Abstract
Background:
The pragmatic management of psychotic disorders is more complex than that delivered in a controlled trial environment. Therefore, this study aims to evaluate the real-world effectiveness of aripiprazole long-acting injectable (ALAI) and compare it with another commonly used long-acting anti-psychotic, once-monthly paliperidone palmitate (PP1M).
Methods:
This naturalistic, independent 4-year mirror image study compared the mean number and length of hospital admissions 2 years before and 2 years after treatment initiation with ALAI. Retention rates, discontinuation reasons and level of adherence were also recorded. Furthermore, indirect comparisons were made between treatment outcomes on ALAI and PP1M.
Results:
A total of 109 eligible patients with a severe mental illness (65% with schizophrenia and 35% with other diagnosis) commenced on ALAI and 173 patients (69% with schizophrenia and 31% with other diagnoses) initiated on PP1M were included. Of these, 37% on ALAI and 34% on PP1M stopped treatment at 2 years; retention rates were most favourable for the schizophrenia group on PP1M. Patients were more likely to discontinue due to lack of effectiveness on ALAI and due to tolerability issues on PP1M. Those who continued for 2 years on ALAI (n = 69), demonstrated an overall decrease of 84% in the mean number and 88% in the mean length of hospital admissions compared with the 2 years before initiation. Although patients on ALAI appeared to have a significantly higher bed occupancy the 2-year period before initiation than patients on PP1M, the reductions in hospitalizations were comparable across both cohorts after 2 years of treatment.
Conclusions:
The introduction of ALAI had a substantial impact on long-term clinical outcomes in this naturalistic cohort; more than half of patients continued treatment and had no admission during 2 years of follow up. There were no significant differences in hospitalisation rates between patients on ALAI and PP1M at 2 years.
Keywords: aripiprazole, compliance, hospitalization, long-acting antipsychotics, paliperidone, schizophrenia
Introduction
Schizophrenia is a chronic and disabling illness, with the majority of patients experiencing multiple relapses during the course of the illness. 1 Each acute episode can have a long-term detrimental impact on individual prognosis and creates a considerable disease burden to society in the form of increasing care and wider societal costs.2,3
Anti-psychotic treatment aims to provide symptom improvement, stabilise remission and prevent relapse. 4 Discontinuity of treatment has found to have a significant effect on the likelihood of relapse and subsequent deterioration. Long-acting injectable antipsychotics (LAIs) have been shown to reduce relapse risk and mortality by addressing issues of non-adherence, facilitating regular contact between the physician and patient, reducing the, albeit rare, risk of overdose while maintaining reliable drug delivery and avoiding the bioavailability issues that occur with oral medication.5,6 It can, therefore, also help clinicians differentiate true treatment resistance to pseudo-resistance caused by pharmo-kinetic factors such as inadequate dosing or undisclosed poor or erratic concordance.
Aripiprazole long-acting injectable (ALAI) is a long acting injectable which has been established in randomised clinical trials.7,8 Aripiprazole is a partial agonist at D2 and 5-HT1A receptors and an antagonist at 5-HT2A receptors. 9 It has been postulated that the partial agonist activity may offer theoretical advantages, particularly in terms of short- and long-term tolerability, and may be associated with a reduced likelihood of long-term dopamine-related neurochemical dysregulation such as dopamine super-sensitivity.10,11
Evidence from randomised clinical trials with strict inclusion and exclusion criteria has demonstrated the safety and efficacy of ALAI. However, pragmatic management of schizophrenia is much more complex, and there is a need for naturalistic data to establish the real world impact. Furthermore, this study aims to compare the real world effectiveness of ALAI with another commonly used second generation long-acting anti-psychotic, Paliperidone palmitate (PP1M), evaluating the comparative effects on treatment continuation and hospital admissions.
Methods
This naturalistic, independent, 4-year mirror image study was carried out in West London NHS Trust (WLHT), a large, urban mental health provider. The study was approved by the WLHT department for audit and naturalistic research (project number 1885).
The cohort for the study was formed from all eligible patients commenced on ALAI between May 2014 and March 2018. This was a within-patient analysis so the comparator group was the same patient, in a different time period. The two time frames were the 2 years before the patient was commenced on ALAI and the 2 years after commencement of ALAI. Data collection was completed in March 2020 allowing for a 2-year post initiation follow up of all patients. Patients were identified from trust community and pharmacy records; information from all sources was cross checked to ensure that duplicate patients were not included.
The electronic clinical records system was used to access the demographical and clinical data needed for the study including primary diagnosis, a recorded secondary diagnosis of substance misuse (ICD 10 F10-19), number and length of hospital admissions in the 2 years prior to and post initiation of ALAI, antipsychotic used immediately prior to ALAI and reason for switch as well as treatment cessation rates and reasons. It was outside of the scope of this study to collect data regarding disease severity although the number of the total previous admissions was recorded as a proxy measure. Information on the level of adherence was also collected by calculating the number of depot injections administered successfully in each 12-month period of treatment. Compliance was divided in three groups: full (no missed dose/year), good or ⩾50% (6–11 injections/year), poor or <50% (1–5 injections/year). Patients were excluded from the study if the full set of information was not available, that is, due to death, loss to follow up or transfer of care outside the service. Inpatients on forensic wards were also excluded due to their often prolonged hospitalisations. After the initial data collection, the data was fully anonymised and held according to local data protection policies.
Patients were included in the analysis on hospitalization rates only if they continued treatment with ALAI for a minimum of 2 years. The number of hospital bed days and admissions before and after treatment with ALAI were compared using a mirror image design over a 4-year period beginning from 2 years before its first imitation to 2 years after in each patient. 12 For patients who initiated ALAI as outpatients in the community, the date of ALAI initiation was the mirror point. For patients who initiated ALAI as inpatients, analysis was completed in order to take into account the time lag from initiation of ALAI to clinical impact so the time from initiation of ALAI to discharge was excluded from the number of bed days. This methodology is consistent with previous studies.13–16
Comparisons were made between treatment outcomes on ALAI and PP1M with a data set collected by the same authors in the same trust between April 2011 and March 2018 (differences in the time periods of data collection between the two cohorts are due mainly due to the earlier introduction of PP1M in clinical practice). The pharmacy records can be linked with the case notes via a unique patient identifier. All patients prescribed PP1M or ALAI were initiated following an independent clinical prescribing decision and received care as usual. The same inclusion and exclusion criteria and methods were used as per the ALAI data collection and analysis.
Statistical analysis
Descriptive statistics were used to summarise information regarding demographics, diagnosis and antipsychotic medication used immediately prior to commencement of ALAI. Means and standard deviations (SD) were calculated for continuous data and frequencies and percentages calculated for categorical data. Discontinuation rates were calculated and primary reasons displayed in a frequency table. Numbers and lengths of inpatient admissions were compared in a within patient analysis. Kolmogorov–Smirnov and Shapiro–Wilks tests revealed that this data was not normally distributed, thus the Wilcoxon signed rank test for paired data was used to compare for admission rates and bed days before and after ALAI and PP1M initiation; p values of 0.05 were used to determine significance. Furthermore, a sub-analysis of patients with a diagnosis of schizophrenia was conducted which is the formal indication for the use of both LAIs. The data was analysed using SPSS Statistics for Windows (IBM, Armonk, NY, USA).
Results
Demographics and continuation rates
A total of 148 patients were identified as having started ALAI between May 2014 and March 2018. Of these, 24 patients were excluded due to insufficient data (incomplete records, lost to follow up, deaths) and 15 because they were forensic inpatients. Of the remaining 109 patients, 71 (65%) patients had a primary diagnosis of schizophrenia and 38 (35%) had schizoaffective disorder, bipolar affective disorder or other diagnosis. The PP1M cohort consisted of 173 patients, 120 (69%) with a diagnosis of schizophrenia and 53 (31%) with other diagnosis. The demographic and clinical characteristics of the total cohorts and the schizophrenia only groups on ALAI and PP1M are included in Table 1. The main significant difference between ALAI and PPM1 groups were the care setting initiation, with ALAI patients more likely to be started in an inpatient environment and PP1M started in an outpatient setting.
Table 1.
Patient demographic and clinical characteristics on ALAI and PP1M.
| Characteristics | ALAI |
PP1M |
X2 (df) or as stated | p value | ALAI |
PP1M |
X2 (df) or as stated | p value |
|---|---|---|---|---|---|---|---|---|
| All patients | All patients | SCZ patients | SCZ patients | |||||
| n = 109 (%) | n = 173 (%) | n = 71 (%) | n = 120 (%) | |||||
| Male | 51.4 | 64.2 | 4.54 (1) | 0.03* | 59.2 | 63.3 | 0.33 (1) | 0.57 |
| Female | 48.6 | 35.8 | 40.8 | 36.7 | ||||
| Age | ||||||||
| Mean (SD), range | 48.39 (15.09), 24–84 | 46.0 (15.9), 21–97 | Mann–Whitney Z Score = −1.44 | 0.15 | 47.17 (16.17), 24–85 | 46.5 (14.9), 21–75 | Mann–Whitney Z Score = −0.37 | 0.71 |
| White | 43.1 | 39.9 | 0.29 (1) | 0.59 | 32.4 | 40.8 | 1.35 (1) | >0.24 |
| Non White | 56.9 | 60.1 | 67.6 | 59.2 | ||||
| Comorbid substance misuse | 20.2 | 19.7 | 0.01 (1) | 0.91 | 19.7 | 19.2 | 0.009 (1) | 0.93 |
| Inpatient | 60.6 | 41.6 | 9.59 (1) | 0.001* | 57.7 | 39.2 | 6.10 (1) | 0.01* |
| Outpatient | 39.4 | 58.4 | 42.3 | 60.8 | ||||
| Number of total previous admissions | ||||||||
| Mean (SD), range | 4.16 (4.54), 0–24 | 3.76 (4.3), 0–31 | Mann-Whitney Z Score = 0.75 | 0.45 | 3.39 (4.06), 0–22 | 3.73 (4.30), 0–31 | Mann-Whitney Z Score = 0.78 | 0.44 |
| Antipsychotic switched from | ||||||||
| None/not known | 3.7 | 0 | Fishers exact test | 0.000* | 5.6 | 0 | Fishers exact test | 0.000* |
| Oral | 67.9 | 49.1 | 63.4 | 40.0 | ||||
| Depot/LAI | 28.4 | 50.9 | 31.0 | 60.0 | ||||
| Reason for switching | ||||||||
| Refusal/compliance | 67.0 | 42.2 | 34.64 (3) | 0.000* | 64.8 | 41.7 | 18.65 (3) | 0.0003* |
| Poor tolerability | 22.0 | 17.3 | 21.1 | 19.2 | ||||
| Ineffectiveness | 8.3 | 13.9 | 9.9 | 14.1 | ||||
| Other/preference for less frequent LAI | 1.8 | 26.5 | 2.8 | 25.0 |
p <0.05.
ALAI, aripiprazole long-acting injectable; LAI, long-acting injectable antipsychotic; PP1M, once-monthly paliperidone palmitate; SD, standard deviation; SCZ, schizophrenia.
Discontinuation rates and reasons are shown in Table 3. Of the total number of patients on ALAI, 37% discontinued at 2 years (28% in the first year and 13% in the second). In the PP1M sample, 34% discontinued at 2 years (23% in the first year and 14% in the second year). For the Schizophrenia groups, 38% stopped treatment at 2 years on ALAI and 31% on PP1M. Patients on ALAI were most likely to discontinue due to ineffectiveness and refusal, and patients on PP1M most likely to stop it due to tolerability issues. The median maintenance dose for ALAI was 400 mg (only available in 300 mg and 400 mg) and for PP1M 100 mg.
Table 3.
Discontinuation rates and reasons on ALAI and PP1M.
| Discontinuation rates | ALAI |
PP1M |
ALAI |
PP1M |
|---|---|---|---|---|
| All patients | All patients | SCZ patients | SCZ patients | |
| N = 109 (%) | N = 173 (%) | N = 71 (%) | N = 120 (%) | |
| Year 1 | 28 | 23 | 28 | 20 |
| Year 2 | 13 | 14 | 14 | 13 |
| Total at 2 years | 37 | 34 | 38 | 31 |
| Discontinuation reasons | ||||
| Refusal/non-adherence | 15 | 11.5 | 15.5 | 10 |
| Ineffectiveness | 16.5 | 8 | 15.5 | 9 |
| Poor tolerability | 4.5 | 14.5 | 5.5 | 11 |
| Other | 1 | 0 | 1.5 | 1 |
ALAI, aripiprazole long-acting injectable; PP1M, once-monthly paliperidone palmitate; SCZ, schizophrenia.
A multivariate logistic analysis was used to calculate whether there were significant differences in demographic and clinical characteristics between the group of patients who continued on ALAI for 2 years versus those who discontinued (Table 4). No significant differences were identified, thus post hoc corrections were not completed.
Table 4.
Demographic and clinical characteristics of patients who continued on ALAI versus patients who discontinued at 2 years.
| Characteristics | ALAI continuers |
ALAI discontinuers |
Coefficient | p value |
|---|---|---|---|---|
| n = 69 | n = 40 | |||
| Male | 50.7% | 52.5% | 0.01 | 0.95 |
| Female | 49.3% | 47.5% | ||
| Age | ||||
| Mean (SD), range | 51.16 (15.42), 24–84 | 43.60 (13.39), 24–74 | −0.00 | 0.14 |
| White | 42% | 45% | −0.1 | 0.29 |
| Non White | 58% | 55% | ||
| Substance misuse | 18.8% | 22.5% | −0.00 | 0.99 |
| Inpatient | 65.2% | 52.5% | 0.18 | 0.06 |
| Outpatient | 34.8% | 47.5% | ||
| Number of total previous admissions | ||||
| Mean (SD), range | 4.06 (4.67), 0–24 | 4.4 (4.27), 0–19 | 0.01 | 0.55 |
| Antipsychotic switched from | ||||
| None/not known | 1.4% | 7.5% | 5.74 | 0.06 |
| Oral | 56.6% | 70.0% | ||
| Depot/LAI | 31.9% | 22.5% | ||
| Reason for switching | ||||
| Refusal/compliance | 65.2% | 70.0% | −0.02 | 0.76 |
| Ineffective | 13.0% | 0% | ||
| Tolerability | 20.3% | 25% | ||
| Other | 1.4% | 5% |
ALAI, aripiprazole long-acting injectable; LAI, long-acting injectable antipsychotic; SD, standard deviation.
Hospitalisation rates
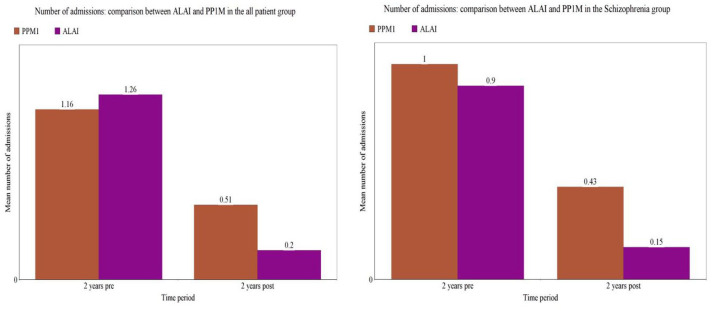
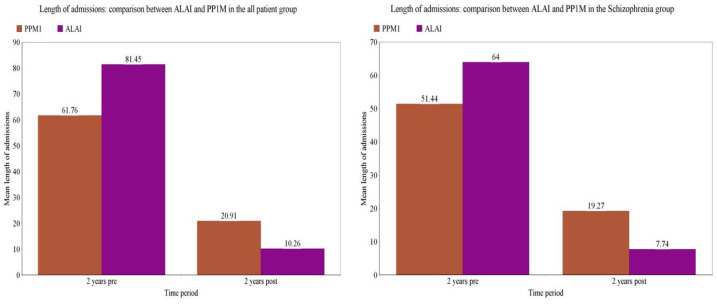
In the patients who continued with ALAI for 2 years (n = 69), the mean number of admissions decreased from 1.2 per patient in the 2 years prior to ALAI initiation to 0.2 per patient in the 2 years post initiation (Table 2 and Figure 1) and the median number of admissions fell from 1 to 0; these reductions were statistically significant (Wilcoxon signed rank p < 0.001). Likewise, the mean number of bed days decreased significantly from 81 days in the 2 years pre-ALAI initiation to 10 bed days in the 2 years post-ALAI initiation (Table 2 and Figure 2) and the median bed days fell from 58 days to 0 days in the same period (Wilcoxon signed rank test p < 0.0001).
Table 2.
Comparison of number and length of admissions 2 years pre and post initiation within and between ALAI and PP1M cohorts for all patients and schizophrenia only groups.
| Comparison of hospitalization rates 2 years pre and post initiation with ALAI and PP1M | ||||||
|---|---|---|---|---|---|---|
| ALAI |
Wilcoxon signed rank | PP1M |
Wilcoxon signed rank | |||
| Total pre | Total post | p value | Total pre | Total post | p value | |
| Number of admissions | ||||||
| SCZ patients | 0.9 | 0.15 | <0.0000* | 1.00 | 0.43 | 0.001* |
| All patients | 1.26 | 0.2 | <0.0001* | 1.16 | 0.51 | 0.001* |
| Length of admissions | ||||||
| SCZ patients | 64.00 | 7.74 | 0.000* | 51.44 | 19.27 | <0.001* |
| All patient | 81.45 | 10.26 | <0.0001* | 61.76 | 20.91 | <0.001* |
| Comparison of hospitalization rates 2 years pre and post initiation between ALAI and PP1M | ||||||
| ALAI pre | PP1M pre | p value | ALAI post | PP1M post | p value | |
| Number of admissions | ||||||
| SCZ patients | 0.9 | 1.0 | 0.426 | 0.15 | 0.43 | 0.143 |
| All patients | 1.26 | 1.16 | 0.004* | 0.2 | 0.51 | 0.078 |
| Length of admissions | ||||||
| SCZ patients | 64.00 | 51.44 | 0.058 | 7.74 | 19.27 | 0.724 |
| All patients | 81.45 | 61.76 | 0.000* | 10.26 | 20.91 | 0.666 |
p <0.05.
ALAI, aripiprazole long-acting injectable; PP1M, once-monthly paliperidone palmitate; SCZ, schizophrenia.
Figure 1.
Mean number of admissions 2 years pre and post initiation of ALAI and PP1M in the all patient and schizophrenia groups.
ALAI, aripiprazole long-acting injectable; PP1M, once-monthly paliperidone palmitate.
Figure 2.
Mean length of admissions 2 years pre and post initiation of ALAI and PP1M in all patient and schizophrenia groups.
ALAI, aripiprazole long-acting injectable; PP1M, once-monthly paliperidone palmitate.
For patients who continued with PP1M for 2 years (n = 112), the mean number of admissions decreased from 1 to 0.4 (Table 2 and Figure 1) and the median from 1 to 0 and the mean length of admissions from 61 to 20 (Table 2 and Figure 2) and the median from 24 to 0 (p < 0.0001, Wilcoxon signed rank test). The schizophrenia patient group who continued for 2 years on both LAI cohorts demonstrated similar statistical significant reductions in bed usage (Table 2, Figures 1 and 2).
Comparison between the patient cohort on ALAI and PP1M for the all patient group and the schizophrenia only group are also detailed in Table 2 and Figures 1 and 2. The all patient group on ALAI had a significantly higher mean number and length of admissions during the 2 years pre-initiation compared with the PPM1 group (p = 0.004 and p = 0.058 Wilcoxon signed rank) but there was no significant difference in the number of admissions and bed days in the 2 years after commencement of either treatment option (p = 0.078 and p = 0.724, Wilcoxon signed rank). In the schizophrenia groups, there were no significant differences in bed occupancy between PP1M and ALAI in the 2 years pre (p = 0.426) or post initiation (p = 0.143).
As an indirect measure of the overall severity of illness prior to LAI initiation, the total number of recorded previous hospital admissions were compared across the two cohorts. Those commenced on ALAI had a mean of 4 and median of 3 total previous admissions and those on PP1M had a mean of 3.95 previous admissions and a mean of 2; this difference was not found to be significant (Wilcoxon signed rank 0.332)
Level of adherence
In the all patient group that continued for 2 years on ALAI, 65% (45/69) received 100% (24/24) injections in the 2-year period, 33% (23/69) received 50% or more and only one patient received less than 50% (<12/24) injections in the 2-year period.
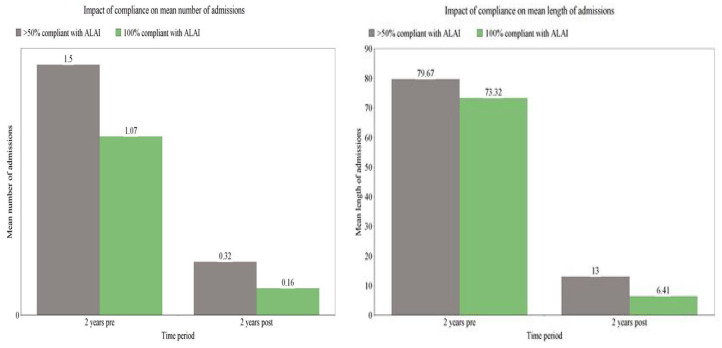
Furthermore, we reviewed the impact of the actual level of adherence on hospital admissions (Figure 3). The fully compliant group (100%) demonstrated a significant reduction in both the number and length of admissions 2 years after compared with 2 years before ALAI initiation (p = 0.00, Wilcoxon signed rank test). The reduction in bed usage, albeit numerically smaller, remained significant for those who were 50% and over compliant (p = 0.003 for number of admissions and p = 0.001 for length of admissions). Statistical tests were not performed for the less than 50% compliant group due to insufficient numbers. Friedman’s test was used to compare number and length of admissions in the 2 years post commencement of ALAI between the fully compliant and over 50% compliant group; differences were found to be statistically not significant (with a chi squared value of 0.5, df 1, asymp 0.480 and 1.33, df 1, asymp 0.248, respectively). The above findings were similar for the schizophrenia only group (data not shown).
Figure 3.
Impact of level of adherence on mean number and length of admissions 2 years pre and post ALAI initiation.
ALAI, aripiprazole long-acting injectable.
Discussion
Bed usage with ALAI
Our naturalistic, mirror-image study showed a substantial reduction in frequency and duration of hospitalisation that was maintained over a 2-year period following the initiation of ALAI (Figures 1 and 2). Patients who continued for 2 years (n = 69), demonstrated an overall reduction of 84% in the mean number of hospital admissions and 88% reduction in mean number of bed days compared with the 2-year period before initiation.
To our knowledge, this is the first mirror image study of ALAI with a 2-year follow-up period. Our results are overall consistent with previous observations from smaller mirror image studies,17,18 though one of these established a rather moderate reduction in bed usage, with the mean number of admissions falling by 36% and the mean number of bed days reducing by 25% the year following initiation of ALAI in 130 patients. 18
The schizophrenia patient group who continued for 2 years (n = 44) demonstrated similar statistical significant drop in hospital bed occupancy to the whole patient group (Table 2). Previous mirror-image studies did not report separately on hospitalization rates of patients with schizophrenia although randomised controlled trial evidence demonstrates that ALAI improves rates of clinical relapse when compared with placebo in this group of patients after 12 months of treatment. 7 Furthermore, another study found that 242 patients who had been commenced on ALAI following treatment with oral aripiprazole showed improvement in psychopathology at 6 months in a real world environment. 19
Regarding pattern of admissions, it was noted that both number and length increased in the year immediately preceding ALAI initiation – a finding consistent with patterns of use of other second generation anti-psychotic depots such as PP1M.15,20 This observation alongside the relatively high total number of hospitalisations pre-LAI initiation may indicate that, due to current perceptions surrounding the use of long acting injectable antipsychotic medication, patients are more likely to be initiated on it following a gradual or prolonged deterioration in their mental health rather than earlier in the course of the illness. 21
Comparison with PP1M
An indirect comparison between the above cohort of patients on ALAI with a different naturalistic cohort of patients treated with PP1M in the same clinical setting found that both demonstrated a statistically significant decrease in number and length of admissions the 2 years before compared with 2 years after LAI initiation without significant differences between the two treatment options with regards to hospitalisation rates in the 2 years after initiation (Table 2).
A smaller naturalistic study with 1 year follow up, comparing ALAI and PP1M22 found similar significant improvements in bed occupancy with both LAIs although statistical comparisons were not made between the two groups. However, a systematic review of short-term, placebo controlled RCTs that attempted an indirect treatment comparison by evaluating their relative efficacy established statistically better results with ALAI compared with PP1M23; the primary endpoint used was the mean change in the Positive and Negative symptom scale total score from baseline between each LAI and placebo.
The total number of previously recorded hospital admissions (an indirect indicator of illness severity prior to LAI initiation) were not found to be significantly different across the two LAI cohorts Table 1). However, in the all diagnoses group treated with ALAI, patients had statistically more frequent and longer admissions in the 2 years prior to treatment compared with patients treated with PP1M which was not the case when comparing the two groups of patients with a diagnosis of schizophrenia only (Table 2). This could indicate that although both medications are being used in a similar fashion for patients with schizophrenia, ALAI may be preferred as an off licence treatment choice for patients with other severe mental illnesses such as bipolar affective disorder requiring prolonged and frequent hospital admissions. Having said that, other studies have shown that clinicians were more likely to prescribe PP1M over ALAI in more symptomatic patients with schizophrenia spectrum disorders 24 and in patients with more frequent and lengthy hospital admissions in the year prior to initiation than those treated with other LAIs. 25 This could mean that prescribing practices may differ across prescribers, settings and formulations and the trans-diagnostic choice of LAIs may be well influenced by factors outside prescribing guidelines.
Finally, in this study patients on ALAI were more likely to be switched from oral medication and commenced in an inpatient setting, while patients on PP1M more likely to be switched from injectable medication in the outpatients; this difference is most likely due to a cohort of stable patients that were switched over to PP1M from the two-weekly risperidone injections.
Continuation rates and level of adherence
A total of 37% of patients initiated on ALAI discontinued treatment at 2 years with 28% stopping in year 1 and 13% in the second year (Table 3). There were no significant differences in demographic and clinical characteristics between those who continued for 2 years on ALAI and those who did not (Table 4).
Continuation rates between patients on ALAI and PP1M in the 2-year follow-up period were broadly similar at 63% and 67%, respectively, with a small difference favouring patients on PP1M with a diagnosis of schizophrenia (62% on ALAI versus 69% on PP1M, Table 3). Higher discontinuation rates have been reported separately for either LAIs in previous naturalistic studies of up to 49% in the first year for ALAI and up to 40% in the first year for PP1M.18,26 Only two other studies have directly compared PP1M and ALAI discontinuation rates: one showed a dropout rate of 14% at 12 months with no difference between PP1M and ALAI and the second reported that 32% of patients on ALAI and 43% patients on PP1M did not complete 28 weeks of treatment.27,28 However, retention rates appear to remain relatively high past the first year of treatment on PP1M in studies reporting on long-term real world treatment outcomes.14,15
Patients on ALAI were most likely to discontinue due to lack of effectiveness (16% versus 8%) and patients with PP1M were most likely to stop taking their medication due to side effects (14.5% versus 4.6%) (Table 3). This is in line with findings from the Qualify study as well as the above mentioned systematic review, whereby a more favourable tolerability profile emerged for ALAI compared with PP1M.23,28
Finally, this is the first study to review the actual level of adherence for patients that continued for 2 years on ALAI and evaluate its impact on hospital admissions. We found that 65% of patients showed full (100%) adherence and 33% good (⩾50%) adherence: only one patient was less than 50% compliant. There were numerical, but not statistically significant, differences in hospitalisation rates between those patients who were fully compliant and those who were partially compliant with ALAI at 2 years (Figure 3). Partial compliance with oral antipsychotic medication has been previously associated with a significant risk of relapse in the long-term treatment of schizophrenia. 29 A study, for example, which used pharmacy records reported that admission rates were lower for those who were fully concordant (14%) than for those who were partially (24%) or non-concordant (35%). 30 The only other study evaluating the impact of partial concordance with a LAI on hospitalisation yielded similar results with fully compliant patients on PP1M showing significantly better outcomes than those with partial or poor adherence at 3 years follow up. 16
It has been hypothesized that long-term anti-psychotic use can cause iatrogenic dopamine hypersensitivity, which is one explanation for the rebound psychosis observed after sometimes only brief cessation of antipsychotics.31,32 Our study demonstrates no significant difference in hospitalisations between patients who are fully and partially compliant with ALAI which may contribute to the early evidence base regarding the benefits of the partial agonist activity of ALAI in the long-term treatment of patients with chronic schizophrenia.
Limitations of the study
This was an independently designed and conducted study that was not externally funded or supported in order to avoid potential bias. Additional strengths include the 2-year period of follow-up. A larger sample size would be desirable, though the relatively high retention rates in the present study increase the validity of the results.
The naturalistic and nonrandomised design of the study may be useful in assessing medication adherence in a ‘real world’ scenario and limiting the influence of the Hawthorne effect when compared with controlled trials, although the implicit limitations of the lack of a randomised control group apply. Hence, while using a mirror-image study design may ensure that some patient specific variables can be controlled and remain constant, it cannot account for other important factors such as changes in socioeconomic variables, increasing age of patients and potential alterations in case management. Additionally, comparisons between PP1M and ALAI were made with cohorts treated in somewhat different time frames, which could also impact on these dynamic factors. In addition, there may have been a shift in the availability of hospital resources during this time as well as prescribing practices including the implementation of shared and supported decision making process. 33 Selection bias was minimised by including a consecutive sample of eligible patients prescribed ALAI and PP1M at the discretion of clinicians in the absence of any restrictive or prescriptive practice.
With regards to data collection, some cases needed to be excluded because of a lack of available information in the electronic records. Data was collected from within a singular hospital trust, which could have led to under-reporting of outcomes in the event that a patient was admitted to an inpatient unit outside of area. Furthermore, using data available from electronic records limited the scope of the data collection, which could have been enhanced by administered questionnaires to ascertain information such as disease severity and current active substance misuse rather than a recorded diagnosis. The number of hospitalisations was used as a proxy measure of illness severity but factors unrelated to disease severity, may impact on the decision to hospitalise; hence, limited conclusions can be drawn regarding the potential benefits of continued treatment on the different levels of disease severity in the absence of validated symptom rating scales.
Conclusion
The introduction of ALAI had a substantial impact on long-term clinical outcomes in this naturalistic cohort. More than half of patients continued treatment and had no admission during 2-year follow-up. Furthermore, there were no major differences in hospitalisation rates at 2 years between ALAI and PP1M.
Footnotes
Author contributions: SP was responsible for the study concept and design. SP, KM and JB were responsible for data extraction and statistical analysis. SP and KM were responsible for drafting the manuscript. All authors were responsible for critical revision of the manuscript and have accepted the final version.
Conflict of interest statement: SP reports grants and honoraria outside the submitted work. KM and JB have nothing to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sofia Pappa  https://orcid.org/0000-0002-6303-1547
https://orcid.org/0000-0002-6303-1547
Contributor Information
Katy Mason, West London NHS Trust, London, UK Lancashire and South Cumbria NHS Foundation Trust.
Joshua Barnett, West London NHS Trust, London, UK.
Sofia Pappa, West London NHS Trust, 43-47 Avenue Road, London, W38NJ, UK Division of Psychiatry, Faculty of Medicine, Imperial College London, London, UK.
References
- 1. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. London: National Institute for Health and Care Excellence, 2014. [Google Scholar]
- 2. Curson DA, Barnes TR, Bamber RW, et al. Long-term depot maintenance of chronic schizophrenic out-patients: the seven-year follow-up of the Medical Research Council fluphenazine/placebo trial. III. Relapse postponement or relapse prevention? The implications for long-term outcome. Br J Psychiatry 1985; 146: 474–480. [DOI] [PubMed] [Google Scholar]
- 3. Emsley R, Chiliza B, Asmal L, et al. The nature of relapse in schizophrenia. BMC Psychiatry 2013; 13: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falkai P, Wobrock T, Lieberman J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: long-term treatment of schizophrenia. World J Biol Psychiatry 2006; 7: 5–40. [DOI] [PubMed] [Google Scholar]
- 5. Correll CU, Lauriello J. Using long-acting injectable antipsychotics to enhance the potential for recovery in schizophrenia. J Clin Psychiatry 2020; 81: MS19053AH5C. [DOI] [PubMed] [Google Scholar]
- 6. Biagi E, Capuzzi E, Colmegna F, et al. Long-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv Ther 2017; 34: 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012; 73: 617–624. [DOI] [PubMed] [Google Scholar]
- 8. Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2014; 75: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 9. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry 2007; 164: 1411–1417. [DOI] [PubMed] [Google Scholar]
- 10. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol 2017; 15: 1192–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs 2015; 29: 773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kishimoto J. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry 2013; 74: 957–965. [DOI] [PubMed] [Google Scholar]
- 13. Taylor D, Olofinjana O. Long-acting paliperidone palmitate – interim results of an observational study of its effect on hospitalization. Int Clin Psychopharmacol 2014; 29: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nikolić N, Page N, Akram A. et al. The impact of paliperidone palmitate long-acting injection on hospital admissions in a mental health setting. Int Clin Psychopharmacol 2017; 32: 95–102. [DOI] [PubMed] [Google Scholar]
- 15. Pappa S, Mason K, Howard E. Long-term effects of paliperidone palmitate on hospital stay and treatment continuation. Int Clin Psychopharmacol 2019; 34: 305–311. [DOI] [PubMed] [Google Scholar]
- 16. Pappa S, Mason K. Partial compliance with long-acting paliperidone palmitate and impact on hospitalization: a 6-year mirror-image study. Ther Adv Psychopharmacol 2020; 10: 2045125320924789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodgson R, Aladakatti C, Kataria N, et al. A mirror image study of the utility of long acting aripiprazole. Eur Psychiatry 2016; 33: S252. [Google Scholar]
- 18. Taylor DM, Sparshatt A, Amin F, et al. Aripiprazole long-acting injection – a mirror image study of its effects on hospitalisation at one year. J Psychopharmacol 2017; 31: 1564–1569. [DOI] [PubMed] [Google Scholar]
- 19. Schöttle D, Janetzky W, Luedecke D, et al. Effectiveness of aripiprazole once-monthly in schizophrenia patients pretreated with oral aripiprazole: a 6-month, real-life non-interventional study [published correction appears in BMC Psychiatry 2018; 18: 389]. BMC Psychiatry 2018; 18: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor DM, Sparshatt A, O’Hagan M, et al. Effect of paliperidone palmitate on hospitalisation in a naturalistic cohort – a four-year mirror image study. Eur Psychiatry 2016; 37: 43–48. [DOI] [PubMed] [Google Scholar]
- 21. Stahl S. Long-acting injectable antipsychotics: shall the last be first? CNS Spectr 2014; 19: 3–5. [DOI] [PubMed] [Google Scholar]
- 22. Hodgson RE. Evaluating the cost and clinical effectiveness of long-acting, injectable aripiprazole and paliperidone palmitate once a month in a real-world setting [published correction appears in Clinicoecon Outcomes Res 2019; 11: dlxvii]. Clinicoecon Outcomes Res 2019; 11: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pae CU, Wang SM, Han C, et al. Comparison between long-acting injectable aripiprazole versus paliperidone palmitate in the treatment of schizophrenia: systematic review and indirect treatment comparison. Int Clin Psychopharmacol 2017; 32: 235–248. [DOI] [PubMed] [Google Scholar]
- 24. Bartoli F, Ostuzzi G, Crocamo C, et al. Clinical correlates of paliperidone palmitate and aripiprazole monohydrate prescription for subjects with schizophrenia-spectrum disorders: findings from the STAR Network Depot Study. Int Clin Psychopharmacol 2020; 35: 214–220. [DOI] [PubMed] [Google Scholar]
- 25. Patel R, Chesney E, Taylor M, et al. Is paliperidone palmitate more effective than other long-acting injectable antipsychotics? Psychol Med 2018; 48: 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whale R, Pereira M, Cuthbert S, et al. Effectiveness and predictors of continuation of paliperidone palmitate long-acting injection treatment: a 12-month naturalistic cohort study. J Clin Psychopharmacol 2015; 35: 591–595. [DOI] [PubMed] [Google Scholar]
- 27. Di Lorenzo R, Ferri P, Cameli M, et al. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: retrospective study in a real-world clinical setting. Neuropsychiatr Dis Treat 2019; 15: 183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res 2015; 168: 498–504. [DOI] [PubMed] [Google Scholar]
- 29. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004; 55: 886–891. [DOI] [PubMed] [Google Scholar]
- 30. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004; 161: 692–699. [DOI] [PubMed] [Google Scholar]
- 31. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol 2017; 15: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull 2012; 38: 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pappa S, Barnett J, Gomme S, et al. Shared and supported decision making in medication in a mental health setting: how far have we come? Community Ment Health J. Epub ahead of print 5 February 2021. DOI: 10.1007/s10597-021-00780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]