Highlights
-
•
Adult Medicaid patients with bipolar I disorder treated with adjunctive oral AAPs.
-
•
Lurasidone had lower hospitalizations vs. olanzapine, quetiapine, and ziprasidone.
-
•
Lurasidone had fewer all-cause hospital days vs. olanzapine and quetiapine.
-
•
Fewer hospitalizations, fewer hospital days could indicate reduced disease burden.
Key words: Atypical antipsychotics, Bipolar I disorder, Hospitalization, lurasidone, Medicaid
Abstract
Background: Atypical antipsychotics (AAPs) with mood stabilizers are recommended as a first-line treatment for patients with bipolar disorder. No studies have compared the inpatient health care resource utilization for patients with bipolar disorder treated with lurasidone as adjunctive therapy with mood stabilizers compared with other oral AAPs.
Objective: To compare the risk of hospitalization for adult Medicaid beneficiaries with bipolar I disorder when treated with lurasidone compared with other oral AAPs as adjunctive therapy with mood stabilizers.
Methods: This retrospective cohort study used the MarketScan Research Databases Multi-State Medicaid Database (IBM, Armonk, NY) claims data to assess patients with bipolar I disorder between January 1, 2014, and June 30, 2019. Adult patients who initiated oral AAP treatment with mood stabilizers (index date) and who were continuously enrolled 12 months before (pre-index) and 24 months after (post-index) the index date were included. Treatment categories assigned by patient-month included lurasidone, aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone with mood stabilizers; no/minimal treatment; AAP monotherapy; and other. Marginal structural models were performed to estimate the all-cause and psychiatric hospitalization rates and hospital length of stay associated with each adjunctive AAP therapy by controlling for both time-invariant and time-varying confounders.
Results: Adults with bipolar I disorder (N = 11,426; mean age = 39.4 years; female=73%) treated with an adjunctive oral AAP with mood stabilizers during the index month were categorized into lurasidone (12%), aripiprazole (17%), olanzapine (7%), quetiapine (32%), risperidone (11%), ziprasidone (7%), or other (15%) treatment groups. The adjusted odds of all-cause and psychiatric hospitalization were significantly higher for olanzapine (all causes: adjusted odds ratio [aOR] = 1.59; 95% CI, 1.13–2.25; psychiatric: aOR = 1.61, 95% CI, 1.12–2.32), quetiapine (all-causes: aOR = 1.27, 95% CI, 1.01–1.58; psychiatric: aOR = 1.28, 95% CI, 1.02–1.59), and ziprasidone (all-causes: aOR = 1.68, 95% CI, 1.05–2.66; psychiatric: aOR = 1.55, 95% CI, 1.02–2.35) compared with lurasidone with mood stabilizers. The adjusted odds of all-cause and psychiatric hospitalizations were numerically lower for lurasidone compared with aripiprazole. The all-cause hospital length of stay per 100 patient-months was significantly higher for olanzapine (20.3 days) and quetiapine (16.0 days) compared with lurasidone (12.2 days, both P values < 0.05).
Conclusions: In a Medicaid population, adults with bipolar I disorder treated with lurasidone as adjunctive therapy with mood stabilizers had significantly lower all-cause and psychiatric hospitalization rates compared with olanzapine, quetiapine, and ziprasidone. Fewer hospitalizations may reduce the economic burden associated with bipolar disorder. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
Introduction
Bipolar disorder is a chronic psychiatric mood disorder with an annual US prevalence of 2.8% among adults.1 Patients with bipolar disorder experience periods without symptoms (euthymia) interspersed with mania (bipolar I disorder) or hypomania (bipolar II disorder) and depression.2 Symptomatic time is more often spent in depressive episodes, which on average last 50% longer than manic or mixed episodes.3,4 Patients with bipolar disorder in depressive episodes (bipolar depression) have an increased risk of attempting suicide,3 treatment noncompliance,5 and hospitalization.6,7
Annual direct health care costs for bipolar disorder are estimated to be $40 to $50 billion in the United States.8 A large proportion (almost 30%) of direct health care costs for patients with bipolar disorder are attributed to inpatient hospitalizations.9,10 High rates of cardiometabolic comorbidities (eg, metabolic disorders, obesity, and diabetes) and psychiatric comorbidities (eg, anxiety and substance abuse) among patients with bipolar disorder contribute to increased all-cause and psychiatric hospitalizations and longer hospital length of stay.11, 12, 13
Atypical antipsychotics (AAPs) and mood stabilizers are first-line treatments for patients with bipolar disorder. Although patients with bipolar disorder often initiate treatment with monotherapy (eg, single AAP or mood stabilizer), up to 70% of patients with bipolar disorder receive adjunctive therapy such as an AAP and a mood stabilizer after 1 year.14 International bipolar disorder treatment guidelines recommend AAPs (eg, quetiapine, aripiprazole, risperidone, and asenapine) with mood stabilizers (eg, lithium and divalproex) for first-line combination treatment of bipolar mania.15 For bipolar depression, lurasidone with mood stabilizers (lithium or divalproex) is the only AAP that is recommended as a first-line adjunctive treatment.15 AAPs may also be prescribed with other mood stabilizers such as lamotrigine and carbamazepine, which are also approved by the Food and Drug Administration for the treatment of bipolar disorder.11,16,17
State Medicaid programs cover a disproportionate share of the adult population with mental illness (21% vs 14% of general population), including bipolar disorder.18 Among adult Medicaid patients with bipolar disorder, approximately 35% of average annual costs (average annual costs = $16,038 [2015 dollars]) are due to inpatient care.19,20 Adult Medicaid patients with bipolar disorder have a similar rate of treatment with adjunctive AAPs as the overall population.14 The 2019-2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults recommend lurasidone with lithium or divalproex as a first-line combination treatment for bipolar depression in patients previously prescribed and optimized on mood stabilizers.21
Previous studies have compared the inpatient health care resource utilization for patients with bipolar disorder treated with lurasidone monotherapy compared with other oral AAPs.22,23 However, no studies have compared the inpatient health care resource utilization for patients with bipolar disorder treated with lurasidone as adjunctive therapy with mood stabilizers compared with other oral AAPs. This retrospective cohort study compared the inpatient health care resource utilization for adult Medicaid patients diagnosed with bipolar I disorder treated with lurasidone compared with other oral AAPs as adjunctive therapy.
Methods
Data source
This retrospective analysis used US Medicaid claims data from the MarketScan Research Databases Multi-State Medicaid Database (IBM, Armonk, NY) from January 1, 2014, to June 30, 2019. The MarketScan data include medical claims, outpatient pharmacy claims, and enrollment data for more than 44 million Medicaid enrollees from geographically dispersed states. The data were de-identified and extracted in compliance with the Health Insurance Portability and Accountability Act of 1996.24 Therefore, institutional review board approval was not required for this study.
Patient selection
Patients were eligible for inclusion in the analysis if they initiated an oral AAP (ie, asenapine, aripiprazole, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, quetiapine, olanzapine, paliperidone, risperidone, or ziprasidone) with ≥24 days of overlap with a mood stabilizer (ie, carbamazepine, lamotrigine, lithium, oxcabazepine, or valproate) during the study period. Adjunctive treatment was defined as having at least 24 days (ie, 80% of days during a 30-day month) of overlap between the oral AAP and a mood stabilizer. No limits on dose ranges for oral AAPs or mood stabilizers were required. The date of the first evidence of an adjunctive oral AAP and mood stabilizer was defined as the index date. Patients were followed for 12 months before the index date (pre-index period) to 24 months after the index date (post-index period).
Patients were included in the analysis in the case that they had a diagnosis of bipolar I disorder (International Classification of Diseases, Ninth Revision, Clinical Modifications [ICD-9-CM] codes: 296.0X, 296.1X, 296.4X, 296.5X, 296.6X, 296.7X, 296.80, and 296.81; 10th revision [ICD-10-CM] codes: F30.XX, F31.0, F31.1X, F31.2, F31.3X, F31.4, F31.5, F31.6X, F31.7X, F31.89, and F31.9) during the pre-index period or on the index date; were adults (age at index date ≥18 years); and were continuously enrolled during the pre-index and post-index periods. Patients were excluded from the analysis in the case that they had a diagnosis of schizophrenia (ICD-9-CM code: 295.X; ICD-10-CM code: F20.X) during the study period; used long-acting injectable AAPs such as the long-acting injectable formulations of aripiprazole Healthcare Common Procedure Coding System (HCPCS: J1942, C9470, J0400, J0401), olanzapine (HCPCS: J2358), paliperidone (HCPCS: J2426), risperidone (HCPCS: J2794, S0163, C9125, C9037), or ziprasidone (HCPCS: J3486, C9204) during the study period; or were pregnant (ICD-9-CM code: 630.xx-679.xx; ICD-10-CM code: O00.xx-O9x.xx) during the study period. The patient inclusion/exclusion criteria are similar to a previous retrospective comparison of oral AAPs and inpatient health care resource utilization.23
Adjunctive oral AAP treatment categories
The primary treatments of interest were oral AAPs with mood stabilizers. Treatment was assigned in 30-day intervals (ie, months) during the post-index period, and the patient treatment-month was the primary unit of analysis. Individual categories of adjunctive therapy were defined as months in which patients received oral lurasidone, aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone with mood stabilizers (≥24 days’ supply and overlap between oral AAP and mood stabilizer). In addition, an other treatment category included oral AAPs with ≥24 days of mood stabilizers with a small sample size (ie, asenapine, brexpiprazole, cariprazine, iloperidone, paliperidone, clozapine); treatment with multiple oral AAPs; 8 to 23 days’ supply of 1 or more oral AAPs; any oral AAP with 8 to 23 days’ supply of mood stabilizers; or monotherapy with mood stabilizers. Additional classifications after the index month included oral AAP monotherapy and no/minimal AAP treatment. AAP monotherapy was defined as ≥24 days of oral AAP treatment during the month without concurrent treatment (≤7 days’ supply) with other oral AAPs or mood stabilizers. The oral AAP monotherapy group was a combination of all oral AAPs in this study (ie, asenapine, aripiprazole, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, quetiapine, olanzapine, paliperidone, risperidone, and ziprasidone). No or minimal AAP treatment was defined as no treatment with oral AAPs or ≤7 days of any oral AAP therapy.
Inpatient health care resource utilization
The primary outcomes of interest were all-cause and psychiatric hospitalization rates per 100 patient months and days hospitalized (hospital length of stay [LOS]) per 100 patient-months. Psychiatric hospitalizations were identified by a mental health disorder diagnosis (see Supplemental Table 1 in the online version at doi.10.1016/j.curtheres.2021.100629) in any diagnosis code field. Hospital LOS included the emergency department visit for patients directly admitted to an inpatient facility.
Demographic characteristics, comorbidities, and other variables
Demographic variables, including age, sex, race/ethnicity (White, Black, Hispanic, other, or missing), and plan type were recorded at the index date. Clinical variables were calculated using claims from the pre-index period. These included the Charlson comorbidity index25; diagnoses of diabetes (ICD-9-CM code: 250.0–250.7; ICD-10-CM code: E10-E14), hyperlipidemia (ICD-9-CM code: 272.0x-272.4x; ICD-10-CM codes: E78.0x - E78.4x, E78.5), hypertension (ICD-9-CM codes: 401.xx - 405.xx, 437.2, 362.11; ICD-10-CM codes: H35.03x, I10.xx -I15.xx, I67.4, N26.2), and obesity (ICD-9-CM codes: 278.0x, V85.3x, V85.4x; ICD-10-CM codes: E66.xx, Z68.3x, Z68.4x); diagnoses of bipolar II disorder, anxiety, major depressive disorder (MDD), and substance abuse (alcohol, opioids, cannabis, cocaine, and other stimulants) (see Supplemental Table 1 in the online version at doi.10.1016/j.curtheres.2021.100629); the pre-index hospitalization rate; the pre-index hospital LOS; and pre-index psychotropic medication use including antidepressants, mood stabilizers, oral AAPs, and anxiolytics. Additional clinical variables were calculated monthly for the post-index period, including the substance abuse indicators and office visits.
Statistical analysis
Descriptive statistics were reported for categorical variables (frequency and percent) and continuous variables (mean and standard deviation) during the pre-index period by the index month treatment category. Statistical significance was tested with t tests for continuous variables and pairwise tests of proportions for categorical variables using lurasidone with mood stabilizers as the reference category.
Marginal structural models (MSMs) were used to estimate the association of each treatment category with the hospitalization rate and hospital LOS. MSMs account for time-varying confounding such as treatment switching by weighting the data by the probability of receiving each treatment in each time period.26 For each month in the post-index period, stabilized inverse probability of treatment weights were calculated to predict assignment to each of the 9 treatment categories using multinomial logistic regressions. Time-invariant covariates included age (restricted cubic spline), sex, race/ethnicity, plan type, pre-index period Charlson comorbidity index indicators, pre-index period comorbidities (diabetes, hypertension, obesity, bipolar II disorder, and substance abuse indictors), the pre-index period dependent variable, pre-index period office visits, index month number of psychiatric ED visits, and index year. Time-varying covariates included the prior-month dependent variable, the prior-month alcohol abuse indicator, the prior-month substance abuse indicator, an indicator for a new diagnosis of anxiety or MDD during either the pre-index period or the prior month that was permanently set to 1 in the following months, the prior-month treatment history indicators (no or minimal treatment, other treatment, or AAP monotherapy), and a restricted cubic spline for time. Separate generalized linear models with logit link and clustering by patient were used to model all-cause and psychiatric hospitalization rates. Zero-inflated Poisson regression models were used to model all-cause and psychiatric hospital LOS. All models were weighted using the inverse probability of treatment weights. Covariates included the treatment category for the current month and all the previously mentioned time-invariant variables.
The statistical analysis was conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) and Stata version 16 (StataCorp, College Station, Texas). Statistical significance was indicated for P < 0.01 and P < 0.05.
Results
Patient characteristics
The analysis included 11,426 adult patients with bipolar I disorder at the index month. Figure 1 shows details of the patient selection process.
Figure 1.
Patient inclusion flow chart. AAP = atypical antipsychotic; LAI = long-acting injectable.
Patient characteristics during the pre-index period and at the index month are reported in Table 1. Patients were assigned to lurasidone (11.6%), aripiprazole (17.0%), olanzapine (6.9%), quetiapine (32.0%), risperidone (10.9%), ziprasidone (6.5%), or other treatment (15.0%) during the index month. The most common mood stabilizer used with the AAPs at the index month was lamotrigine (47.8%) followed by lithium (22.3%), oxcarbazepine (14.5%), carbamazepine (8.8%), multiple mood stabilizers (6.1%), and valproate (0.4%) (see Supplemental Table 2 in the online version at doi.10.1016/j.curtheres.2021.100629).
Table 1.
Pre-index patient demographic characteristics, comorbidities, and health care utilization by treatment group at index.*
| Lurasidone† | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | Other treatment | |
|---|---|---|---|---|---|---|---|
| Sample size at index‡ | 1330 (11.6) | 1947 (17.0) | 792 (6.9) | 3652 (32.0) | 1248 (10.9) | 746 (6.5) | 1711 (15.0) |
| Age (y)§ | 39.0 (10.5) | 38.8 (12.0) | 39.1 (12.4) | 40.7 (11.8)|| | 38.7 (12.5) | 39.2 (12.1) | 38.3 (11.8) |
| Female‡ | 1110 (83.5) | 1450 (74.5)|| | 484 (61.1)|| | 2710 (74.2)|| | 837 (67.1)|| | 597 (80.0) | 1163 (68.0)|| |
| Race‡ | |||||||
| White | 1084 (81.5) | 1572 (80.7) | 591 (74.6)|| | 2722 (74.5)|| | 874 (70.0)|| | 577 (77.3)¶ | 1337 (78.1)¶ |
| Black | 127 (9.5) | 172 (8.8) | 105 (13.3)¶ | 472 (12.9)|| | 193 (15.5)|| | 53 (7.1)¶ | 179 (10.5) |
| Hispanic | nr | 16 (0.8) | nr | 59 (1.6)|| | 15 (1.2)¶ | nr | 14 (0.8) |
| Other | nr | 45 (2.3) | nr | 39 (1.1) | 19 (1.5) | nr | 29 (1.7) |
| Missing | 91 (6.8) | 142 (7.3) | 72 (9.1) | 360 (9.9)|| | 147 (11.8)|| | 94 (12.6)|| | 152 (8.9)¶ |
| Index year‡ | |||||||
| 2015 | 815 (61.3) | 1344 (69.0)|| | 484 (61.1) | 2497 (68.4)|| | 908 (72.8)|| | 542 (72.7)|| | 1098 (64.2) |
| 2016 | 419 (31.5) | 491 (25.2)|| | 240 (30.3) | 955 (26.2)|| | 278 (22.3)|| | 165 (22.1)|| | 496 (29.0) |
| 2017 | 96 (7.2) | 112 (5.8) | 68 (8.6) | 200 (5.5)¶ | 62 (5.0)¶ | 39 (5.2) | 117 (6.8) |
| Plan type‡ | |||||||
| HMO | 825 (62.0) | 1262 (64.8) | 440 (55.6)|| | 2301 (63.0) | 722 (57.9)¶ | 445 (59.7) | 984 (57.5)¶ |
| Comprehensive | 505 (38.0) | 685 (35.2) | 352 (44.4)|| | 1351 (37.0) | 526 (42.1)¶ | 301 (40.3) | 727 (42.5)¶ |
| CCI§ | 0.8 (1.2) | 0.8 (1.3) | 0.8 (1.4) | 0.9 (1.3) | 0.8 (1.2) | 0.8 (1.2) | 0.9 (1.4) |
| Psychiatric comorbidities‡ | |||||||
| Anxiety | 807 (60.7) | 1077 (55.3)|| | 405 (51.1)|| | 2077 (56.9)¶ | 602 (48.2)|| | 391 (52.4)|| | 910 (53.2)|| |
| Major depressive disorder | 744 (55.9) | 1032 (53.0) | 397 (50.1)|| | 1961 (53.7) | 580 (46.5)|| | 350 (46.9)|| | 844 (49.3)|| |
| Substance abuse# | 368 (27.7) | 471 (24.2)¶ | 253 (31.9)¶ | 1121 (30.7)¶ | 294 (23.6)¶ | 173 (23.2)¶ | 476 (27.8) |
| Physical comorbidities‡ | |||||||
| Hypertension | 499 (37.5) | 699 (35.9) | 303 (38.3) | 1477 (40.4) | 448 (35.9) | 283 (37.9) | 645 (37.7) |
| Hyperlipidemia | 393 (29.5) | 567 (29.1) | 217 (27.4) | 1127 (30.9) | 377 (30.2) | 242 (32.4) | 546 (31.9) |
| Obesity | 446 (33.5) | 607 (31.2) | 150 (18.9)|| | 930 (25.5)|| | 315 (25.2)|| | 264 (35.4) | 506 (29.6)¶ |
| Diabetes | 289 (21.7) | 367 (18.8)¶ | 121 (15.3)|| | 638 (17.5)|| | 226 (18.1)¶ | 157 (21.0) | 307 (17.9)|| |
| Psychotropic medication use‡ | |||||||
| Antidepressants | 1047 (78.7) | 1583 (81.3) | 568 (71.7)|| | 2816 (77.1) | 921 (73.8)|| | 581 (77.9) | 1309 (76.5) |
| Mood stabilizers | 1057 (79.5) | 1571 (80.7) | 590 (74.5)|| | 2637 (72.2)|| | 982 (78.7) | 597 (80.0) | 1239 (72.4)|| |
| Oral AAPs | 1001 (75.3) | 1571 (80.7)|| | 583 (73.6) | 2907 (79.6)|| | 978 (78.4) | 618 (82.8)|| | 1470 (85.9)|| |
| Anxiolytics | 646 (48.6) | 851 (43.7)|| | 323 (40.8)|| | 1578 (43.2)|| | 453 (36.3)|| | 349 (46.8) | 767 (44.8)¶ |
| Hospitalization rate per 100 patient-months | |||||||
| All causes | 2.54 | 2.52 | 3.20|| | 2.69 | 2.34 | 2.40 | 2.78 |
| Psychiatric | 2.35 | 2.34 | 2.99|| | 2.52 | 2.14 | 2.25 | 2.55 |
| Hospital LOS days per 100 patient-months | |||||||
| All causes | 20.76 | 20.54 | 35.51|| | 24.86 | 23.45 | 19.48 | 29.00|| |
| Psychiatric | 17.64 | 18.57 | 32.22|| | 22.43|| | 18.97 | 16.89 | 26.36|| |
AAP = atypical antipsychotics; CCI = Charlson comorbidity index; HMO = health maintenance organization; nr = not reported.
Boldface type indicates significant differences between lurasidone and other oral atypical antipsychotics or other treatment at P < 0.05. Cells with <11 patients are not reported in accordance with Centers for Medicare and Medicaid Services cell size suppression policy.
Reference category.
Values are presented as n (%).
Values are presented as mean (standard deviation).
Indicates significance versus lurasidone at P < 0.01.
Indicates significance versus lurasidone at P < 0.05.
Substance abuse includes alcohol, opioids, cannabis, cocaine, hallucinogens, sedatives, inhalants, and other stimulants (eg, amphetamine and psychostimulant) abuse.
The average age for patients initiating an adjunctive oral AAP with mood stabilizers was 39.4 years (lurasidone mean age = 39.0 years vs aripiprazole = 38.8 years [P ≥ 0.05] vs olanzapine = 39.1 years [P ≥ 0.05] vs quetiapine = 40.7 years [P < 0.01] vs risperidone = 38.7 years [P ≥ 0.05] vs ziprasidone = 39.2 years [P ≥ 0.05]). Compared with patients who were treated with other oral AAPs, a significantly higher proportion of patients who were treated with lurasidone were female (lurasidone = 83.5% vs aripiprazole = 74.5% [P < 0.01] vs olanzapine = 61.1% [P < 0.01] vs quetiapine = 74.2% [P < 0.01] vs risperidone = 67.1% [P < 0.01]) and White (lurasidone = 81.5% vs olanzapine = 74.6% [P < 0.01] vs quetiapine = 74.5% [P < 0.01] vs risperidone = 70.0% [P < 0.01] vs ziprasidone = 77.3% [P < 0.05]).
During the pre-index period, patients who were treated with lurasidone compared with other AAPs were significantly more likely to have a history of anxiety diagnoses (lurasidone = 60.7% vs aripiprazole = 55.3% [P < 0.01] vs olanzapine = 51.1% [P < 0.01] vs quetiapine = 56.9% [P < 0.05] vs risperidone = 48.2% [P < 0.01] vs ziprasidone = 52.4% [P < 0.01]). The proportion of patients with substance abuse was significantly higher for patients treated with lurasidone (27.7%) compared with aripiprazole (24.2% [P < 0.05]), risperidone (23.6% [P < 0.05]), and ziprasidone (23.2% [P < 0.05]) but lower compared with olanzapine (31.9% [P < 0.05]) and quetiapine (30.7% [P < 0.05]). A significantly higher percentage of patients treated with lurasidone had been diagnosed with obesity (lurasidone = 33.5% vs olanzapine = 18.9% [P < 0.01] vs quetiapine = 25.5% [P < 0.01] vs risperidone = 25.2% [P < 0.01]) and diabetes (lurasidone = 21.7% vs aripiprazole = 18.8% [P < 0.05] vs olanzapine = 15.3% [P < 0.01] vs quetiapine = 17.5% [P <0.01] vs risperidone = 18.1% [P < 0.05]).
A significantly higher proportion of patients treated with lurasidone had at least 1 prescription for anxiolytics (lurasidone = 48.6% vs aripiprazole = 43.7% [P < 0.01] vs olanzapine = 40.8% [P < 0.01] vs quetiapine = 43.2% [P < 0.01] vs risperidone = 36.3% [P < 0.01]) during the pre-index period. The proportion of patients with at least 1 prescription for antidepressant agents during the pre-index period was significantly higher for patients treated with lurasidone (78.7%) compared with olanzapine (71.7% [P < 0.01]) and risperidone (73.8% [P < 0.01]).
Unadjusted hospitalization rate and hospital LOS during the 24-month post-index period
The unadjusted hospitalization rate and hospital LOS by treatment group during the post-index period are reported in Table 2.
Table 2.
Unadjusted all-cause and psychiatric hospitalizations and hospital length of stay (LOS) during the 24-month follow-up period.*
| Lurasidone† | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | No/Minimal treatment | Other Treatment | AAP Monotherapy | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment months | 10,863 | 17,554 | 7028 | 32,813 | 11,355 | 7600 | 59,370 | 87,402 | 28,813 |
| Hospitalizations rate, per 100 patient months | |||||||||
| All-cause | 2.12 | 2.21 | 2.96‡ | 2.66‡ | 1.60‡ | 2.21 | 2.35 | 3.29§ | 3.26§ |
| Psychiatric | 1.78 | 1.85 | 2.40‡ | 2.24‡ | 1.30‡ | 1.91 | 2.08 | 2.91§ | 2.77§ |
| Hospital LOS, per 100 patient months | |||||||||
| All-cause | 10.15 | 11.50 | 16.70§ | 13.64‡ | 8.80 | 10.88 | 15.18§ | 18.62§ | 18.54§ |
| Psychiatric | 8.75 | 8.73 | 12.66‡ | 10.93 | 6.75 | 9.03 | 12.78§ | 16.09§ | 14.93§ |
AAP = atypical antipsychotics.
Boldface type indicates significance of outcomes in comparison to lurasidone at P < 0.05.
Reference category.
Indicates significance versus lurasidone at P < 0.05.
Indicates significance versus lurasidone at P < 0.01.
During the 24-month post-index period, the unadjusted all-cause and psychiatric hospitalization rates per 100 patient-months were significantly lower for lurasidone (all-cause hospitalization rate = 2.12 and psychiatric hospitalization rate = 1.78) compared with olanzapine (all-cause hospitalization rate = 2.96 [P < 0.05] and psychiatric hospitalization rate = 2.40 [P < 0.05]), quetiapine (all-cause hospitalization rate = 2.66 [P <0.05] and psychiatric hospitalization rate = 2.24 [P < 0.05]), and AAP monotherapy (all cause hospitalization rate = 3.26 [P < 0.01] and psychiatric hospitalization rate = 2.77 [P < 0.01]). The hospitalization rates were significantly higher for lurasidone compared with risperidone (all-cause hospitalization rate = 1.60 [P < 0.05] and psychiatric hospitalization rate = 1.30 [P < 0.05]).
The unadjusted all-cause hospital LOS per 100 patient-months was significantly shorter for lurasidone (10.2 days) compared with olanzapine (16.7 days [P < 0.01]), quetiapine (13.6 days [P < 0.05]), and AAP monotherapy (18.6 days [P < 0.01]). Similarly, the unadjusted psychiatric hospital LOS was significantly shorter for lurasidone (8.8 days) compared with olanzapine (12.7 days [P < 0.05]) and AAP monotherapy (14.9 days [P < 0.01]).
MSMs
The MSM adjusted odds of hospitalization and risk of hospital LOS are presented in Figure 2 and Figure 3, respectively. Table 3 shows the adjusted hospitalization rate and hospital LOS controlling for time-invariant and time-varying covariates during the 24-month post-index period.
Figure 2.
Marginal structural model-adjusted risk of all-cause and psychiatric hospitalizations during 24-month follow-up period. Adjusted rates control for patient demographic characteristics, clinical characteristics, and health care utilization as well as time-varying indicators of key clinical characteristics, health care utilization, and time trends. Bold text indicates statistical significance based on 95% CI. OR = odds ratio.
Figure 3.
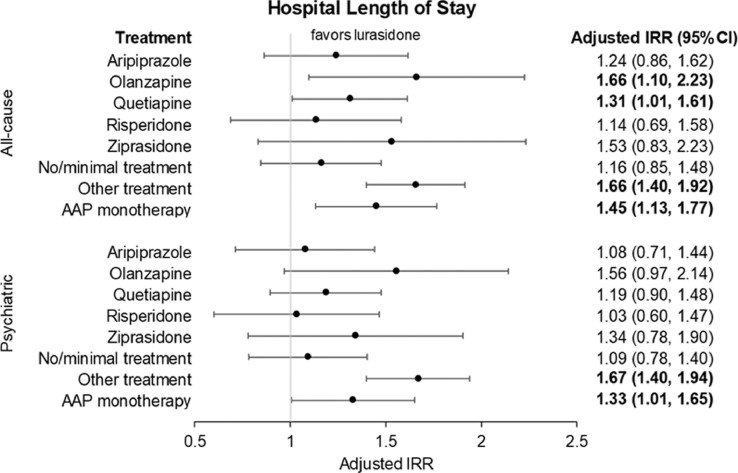
Marginal structural model-adjusted risk of all-cause and psychiatric hospital length of stay during 24-month follow-up period. Adjusted rates control for patient demographic characteristics, clinical characteristics, and health care utilization as well as time-varying indicators of key clinical characteristics, health care utilization, and time trends. Bold text indicates statistical significance based on 95% CI. IRR = incidence rate ratio.
Table 3.
Adjusted risk of all-cause and psychiatric hospitalizations and hospital length of stay (LOS) during the 24-month follow-up period.*
| Lurasidone† | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Ziprasidone | No/minimal treatment | Other treatment | AAP monotherapy | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment months | 10,863 | 17,554 | 7028 | 32,813 | 11,355 | 7600 | 59,370 | 87,402 | 28,813 |
| Hospitalizations rate, per 100-patient mo | |||||||||
| All causes | 2.36 | 2.70 | 3.67‡ | 2.96§ | 2.30 | 3.85§ | 2.19 | 3.52‡ | 3.03§ |
| Psychiatric | 1.97 | 2.24 | 3.11§ | 2.49§ | 1.96 | 2.99§ | 1.84 | 3.13‡ | 2.55§ |
| Hospital LOS, per 100-patient mo | |||||||||
| All causes | 12.21 | 15.14 | 20.29§ | 16.02§ | 13.86 | 18.70 | 14.19 | 20.25‡ | 17.72‡ |
| Psychiatric | 10.51 | 11.32 | 16.35 | 12.48 | 10.86 | 14.11 | 11.48 | 17.54‡ | 13.96§ |
AAP = atypical antipsychotics.
Boldface type indicates significance of outcomes in comparison to lurasidone at P < 0.05. Adjusted rates control for patient demographic characteristics, clinical characteristics, and health care utilization as well as time-varying indicators of key clinical characteristics, health care utilization, and time trends. Adjusted hospitalization rates will not precisely match adjusted odds ratios. Adjusted rates are calculated at the patient-level from each patient's predicted log odds, then averaged across the sample. The nonlinear conversion from log odds to predicted rates leads to minor differences if adjusted odds ratios are then back-calculated from the predicted rates.
Reference category.
Indicates significance versus lurasidone at P < 0.01.
Indicates significance versus lurasidone at P < 0.05.
After adjusting for time-invariant and time-varying covariates, the all-cause hospitalization rates per 100 patient-months remained significantly lower for lurasidone (2.36) compared with olanzapine (3.67) (adjusted odds ratio [aOR] = 1.59; 95% CI, 1.13–2.25; P < 0.01), quetiapine (2.96) (aOR = 1.27; 95% CI, 1.01–1.58; P < 0.05), and AAP monotherapy (3.03) (aOR = 1.30; 95% CI, 1.02–1.65; P < 0.05). The all-cause hospitalization rate for lurasidone (2.36) became statistically significantly lower compared with ziprasidone (3.85) (aOR = 1.68; 95% CI, 1.05–2.66; P < 0.05).
The psychiatric hospitalization rate remained significantly lower for lurasidone (1.97) compared with olanzapine (3.11) (aOR = 1.61; 95% CI, 1.12–2.32; P < 0.05), quetiapine (2.49) (aOR = 1.28; 95% CI, 1.02–1.59; P < 0.05), and AAP monotherapy (2.55) (aOR = 1.31; 95% CI, 1.03–1.66; P < 0.05) after controlling for time-invariant and time varying covariates. The psychiatric hospitalization rate for lurasidone (1.97) became statistically significantly lower compared with ziprasidone (2.99) (aOR = 1.55; 95% CI, 1.02–2.35; P < 0.05). The results for lurasidone compared with risperidone were no longer significantly different after controlling for time-invariant and time-varying covariates.
The all-cause hospital LOS remained significantly shorter for lurasidone (12.2 days) compared with olanzapine (20.3 days) (adjusted incidence rate ratio [aIRR] = 1.66; 95% CI, 1.10–2.23; P < 0.05), quetiapine (16.0 days) (aIRR = 1.31, 95% CI, 1.01–1.61; P < 0.05), and AAP monotherapy (17.7 days) (aIRR = 1.45, 95% CI, 1.13–1.77; P < 0.01). The psychiatric hospital LOS remained significantly shorter for lurasidone (10.5 days) compared with AAP monotherapy (14.0 days) (aIRR = 1.33; 95% CI, 1.01–1.65; P < 0.05).
Discussion
This retrospective claims database analysis is the first study to compare hospitalization risk among adult Medicaid patients with bipolar I disorder treated with lurasidone as adjunctive therapy with mood stabilizers versus other adjunctive oral AAPs. During 24-months of follow-up, adult Medicaid patients with bipolar I disorder treated with lurasidone had statistically significantly lower all-cause and psychiatric hospitalization rates compared with those who were treated with olanzapine, quetiapine, or ziprasidone. In addition, treatment with lurasidone was also associated with significantly shorter all-cause hospital LOS compared with olanzapine or quetiapine.
The results from this study are consistent with 2 earlier studies of patients with bipolar I disorder treated with lurasidone monotherapy compared with other oral AAPs.22,23 In a Medicaid population, the odds of all-cause hospitalizations per 100 patient-months were significantly higher for olanzapine and quetiapine compared with lurasidone monotherapy.23 In a commercially insured population, the odds of psychiatric hospitalizations per 100 patient-months were significantly higher for olanzapine, quetiapine, risperidone, and ziprasidone compared with lurasidone monotherapy.22 The odds of hospitalization for aripiprazole, olanzapine, quetiapine, and ziprasidone with mood stabilizers compared with lurasidone were directionally the same (favoring lurasidone) in this study.
Treatment switching is frequent in patients receiving antipsychotic agents and complicates the estimation of the association of treatments with outcomes when using an intent-to-treat approach. The MSM methods used in this study reduced potential confounding from treatment switching by not only adjusting for the time-invariant variables, including patient demographic characteristics, clinical characteristics, and health care resource utilization before treatment initiation, but also accounting for time-varying variables, including clinical characteristics and health care utilization that may have an influence on treatment selection over time.26 In addition, by requiring a medication possession ratio ≥80% (ie, 24 days out of 30 days in the patient treatment-month), the potential confounding from treatment noncompliance, which has been associated with increased hospitalizations,27,28 was also reduced.
The safety and efficacy of lurasidone with lithium or divalproex for the treatment of bipolar depression has been established in short- and long-term trials.29, 30, 31 However, the comparative efficacy of lurasidone with mood stabilizers compared with other AAPs with mood stabilizers has not been directly studied in patients with bipolar disorder. Indirect comparisons, such as network meta-analyses, have focused on monotherapy treatment and found that patients treated with lurasidone monotherapy (without mood stabilizers) have greater odds of response and remission compared with aripiprazole, olanzapine, and quetiapine monotherapy32,33 and significantly less weight gain compared with olanzapine and quetiapine,32 which may help explain the lower hospitalization rates for patients treated with lurasidone compared with aripiprazole, olanzapine, and quetiapine. Lurasidone has also been found to be associated with a relatively low risk for developing metabolic syndrome,34 which may help explain the shorter hospital LOS for patients treated with lurasidone compared with olanzapine or quetiapine.12
In this study, the adjusted psychiatric hospitalization rate accounted for approximately 80% of the all-cause hospitalization rate across treatment cohorts. Inpatient hospitalizations are a high-cost component of bipolar disorder care.35 In addition to cost, greater recurrence of mood episodes has been associated with higher odds of psychiatric hospitalizations for patients with bipolar I disorder in a community sample and prospectively associated with greater risk of disability, unemployment, and poor functioning in the same sample.7 Reductions in psychiatric hospitalizations among patients with bipolar disorder could reduce health care resource utilization and costs and be associated with improvements in health outcomes for patients with bipolar disorder.
There are several limitations to this study. First, this study used administrative health care claims data. The primary purpose of claims data is for billing, and it does not capture information about symptoms, severity of illness, chronicity, and functional status and may include coding errors and misclassifications. Second, the study results may not be generalizable to populations other than adult US Medicaid beneficiaries with bipolar I disorder such as patients outside the United States, with commercial insurance, with Medicare coverage, or without health insurance. Third, unobserved confounders such as socioeconomic status and severity of disease could still be different between the treatment cohorts and have an influence on the outcomes. To minimize this possibility, a large number of observable time-invariant and time-varying variables known to be associated with inpatient health care resource utilization and severity of disease, including psychiatric-related emergency department use, pre-index office visits, and the prior month-dependent variable among patients with bipolar disorder were controlled for using marginal structural models. Fourth, although a new diagnosis of anxiety or MDD during each month was used as a proxy for other medication use and was controlled for in the outcome models, concomitant anxiolytic or antidepressant use may confound the association of oral AAPs with hospitalizations in the study population. Fifth, the focus of this study was on all-cause and psychiatric hospitalizations. Further studies could look at phase-specific hospitalizations for bipolar disorder. Finally, all pharmacotherapy combinations used by patients with bipolar I disorder could not be analyzed in this study due to sample size considerations. However, the use of AAPs with mood stabilizers is a large proportion of bipolar I disorder treatment strategies.
Conclusions
In a Medicaid population, adults with bipolar I disorder treated with lurasidone with mood stabilizers had significantly lower all-cause and psychiatric hospitalization rates compared with those treated with olanzapine, quetiapine, and ziprasidone with mood stabilizers. Reducing the hospitalization rates could help reduce economic burden for payers and patients with bipolar disorder.
Conflicts of Interest
This study was funded by Sunovion Pharmaceuticals Inc. X. Niu, C. Dembek, K. Laubmeier, G. R. Williams, and M. Tocco are employees of Sunovion Pharmaceuticals Inc. S. Dennen, Y. Liu, and P. Veeranki are employees of PRECISIONheor, which received funding from Sunovion Pharmaceuticals Inc to conduct this study. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank Barbara Blaylock, PhD, from Blaylock Health Economics LLC for providing medical writing support.
All authors approved the final manuscript. X. Niu, C. Dembek, K. Laubmeier, G. R. Williams, and M. Tocco were involved in the study conception and design, interpretation of study findings, drafting/editing the manuscript, and providing final approval. S. Dennen and Y. Liu were involved in the study conception and design, conducting the analyses, interpretation of study findings, drafting/editing the manuscript, and providing final approval. P. Veeranki oversaw the data analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2021.100629.
Appendix. Supplementary materials
References
- 1.Merikangas KR, Jin R, He JP. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. Mar 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . 5th ed. 2013. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA. [Google Scholar]
- 3.McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. Nov 2019;35(11):1993–2005. doi: 10.1080/03007995.2019.1636017. [DOI] [PubMed] [Google Scholar]
- 4.Miller S, Dell'Osso B, Ketter TA. The prevalence and burden of bipolar depression. J Affect Disord. Dec 2014;169(Suppl 1):S3–S11. doi: 10.1016/S0165-0327(14)70003-5. [DOI] [PubMed] [Google Scholar]
- 5.García S, Martínez-Cengotitabengoa M, López-Zurbano S. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. Aug 2016;36(4):355–371. doi: 10.1097/JCP.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlick DA, Rosenheck RA, Clarkin JF. Symptoms predicting inpatient service use among patients with bipolar affective disorder. Psychiatr Serv. Jun 1999;50(6):806–812. doi: 10.1176/ps.50.6.806. [DOI] [PubMed] [Google Scholar]
- 7.Peters AT, West AE, Eisner L. The burden of repeated mood episodes in bipolar I disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions. J Nerv Ment Dis. Feb 2016;204(2):87–94. doi: 10.1097/NMD.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bessonova L, Ogden K, Doane MJ. The economic burden of bipolar disorder in the United States: a systematic literature review. ClinicoEconomics and Outcomes Research. 2020;12:481–497. doi: 10.2147/CEOR.S259338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleine-Budde K, Touil E, Moock J. Cost of illness for bipolar disorder: a systematic review of the economic burden. Bipolar Disord. Jun 2014;16(4):337–353. doi: 10.1111/bdi.12165. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier M, Greene M, Guerin A. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord. Jan 15 2018;226:45–51. doi: 10.1016/j.jad.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho AF, Firth J, Vieta E. Bipolar disorder. N Engl J Med. Jul 2 2020;383(1):58–66. doi: 10.1056/NEJMra1906193. [DOI] [PubMed] [Google Scholar]
- 12.Correll CU, Ng-Mak DS, Stafkey-Mailey D. Cardiometabolic comorbidities, readmission, and costs in schizophrenia and bipolar disorder: a real-world analysis. Ann Gen Psychiatry. 2017;16:9. doi: 10.1186/s12991-017-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan T, Greene M, Chang E. Hospitalization risk factors in antipsychotic-treated schizophrenia, bipolar I disorder or major depressive disorder. J Comp Eff Res. Mar 2019;8(4):217–227. doi: 10.2217/cer-2018-0090. [DOI] [PubMed] [Google Scholar]
- 14.Greene M, Paladini L, Lemmer T. Systematic literature review on patterns of pharmacological treatment and adherence among patients with bipolar disorder type I in the USA. Neuropsychiatr Dis Treat. 2018;14:1545–1559. doi: 10.2147/NDT.S166730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yatham LN, Kennedy SH, Parikh SV. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. Mar 2018;20(2):97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeberle A, Greil W, Russmann S. Mono- and combination drug therapies in hospitalized patients with bipolar depression. Data from the European drug surveillance program AMSP. BMC Psychiatry. Sep 21 2012;12:153. doi: 10.1186/1471-244X-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornaro M, De Berardis D, Koshy AS. Prevalence and clinical features associated with bipolar disorder polypharmacy: a systematic review. Neuropsychiatr Dis Treat. 2016;12:719–735. doi: 10.2147/NDT.S100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser Family Foundation . 2017. Medicaid's role in financing behavioral health services for low-income individuals. Available from: https://www.kff.org/medicaid/issue-brief/medicaids-role-in-financing-behavioral-health-services-for-low-income-individuals/ [Google Scholar]
- 19.Guo JJ, Keck PE, Li H. Treatment costs related to bipolar disorder and comorbid conditions among Medicaid patients with bipolar disorder. Psychiatr Serv. Aug 2007;58(8):1073–1078. doi: 10.1176/ps.2007.58.8.1073. [DOI] [PubMed] [Google Scholar]
- 20.Chapel JM, Ritchey MD, Zhang D. Prevalence and medical costs of chronic diseases among adult Medicaid beneficiaries. Am J Prev Med. Dec 2017;53(6s2) doi: 10.1016/j.amepre.2017.07.019. S143-S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florida Medicaid Drug . University of South Florida; 2020. Therapy Management Program for Behavioral Health. 2019–2020 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. [Google Scholar]
- 22.Ng-Mak D, Halpern R, Rajagopalan K. Hospitalization risk in bipolar disorder patients treated with lurasidone versus other atypical antipsychotics. Curr Med Res Opin. Feb 2019;35(2):211–219. doi: 10.1080/03007995.2018.1462787. [DOI] [PubMed] [Google Scholar]
- 23.Niu X, Veeranki P, Dennen S. Hospitalization risk among adults with bipolar I disorder treated with oral atypical antipsychotics: a long-term retrospective analysis of Medicaid claims data. Psych Congress 2020; September 10-13, Virtual Conference, USA; 2020. [Google Scholar]
- 24.United States Congress. Health Insurance Portability and Accountability Act of 1996. 104-191 Aug 21, 1996. Available from: https://www.gpo.gov/fdsys/pkg/PLAW-104publ191/html/PLAW-104publ191.htm [PubMed]
- 25.Quan H, Sundararajan V, Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. Nov 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26.Faries DE, Kadziola ZA. SAS Institute.; Cary, NC: 2010. Analysis of longitudinal observational data using marginal structural models. Analysis of observational health care data using SAS; pp. 211–219. [Google Scholar]
- 27.Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry. Feb 18 2009;8:7. doi: 10.1186/1744-859X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broder MS, Greene M, Chang E. Atypical antipsychotic adherence is associated with lower inpatient utilization and cost in bipolar I disorder. J Med Econ. Jan 2019;22(1):63–70. doi: 10.1080/13696998.2018.1543188. [DOI] [PubMed] [Google Scholar]
- 29.Loebel A, Cucchiaro J, Silva R. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. Feb 2014;171(2):169–177. doi: 10.1176/appi.ajp.2013.13070985. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese JR, Pikalov A, Streicher C. Lurasidone in combination with lithium or valproate for the maintenance treatment of bipolar I disorder. Eur Neuropsychopharmacol. Sep 2017;27(9):865–876. doi: 10.1016/j.euroneuro.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Pikalov A, Tsai J, Mao Y. Long-term use of lurasidone in patients with bipolar disorder: safety and effectiveness over 2 years of treatment. Int J Bipolar Disord. Dec 2017;5(1):9. doi: 10.1186/s40345-017-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostacher M, Ng-Mak D, Patel P. Lurasidone compared to other atypical antipsychotic monotherapies for bipolar depression: A systematic review and network meta-analysis. World J Biol Psychiatry. Dec 2018;19(8):586–601. doi: 10.1080/15622975.2017.1285050. [DOI] [PubMed] [Google Scholar]
- 33.Bahji A, Ermacora D, Stephenson C. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: A systematic review and network meta-analysis. J Affect Disord. May 15 2020;269:154–184. doi: 10.1016/j.jad.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Tocco M, Newcomer JW, Mao Y. 160 Lurasidone and metabolic syndrome: results from short- and long-term clinical studies in patients with bipolar depression. CNS Spectrums. 2020;25(2):302–303. [Google Scholar]
- 35.Stensland M, Watson PR, Grazier KL. An examination of costs, charges, and payments for inpatient psychiatric treatment in community hospitals. Psychiatr Serv. Jul 2012;63(7):666–671. doi: 10.1176/appi.ps.201100402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.