Abstract

Lean premixed combustion is one of the most effective methods to constrain pollutant emissions for modern industrial gas turbines. An experimental study was performed on its propagation speed and internal structure at engine-relevant temperatures. A Bunsen burner was employed for the measurement with an optical schlieren system. The results show that the increase of preheating temperature dramatically accelerates the propagation of methane flames. The numerical results predicted by GRI-Mech 3.0, FFCM-1, and USC Mech II were also compared. The GRI-Mech 3.0 seems to overestimate the laminar flame speed at high operating conditions, while FFCM-1 underestimates the laminar flame speed compared to the present experimental data. The prediction by FFCM-1 shows good agreement with the overall existing data. The USC Mech II seems to overestimate the laminar flame speed at fuel-lean conditions while shows good agreement with present experimental measurements at stoichiometric conditions when the inlet temperature increases. It is also indicated that the flame is thinned at high-temperature conditions and the importance of CO production to the propagation speed increases. Finally, based on the experimental data, an empirical correlation of the laminar flame speed was developed in the range of Tu = 300–800 K and ϕ = 0.7–1.0, the maximum deviation of which was less than 8%. The results of this study may contribute to the optimization of advanced gas turbine combustors.
1. Introduction
Methane is one of the most important energy sources nowadays and the main fuel for modern industrial gas turbines. To avoid excessive NOx emissions, lean premixed flames have been widely adopted in combustors under elevated temperature and pressure conditions. For a typical H class gas turbine, the pressure ratio of the compressor is 19–23,1−3 and therefore the inlet temperature to the combustor is about 690–750 K when the air compression process is regarded as an isentropic process. Much attention has been paid to the topic of the laminar flame speed of methane–air mixtures since it is an important factor in the combustor design. For an ideal low emission combustion process, the flame propagation speed must match the local flow speed. Fail to do so, the combustor may experience some problems, e.g., dynamic instability, flashback, blowoff,4,5 or high CO emissions.6−8
The laminar flame speed (SL) is defined as the propagation speed of a planar, unstretched, adiabatic, premixed flame.9,10 It is a key parameter and of great importance for a combustible mixture because it governs many properties of combustion such as the shape and stabilization of a premixed flame. Extensive experiments have been conducted to measure SL of methane–air mixtures using various apparatuses. Gu et al.11 measured SL of a methane–air mixture between 300 and 400 K using spherically expanding flames. Correlations between SL and equivalence ratio were empirically given as an exponential function within the range of ϕ = 0.8–1.2. Hu et al.12 measured SL of methane–air mixtures up to 443 K. Ogami and Kobayashi13 measured SL of stoichiometric methane–air mixtures diluted by helium up to 600 K. More recently, SL of methane–air mixtures has been measured at 300–573 K by Mohammad et al.,14 489–573 K by Ferris et al.,15 and 300–650 K by Varghese et al.16 Egolfopoulos and co-workers17 measured SL for C1–C4 hydrocarbons at 400–520 K and 0.8–3.0 MPa. Along with theoretical and numerical studies, it is unequivocal that the flame propagation speed and its gradient to temperature increase with the preheating temperature. However, this dependence has not been quantificationally measured at engine-relevant conditions, which leaves a major shortcoming in the fundamental database. As summarized by Konnov,18 previous data were performed over a wide range of pressure (up to 6 MPa), while the preheating temperature was mostly below 500 K. Furthermore, large discrepancies still lie between existing literature for elevated temperature conditions.
The main experimental approaches to determine the flame speed are Bunsen burner,6−8,10,13 spherically expanding bomb,10,11,19−25 heat flux,26−30 and stagnation flame.31−33 Detailed advantages and limitations of these methods were reviewed by Egolfopoulos et al.34 The Bunsen burner is deemed as a suitable choice to investigate the flame speed under elevated temperatures and ambient pressure. This approach is feasible to control the preheating process and form an unstretched, quasi-adiabatic premixed flame. The shortcoming of this method is that the flame shape is strongly affected by the heat loss at the nozzle rim, curvature on the flame tip, and the equivalence ratio discrepancy due to fresh air entrainment around the flame. To avoid these effects, the Angle method13,35−37 is usually employed, which measures the slope of the flame front and excludes the nonlinear sections, i.e., the tip and base of the flame.
In addition, numerical simulations also play an important role in combustion modeling and engineering design. For methane, many detailed chemical mechanisms have been developed. GRI-Mech 3.038 is a well-developed mechanism that consists of 53 species and 325 reactions. It has been optimized using experimental laminar flame speeds of methane up to 600 K. FFCM-139 is one of the most advanced mechanisms that consists of 37 species and 291 reactions. USC Mech II40 is a high-temperature combustion model of H2/CO/C1–C4 compounds, which consists of 111 species and 784 reactions and was subject to validation tests against reliable combustion data. Mechanisms mentioned above have been used to model the laminar methane–air flames.
The objective of this work is to investigate the premixed laminar flame speed of lean methane–air mixtures at preheating temperatures up to 800 K, which covers the inlet temperature range of modern industrial gas turbine combustors. The remainder of this paper is organized as follows. Section 2 presents the experimental and numerical details. Section 3 presents the results and discusses the response of the laminar flame structure to different preheating temperatures. Finally, in Section 4, some important conclusions are summarized.
2. Experimental and Numerical Details
The experimental setup is shown in Figure 1, including a Bunsen burner and an optical schlieren system.
Figure 1.

Experimental setup.
2.1. Bunsen Burner
An axisymmetric premixed burner was designed and developed to generate a steady conical laminar premixed flame stabilized on the outlet of a contracting nozzle, and the diameter (d) and the contraction ratio of which are 10 mm and 2.25, respectively. The height of the Bunsen burner is 700 mm, which is long enough to mix the fuel with an air stream. Two K-type thermocouples were placed, one downstream of the gas inlet, while the other upstream of the contracting nozzle, to monitor temperatures throughout the Bunsen burner. The preheating temperature of the unburned mixture (Tu) was determined by the latter. During each measurement, the fluctuation of temperature was controlled within ±5 K.
2.2. Optical Diagnostics and Determination of the Laminar Flame Speed
As shown in Figure 1, the optical system consists of a tungsten–halogen lamp, a pinhole, a set of lenses, and a camera. In the experiments, the light through the pinhole was collimated by a convex lens. The collimated light passed through the Bunsen flame before it was focused on a vertically installed knife edge. The schlieren images were taken using a digital CMOS camera (FE 1.8/50, 2768 × 1560).
As shown in Figure 2a, the brightness of the schlieren image represents the corresponding temperature gradient in the Bunsen flame field. The line formed by the brightest pixel in the direction perpendicular to the knife edge is determined as the flame front. The Angle method13,35−37 was chosen to calculate the flame speed from schlieren images. Other than the area-average method,6−8,10 this approach only uses data in the middle section of the flame front, where it is free from the adverse effects such as flame stretch and curvature.
Figure 2.
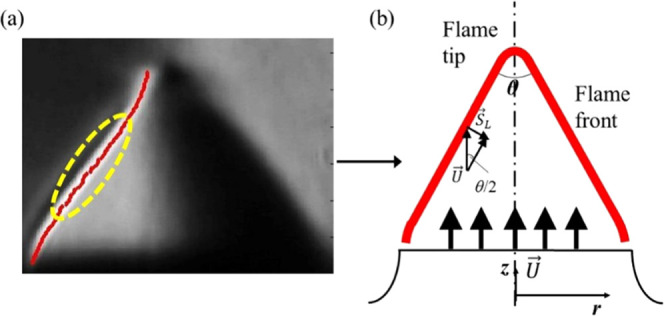
(a) Flame front obtained by the optical method. (b) Angle method for determination of SL.
As shown in Figure 2b, the flame propagates toward the unburned mixture at an angle θ/2. The laminar flame speed (SL) is determined to be equal to the velocity component of the unburned mixture, which is normal to the flame front, and therefore, SL is calculated as follows
| 1 |
| 2 |
where U is the average velocity of the unburned mixture jet, and QV is the volumetric flow rate of the unburned methane–air flow.
The uncertainty of our measurements was estimated from two main
sources: the uncertainty of the volumetric flow rate of the unburned
methane–air flow (UQV) and the uncertainty of the calculated slope of
the flame front (Usin θ). UQV came from the
mass flow controller uncertainty, which was estimated to be ∼1.4%,
and Usin θ came from the
postprocess of the schlieren method. Then, the overall uncertainties
were estimated from 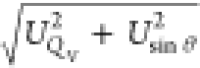 .
.
2.3. Numerical Setup
The simulation of the one-dimensional premixed flame was carried out using the PREMIX code41 of the CHEMKIN package42 with the GRI-Mech 3.0,38 FFCM-1,39 and USC Mech II40 mechanisms.
3. Results and Discussion
3.1. System Validation
To validate the present measurement system, the methane–air flame speed at 300 and 373 K were measured and compared with existing literature,6−8,14,16,20,27−29,43−45 as shown in Figure 3. Each data point was obtained by averaging the results of 24 schlieren images, and the error bars represent the scale of one standard deviation. Error bars of the equivalence ratio are also plotted in Figure 3, which represent the standard deviation of equivalence ratio fluctuation during measurements. Furthermore, the simulation results were plotted for comparison. The present measurements agree well with the existing data. SL increases with the equivalence ratio and the preheating temperature. The laminar flame speeds of the stoichiometric mixture at 300 and 373 K are 35.9 and 52.3 cm/s, respectively. The standard deviation of data at 373 K is larger than that at 300 K. This may be due to the noises on schlieren images, which were caused by the natural convection between the heated experiment rig and the ambient environment. It is also seen that the numerical results by GRI-Mech 3.0 quantificationally agree with present measurements, while FFCM-1 slightly underestimates the flame speed. Predictions for 373 K by GRI-Mech 3.0 seem to be a little overshooting near fuel stoichiometric conditions. As for USC Mech II, at 300 and 373 K, it seems to slightly overestimate the flame speed at lean conditions compared to the overall existing data. The maximum overall uncertainties of the flame speed are estimated to be ∼4.7% at 300 K and ∼4.2% at 373 K, respectively.
Figure 3.

Laminar flame speed of methane–air flames at 300 and 373 K.
3.2. Experimental Results
The measurements of laminar flame speeds were performed at ambient pressure in the temperature range of 350–800 K. The present experimental data can be seen in Table A1 in Appendix A. Figure 4 shows the comparison of the present experimental data with existing SL data14,16,31,46−48 at elevated preheating temperatures. The results indicate that GRI-Mech 3.0 overestimates the SL while FFCM-1 shows a good agreement with existing data at Tu ≤ 550 K. The USC Mech II mechanism shows a good agreement with present experimental measurements at stoichiometric conditions when the inlet temperature elevates but still slightly overestimates the flame speed at lean conditions as previously mentioned. GRI-Mech 3.0 and FFCM-1 have a tendency to overestimate when the preheating temperature increases (Tu > 550 K).
Table A1. Experimental Data of the Laminar Flame Speed of the Methane–Air Mixture.
| Tu (K) | ϕ | SL (cm/s) | √σ (cm/s) |
|---|---|---|---|
| 299 | 0.697 | 19.3 | 0.174 |
| 302 | 0.792 | 26.1 | 0.668 |
| 303 | 0.898 | 33.4 | 1.00 |
| 303 | 0.951 | 34.0 | 0.867 |
| 303 | 1.00 | 34.8 | 1.13 |
| 304 | 0.896 | 32.0 | 1.03 |
| 305 | 0.998 | 37.0 | 0.363 |
| 305 | 0.850 | 29.3 | 1.01 |
| 306 | 0.794 | 25.7 | 0.924 |
| 307 | 0.753 | 23.0 | 0.932 |
| 308 | 0.694 | 18.7 | 0.850 |
| 345 | 1.00 | 46.8 | 1.39 |
| 346 | 0.998 | 46.8 | 0.495 |
| 346 | 1.00 | 47.5 | 1.45 |
| 346 | 0.951 | 44.9 | 1.89 |
| 347 | 0.951 | 47.4 | 1.84 |
| 347 | 0.897 | 42.3 | 1.10 |
| 348 | 0.950 | 46.0 | 0.858 |
| 348 | 0.897 | 45.2 | 1.04 |
| 348 | 0.850 | 38.1 | 0.711 |
| 350 | 0.896 | 43.0 | 1.26 |
| 350 | 0.850 | 39.5 | 2.44 |
| 350 | 0.794 | 34.2 | 0.780 |
| 352 | 0.850 | 39.8 | 1.16 |
| 352 | 0.794 | 36.7 | 1.85 |
| 352 | 0.753 | 30.8 | 0.757 |
| 354 | 0.796 | 36.0 | 1.12 |
| 354 | 0.750 | 32.6 | 0.919 |
| 354 | 0.695 | 26.9 | 0.779 |
| 357 | 0.753 | 30.8 | 1.15 |
| 357 | 0.662 | 23.2 | 0.561 |
| 358 | 0.695 | 28.7 | 0.963 |
| 359 | 0.695 | 26.6 | 1.15 |
| 362 | 0.666 | 23.1 | 1.17 |
| 362 | 0.644 | 22.7 | 2.34 |
| 366 | 1.00 | 53.8 | 1.20 |
| 367 | 1.00 | 52.1 | 1.40 |
| 367 | 0.951 | 53.7 | 1.54 |
| 368 | 0.950 | 50.1 | 1.51 |
| 368 | 0.896 | 50.4 | 1.70 |
| 368 | 1.00 | 51.0 | 1.09 |
| 369 | 0.895 | 50.0 | 0.851 |
| 369 | 0.850 | 47.0 | 1.07 |
| 369 | 0.950 | 49.3 | 1.73 |
| 370 | 0.849 | 45.9 | 1.13 |
| 370 | 0.794 | 42.9 | 0.787 |
| 370 | 0.897 | 46.2 | 0.752 |
| 371 | 0.794 | 41.7 | 1.47 |
| 371 | 0.751 | 37.6 | 1.35 |
| 371 | 0.850 | 42.1 | 1.05 |
| 372 | 0.750 | 36.7 | 0.815 |
| 372 | 0.795 | 37.9 | 1.19 |
| 373 | 0.693 | 30.3 | 1.23 |
| 373 | 0.695 | 32.4 | 1.03 |
| 373 | 0.751 | 34.3 | 0.895 |
| 374 | 0.695 | 29.3 | 1.03 |
| 375 | 0.661 | 26.1 | 0.631 |
| 375 | 0.656 | 28.6 | 0.679 |
| 376 | 0.660 | 25.2 | 0.986 |
| 395 | 1.00 | 56.9 | 1.11 |
| 396 | 1.00 | 59.1 | 1.28 |
| 396 | 1.00 | 58.0 | 1.83 |
| 396 | 0.951 | 54.9 | 1.48 |
| 397 | 0.950 | 56.6 | 1.99 |
| 397 | 0.896 | 50.9 | 0.931 |
| 398 | 0.950 | 57.3 | 1.00 |
| 398 | 0.896 | 55.3 | 2.04 |
| 398 | 0.851 | 49.0 | 1.18 |
| 399 | 0.850 | 51.0 | 1.36 |
| 400 | 0.895 | 55.2 | 1.53 |
| 400 | 0.794 | 47.8 | 1.12 |
| 400 | 0.794 | 43.0 | 1.44 |
| 401 | 0.751 | 38.2 | 0.857 |
| 402 | 0.849 | 50.8 | 2.03 |
| 402 | 0.751 | 41.3 | 1.67 |
| 403 | 0.695 | 32.2 | 1.13 |
| 404 | 0.794 | 45.6 | 1.82 |
| 404 | 0.695 | 36.5 | 1.42 |
| 405 | 0.661 | 29.5 | 0.703 |
| 406 | 0.750 | 40.2 | 0.659 |
| 406 | 0.656 | 29.9 | 0.738 |
| 407 | 0.693 | 34.5 | 1.27 |
| 409 | 0.656 | 28.1 | 0.970 |
| 445 | 1.00 | 70.2 | 0.945 |
| 445 | 1.00 | 69.7 | 1.71 |
| 446 | 1.00 | 72.0 | 2.69 |
| 446 | 0.950 | 70.1 | 2.07 |
| 447 | 0.896 | 69.4 | 2.46 |
| 447 | 0.950 | 67.3 | 2.57 |
| 448 | 0.896 | 67.4 | 1.48 |
| 448 | 0.950 | 71.1 | 1.91 |
| 448 | 0.850 | 64.3 | 2.52 |
| 448 | 0.896 | 64.4 | 1.33 |
| 449 | 0.850 | 65.2 | 1.08 |
| 450 | 0.795 | 58.4 | 1.69 |
| 450 | 0.795 | 58.6 | 3.29 |
| 450 | 0.850 | 59.9 | 0.656 |
| 451 | 0.751 | 52.4 | 2.61 |
| 452 | 0.751 | 50.2 | 1.84 |
| 452 | 0.694 | 45.9 | 1.88 |
| 452 | 0.795 | 55.0 | 1.79 |
| 453 | 0.656 | 38.7 | 1.08 |
| 454 | 0.751 | 50.2 | 1.49 |
| 455 | 0.694 | 43.4 | 1.73 |
| 455 | 0.694 | 42.7 | 0.758 |
| 457 | 0.656 | 36.3 | 0.615 |
| 458 | 0.656 | 36.5 | 0.841 |
| 468 | 1.00 | 81.5 | 2.51 |
| 468 | 1.00 | 77.4 | 2.77 |
| 469 | 0.950 | 80.3 | 2.88 |
| 469 | 0.950 | 73.9 | 1.16 |
| 470 | 0.850 | 72.9 | 1.84 |
| 470 | 0.896 | 78.5 | 2.49 |
| 470 | 0.897 | 72.2 | 2.05 |
| 471 | 0.795 | 68.2 | 1.17 |
| 472 | 0.950 | 78.5 | 2.49 |
| 472 | 1.00 | 82.2 | 2.20 |
| 472 | 0.751 | 61.6 | 1.88 |
| 472 | 0.849 | 66.6 | 1.59 |
| 473 | 0.897 | 76.5 | 1.96 |
| 473 | 0.694 | 52.7 | 1.86 |
| 473 | 0.795 | 62.1 | 1.22 |
| 474 | 0.850 | 72.8 | 2.44 |
| 474 | 0.656 | 45.7 | 1.61 |
| 474 | 0.751 | 54.0 | 0.897 |
| 475 | 0.795 | 65.5 | 2.76 |
| 475 | 0.694 | 47.2 | 0.984 |
| 476 | 0.751 | 61.0 | 1.79 |
| 477 | 0.696 | 51.9 | 2.05 |
| 477 | 0.661 | 40.8 | 1.25 |
| 478 | 0.656 | 44.3 | 1.81 |
| 495 | 1.00 | 86.0 | 1.91 |
| 495 | 1.00 | 83.0 | 2.40 |
| 496 | 0.950 | 85.1 | 2.87 |
| 496 | 0.950 | 81.0 | 1.33 |
| 497 | 0.896 | 83.2 | 2.98 |
| 497 | 0.896 | 79.4 | 1.78 |
| 498 | 1.00 | 86.7 | 2.04 |
| 498 | 0.850 | 72.8 | 2.18 |
| 499 | 0.950 | 84.5 | 1.82 |
| 499 | 0.850 | 78.2 | 3.32 |
| 500 | 0.897 | 83.8 | 2.84 |
| 500 | 0.795 | 71.7 | 3.50 |
| 500 | 0.795 | 66.7 | 3.13 |
| 501 | 0.850 | 77.7 | 3.47 |
| 501 | 0.751 | 64.6 | 1.72 |
| 501 | 0.751 | 62.2 | 1.51 |
| 502 | 0.795 | 71.1 | 2.86 |
| 502 | 0.694 | 56.7 | 1.62 |
| 502 | 0.694 | 53.4 | 1.60 |
| 503 | 0.751 | 64.6 | 1.42 |
| 503 | 0.656 | 47.5 | 1.48 |
| 503 | 0.657 | 45.9 | 1.20 |
| 504 | 0.696 | 55.3 | 2.09 |
| 505 | 0.656 | 47.1 | 1.97 |
| 545 | 1.00 | 103 | 3.97 |
| 545 | 0.998 | 102 | 3.56 |
| 546 | 0.950 | 102 | 3.64 |
| 546 | 0.950 | 98.0 | 4.10 |
| 547 | 0.896 | 93.8 | 3.45 |
| 548 | 0.896 | 97.8 | 3.02 |
| 548 | 0.794 | 82.6 | 3.22 |
| 548 | 0.848 | 90.6 | 3.78 |
| 549 | 0.848 | 96.6 | 4.20 |
| 549 | 0.695 | 64.7 | 2.12 |
| 549 | 0.751 | 76.1 | 3.85 |
| 550 | 0.796 | 87.5 | 3.75 |
| 550 | 0.664 | 52.3 | 1.72 |
| 550 | 0.677 | 59.3 | 3.02 |
| 552 | 0.751 | 78.6 | 1.80 |
| 553 | 0.695 | 70.4 | 2.11 |
| 554 | 0.655 | 59.4 | 2.05 |
| 555 | 0.596 | 50.6 | 1.33 |
| 568 | 1.00 | 117 | 2.70 |
| 568 | 1.00 | 109 | 3.77 |
| 569 | 0.949 | 114 | 3.16 |
| 569 | 0.949 | 106 | 3.47 |
| 570 | 0.896 | 113 | 3.97 |
| 570 | 0.895 | 102 | 3.24 |
| 571 | 0.847 | 104 | 3.05 |
| 571 | 0.848 | 97.0 | 3.04 |
| 572 | 0.795 | 87.5 | 3.68 |
| 573 | 0.793 | 99.8 | 2.12 |
| 573 | 0.750 | 78.8 | 2.94 |
| 574 | 0.750 | 91.6 | 2.36 |
| 575 | 0.695 | 81.9 | 3.16 |
| 575 | 0.698 | 72.0 | 2.66 |
| 576 | 0.652 | 71.8 | 1.76 |
| 576 | 0.674 | 64.4 | 3.00 |
| 578 | 0.600 | 57.5 | 1.77 |
| 578 | 0.650 | 57.2 | 2.80 |
| 595 | 1.00 | 122 | 2.68 |
| 595 | 1.00 | 121 | 4.40 |
| 596 | 0.949 | 121 | 3.50 |
| 596 | 0.949 | 117 | 4.78 |
| 597 | 0.896 | 121 | 3.83 |
| 597 | 0.847 | 105 | 3.92 |
| 597 | 0.897 | 111 | 3.30 |
| 598 | 0.847 | 114 | 4.05 |
| 598 | 0.750 | 89.0 | 3.33 |
| 598 | 0.793 | 98.3 | 3.40 |
| 599 | 0.695 | 78.9 | 3.07 |
| 600 | 0.793 | 105 | 3.71 |
| 600 | 0.654 | 65.1 | 2.86 |
| 600 | 0.670 | 72.0 | 2.77 |
| 601 | 0.750 | 95.7 | 4.52 |
| 602 | 0.695 | 85.4 | 2.00 |
| 603 | 0.652 | 76.6 | 2.48 |
| 604 | 0.600 | 63.5 | 3.36 |
| 644 | 1.00 | 147 | 5.36 |
| 645 | 0.949 | 145 | 6.76 |
| 645 | 1.00 | 142 | 4.83 |
| 646 | 0.949 | 138 | 5.45 |
| 647 | 0.895 | 141 | 6.20 |
| 647 | 0.895 | 135 | 3.64 |
| 648 | 0.848 | 136 | 5.86 |
| 648 | 0.848 | 126 | 5.30 |
| 649 | 0.794 | 116 | 5.35 |
| 650 | 0.794 | 131 | 3.00 |
| 650 | 0.749 | 105 | 4.22 |
| 651 | 0.695 | 99.4 | 4.16 |
| 652 | 0.749 | 117 | 3.65 |
| 652 | 0.678 | 85.6 | 3.79 |
| 653 | 0.695 | 105 | 3.13 |
| 654 | 0.654 | 94.4 | 3.75 |
| 654 | 0.655 | 79.3 | 4.05 |
| 656 | 0.596 | 81.3 | 1.62 |
| 695 | 1.00 | 167 | 7.01 |
| 695 | 1.00 | 164 | 3.26 |
| 696 | 0.948 | 165 | 5.40 |
| 696 | 0.948 | 162 | 4.07 |
| 697 | 0.895 | 163 | 7.71 |
| 698 | 0.847 | 157 | 4.13 |
| 698 | 0.895 | 156 | 5.19 |
| 699 | 0.795 | 149 | 6.62 |
| 699 | 0.847 | 152 | 6.46 |
| 700 | 0.749 | 142 | 5.01 |
| 700 | 0.749 | 135 | 6.41 |
| 700 | 0.795 | 140 | 6.63 |
| 701 | 0.695 | 128 | 4.01 |
| 701 | 0.704 | 117 | 4.76 |
| 702 | 0.681 | 105 | 4.98 |
| 703 | 0.651 | 112 | 4.21 |
| 703 | 0.657 | 99.6 | 3.72 |
| 705 | 0.597 | 96.8 | 3.02 |
| 745 | 1.00 | 196 | 7.86 |
| 745 | 1.00 | 191 | 8.75 |
| 746 | 0.948 | 192 | 7.24 |
| 746 | 0.950 | 188 | 6.09 |
| 747 | 0.897 | 188 | 8.92 |
| 747 | 0.897 | 187 | 8.13 |
| 748 | 0.848 | 186 | 7.07 |
| 748 | 0.848 | 177 | 7.11 |
| 749 | 0.795 | 176 | 6.64 |
| 749 | 0.797 | 164 | 8.29 |
| 750 | 0.749 | 164 | 5.90 |
| 750 | 0.749 | 154 | 10.5 |
| 751 | 0.705 | 140 | 5.89 |
| 752 | 0.696 | 149 | 4.27 |
| 753 | 0.684 | 130 | 7.48 |
| 754 | 0.652 | 133 | 4.97 |
| 755 | 0.653 | 122 | 8.03 |
| 757 | 0.598 | 119 | 6.79 |
| 795 | 1.00 | 226 | 5.47 |
| 795 | 1.00 | 221 | 12.0 |
| 796 | 0.949 | 225 | 4.44 |
| 796 | 0.949 | 216 | 10.2 |
| 797 | 0.896 | 224 | 7.42 |
| 797 | 0.896 | 211 | 14.6 |
| 798 | 0.848 | 212 | 7.23 |
| 798 | 0.848 | 204 | 13.4 |
| 799 | 0.795 | 206 | 7.21 |
| 799 | 0.795 | 193 | 14.1 |
| 800 | 0.749 | 192 | 5.83 |
| 800 | 0.749 | 180 | 12.0 |
| 801 | 0.696 | 182 | 7.69 |
| 801 | 0.707 | 169 | 9.79 |
| 802 | 0.651 | 162 | 9.08 |
| 802 | 0.688 | 147 | 8.04 |
| 803 | 0.598 | 142 | 4.30 |
| 803 | 0.667 | 139 | 6.03 |
Figure 4.
Comparisons between present experimental and existing SL data at 0.1 MPa and elevated preheating temperatures.
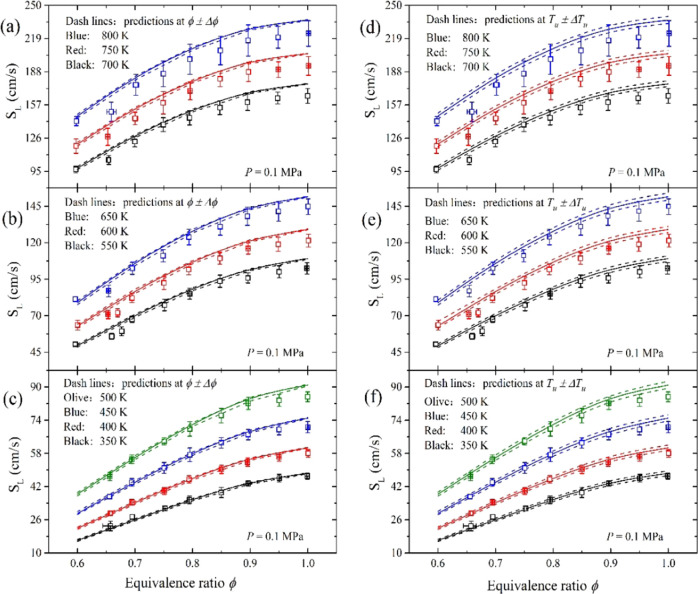
Figure 5 shows the present experimental SL data with numerical predictions of GRI-Mech 3.0 at 350–800 K. In addition, the uncertainties of the mass flow rate and temperature measurements were also considered in numerical simulations. Each pair of dashed lines in Figure 5 represents the flame speed corresponding to the upper and lower error limits of equivalence ratio (left column) and preheating temperature (right column) measurements, respectively. It is indicated that the impact of temperature uncertainty on the measurement is about 3 times larger than that of the equivalence ratio. The propagation speed of the lean premixed flame monotonously increases with the preheating temperature and equivalence ratio. At 800 K, the flame speed of the stoichiometric mixture is 223.8 cm/s, which is about 6 times larger compared to that at 300 K. Interference between the preheated mixture jet and the ambient environment was encountered. There were noises in the schlieren images due to the vortices caused by the natural convection. As the preheating temperature increases from 350 to 800 K, those noises escalated the measuring error of SL. Nevertheless, the measuring error in this study was still under ±7%. The overall uncertainty of the flame speed at 800 K was estimated to be ∼7.1%, which is about 2 times larger compared to that at 300 K.
Figure 5.
Laminar flame speeds from simulations and experiments at different preheating temperatures. Solid lines: predictions at Tu and ϕ. Dashed lines in (a)–(c): predictions at ϕ ± Δϕ. Dashed lines in (d)–(f): predictions at Tu ± ΔTu.
The numerical results predicted by GRI-Mech 3.0 agree well with the measurements at low preheating temperatures (below 400 K). Between 450 and 500 K, quantitative prediction is made at fuel-lean conditions (ϕ ≤ 0.90). For cases with a higher temperature or equivalence ratio, the flame speed is constantly overpredicted by the numerical method. At 800 K, this discrepancy is 6% for the stoichiometric mixture. One of the reasons might be the thermal radiation in the experiments.
To evaluate the impact of thermal radiation on the experiments, an Optical Thin (OPT) model was used with the PREMIX code to predict the laminar flame speed of methane. As shown in Figure 6, the numerical results were plotted with the present experimental results. The results indicate that the effect of thermal radiation is negligible.
Figure 6.
Numerical predictions of SL at 350–800 K using Optical Thin model.
3.3. Numerical Simulation
Since the GRI-Mech
3.0 mechanism has been optimized using experimental data up to 600
K, it was chosen to simulate the methane–air flames structure
at elevated preheating temperatures. Sensitivity analysis was performed
to determine the importance of each specific elementary reaction to
the flame propagation speed at preheating conditions. The sensitivity
coefficients are defined as 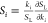 , where ki is the reaction
rate for the ith elementary
reaction.12 Nine elementary reactions with
the largest magnitude of the sensitivity coefficient are shown in Figure 7. It is shown that
the flame speed is most sensitive to the chain-branching reaction
R38, and the coefficient of which is much greater than the others.
For most reactions, the magnitude of the coefficient decreases with
increasing temperature. However, for R166, R167, and R284, the sensitivity
coefficient changes otherwise, which may suggest that the importance
of CO production elevates. For the fuel-lean scenario, the importance
of R35 and R99 increases since the oxygen is sufficient in the reaction
system, which can promote the oxidation of CO.
, where ki is the reaction
rate for the ith elementary
reaction.12 Nine elementary reactions with
the largest magnitude of the sensitivity coefficient are shown in Figure 7. It is shown that
the flame speed is most sensitive to the chain-branching reaction
R38, and the coefficient of which is much greater than the others.
For most reactions, the magnitude of the coefficient decreases with
increasing temperature. However, for R166, R167, and R284, the sensitivity
coefficient changes otherwise, which may suggest that the importance
of CO production elevates. For the fuel-lean scenario, the importance
of R35 and R99 increases since the oxygen is sufficient in the reaction
system, which can promote the oxidation of CO.
Figure 7.
Effects of different preheating temperatures on sensitivity coefficients.
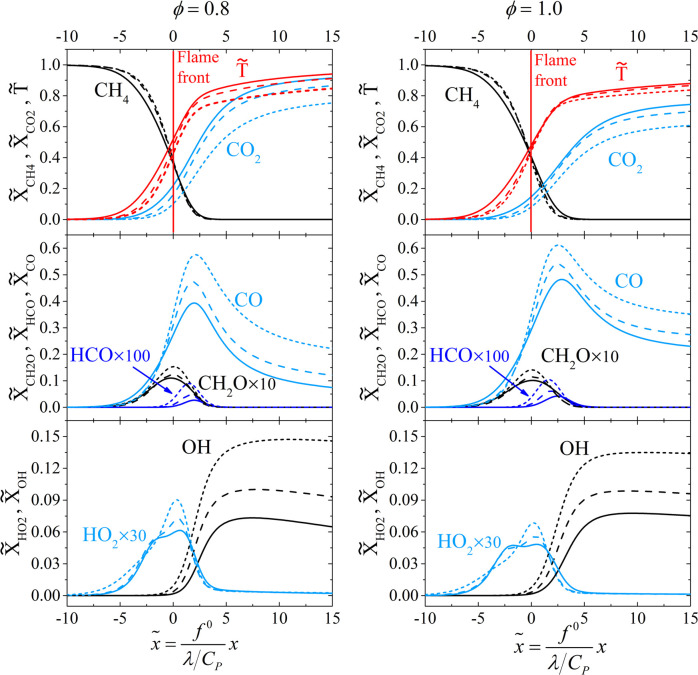
Figure 8 shows the computed methane premixed flame structure by GRI-Mech 3.0 under the flame coordinate normalized by the characteristic flame thickness (λ/CP)/f0. For comparison, a set of nondimensional variables is defined as follows
| 3 |
where subscripts u and b represent the unburned mixture and the burned mixture, respectively. λ is the thermal conductivity, CP is the specific heat, ρu is the density, and XB is the mole fraction of species B. The flame front (x̃ = 0) is defined as the position with the maximum temperature gradient.
Figure 8.
Computed flame structure. Solid lines: Tu = 300 K; dashed lines: Tu = 500 K; and short dashed lines: Tu = 800 K.
The numerical results show that the nondimensional temperature gradient increases with the preheating temperature. This indicates that the thickness of the flame reduces due to the change of chemistry and radical distribution. For the conditions ϕ = 0.8 and 1.0, the flame thicknesses (δT) at Tu = 800 K are 58% and 63% of that at 300 K, respectively. δT is calculated based on the maximum gradient of the temperature profile (dT/dx)max as follows
| 4 |
Figure 8 also shows that the peak concentration of the minor species increases with the preheating temperature, which suggests that the reactivity of the mixture is amplified. In the typical preheating zone of the flame (x̃ ∼ −5), the concentrations of CO and CH2O decrease with the inlet temperature, while the concentration of HO2 notably increases. R119 (HO2 + CH3 = OH + CH3 O) is a dominant step for methyl oxidation in the autoignition process.4 The high concentration of HO2 in front of the flame indicates that the ignition process is activated ahead of the reaction layer at high preheating temperatures.
Hence, it is suggested that with increasing inlet temperature, the flame is accelerated and thinned not only due to the reactivity elevation in the reaction layer but also due to that in the preheating zone.
3.4. Empirical Correlation
An empirical correlation was developed based on the present measurements, the form of which was chosen as follows
| 5 |
where a, b, c, n1, and n2 are independent constants. This function is an extension of the previous analysis conducted by Yu49 (eq 24). Two exponents, n1 and n2, were added to mimic the nonlinear effects and improve the accuracy in data fitting. In the range of Tu = 300–800 K and ϕ = 0.7–1.0, the flame speed is
| 6 |
The comparison between these correlations and experimental results is shown in Figure 9. It is suggested that the results increase monotonously with the preheating temperature and the equivalence ratio, the uncertainty of which is within ±8%.
Figure 9.
Comparison between the empirical correlations and the present experimental results.
In the existing literatures,12,48,50−56 power-law correlations were used to fit the stoichiometric methane–air laminar flame speed at 0.1 MPa. However, as shown in Figure 10, the discrepancy is quite large when the preheating temperature is high (Tu > 550 K). Correlations by Iijima and Takeno, Hill and Hung, Akram, and Amirante et al. show underestimation, while the correlation by Wang et al. shows overestimation at Tu > 550 K. Correlations by Elia et al., Rahim, Hu et al. and Hinton et al. show relatively good agreement with present experiments.
Figure 10.

Correlations of stoichiometric methane–air laminar flame speed at 0.1 MPa.
4. Conclusions
The laminar flame speed of a methane–air mixture was measured using a Bunsen burner at Tu = 300–800 K, ϕ = 0.7–1.0, and ambient pressure. A numerical investigation was also conducted to gain an understanding of the impact of preheating temperature on the propagation and structure of premixed flames. The main conclusions are as follows.
-
(1)
The increase of preheating temperature dramatically accelerates the flame propagation, while the reduction of the flame thickness is relatively moderate. At 800 K, the flame speed of the stoichiometric mixture is about 6 times larger compared to that at 300 K, and the flame thickness is 63% of that at 300 K. It indicates that the importance of CO production to the flame propagation elevates with the preheating temperature.
-
(2)
Predictions made by GRI-Mech 3.0 generally agree well with the experimental measurements. At high operating conditions, i.e., Tu > 550 K or ϕ > 0.90, the numerical results by GRI-Mech 3.0 incline to overshooting. FFCM-1 seems to underestimate the flame speed compared to the present experiments; however, it is suggested that FFCM-1 agrees well with the overall existing data. Predictions of USC Mech II also agree well with present experiments at stoichiometric conditions; however, USC Mech II seems to overestimate the flame speed at fuel-lean conditions.
-
(3)
An empirical correlation of the laminar flame speed was developed in the range of Tu = 300–800 K and ϕ = 0.7–1.0, as shown in eq 6, the maximum deviation of which was less than 8%.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51406200).
Appendix A
The authors declare no competing financial interest.
References
- Huth M.; Gruschka U.; Janus B.; Meisl J.; Wasif A. S. In ULN System for the New SGT5-8000H Gas Turbine: Design and High Pressure Rig Test Results., Turbo Expo: Power for Land, Sea, and Air; ASME, 2008; pp 913–919.
- Ulitz F. E.; Kuesters B.; Mildner F.; Mittelbach M.; Wasdell D. In Design and Validation of a Compressor for a New Generation of Heavy-Duty Gas Turbines, ASME Power Conference, 2007; pp 653–663.
- Matta R. K.; Mercer G. D.; Tuthill R. S.. Power Systems for the 21st Century-‘‘H’’ Gas Turbine Combined-Cycles; Report No. GER-3935B; GE Power Systems: Schenectady, NY, 2000.
- Law C.Combustion Physics. Cambridge University Press: New York, USA, 2006. [Google Scholar]
- Lieuwen T.Unsteady Combustor Physics. Cambridge University Press: New York, USA, 2012. [Google Scholar]
- Ouimette P.; Seers P. Numerical comparison of premixed laminar flame velocity of methane and wood syngas. Fuel 2009, 88, 528–533. 10.1016/j.fuel.2008.10.008. [DOI] [Google Scholar]
- Wu Y.; Modica V.; Rossow B.; Grisch F. Effects of pressure and preheating temperature on the laminar flame speed of methane/air and acetone/air mixtures. Fuel 2016, 185, 577–588. 10.1016/j.fuel.2016.07.110. [DOI] [Google Scholar]
- Li W.; Jiang Y.; Jin Y.; Zhu X. Investigation of the influence of DMMP on the laminar burning velocity of methane/air premixed flames. Fuel 2019, 235, 1294–1300. 10.1016/j.fuel.2018.08.099. [DOI] [Google Scholar]
- Andrews G. E.; Bradley D. Determination of burning velocities: A critical review. Combust. Flame 1972, 18, 133–153. 10.1016/S0010-2180(72)80234-7. [DOI] [Google Scholar]
- Mazas A. N.; Fiorina B.; Lacoste D. A.; Schuller T. Effects of water vapor addition on the laminar burning velocity of oxygen-enriched methane flames. Combust. Flame 2011, 158, 2428–2440. 10.1016/j.combustflame.2011.05.014. [DOI] [Google Scholar]
- Gu X.; Haq M.; Lawes M.; Woolley R. Laminar burning velocity and markstein lengths of methane-air mixtures. Combust. Flame 2000, 121, 41–58. 10.1016/S0010-2180(99)00142-X. [DOI] [Google Scholar]
- Hu E.; Li X.; Meng X.; Chen Y.; Cheng Y.; Xie Y.; Huang Z. Laminar flame speeds and ignition delay times of methane–air mixtures at elevated temperatures and pressures. Fuel 2015, 158, 1–10. 10.1016/j.fuel.2015.05.010. [DOI] [Google Scholar]
- Ogami Y.; Kobayashi H. laminar burning velocity of stoichiometric CH4/air premixed flames at high pressure and high temperature. JSME Int. J., Ser. B 2005, 48, 603–609. 10.1299/jsmeb.48.603. [DOI] [Google Scholar]
- Mohammad A.; Juhany K. A. Laminar burning velocity and flame structure of DME/methane + air mixtures at elevated temperatures. Fuel 2019, 245, 105–114. 10.1016/j.fuel.2019.02.085. [DOI] [Google Scholar]
- Ferris A. M.; Susa A.; Davidson D.; Hanson R. High-temperature laminar flame speed measurements in a shock tube. Combust. Flame 2019, 205, 241–252. 10.1016/j.combustflame.2019.04.007. [DOI] [Google Scholar]
- Varghese R. J.; Kolekar H.; Kishore V. R.; Kumar S. Measurement of laminar burning velocities of methane-air mixtures simultaneously at elevated pressures and elevated temperatures. Fuel 2019, 257, 116120 10.1016/j.fuel.2019.116120. [DOI] [Google Scholar]
- Movaghar A.; Lawson R.; Egolfopoulos F. N. Confined spherically expanding flame method for measuring laminar flame speeds: Revisiting the assumptions and application to C1-C4 hydrocarbon flames. Combust. Flame 2020, 212, 79–92. 10.1016/j.combustflame.2019.10.023. [DOI] [Google Scholar]
- Konnov A. A.; Mohammad A.; Kishore V. R.; Kim N. I.; Prathap C.; Kumar S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. 10.1016/j.pecs.2018.05.003. [DOI] [Google Scholar]
- Rozenchan G.; Zhu D. L.; Law C. K.; Tse S. D. Outward propagation, burning velocities, and chemical effects of methane flames up to 60 ATM. Proc. Combust. Inst. 2002, 29, 1461–1469. 10.1016/S1540-7489(02)80179-1. [DOI] [Google Scholar]
- Tahtouh T.; Halter F.; Mounaïm-Rousselle C. Measurement of laminar burning speeds and Markstein lengths using a novel methodology. Combust. Flame 2009, 156, 1735–1743. 10.1016/j.combustflame.2009.03.013. [DOI] [Google Scholar]
- Wang S. F.; Zhang H.; Jarosinski J.; Gorczakowski A.; Podfilipski J. Laminar burning velocities and Markstein lengths of premixed methane/air flames near the lean flammability limit in microgravity. Combust. Flame 2010, 157, 667–675. 10.1016/j.combustflame.2010.01.006. [DOI] [Google Scholar]
- Qin X.; Ju Y. Measurements of burning velocities of dimethyl ether and air premixed flames at elevated pressures. Proc. Combust. Inst. 2005, 30, 233–240. 10.1016/j.proci.2004.08.251. [DOI] [Google Scholar]
- Halter F.; Chauveau C.; Djebaïli-Chaumeix N.; Gökalp I. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane–hydrogen–air mixtures. Proc. Combust. Inst. 2005, 30, 201–208. 10.1016/j.proci.2004.08.195. [DOI] [Google Scholar]
- Mitu M.; Giurcan V.; Razus D.; Oancea D. Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. 10.1016/j.jhazmat.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Qin X.; Ju Y.; Zhao Z.; Chaos M.; Dryer F. L. High temperature ignition and combustion enhancement by dimethyl ether addition to methane–air mixtures. Proc. Combust. Inst. 2007, 31, 1215–1222. 10.1016/j.proci.2006.07.177. [DOI] [Google Scholar]
- Bosschaart K. J.; De Goey L. P. H. Detailed analysis of the heat flux method for measuring burning velocities. Combust. Flame 2003, 132, 170–180. 10.1016/S0010-2180(02)00433-9. [DOI] [Google Scholar]
- Han X.; Wang Z.; Wang S.; Whiddon R.; He Y.; Lv Y.; Konnov A. A. Parametrization of the temperature dependence of laminar burning velocity for methane and ethane flames. Fuel 2019, 239, 1028–1037. 10.1016/j.fuel.2018.11.118. [DOI] [Google Scholar]
- Nilsson E. J. K.; van Sprang A.; Larfeldt J.; Konnov A. A. Effect of natural gas composition on the laminar burning velocities at elevated temperatures. Fuel 2019, 253, 904–909. 10.1016/j.fuel.2019.05.080. [DOI] [Google Scholar]
- Goswami M.; Derks S. C. R.; Coumans K.; Slikker W. J.; de Andrade Oliveira M. H.; Bastiaans R. J. M.; Luijten C. C. M.; de Goey L. P. H.; Konnov A. A. The effect of elevated pressures on the laminar burning velocity of methane+air mixtures. Combust. Flame 2013, 160, 1627–1635. 10.1016/j.combustflame.2013.03.032. [DOI] [Google Scholar]
- Dirrenberger P.; Le Gall H.; Bounaceur R.; Herbinet O.; Glaude P.-A.; Konnov A.; Battin-Leclerc F. Measurements of Laminar Flame Velocity for Components of Natural Gas. Energy Fuels 2011, 25, 3875–3884. 10.1021/ef200707h. [DOI] [Google Scholar]
- Wu C. K.; Law C. K. On the determination of laminar flame speeds from stretched flames. Symp. (Int.) Combust. 1985, 20, 1941–1949. 10.1016/S0082-0784(85)80693-7. [DOI] [Google Scholar]
- Li Z.; Cheng X.; Wei W.; Qiu L.; Wu H. Effects of hydrogen addition on laminar flame speeds of methane, ethane and propane: Experimental and numerical analysis. Int. J. Hydrogen Energy 2017, 42, 24055–24066. 10.1016/j.ijhydene.2017.07.190. [DOI] [Google Scholar]
- Salusbury S. D.; Bergthorson J. M. Maximum stretched flame speeds of laminar premixed counter-flow flames at variable Lewis number. Combust. Flame 2015, 162, 3324–3332. 10.1016/j.combustflame.2015.05.023. [DOI] [Google Scholar]
- Egolfopoulos F. N.; Hansen N.; Ju Y.; Kohse-Höinghaus K.; Law C. K.; Qi F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Prog. Energy Combust. Sci. 2014, 43, 36–67. 10.1016/j.pecs.2014.04.004. [DOI] [Google Scholar]
- Lewis B.; Von Elbe G.. Combustion Flames and Explosions of Gases.; Academic Press: London, UK, 1987. [Google Scholar]
- Andrews G. E.; Bradley D. The Burning Velocity of Methane-Air Mixtures. Combust. Flame 1972, 19, 275–288. 10.1016/S0010-2180(72)80218-9. [DOI] [Google Scholar]
- Echeverri-Uribe C.; Amell A. A.; Rubio-Gaviria L. M.; Colorado A.; McDonell V. Numerical and experimental analysis of the effect of adding water electrolysis products on the laminar burning velocity and stability of lean premixed methane/air flames at sub-atmospheric pressures. Fuel 2016, 180, 565–573. 10.1016/j.fuel.2016.04.041. [DOI] [Google Scholar]
- Gregory D.M.G.; Smith P.; Frenklach M.; Moriarty N. W.; Eiteneer B.; Goldenberg M.; Bowman C. T.; Hanson R. K.; Song S.; Gardiner W. C. Jr.; Lissianski V. V.; Qin Z.. http://www.me.berkeley.edu/gri_mech.
- Smith G.; Tao Y.; Wang H.. Foundational Fuel Chemistry Model (FFCM-1), Version 1.0. http://nanoenergy.stanford.edu/ffcm1.
- Wang H.; You X.; Joshi A. V.; Davis S. G.; Laskin A.; Egolfopoulos F.; Law C. K.. USC Mech Version II. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds. http://ignis.usc.edu/USC_Mech_II.htm (May 2007).
- Kee R. J.; Grcar J. F.; Smooke M. D.; Miller J. A.; Meeks E.. PRMIX: A Fortran Program for Modeling Steady Laminar One-Dimensional Premixed Flames; SAND85-8249; Sandia National Laboratory: Albuquerque, NM, 1985.
- Kee R. J.; Rupley F. M.; Miller J. A.. CHEMKIN-II: A Fortran Chemical Kinetics Package for the Analysis of Gas-Phase Chemical Kinetics; SAND89-8009; Sandia National Laboratories: Albuquerque, NM, 1989.
- Duva B. C.; Wang Y. C.; Chance L. E.; Toulson E. Correlations for the laminar burning velocity and burned gas Markstein length of methane-air mixtures diluted with flue gases at high temperatures and pressures. Fuel 2020, 281, 118721 10.1016/j.fuel.2020.118721. [DOI] [Google Scholar]
- Duva B. C.; Chance L. E.; Toulson E. The critical lower radius limit approach for laminar flame speed measurement from spherically expanding stretched flames. Exp. Therm. Fluid Sci. 2021, 121, 110284 10.1016/j.expthermflusci.2020.110284. [DOI] [Google Scholar]
- Liu Y.; Wang J.; Gu W.; Ma H.; Zeng W. Effects of CH4 mixing on the laminar burning velocity and Markstein length of RP-3/air premixed flame. Fuel 2021, 289, 119761 10.1016/j.fuel.2020.119761. [DOI] [Google Scholar]
- Akram M.; Saxena P.; Kumar S. Laminar Burning Velocity of Methane–Air Mixtures at Elevated Temperatures. Energy Fuels 2013, 27, 3460–3466. 10.1021/ef4009218. [DOI] [Google Scholar]
- Dirrenberger P.; Le Gall H.; Bounaceur R.; Glaude P.-A.; Battin-Leclerc F. Measurements of Laminar Burning Velocities above Atmospheric Pressure Using the Heat Flux Method—Application to the Case of n-Pentane. Energy Fuels 2015, 29, 398–404. 10.1021/ef502036j. [DOI] [Google Scholar]
- Akram M.; Kumar S. Experimental studies on dynamics of methane–air premixed flame in meso-scale diverging channels. Combust. Flame 2011, 158, 915–924. 10.1016/j.combustflame.2011.02.011. [DOI] [Google Scholar]
- Yu Z.; Ai Y.; Wang Y.; Luo C. Thermoacoustic instability analysis of a laminar lean premixed flame under autoignitive conditions. Combust. Flame 2021, 225, 513–523. 10.1016/j.combustflame.2020.11.032. [DOI] [Google Scholar]
- Iijima T.; Takeno T. Effects of temperature and pressure on burning velocity. Combust. Flame 1986, 65, 35–43. 10.1016/0010-2180(86)90070-2. [DOI] [Google Scholar]
- Hill P. G.; Hung J. Laminar Burning Velocities of Stoichiometric Mixtures of Methane with Propane and Ethane Additives. Combust. Sci. Technol. 1988, 60, 7–30. 10.1080/00102208808923973. [DOI] [Google Scholar]
- Elia M.; Ulinski M.; Metghalchi M. Laminar burning velocity of methane-air-diluent mixtures. J. Eng. Gas Turbines Power 2001, 123, 190–196. 10.1115/1.1339984. [DOI] [Google Scholar]
- Rahim F.Determination of burning speed for methane/oxidizer/diluent mixtures; Ph.D. Thesis; Northeastern University: Boston, USA, 2002. [Google Scholar]
- Amirante R.; Distaso E.; Tamburrano P.; Reitz R. D. Laminar flame speed correlations for methane, ethane, propane and their mixtures, and natural gas and gasoline for spark-ignition engine simulations. Int. J. Engine Res. 2017, 18, 951–970. 10.1177/1468087417720018. [DOI] [Google Scholar]
- Hinton N.; Stone R.; Cracknell R. Laminar burning velocity measurements in constant volume vessels – Reconciliation of flame front imaging and pressure rise methods. Fuel 2018, 211, 446–457. 10.1016/j.fuel.2017.09.031. [DOI] [Google Scholar]
- Wang Y.; Movaghar A.; Wang Z.; Liu Z.; Sun W.; Egolfopoulos F. N.; Chen Z. Laminar flame speeds of methane/air mixtures at engine conditions: Performance of different kinetic models and power-law correlations. Combust. Flame 2020, 218, 101–108. 10.1016/j.combustflame.2020.05.004. [DOI] [Google Scholar]