Abstract

Few natural, biocompatible, and inexpensive emulsifiers are available because such emulsifiers must satisfy severe requirements, be produced synthetically rather than naturally, be nontoxic, and require minimal effort to produce. Therefore, the synthesis of food-grade and biocompatible nanoparticles as an alternative to surfactants has recently received attention in the industry. However, many previous efforts involved chemical modification of materials or the introduction of secondary cocomponents for emulsion formation. To achieve the goal of simple preparation, we consider here chitosan nanoparticles to prepare Pickering emulsions of food-grade oil through the control of pH, without further chemical modification or extra additives. A mild process can prepare nanoparticles from chitosan by simply increasing the pH from 3.0 to 6.0. The results showed that the average radius of chitosan at pH 6.0 was 170 nm, while large aggregates were formed at pH 6.5. These nanoparticles were utilized to prepare the Pickering emulsion. The average size of emulsion droplets decreased upon increasing the pH from 3.0 to 6.0. Moreover, Pickering emulsions at different oil fractions and nanoparticle concentrations were stable and showed a low creaming index for 45 days. The emulsions were stable against coalescence and flocculation and behaved rheologically as gel-like, shear-thinning fluids (G′ > G″). Pickering emulsion prevents the growth of the microorganism (Staphylococcus aureus) at different pH values and chitosan concentrations. These results demonstrate that chitosan nanoparticles could be a cost-effective and biocompatible emulsifier for the food or pharmaceutical industry for encapsulation and bioactive compounds, and Pickering emulsions have promising antibacterial effects for further applications.
1. Introduction
More than a century ago, Ramsden and Pickering used colloidal particles to stabilize the emulsions by the adsorption of particles between two immiscible liquids, which are generally called Pickering emulsions.1−5 The particles in a Pickering emulsion stabilize the emulsion droplets and trap the oil inside a solid particle layer.6,7 The particles mainly form a steric barrier at the oil–water interface and prevent the droplets from coalescence, making the emulsion more stable.8,9 Pickering emulsions can be prepared by functional materials such as hybrid colloidal assemblies,10 Janus particles,11,12 foams, and hollow permeable structures.13,14 The application and preparation of Pickering emulsions from food-grade and stimulus-responsive particles as particulate emulsifiers have received significant attention over the last two decades in many industrial sectors such as food, personal care, agrochemical, and pharmaceutical.15,16 Various particles have been synthesized which respond to stimuli like temperature,16−19 pH,20−25 and magnetic fields26−28 or the addition of salts.29 Special consideration has been given to pH-responsive particulate emulsifiers, such as microgels,30,31 inorganic particles,32 and polymer particles,26 to control the preparation of Pickering emulsions. Many reported microgel and polymer particle emulsifiers are based on synthetic polymers, but they are not biocompatible, which may cause some problems.33 Therefore, there is the need for the bioeffective and food-grade stimulus-responsive emulsifier for emulsions.34,35
Among various naturally occurring polymers for the potential emulsion application,36−39 chitosan is one of the most abundant, and it can be used as a particulate emulsifier for Pickering emulsions.40,41 Chitosan is a biodegradable, renewable, nontoxic, and naturally occurring polycationic polysaccharide obtained from chitin by deacetylation of the amino groups. It contains β-[1 → 4]-linked 2-acetamido-2-deoxy-d-glucopyranose and 2-amino-2-deoxy-d-glucopyranose units.42 The weakly basic amino groups (pKa ≈ 6.5) allow chitosan to dissolve in water at pH < 6.5 due to protonation, while it becomes insoluble at pH values higher than pKa. Under these conditions, chitosan molecules form aggregates due to the loss of charge.43 In the previous research, chitosan is employed to form Pickering emulsions, but the system needs the chemical modification of chitosan or needs a secondary additive. İlyasoğlu and Marcin graft caffeic acid groups on the chitosan chain to realize the formation of Pickering emulsions.44 Studart et al. introduce silica nanoparticles to realize the stabilization of emulsion.45 Although previous studies have successfully produced Pickering emulsions using simple chitosan. Pickering emulsions without further chemical reactions or special second matching pairs are still studied only in a few reports. Here, in this paper, we employ food-grade chitosan to form aggregated nanoparticles to stabilize further food oil forming Pickering emulsion through pH control. Moreover, the effect of factors such as the oil-to-water ratio, pH, and chitosan concentrations on emulsion stability (creaming and coalescence) were systematically studied. The rheological behavior of the resulting emulsions was also characterized. Furthermore, Pickering emulsions could prevent the growth of microorganisms like S. aureus. Our results certify that a simple chitosan system is suitable for forming NPs to prepare Pickering emulsions with good stability. The developed strategy could be helpful for the practical application of chitosan-based Pickering emulsions.
2. Results and Discussion
2.1. Behavior of Chitosan at Different pH Values
Chitosan molecules are relatively hydrophilic with a low surface activity toward oil, at the lowest pH, while their hydrophobicity increases upon increasing pH.46 This pH-dependent property makes chitosan stimulus-responsive. The responsive assembly of food-grade chitosan to pH to form colloidal particles is investigated first (Scheme 1). As shown in Figure 1a, the chitosan solutions are transparent from 3.0 to 6.0 pH with about 97% transmittance. At low pH (<pKa 6.5), chitosan dissolves completely due to the protonation of its amino groups.35 The size of chitosan molecules increases gradually from 113.3 to 170.5 nm as pH increased from 3.0 to 6.0 (Figure 1c). TEM micrographs show nano-sized chitosan particles (Figure 1d). At pH 6.5, the transmittance of chitosan sharply decreased to 74.3% due to the formation of large particles, with a radius around 582.6 nm (Figure 1c, diameter larger than 1000 nm). The amine groups are deprotonated with increasing pH, which leads to an increase in the turbidity of solutions with decreasing solubility, indicating that chitosan molecules were converted into large particles. Changes in electrophoretic mobility accompanied this chitosan behavior (expressed as zeta potential, ζ), as demonstrated in Figure 1b. The zeta potential decreased from 38.3 to 9.2 mV as the chitosan pH increased from 3.0 to 6.0. The zeta potential gradually reduced from 1.3 to 0.3 mV as the pH increased from 6.5 to 9.0. The clustering of the partially charged chitosan molecules leads to gel-like particles with a diameter larger than 1000 nm, from pH 6.5 to 10. Hence, pH 6.0 is the turning point for the formation of large aggregates.
Scheme 1. pH-Responsive Assembly of Chitosan to form Colloid Particles.
Figure 1.
Transmittance (a), zeta potential (b), and size (c) of chitosan nanoparticles at different pH values. TEM of chitosan nanoparticles at pH = 6.0 (d). The concentration of chitosan was 0.5 wt %.
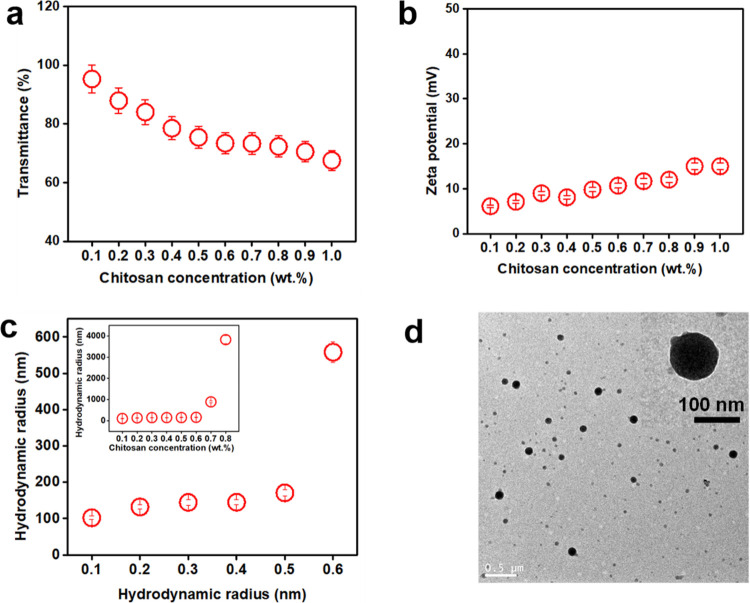
The effect of chitosan concentration was investigated, as shown in Figure 2. The transmittance sharply decreases from 97 to 70% and remains around 70% ± 2.6 when the concentration was increased from 0.5 to 1.0 wt % (Figure 2a). However, the zeta potential gradually increases from 6 mV to 15 mV with increasing the chitosan concentration (Figure 2b). For the NP sizes, chitosan concentration of 0.5 wt % is the turning point; below this concentration, particles are in nanoscale, while above, they are micron-sized, as shown in Figure 2c. However, TEM micrographs show nano-sized chitosan particles at this concentration of 0.5 wt % (Figure 2d).
Figure 2.
Effect of chitosan concentration on transmittance (a), zeta potential (b), radius of particles (c), and the TEM (d) image of chitosan nanoparticles (0.5 wt %) at pH 6.0.
2.2. Effect of the Oil Ratio
The macroscopic appearance of Pickering emulsions prepared with various oil ratios is shown in Figure 3. The increasing oil ratio led to a higher emulsified phase volume as observed visually. The average emulsion droplet size elevated from 0.92 to 4.5 μm when the oil fraction increased from 10 to 50% (Figure 3b and Figure S1). At an oil fraction of 60%, it can be observed that the Pickering emulsion was not stable, and the creaming was found on the first day (Figure 3a). Meanwhile, the creaming index decreases (Figure S1b). The phase separation was improved as the oil ratio increased, which could be explained by the crowding effect. They owe their stability against creaming to the very high-volume fraction (50%) at which the droplets were essentially jammed. The longer stability was observed 1 week after the 45th day (Figure S2), and the phenomenon was almost the same as with the 45th day of storage (Figure 3a). In addition, a fluorescence microscope is used to observe the location of the chitosan nanoparticle on the oil, and fluorescein isothiocyanate (FITC) is encapsulated inside the chitosan nanoparticle. As shown in Figure S3, the green fluorescent circle indicates chitosan nanoparticle coverage on the oil surface. TEM micrographs (Figure S4) revealed spherical droplets consistent with the optical and FITC micrograph results.
Figure 3.

Macroscopic view (a), and the microstructure (b) of Pickering emulsions prepared by chitosan nanoparticles at pH 6.0 with different oil ratios and 0.5 wt % of chitosan concentration.
The amount of chitosan is limiting at a higher fraction of oil, causing instability in emulsions with fast coalescing of droplets, providing coarser emulsions. The visual observation was consistent with the microscopic observations (Figure 3b). The rheology of Pickering emulsion can further explain this. In emulsions with a 60% oil ratio, moduli were lower than the emulsions with 50% oil fractions at the same frequency. This finding indicates that the reduction in elasticity of emulsions with an increasing oil ratio may be due to the bulk coalescence of droplets.47 For oil fraction less than 50%, the shear viscosity of all emulsions having different oil content fractions decreased with the shear rate increase, indicating shear thinning behavior.
The shear viscosity of the Pickering emulsions increased with the oil fraction up to 50%. This behavior suggests that with increasing dispersed phase droplet fractions, the stability of emulsions improved.47 Viscoelastic results showed that G′ of all emulsions remained higher than the G″, suggesting that the systems show gel-like characteristics throughout the applied frequencies (Figure 4). Our results are consistent with Arditty et al., who found that gel-like emulsions had been produced by particle interactions with increasing oil fraction.48 The droplets may be densely packed and jammed during such circumstances, leading to higher viscosity and may have resisted droplet coalescence. In emulsions with a 10% oil fraction, G″ moduli is higher than G′ indicating that emulsions behave as liquid-like materials.47 For the experiments described in the following sections, the oil fraction was fixed at 50%.
Figure 4.
(a) Flow profiles (shear rate over the shear viscosity), (b) elastic moduli G′, and viscous moduli G″ of the Pickering emulsion stabilized by chitosan particles. The emulsions were produced with 0.5 (w/v %) chitosan concentration at pH 6.0.
2.3. Effect of pH
The effect of pH on the chitosan-based Pickering emulsion was examined and displayed in Figure 5. Although the fresh emulsion did not show any phase separation behavior on the first day, the emulsions formed below pH 6.0 were not stable for 45 days of storage. Under such conditions (pH 3.0 or 4.5), most of the chitosan molecules were protonated and separately dissolve in the aqueous solution (Scheme 1), forming limited chitosan aggregated particles, which may not be enough to minimize the size and to stabilize the emulsion droplet. At pH 6.0, there is a balance between protonation and antiprotonation of chitosan, leading to the formation of sufficient nano-sized aggregated particles to stabilize the emulsion droplet. Figure 5b confirms that the droplet size at pH 3.0 and 4.5 is larger than the droplet size at pH 6.0 (Figure S5). The emulsification capacity of chitosan at higher pH improved, and emulsion droplets with smaller average size were produced. Especially at pH 6.0, emulsion droplets of smaller size (0.8 μm) and narrow distribution were created without any droplet flocculation. After storage, the emulsion at pH 3.0 and 4.5, separated into two layers (Figure 5a), indicating flocculation of emulsions due to the cracking of chitosan particles’ layer, resulting coalescence and instability in emulsions. Consequently, oil and water separation was found in the acidic medium because particles become more hydrophilic at the lowest pH value.49
Figure 5.

Visual appearance (a) and average droplet size (b) of Pickering emulsions prepared by chitosan nanoparticles (0.5 wt %) at different pH values and fixed oil ratio (50%).
2.4. Effect of Chitosan Concentration
The unstable emulsion is also observed at a low concentration of chitosan particles (Figure 6a). Emulsion stabilized by particles at low concentration (0.1 wt % and 0.3 wt %), the number of chitosan particles were insufficient to homogenize and stabilize extra oil droplets, which results in loss of original equilibrium (emulsion failed to produce), resulting in unstable emulsion. This shows that chitosan molecules at 0.5 wt % (pH 6.0) with a compact and flexible chain structure readily adsorbed at the oil/water interface with a low steric hindrance value and formed a shell around the oil which supported the formation of stable Pickering emulsions with lower average size droplets (Figure 6). It showed that the average emulsion droplet size decreased from 35.5 ± 3.12 to 0.9 ± 3.1 μm (Figure 6b and Figure S6).
Figure 6.

Visual appearance (a) and average droplet size (b) of Pickering emulsions prepared by chitosan nanoparticles (0.1–0.5 wt %, 6.0 pH) and the oil ratio is 50%
2.5. Antimicrobial Properties of Pickering Emulsions
Gram-positive (S. aureus) bacteria were used to test the possible antimicrobial activity of the Pickering emulsion stabilized by chitosan nanoparticles, as shown in Figure 7. All the culture media containing Pickering emulsions presented transparent inhibition zones with different pH values. In particular, samples with pH 3.5 and 4.5 show remarkable antibacterial effects (Figure 7a).
Figure 7.

(a) Inhibition zone of Pickering emulsions stabilized by chitosan nanoparticles (0.5 wt %) at the emulsion of pH 3.0 (A), an emulsion of pH 4.5 (B), an emulsion of pH 6.0(C); sample (O) is only soybean oil without chitosan particles, and sample (W) is the control containing only water. The ratio of oil is 50% with 0.5 wt % concentration of chitosan particles. (b) Inhibition zone of Pickering emulsions at emulsion of 0.1 wt % NPs (A), emulsion of 0.3 wt % NPs (B), emulsion of 0.5 wt % NPs (C); sample (O) is only soybean oil without chitosan particles, and sample (W) is the control containing only water. The ratio of oil is 50% with pH 6.
Some researchers proved that the antibacterial activity of chitosan at a low pH value was related to the effect of the amino groups (hydrophilic), which can interact with the bacterial cell wall and make intracellular components leak by increasing their penetrability.50,51 Moreover, the colony-forming units (CFU) are counted, and the antibacterial activities are calculated:52 the killing percentage of chitosan nanoparticles at pH 3.0 and pH 4.5 is around 100%, while chitosan nanoparticles at pH 6.0 also show a high killing percentage at 82% (Figure S7). In addition, chitosan nanoparticles could damage the cell membranes of a microorganism after incubation with S. aureus, which is confirmed by SEM in Figure S8.50,53 The effect of chitosan concentration on the antibacterial activity is shown in Figure 7b. The transparent inhibition zones of Pickering emulsions with different chitosan particle concentrations (0.1–0.5 wt %) have a similar effect.
3. Conclusions
In conclusion, a pH-responsive biocompatible food-grade nanoparticle was produced from chitosan at pH 6.0. The nanoparticles for emulsions can be modified from surface-inactive to surface-active and vice versa by tuning the pH. These nanoparticles were used as an emulsifier for the stabilization of emulsions. pH was used to control the stability of the emulsion. Other parameters, such as effects of nanoparticle concentration, oil fraction on droplet size, and emulsion stability, were also studied. The parametric study shows that the emulsion was stable against coalescence and flocculation and revealed the shear thinning behavior (G′ > G″, gel-like structure). The main advantage of nanoparticles for Pickering emulsions relative to other more traditional stabilization methods is its surfactant-free preparation, green, and economical. The antimicrobial properties of Pickering emulsions were tested at different pH values and concentrations. Pickering emulsion indicates remarkable antibacterial effects, especially at pH 3.5 and 4.5. These results open the door of chitosan being applied for Pickering emulsions in various domains, including food, flavor/fragrance, cosmetic, and medical applications. Pickering emulsions have promising antibacterial effects that can improve the stability and quality of food in daily life.
4. Materials and Methods
4.1. Materials
Medium molecular weight chitosan (190–310 kDa, the viscosity of 200–800 cPs, and deacetylation degree of 75–85%,) acetic acid, hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (Shanghai, China). Soybean oil was bought from a supermarket in Shanghai, China. All solutions were prepared with Milli-Q water. All other reagents used were of analytical grade, and deionized water was used throughout unless stated otherwise.
4.2. Chitosan Solution Preparation
Stock chitosan solutions (1 w/v %) were produced by dissolving an appropriate amount of dry powder into a 1 w/v % acetic acid solution. The mixture was stirred overnight at 450 rpm and ambient temperature. Impurities were filtered from the resulting chitosan solution before using it. The pH of chitosan solutions was adjusted using a HCl/NaOH (0.1–0.5 M) solution. Solutions of chitosan of different concentrations were prepared by dilution of stock solution.
4.3. Preparation of Pickering Emulsions
All emulsions were prepared at a total volume of 5 mL using a range of oil ratios (10% to 60%) via mixing the chitosan solution (0.1–0.5 wt %) at different pH values (3.0, 4.5, and 6.0). The mixture was homogenized by an ultrasonic homogenizer (Scientz Biotechnology Co., Ltd., Ningbo). The head of the titanium probe (6 mm, diameter) was dipped into a tube containing the mixture. The power was 100 W, and the mixing time 2 min, with 5 s pulse on, 2 s pulse off. A stirred ice water bath was used to reduce heating effects; a small increase in temperature <20 °C was observed during sonication. The chitosan concentration, oil ratio, and pH on emulsions properties were examined.
4.4. Antibacterial Activity and Killing Efficiency
The Oxford cup method was used to assess antibacterial activity. Gram-positive Staphylococcus aureus (S. aureus) microorganisms were chosen for antimicrobial activities of the Pickering emulsion. The sterile nutrient agar surface was covered with an Oxford cup (outer diameter 8.0 0.1 mm, inner diameter 6.0 0.1 mm). Suspensions of inoculated microorganisms were taken from a nutrient broth medium that had been diluted. The petri dishes were filled with nutrient broth containing inoculated microorganisms. The suspension ratio of S. aureus to nutrient broth was about 1:10. The suspensions of S. aureus had concentrations of around 108 CFU/mL. The Oxford cup was then filled with 40 μL of Pickering emulsions. For the control experiment, physiological saline (0.9% NaCl solution) was used instead of Pickering emulsions in the same volume. After removing the Oxford cup, the petri dishes were incubated at 37 °C for 24 h. Antibacterial activity was measured using the inhibition zone.
From a solid Luria–Bertani agar plate, a single colony of S. aureus bacteria was transferred to 10 mL of liquid Luria–Bertani culture medium in the presence of 50 μg/mL ampicillin and cultured overnight at 37 °C with shaking. S. aureus was collected via centrifugation and washed three times in PBS before being suspended in the solution. The S. aureus bacterial suspension’s optical density (OD = 600) was set to 1.0 to obtain a concentration of 5 × 108 cells/mL, which was then diluted fivefold in PBS. In a 24-well plate, the sterilized polymer surfaces were incubated with 10 μL of S. aureus bacterial suspension. Bacterial suspensions were extracted after 30 min of incubation at 37 °C and serially diluted (10,000-fold) in PBS. Each 100 μL dilution was plated in triplicate on a solid Luria–Bertani agar plate and incubated for 16 h at 37 °C. After incubation, the number of CFU on each plate was counted, and the average concentration (in CFU/mL) was calculated. Each test was carried out three times. The killing efficiency (KE) was estimated using the following formula:52,54,55
where NCFU,sample and NCFU,control are the numbers of colony-forming units in the sample and control, respectively.
4.5. Measurements and Characterization
4.5.1. pH of Solutions
pH of the chitosan solution was measured using a digital pH meter (Mettler Toledo, FE220) at 20 ± 0.5 °C.
4.5.2. Zeta Potential and Size of Nanoparticles
The electrophoretic mobility of emulsion droplets was determined at room temperature by a Zeta Master (Malvern Instruments, ZEM5003, Worcs., UK). The sizes of nanoparticles were measured by dynamic light scattering (DLS). DLS was performed at 25 °C with an ALV-CGS3 light scattering apparatus, operating at a wavelength of 632.8 nm. The mean Rh (hydrodynamic radius) of NPs was measured at a fixed angle of 90°. The CONTIN method was used to analyze the size distribution of the particle radius. For data processing, average and standard deviations were obtained from six duplicates with each acquisition times of 10 s.
4.5.3. UV–vis Spectroscopy
The transmittance (turbidity) of chitosan solutions was measured by a UV–vis spectrophotometer (Shimadzu UV-1800) in a transmittance mode (%T) at 633 nm. Each result was calculated from the average of three repeated measurements.
4.5.4. Transmission Electron Microscopy
Morphology of particles and emulsion droplets was observed by transmission electron microscopy (TEM, Hitachi, H-700 Tokyo, Japan). Samples for TEM analysis were prepared by dropping 10 μL of chitosan solution (6.0 pH) and the emulsion (diluted 1000 times) on a carbon-coated copper grid; after 30 s, the excess sample was removed by a filter paper, and the grid was dried at room temperature for 1 hr.
4.5.5. Optical Microscopy
The microstructure of emulsions was observed with an optical microscope (Leica Microsystems Inc., Wetzlar, Germany). A drop of the emulsion was put on a microscope slide, covered with a cover glass, and observed through a microscope.
4.5.6. Creaming Index
The stability of the emulsion was determined by determining the creaming index (CI). The total height of emulsions (Ht) and the height of the serum (Hs) (i.e., the sum of the turbid layer and/or the transparent layer of the vessel at ambient temperature) were measured, and the CI percentage (CI %) was calculated as (Hs/Ht) × 100. The average of three measurements was reported.
4.5.7. Rheological Measurement of the Emulsion
The rheological behavior of the Pickering emulsion was measured by an MCR501 rheometer (Anton Paar, Germany) using parallel plates (PP25) at a 0.5 mm gap. The temperature was controlled by a Peltier element. The shear viscosity of the emulsion was determined at increasing shear rates, from 0.01 to 100 s–1. After this, the loss (G″) and storage (G′) moduli were measured as a function of frequency from 0.1 to 100 rad/s, at 1.0% constant strain (linear region). The average of three measurements were presented.
Acknowledgments
We gratefully thank the financial support from the NSFC (National Natural Science Foundation of China) for Young Scholars (21706074), Fundamental Research Funds for the Central Universities (222201814007), and Key Scientific and Technological Project of Xinjiang Bingtuan (2018AB025, 2017BTRC002).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01490.
The average droplet size and creaming index of Pickering emulsions at different oil ratios (Figure S1); macroscopic view of Pickering emulsions prepared by chitosan nanoparticles at pH 6.0 with varying ratios of oil (Figure S2); fluorescence microscopy of oil stabilized by chitosan nanoparticles (Figure S3); TEM micrographs of emulsion droplets (Figure S4); average droplet size of Pickering emulsions prepared by chitosan nanoparticles at different pH values and fixed oil ratio (Figure S5); average droplet size of Pickering emulsions prepared at different concentrations and fixed oil ratio (Figure S6); CFU of control of S. aureus and chitosan NPs at pH 3.0, 4.5, and 6.0 (Figure S7); and SEM images of control of S. aureus and after incubation with chitosan NPs at pH 6.0 (Figure S8) (PDF)
Author Contributions
∇ R.A. and M.W. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Ramsden W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation). Proc.R. Soc. Lon. 1903, 72, 156–164. 10.1098/rspl.1903.0034. [DOI] [Google Scholar]
- Pickering S. U. CXCVI.—Emulsions. J. Chem. Soc., Trans. 1907, 91, 2001–2021. 10.1039/CT9079102001. [DOI] [Google Scholar]
- Bao Y.; Zhang Y.; Liu P.; Ma J.; Zhang W.; Liu C.; Simion D. Novel fabrication of stable Pickering emulsion and latex by hollow silica nanoparticles. J. Colloid Interface Sci. 2019, 553, 83–90. 10.1016/j.jcis.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Briggs N.; Raman A. K. Y.; Barrett L.; Brown C.; Li B.; Leavitt D.; Aichele C. P.; Crossley S. Stable pickering emulsions using multi-walled carbon nanotubes of varying wettability. Colloids Surf., A 2018, 537, 227–235. 10.1016/j.colsurfa.2017.10.010. [DOI] [Google Scholar]
- Sabri F.; Raphael W.; Berthomier K.; Fradette L.; Tavares J. R.; Virgilio N. One-step processing of highly viscous multiple Pickering emulsions. J. Colloid Interface Sci. 2020, 560, 536–545. 10.1016/j.jcis.2019.10.098. [DOI] [PubMed] [Google Scholar]
- Dickinson E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. 10.1016/j.cocis.2009.11.001. [DOI] [Google Scholar]
- Dickinson E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci. Technol. 2012, 24, 4–12. 10.1016/j.tifs.2011.09.006. [DOI] [Google Scholar]
- Yu X.; Wen T.; Cao P.; Shan L.; Li L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J. Colloid Interface Sci. 2019, 556, 258–265. 10.1016/j.jcis.2019.08.027. [DOI] [PubMed] [Google Scholar]
- Yu M.; Zhou L.; Zhang J.; Yuan P.; Thorn P.; Gu W.; Yu C. A simple approach to prepare monodisperse mesoporous silica nanospheres with adjustable sizes. J. Colloid Interface Sci. 2012, 376, 67–75. 10.1016/j.jcis.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Liu H.; Wang C.; Gao Q.; Liu X.; Tong Z. Magnetic hydrogels with supracolloidal structures prepared by suspension polymerization stabilized by Fe(2)O(3) nanoparticles. Acta Biomater. 2010, 6, 275–281. 10.1016/j.actbio.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Daisuke Suzuki S. T.; Kawaguchi H.; Microgels J. Prepared by Surfactant-Free Pickering Emulsion-Based Modification and Their Self-Assembly. J. Am. Chem. Soc. 2007, 129, 8088–8089. 10.1021/ja072258w. [DOI] [PubMed] [Google Scholar]
- Liang Hong S. J. Steve Granick, Simple Method to Produce Janus Colloidal Particles in Large Quantity. Langmuir 2006, 22, 9495. 10.1021/la062716z. [DOI] [PubMed] [Google Scholar]
- Stocco A.; Drenckhan W.; Rio E.; Langevin D.; Binks B. P. Particle-stabilised foams: an interfacial study. Soft Matter 2009, 5, 2215. 10.1039/b901180c. [DOI] [Google Scholar]
- Bon P. J. C. Cellular Polymer Monoliths Made via Pickering High Internal Phase Emulsions. Chem. Mater. 2007, 19, 1537–1539. 10.1021/cm0628810. [DOI] [Google Scholar]
- Mwangi W. W.; Lim H. P.; Low L. E.; Tey B. T.; Chan E. S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. 10.1016/j.tifs.2020.04.020. [DOI] [Google Scholar]
- Sharkawy A.; Barreiro M. F.; Rodrigues A. E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885 10.1016/j.carbpol.2020.116885. [DOI] [PubMed] [Google Scholar]
- Wang L. J.; Hu Y. Q.; Yin S. W.; Yang X. Q.; Lai F. R.; Wang S. Q. Fabrication and characterization of antioxidant pickering emulsions stabilized by zein/chitosan complex particles (ZCPs). J. Agric. Food Chem. 2015, 63, 2514–2524. 10.1021/jf505227a. [DOI] [PubMed] [Google Scholar]
- Ngai T.; Behrens S. H.; Auweter H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem. Commun. 2005, 331–333. 10.1039/b412330a. [DOI] [PubMed] [Google Scholar]
- To Ngai H. A.; Behrens S. H. Environmental Responsiveness of Microgel Particles and Particle-Stabilized Emulsions. Macromolecules 2006, 39, 8171–8177. 10.1021/ma061366k. [DOI] [Google Scholar]
- Kalliola S.; Repo E.; Srivastava V.; Heiskanen J. P.; Sirvio J. A.; Liimatainen H.; Sillanpaa M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloids Surf. B. Biointerfaces 2017, 153, 229–236. 10.1016/j.colsurfb.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Cong Y.; Chen K.; Zhou S.; Wu L. Synthesis of pH and UV dual-responsive microcapsules with high loading capacity and their application in self-healing hydrophobic coatings. J. Mater. Chem. A 2015, 3, 19093–19099. 10.1039/C5TA04986E. [DOI] [Google Scholar]
- Dararatana N.; Seidi F.; Hamel J.; Crespy D. Controlling release kinetics of pH-responsive polymer nanoparticles. Polym. Chem. 2020, 11, 1752–1762. 10.1039/C9PY01946D. [DOI] [Google Scholar]
- Kong M.; Peng X.; Cui H.; Liu P.; Pang B.; Zhang K. pH-responsive polymeric nanoparticles with tunable sizes for targeted drug delivery. RSC Adv. 2020, 10, 4860–4868. 10.1039/C9RA10280A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierszewska M.; Ostrowska-Czubenko J.; Chrzanowska E. pH-responsive chitosan/alginate polyelectrolyte complex membranes reinforced by tripolyphosphate. Eur. Polym. J. 2018, 101, 282–290. 10.1016/j.eurpolymj.2018.02.031. [DOI] [Google Scholar]
- Li J.; Stöver H. D. H. Doubly pH-Responsive Pickering Emulsion. Langmuir 2008, 24, 13237–13240. 10.1021/la802619m. [DOI] [PubMed] [Google Scholar]
- Nallamilli T.; Binks B. P.; Mani E.; Basavaraj M. G. Stabilization of Pickering Emulsions with Oppositely Charged Latex Particles: Influence of Various Parameters and Particle Arrangement around Droplets. Langmuir 2015, 31, 11200–11208. 10.1021/acs.langmuir.5b02443. [DOI] [PubMed] [Google Scholar]
- Brugger B.; Richtering W. Magnetic, Thermosensitive Microgels as Stimuli-Responsive Emulsifiers Allowing for Remote Control of Separability and Stability of Oil in Water-Emulsions. Adv. Mater. 2007, 19, 2973–2978. 10.1002/adma.200700487. [DOI] [Google Scholar]
- Sonia Melle M. L.; Fuller G. G. Pickering Emulsions with Controllable Stability. Langmuir 2005, 6, 2158–2162. [DOI] [PubMed] [Google Scholar]
- Yang F.; Liu S.; Xu J.; Lan Q.; Wei F.; Sun D. Pickering emulsions stabilized solely by layered double hydroxides particles: the effect of salt on emulsion formation and stability. J. Colloid Interface Sci. 2006, 302, 159–169. 10.1016/j.jcis.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Liu K.; Jiang J.; Cui Z.; Binks B. P. pH-Responsive Pickering Emulsions Stabilized by Silica Nanoparticles in Combination with a Conventional Zwitterionic Surfactant. Langmuir 2017, 33, 2296–2305. 10.1021/acs.langmuir.6b04459. [DOI] [PubMed] [Google Scholar]
- Gujarathi N. A.; Rane B. R.; Patel J. K. pH sensitive polyelectrolyte complex of O-carboxymethyl chitosan and poly (acrylic acid) cross-linked with calcium for sustained delivery of acid susceptible drugs. Int. J. Pharm. 2012, 436, 418–425. 10.1016/j.ijpharm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Jaemyung Kim L. J. C.; Kim F.; Yuan W.; Shull K. R.; Huang J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180. 10.1021/ja102777p. [DOI] [PubMed] [Google Scholar]
- Tan C.; Pajoumshariati S.; Arshadi M.; Abbaspourrad A. A simple route to renewable high internal phase emulsions (HIPEs) strengthened by successive cross-linking and electrostatics of polysaccharides. Chem. Commun. 2019, 55, 1225–1228. 10.1039/C8CC09683J. [DOI] [PubMed] [Google Scholar]
- Paunov V. N.; Cayre O. J.; Noble P. F.; Stoyanov S. D.; Velikov K. P.; Golding M. Emulsions stabilised by food colloid particles: role of particle adsorption and wettability at the liquid interface. J. Colloid Interface Sci. 2007, 312, 381–389. 10.1016/j.jcis.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Wang X. Y.; Heuzey M. C. Chitosan-Based Conventional and Pickering Emulsions with Long-Term Stability. Langmuir 2016, 32, 929–936. 10.1021/acs.langmuir.5b03556. [DOI] [PubMed] [Google Scholar]
- Bjorkegren S.; Nordstierna L.; Torncrona A.; Palmqvist A. Hydrophilic and hydrophobic modifications of colloidal silica particles for Pickering emulsions. J. Colloid Interface Sci. 2017, 487, 250–257. 10.1016/j.jcis.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Ruiyi L.; Zaijun L.; Junkang L. Histidine-functionalized carbon-based dot-Zinc(II) nanoparticles as a novel stabilizer for Pickering emulsion synthesis of polystyrene microspheres. J. Colloid Interface Sci. 2017, 493, 24–31. 10.1016/j.jcis.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Lamont K.; Pensini E.; Marangoni A. G. Gelation on demand using switchable double emulsions: A potential strategy for the in situ immobilization of organic contaminants. J. Colloid Interface Sci. 2020, 562, 470–482. 10.1016/j.jcis.2019.11.090. [DOI] [PubMed] [Google Scholar]
- Miras J.; Vilchez S.; Solans C.; Esquena J. Chitosan macroporous foams obtained in highly concentrated emulsions as templates. J. Colloid Interface Sci. 2013, 410, 33–42. 10.1016/j.jcis.2013.07.072. [DOI] [PubMed] [Google Scholar]
- Perrin E.; Bizot H.; Cathala B.; Capron I. Chitin nanocrystals for Pickering high internal phase emulsions. Biomacromolecules 2014, 15, 3766–3771. 10.1021/bm5010417. [DOI] [PubMed] [Google Scholar]
- Nilsen-Nygaard J.; Strand S.; Vårum K.; Draget K.; Nordgård C. Chitosan: Gels and Interfacial Properties. Polymer 2015, 7, 552–579. 10.3390/polym7030552. [DOI] [Google Scholar]
- Desbrièresa J.; M C.; Rinaudo M. Hydrophobic derivatives of chitosan Characterization and rheological behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. 10.1016/0141-8130(96)01095-1. [DOI] [PubMed] [Google Scholar]
- Kalliola S.; Repo E.; Srivastava V.; Zhao F.; Heiskanen J. P.; Sirvio J. A.; Liimatainen H.; Sillanpaa M. Carboxymethyl Chitosan and Its Hydrophobically Modified Derivative as pH-Switchable Emulsifiers. Langmuir 2018, 34, 2800–2806. 10.1021/acs.langmuir.7b03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İlyasoğlu H. N.; Marcin Z. Caffeic acid grafted chitosan as a novel dual-functional stabilizer for food-grade emulsions and additive antioxidant property. Food Hydrocoll. 2019, 95, 168–176. 10.1016/j.foodhyd.2019.04.043. [DOI] [Google Scholar]
- Alison L.; Demirors A. F.; Tervoort E.; Teleki A.; Vermant J.; Studart A. R. Emulsions Stabilized by Chitosan-Modified Silica Nanoparticles: pH Control of Structure-Property Relations. Langmuir 2018, 34, 6147–6160. 10.1021/acs.langmuir.8b00622. [DOI] [PubMed] [Google Scholar]
- Wei Z.; Wang C.; Zou S.; Liu H.; Tong Z. Chitosan nanoparticles as particular emulsifier for preparation of novel pH-responsive Pickering emulsions and PLGA microcapsules. Polymer 2012, 53, 1229–1235. 10.1016/j.polymer.2012.02.015. [DOI] [Google Scholar]
- Ge S.; Xiong L.; Li M.; Liu J.; Yang J.; Chang R.; Liang C.; Sun Q. Characterizations of Pickering emulsions stabilized by starch nanoparticles: Influence of starch variety and particle size. Food Chem. 2017, 234, 339–347. 10.1016/j.foodchem.2017.04.150. [DOI] [PubMed] [Google Scholar]
- Arditty S.; Schmitt V.; Giermanska-Kahn J.; Leal-Calderon F. Materials based on solid-stabilized emulsions. J. Colloid Interface Sci. 2004, 275, 659–664. 10.1016/j.jcis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Mwangi W. W.; Ho K.-W.; Tey B.-T.; Chan E.-S. Effects of environmental factors on the physical stability of pickering-emulsions stabilized by chitosan particles. Food Hydrocoll. 2016, 60, 543–550. 10.1016/j.foodhyd.2016.04.023. [DOI] [Google Scholar]
- Wu Z.; Zhou W.; Pang C.; Deng W.; Xu C.; Wang X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. 10.1016/j.foodchem.2019.05.114. [DOI] [PubMed] [Google Scholar]
- Fereidoon Shahidi J. K. V. A. You-JinJeon, Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. 10.1016/S0924-2244(99)00017-5. [DOI] [Google Scholar]
- Chen T.; Yang H.; Wu X.; Yu D.; Ma A.; He X.; Sun K.; Wang J. Ultrahighly Charged Amphiphilic Polymer Brushes with Super-Antibacterial and Self-Cleaning Capabilities. Langmuir 2019, 35, 3031–3037. 10.1021/acs.langmuir.8b04187. [DOI] [PubMed] [Google Scholar]
- Sarhan W. A.; Azzazy H. M.; El-Sherbiny I. M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. 10.1021/acsami.6b00739. [DOI] [PubMed] [Google Scholar]
- Wang H.; Shi X.; Yu D.; Zhang J.; Yang G.; Cui Y.; Sun K.; Wang J.; Yan H. Antibacterial Activity of Geminized Amphiphilic Cationic Homopolymers. Langmuir 2015, 31, 13469–13477. 10.1021/acs.langmuir.5b03182. [DOI] [PubMed] [Google Scholar]
- Zhou C.; Wang F.; Chen H.; Li M.; Qiao F.; Liu Z.; Hou Y.; Wu C.; Fan Y.; Liu L.; Wang S.; Wang Y. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. 10.1021/acsami.5b12688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.