 2019 WCLC. 26 Twenty-two enrolled patients received a fixed-dose intravenous injection of 200 mg of sintilimab every 3 weeks, and 12 mg of anlotinib was given orally on days 1–14 once a day for a treatment cycle of 3 weeks. Combination therapy continued until disease progression or observation of unacceptable toxicity. At the time of data analysis in July 2019, 16 of the 22 patients achieved a partial response (PR), the ORR was 72.7%, and the DCR was 100%. The incidence of treatment-related (tr) AEs was 100%, and the incidence of grade ⩾3 trAEs was 27%. In 31% of the patients, dose adjustment or drug interruption was required owing to AEs, and patients with pneumonitis eventually died. Research progress on sintilimab in the field of neoadjuvant therapy was updated successively at the ASCO meeting and the WCLC in 2019. 27 Preliminary results on the efficacy and safety of sintilimab in a single-center, single-arm, Ib phase study as a neoadjuvant therapy for resectable squamous NSCLC were presented as a poster by Li Ning (He Jie team, Department of Thoracic Surgery, Cancer Hospital of the Chinese Academy of Medical Sciences). The enrolled patients underwent two courses of monotherapy with sintilimab, 200 mg fixed-dose intravenous injection every 3 weeks. Surgical treatment was performed between day 29–43 after the initial administration and the patients were randomly assigned to conventional chemotherapy, sintilimab monotherapy, and chemotherapy combined sintilimab for adjuvant therapy. When feasible, sintilimab was used as an adjuvant therapy at 200 mg every 4 weeks. Twenty-two patients with resectable squamous NSCLC were enrolled in the study by January 28, 2019. Ten patients achieved major pathological remission (MPR), with an MPR rate of 45.5%, among whom four patients achieved pathological complete remission. Image evaluation of three patients with PR revealed one case of progressive disease and 18 cases of stable disease, resulting in an ORR of 13.6%. The incidence of trAEs was 54.4%, and the incidence of grade 3–4 AEs (elevated γ-glutamyl transferase) was 4.5%.
At WCLC 2019, the adjuvant therapy group was changed to routine chemotherapy ± radiotherapy. 27 Moreover, there was no defined tissue type for the enrolled NSCLC patients at the subsequent WCLC 2019 conference when treatment-naïve resectable sqNSCLC (stage IB-IIIA) that was confirmed by histopathology at ASCO 2019. Subsequent analysis presenting at WCLC 2019 showed that a total of 40 patients enrolled in the study and the data were analyzed by June 15, 2019. Among 37 assessable patients, eight patients achieved a radiological PR, resulting in an ORR of 21.6% according to the RECIST 1.1 guidelines. Fifteen (40.5%, 30.2–66.9%) patients achieved MPR, and six (16.2%, Cl: 6.2–32.0%) patients had a complete pathologic response. The primary endpoint was adjusted from disease-free survival to AEs within 90 days or 30 days after the first administration of sintilimab. The incidence of trAEs of all grades was 52.5%, which was similar to the data reported at ASCO 2019. The incidence rate of grade ⩾3 AEs was 10%. Two patients developed severe trAEs, namely neoadjuvant treatment-associated pneumonitis (grades 3 and 5, respectively). The rate of non-delayed operation was 82.5%, and the delayed operation of two patients was mainly due to a rise in the levels of alanine transaminase and aspartate aminotransferase (grade 2) after neoadjuvant therapy. Postoperative complications occurred in four patients (10.0%) and included hemoptysis (grade 1), postoperative incision infection (grade 2), pain (grade 1), and fever (grade 2). The final detailed results were reported in January 2020. 28
ORIENT-11 was a randomized, double-blind, placebo-controlled phase III clinical study that compared sintilimab or placebo plus pemetrexed and platinum in treatment-naïve patients with locally advanced or metastatic non-squamous NSCLC and without sensitizing EGFR/ALK mutation. The main results of this study were first disclosed in the form of an oral report at the ESMO Virtual Congress 2020. 29 The combination therapy with sintilimab significantly prolonged the PFS, reduced the risk of disease progression by 52%, and tended to improve OS. Based on these results, the National Drug Administration officially accepted the new indication application in April 2020. Results published in October 2020 after a median follow-up of 8.9 months showed that the median PFS was significantly longer in the sintilimab-combination group than in the placebo-combination group (8.9 months versus 5.0 months; HR: 0.48, CI: 0.36–0.64, p < 0.001). The confirmed ORR was 51.9% (45.7–58.0%) in the sintilimab-combination group and 29.8% (22.1–38.4%) in the placebo-combination group. 30 The ORIENT-12 (NCT03629925) study, 31 a randomized, double-blind, phase III controlled clinical study led by Professor Zhou (Shanghai Lung Hospital Affiliated to Tongji University) enrolled 357 newly diagnosed patients with either locally advanced/metastatic squamous NSCLC. The patients were randomly treated with sintilimab plus gemcitabine and platinum or placebo combined with gemcitabine and platinum as a first-line treatment. At the ESMO 2020 congress, the combination was shown to improve the median PFS (5.9 months versus 4.9 months; HR: 0.57, CI: 0.44–0.76, p < 0.001), with an acceptable safety profile. The median OS in the two groups has not yet been reported. Sintilimab is currently used in seven clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China.
Camrelizumab (Hengrui medicine)
Camrelizumab, also known as SHR-1210, is a humanized anti-PD-1 antibody. Research data on camrelizumab in the treatment of lung cancer were reported in four studies: a phase II study of camrelizumab as a second-line monotherapy in NSCLC, 32 a phase III study of camrelizumab in combination with chemotherapy in non-squamous NSCLC, 33 a phase Ib/II study of camrelizumab in combination with apatinib (a small-molecule tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor-2) in NSCLC, 34 and a phase II study of camrelizumab in extensive-stage SCLC. 35
The results from the phase II study on second-line camrelizumab monotherapy of Chinese patients with advanced/metastatic NSCLC with different levels of PD-L1 expression were presented at the WCLC 2019. The primary endpoint ORR was 18.5%. The median PFS was 3.2 months (2.0–3.4) and the median OS was 19.4 months (11.6–not reached). Treatment-related AEs (all grades) occurred in 87.7% of patients.
The phase III study, in which camrelizumab was combined with pemetrexed/carboplatin as a first-line treatment of oncogenic driver mutation-negative advanced/metastatic non-squamous NSCLC, was reported at the WCLC 2019 36 and was published on December 18, 2020. The combined chemotherapy regimen in the control group was carboplatin plus pemetrexed. Crossover to the camrelizumab arm was permitted for patients in the chemotherapy arm who had confirmed disease progression. After a median follow-up of 11.9 months, the median PFS was 11.3 months in the camrelizumab plus chemotherapy arm and 8.3 months in the chemotherapy arm (HR: 0.61, CI: 0.46–0.80, p < 0.001). The OS with camrelizumab plus chemotherapy was superior to chemotherapy within the follow-up: 17.1 versus 14.2 months (p = 0.03). Safety profiles were acceptable. Based on these data, camrelizumab was approved by the China FDA for the first-line treatment of non-squamous NSCLC patients without EGFR and ALK mutations in June 2020. Long-term prognosis data are still being collected and the PFS of PD-L1-positive patients will be further evaluated.
The results from the phase I/II study of a combination of camrelizumab and apatinib for the treatment of advanced NSCLC were presented at the 2019 ASCO meeting. The study included phase Ib apatinib dose-escalation and phase II expansion cohorts. Combined apatinib and camrelizumab showed encouraging antitumor activity and acceptable toxicity in chemotherapy-pretreated patients with advanced non-squamous NSCLC, with a median PFS of 5.7 months (4.5–8.8 months) and an OS of 15.5 months (10.9–24.5 months). Unexpected AEs were not observed.
The PASSION study, a multicenter, two-stage, phase II trial, evaluated camrelizumab plus apatinib in extensive-disease SCLC after platinum-based chemotherapy in patients with extensive-stage SCLC who failed platinum-based chemotherapy. Patients were divided into a chemotherapy-sensitive group and a chemotherapy-resistant group (defined as patients with disease relapse ⩾90 and <90 days after platinum-based chemotherapy, respectively). The sensitive and resistant groups had a comparable confirmed ORR (37.5% versus 32.3%), median PFS (3.6 versus 2.7 months), and median OS (9.6 versus 8.0 months). These phase II data warrant further clinical studies of camrelizumab plus apatinib in SCLC. Apatinib is currently used in four clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China.
Tislelizumab (BeiGene)
Tislelizumab, also known as BGB-A317, is an investigational humanized IgG4 monoclonal antibody that binds to the extracellular domain of human PD-1 with high specificity. The tislelizumab domain binding to PD-1 was specifically engineered to minimize FcγR binding on macrophages, thereby abrogating antibody-dependent phagocytosis, which could induce T-cell clearance and resistance to anti-PD-1 therapy. 37
The BGB-A317-102 study, a phase I/II study, evaluated the safety, tolerability, pharmacokinetics, and preliminary antitumor activities of tislelizumab in Chinese participants with advanced solid tumors. 38 NSCLC patients were divided into PD-L1-positive (PD-L1 expression in >10% of tumor cells) and PD-L1-negative (PD-L1 expression in <10% of tumor cells) groups in the expansion phase. Preliminary results after a median fellow-up of 8.4 months demonstrated that tislelizumab was well tolerated by advanced NSCLC patients and showed some antitumor activity, with an ORR of 11.8% in the PD-L1+ group and of 47.1% in the PD-L1– group and a DCR of 11.8% in the PD-L1+ group and of 64.0% in the PD-L1– group.
BGB-A317-206 was a phase II, open-label, multi-cohort trial to investigate the preliminary antitumor activity, safety, and pharmacokinetics of tislelizumab in combination with chemotherapy as a first-line treatment in Chinese patients with locally advanced or metastatic lung cancer. The study population was divided into four cohorts: a non-squamous NSCLC cohort (tislelizumab combined with pemetrexed + carboplatin/cisplatin), a squamous NSCLC cohort A (tislelizumab combined with paclitaxel + cisplatin/carboplatin), a squamous NSCLC cohort B (tislelizumab combined with gemcitabine + cisplatin/carboplatin), and a SCLC cohort (tislelizumab combined with etoposide + cisplatin/carboplatin). Results presented at the 2019 ASCO meeting showed that the ORR and DCR were 43.8% and 93.8%, respectively, in the non-squamous NSCLC cohort (n = 16); 80% and 93%, respectively, in the squamous NSCLC cohort A (n = 15); 66.7% and 83%, respectively, in the squamous NSCLC cohort B (n = 6); and 77% and 88%, respectively, in the SCLC cohort (n = 17). The median PFS in the four cohorts was 9.0 months, 7.0 months, not reached, and 6.9 months, respectively. The median OS was not reached in all cohorts, except for the SCLC cohort, in which it was 15.6 months.
RATIONALE 304 (NCT03663205) was a phase III clinical study in a Chinese population for the first-line treatment of non-squamous NSCLC with tislelizumab combined with pemetrexed/platinum versus pemetrexed/platinum alone. Mid-term analysis results presented at the ESMO 2020 meeting showed that tislelizumab plus chemotherapy significantly improved the PFS compared with the placebo (9.7 months versus 7.6 months; HR: 0.65, CI: 0.42–0.90, p = 0.004).
RATIONALE 307, an open, multicenter phase III study evaluated tislelizumab combined chemotherapy versus chemotherapy alone in the first-line treatment of advanced squamous NSCLC. 39 In this study, the efficacy and safety of a combination therapy of tislelizumab and chemotherapy as a first-line treatment of squamous NSCLC were evaluated. The study enrolled 360 Chinese patients with IIIB or IV squamous NSCLC, who were randomly divided into three groups at a 1:1:1 ratio. Group A received tislelizumab combined with paclitaxel + carboplatin, group B received tislelizumab combined with albumin-bound paclitaxel + carboplatin, and group C received paclitaxel + carboplatin for 4–6 cycles. Subsequently, patients in all groups received maintenance therapy with tislelizumab. The median PFS of the three groups was 7.6 months (CI: 6.0–9.8), 7.6 months (CI: 5.8–11.0), and 5.5 months (CI: 4.2–5.7), respectively, and the stratified HRs were 0.524 (CI: 0.37–0.74; p < 0.001) between arms A and C and 0.478 (CI: 0.336–0.679; p < 0.001) between arms B and C. The median OS was not reached in all groups. The ORR was 72.5% (63.6–80.3%), 74.8% (66.0–82.3%), and 49.6% (40.4–58.8%), respectively, and the median DCR was 8.2 months (CI: 5.0–not estimable), 8.6 months (CI: 6.3–not estimable), and 4.2 months (CI: 2.8–4.9), respectively. The drug withdrawal rate due to AEs in the three groups was 12.5%, 29.7% and 15.4%, respectively, and the incidence of severe AEs was 37.5%, 38.9%, and 23.6%, respectively. Tislelizumab is currently used in six clinical studies registered at the Drug Evaluation Center of the State Drug Administration in China.
Analysis of PFS in subgroups stratified based on PD-L1 expression showed that PFS was improved irrespective of the PD-L1 expression level.
Bi-specific antibodies
As of March 2019, a total of 85 bi-specific antibodies 40 have entered the commercial or clinical research and development stage. Approximately 86% of the studies on bi-specific antibodies are antitumor studies, with phase I exploration studies accounting for the vast majority. A bi-specific antibody targets one of the antigens of the PD-1–PD-L1 axis, which include cytotoxic T-lymphocyte-associated protein 4, lymphocyte activation gene 3, or T-cell immunoglobulin mucin 3. Dual-target inhibitors have proven highly efficient as a monotherapy or in combination with chemotherapy in recent in preclinical and clinical studies. TIGIT, a novel immune-suppressive checkpoint protein T-cell immune-receptor with Ig and immune-receptor tyrosine-based inhibitory motif domains expressed on immune cells (including T cells and natural killer cells), was also regarded as an efficient target of immunotherapy recently.
KN046 blocks both the CTLA-4 and the PD-L1 pathways, with a stronger affinity for PD-L1. It targets and accumulates in tumor microenvironments with high PD-L1 expression, with limited peripheral distribution in the lungs, thus reducing possible side effects on the peripheral system. Currently, two phase II studies are being conducted in China. These include a study evaluating the efficacy and safety of KN046 combined with platinum-containing dual-drug chemotherapy in patients with NSCLC (CTR20191219) and a study evaluating the efficacy and safety of KN046 at different doses (CTR20190195).
A phase Ib/II clinical trial of a novel anti-PD-1/CTLA-4 bi-specific antibody drug, AK104, in China was started on March 1, 2019. At the 2019 Society for Immunotherapy of Cancer meeting, partial data of a clinical trial of AK104 in Australia were released, which suggested that it not only had a better curative effect but also a theoretically lower toxicity than the combined use of immune dual drugs.
Besides agents targeting PD-1 and CTLA-4, dual targeting of transforming growth factor beta (TGF-β) and PD-1 currently has substantial research interest. TGF-β promotes fibrosis, angiogenesis, and metastasis and induces immune tolerance, creating a good microenvironment for tumor growth and metastasis. Results from various phase II clinical studies have shown good antitumor activity of TGF-β inhibitors. From a mechanistic point of view, dual-target antibodies have stronger antitumor activity as they activate both innate and acquired immunity. M7824 is an innovative first-in-class bifunctional fusion protein, which is composed of a human IgG1 monoclonal antibody against PD-L1 fused to the extracellular domain of TGF-β receptor II. 41 In mouse models, M7824 showed a stronger antitumor activity than monotherapy with a PD-L1 inhibitor or TGF-β trap and provided long-term immune protection, thereby prolonging survival. An international multicenter phase III clinical trial conducting a head-to-head comparison of M7824 and pembrolizumab is currently being carried out in China. Newly treated patients with stage IV NSCLC and high PD-L1 expression (defined as PD-L1 ⩾80% as detected with a 73-10 kit and TPS of ⩾50% as detected with a 22C3 kit) will be randomly assigned (1:1) to either M7824 (1200 mg every 2 weeks) or pembrolizumab (200 mg every 3 weeks) treatment. The primary endpoints are PFS and OS and will be evaluated by an independent blind review committee. The secondary endpoints are ORR, DCR, pharmacokinetics, adenosine dehydrogenase level, and trAE incidence, and will evaluate by the same committee. The study is currently recruiting patients. TIGIT is a new type of immune-suppressive checkpoint that exists on activated T cells and natural killer cells in various cancers, including NSCLC. The CITYSCAPE trial 42 presented at ASCO Virtual Congress 2020 evaluated the efficacy and safety of tiragolumab (a novel cancer immunotherapy designed to bind to TIGIT) + atezolizumab versus placebo + atezolizumab as a first-line treatment for patients with advanced NSCLC. Compared with atezolizumab monotherapy, tiragolumab + atezolizumab significantly improved the ORR (37.3% versus 20.6%) and PFS (5.6 months versus 3.9 months) of the intention-to-treat analysis population, and the subgroup with PD-L1 TPS of ⩾50% showed the greatest improvement. The safety of the both treatments was similar.
Conclusions and future directions
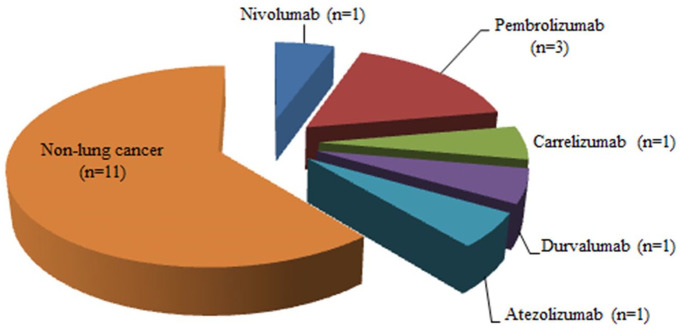
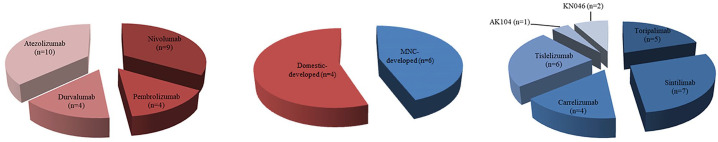
Immunotherapy activating antitumor immunity to target and kill cancer cells has revolutionized the lung cancer treatment landscape in China. Combinations of immunotherapy with other therapies, such as chemotherapy, radiotherapy, and targeted therapy, represent a new modality for treating lung cancer that can achieve greater therapeutic efficacy via multiple synergistic mechanisms. At present, eight PD-1/PD-L1 ICIs are approved for 18 indications in China, of which five for 7 indications in lung cancer (Figure 1). The domestic PD-1 antibodies toripalimab, sintilimab, camrelizumab, and tislelizumab have not yet obtained approval. These novel domestic drugs are “me-too drugs” that have achieved better or equivalent results compared with those of the prototypes in various clinical studies. A phase I trial evaluated the safety, antitumor activity, and pharmacokinetics of toripalimab in patients with advanced NSCLC; trAEs occurred in 43.9% of the patients. 43 Sintilimab-related AEs were observed in 71.4% of non-squamous NSCLC patients and 65.0% of squamous NSCLC patients in two cohorts of an open-label, phase Ib study evaluating the efficacy and safety of sintilimab in combination with chemotherapy in previously untreated patients with advanced or metastatic non-squamous or squamous NSCLC. No grade 5 AEs or AE-related deaths were reported. 44 One study assessing the efficacy activity and safety of camrelizumab in patients with previously treated, advanced NSCLC negative for oncogenic drivers reported trAEs (all grades) in 69.0% of the 25 enrolled patients. 45 The phase III study 39 of tislelizumab plus chemotherapy versus chemotherapy alone as a first-line treatment for advanced squamous NSCLC demonstrated trAEs in 12.5%, 29.7%, and 15.4% of patients in the tislelizumab + paclitaxel + carboplatin, tislelizumab + nab-paclitaxel + carboplatin, and paclitaxel + carboplatin arms, respectively. No new safety signals were identified upon addition of tislelizumab to chemotherapy. Overall, the safety profiles of tislelizumab and toripalimab seemed to be more acceptable than that of chemotherapy. The Fc region of the tislelizumab antibody is engineered to avoid T-cell depletion caused by antibody-mediated phagocytosis. Given this unique feature and the low prevalence of treatment-associated AEs, tislelizumab has better innovation potential than the other PD-1 antibodies. Four of the 18 approved indications pertain to first-line treatment. Pembrolizumab has been approved for the first-line monotherapy of locally advanced/metastatic EGFR/ALK-negative NSCLC patients with PD-L1 TPS of ⩾1, and subgroup analysis revealed that patients with TPS of ⩾50% benefited more from pembrolizumab than those with TPS of 1–49% (HR for OS, 0.69 versus 0.92, p < 0.001). 4 Pembrolizumab combined with pemetrexed and a platinum-based drug has been approved for the first-line treatment of patients with metastatic non-squamous NSCLC, regardless of tumor PD-L1 expression. 16 Pembrolizumab combined with carboplatin and paclitaxel is approved as a first-line treatment for patients with metastatic squamous NSCLC. 17 Moreover, atezolizumab in combination with carboplatin and etoposide is indicated as a first-line treatment for patients with extensive-stage SCLC. Clinical studies for lung cancer currently ongoing in China are shown in Figure 2, and detailed information of each trial is presented in Table 1.
Figure 1.
Programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors (ICIs) approved for the treatment of lung cancer in China and their respective approved indications.
Figure 2.
Currently ongoing clinical studies on immune checkpoint inhibitors (ICIs) developed by multinational corporations (MNCs) and by domestic companies for the treatment of lung cancer in Chinese patients. Among the 52 clinical studies on ICIs, 27 studies assessed six MNC-developed ICIs in Chinese patients (red pie slice) and 25 studies evaluated eight domestically developed ICIs (blue pie slice).
The RATIONALE-307 study demonstrated that tislelizumab combined with chemotherapy is a new option for the first-line standard treatment of advanced squamous NSCLC, regardless of PD-L1 expression. The development of new antitumor drugs and exploration of better therapeutic options for clinical decision-making in China are urgently needed. Further, it is necessary to continue to explore better treatment schemes, especially, the selection of combined regimens, dosage, timing, and duration of administration, to ensure effective control of toxicity while increasing the efficacy. Clinicians could make reasonable choice for different patients groups according to the results of clinical trials of each drug in the decision-making process.
To the best of our knowledge, this review is the first to summarize current research and ongoing trials of various ICIs and to discuss the outcomes of treatments with these ICIs for the above indications in China. Additional studies are needed to analyze the clinical trial results in more depth and understand which patients are more likely to benefit from the various treatments. We expect that immunotherapy will inevitably be part of the treatment arsenal for lung cancer in the near future.
Footnotes
Author contributions: Conception/design: Jiadi Gan, Yihua Huang, Wenfeng Fang, Li Zhang
Manuscript writing: Jiadi Gan, Yihua Huang, Wenfeng Fang, Li Zhang
Final approval of the manuscript: Jiadi Gan, Yihua Huang, Wenfeng Fang, Li Zhang
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Chinese National Natural Science Foundation Project (81972556, 81772476, and 81872499).
Contributor Information
Jiadi Gan, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou 510060, China.
Yihua Huang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou 510060, China.
Wenfeng Fang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong 510060, China.
Li Zhang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China.
References
- 1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021; 71: 7–33. [DOI] [PubMed] [Google Scholar]
- 2. Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014; 515: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Y, Chang J, Zhang L, et al. OA10 CheckMate 078: patient-reported outcomes (PROs) with nivolumab vs docetaxel in advanced non-small cell lung cancer (NSCLC). J Thorac Oncol 2018; 13: S1047–S1048. [Google Scholar]
- 4. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 5. Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol 2020; 15: 288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horn L, Reck M, Mok T, et al. IMpower133: a phase I/III study of 1L atezolizumab with carboplatin and etoposide in patients with extensive-stage SCLC. J Thorac Oncol 2016; 11: S305–S306. [Google Scholar]
- 7. Rajan A, Kim C, Heery CR, et al. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: role in advanced cancers. Hum Vaccin Immunother 2016; 12: 2219–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. New Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 35: 3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14: 867–875. [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Lu S, Zhou C, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078). Paper presented at CSCO Annual Meeting, 18–22 September 2019, Xiamen, China. [DOI] [PubMed] [Google Scholar]
- 14. Gallego J, Juárez M, Paz-Ares L. The safety and efficacy of pembrolizumab for the treatment of non-small cell lung cancer. Expert Opin Drug Saf 2020; 19: 233–242. [DOI] [PubMed] [Google Scholar]
- 15. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. New Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 16. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. New Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 17. Wu Y, Zhang L, Fan Y, et al. KEYNOTE-042 China study: first-line pembrolizumab vs chemotherapy in Chinese patients with advanced NSCLC with PD-L1 TPS ⩾ 1%. J Thorac Oncol 2019; 14: S290–S291. [Google Scholar]
- 18. Ettinger D S, Wood D E, Aggarwal C, et al. NCCN guidelines insights: non–small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 2019; 17: 1464–1472. [DOI] [PubMed] [Google Scholar]
- 19. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 2020; 38: 1505–1517. [DOI] [PubMed] [Google Scholar]
- 20. Cheng Y, Zhang L, Hu J, et al. Keynote-407 China extension study: pembrolizumab (pembro) plus chemotherapy in Chinese patients with metastatic squamous NSCLC. Ann Oncol 2019; 30: ix201–ix202. [Google Scholar]
- 21. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. New Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 22. Vansteenkiste JF, Naidoo J, Faivre-Finn C, et al. Efficacy of durvalumab in patients with stage III NSCLC who experience pneumonitis (PACIFIC). Ann Oncol 2019; 30: v592–v593. [Google Scholar]
- 23. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. New Engl J Med 2018; 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 24. Reck M, Liu SV, Mansfield AS, et al. IMpower133: updated overall survival (OS) analysis of first-line (1L) atezolizumab (atezo) plus carboplatin plus etoposide in extensive-stage SCLC (ES-SCLC). Ann Oncol 2019; 30: v710–v717. [Google Scholar]
- 25. Fu J, Wang F, Dong L-H, et al. Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001, a programmed cell death protein-1 (PD-1) monoclonal antibody. Acta Pharmacol Sin 2017; 38: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han B, Chu T, Zhong R, et al. JCSE01.11 Efficacy and safety of sintilimab with anlotinib as first-line therapy for advanced non-small cell lung cancer (NSCLC). J Thorac Oncol 2019; 14: S129. [Google Scholar]
- 27. Li N, Ying J, Tao X, et al. Efficacy and safety of neoadjuvant PD-1 blockade with sintilimab in resectable squamous non-small cell lung cancer (sqNSCLC). J Thorac Oncol 2019; 14: S128–S129. [Google Scholar]
- 28. Gao SG, Li N, Gao SY, et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol 2020; 15: 816–826. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Yang Y, Wang Z, et al. ORIENT-11: sintilimab plus pemetrexed plus platinum as first-line therapy for locally advanced or metastatic non-squamous NSCLC. J Thorac Oncol 2020; 15: E41. [DOI] [PubMed] [Google Scholar]
- 30. Yang Y, Wang Z, Fang J, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 2020; 15: 1636–1646. [DOI] [PubMed] [Google Scholar]
- 31. Zhou C, Wu L, Fan Y, et al. ORIENT-12: sintilimab plus gemcitabine and platinum (GP) as first-line (1L) treatment for locally advanced or metastatic squamous non-small-cell lung cancer (sqNSCLC). Ann Oncol 2020; 31: S1186–S1186. [Google Scholar]
- 32. Wu Y, Huang C, Fan Y, et al. A phase II umbrella study of camrelizumab in different PD-L1 expression cohorts in pre-treated advanced/metastatic non-small cell lung cancer. J Thorac Oncol 2019; 14: S382–S383. [Google Scholar]
- 33. Zhou C, Chen G, Huang Y, et al. A randomized phase 3 study of camrelizumab plus chemotherapy as 1st line therapy for advanced/metastatic non-squamous non-small cell lung cancer. J Thorac Oncol 2019; 14: S215–S216. [Google Scholar]
- 34. Zhou C, Wang Y, Zhao J, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced non-squamous NSCLC previously treated with chemotherapy. Clin Cancer Res 2020; 15: 1636–1646. [DOI] [PubMed] [Google Scholar]
- 35. Fan Y, Zhao J, Wang Q, et al. Camrelizumab plus apatinib in extensive-stage small-cell lung cancer (PASSION): a multicenter, two-stage, phase 2 trial. J Thorac Oncol 2020; 16: 299–309. [DOI] [PubMed] [Google Scholar]
- 36. Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021; 9: p305–p314. [DOI] [PubMed] [Google Scholar]
- 37. Shen L, Yuan Y, Liu T, et al. Clinical profile of tislelizumab in Chinese patients with MSI-H or dMMR solidtumors: preliminary results from an indication-expansion cohort. Paper presented at 21st Annual Chinese Society for Clinical Oncology Congress, 19–22 September 2018, Xiamen, China. [Google Scholar]
- 38. Shen L, W YL, G J, et al. Preliminary results of a phase 1/2 study of BGB-A317, an anti-PD1 mAb in Chinese patients with advanced tumors. Paper presented at 20th Annual Chinese Society for Clinical Oncology, 26–30 September 2017, Xiamen International Conference and Exhibition Center, China. [Google Scholar]
- 39. Wang J, Lu S, Yu X, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2021; 7: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Labrijn AF, Janmaat ML, Reichert JM, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 2019; 18: 585–608. [DOI] [PubMed] [Google Scholar]
- 41. Strauss J, Heery C, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res 2018; 24: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodriguez-Abreu D, Johnson ML, Hussein MA, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol 2020; 38(Suppl. 15): 9503. [Google Scholar]
- 43. Wang Z, Ying J, Xu J, et al. Safety, antitumor activity, and pharmacokinetics of toripalimab, a programmed cell death 1 inhibitor, in patients with advanced non-small cell lung cancer: a phase 1 trial. JAMA Netw Open 2020; 3: e2013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang H, Zheng Y, Qian J, et al. Efficacy and safety of sintilimab in combination with chemotherapy in previously untreated advanced or metastatic nonsquamous or squamous NSCLC: two cohorts of an open-label, phase 1b study. Cancer Immunol Immunother 2021; 70: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun GG, Jia JH, Gao P, et al. 440 activity and safety of camrelizumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced non-small-cell lung cancer. J ImmunoTher Cancer 2020; 8: A466. [Google Scholar]