Abstract
Background
Isocorydine (ICD) has anticancer effects; however, its suboptimal bioactivity has driven the production of ICD derivatives, including 8-amino-isocorydine (8-NICD).
Objective
This study explored the antitumor effects of 8-NICD on a variety of tumor cell lines to detect tumors sensitive to 8-NICD and investigated the mechanisms by which it suppresses tumor cell growth.
Methods
Human gastric carcinoma (GC) cells (MGC-803) were used to evaluate the effects of 8-NICD on cell proliferation and apoptosis. The in vivo action of 8-NICD in a nude mouse xenograft model was also investigated. The antioxidant activity of 8-NICD was evaluated using a 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay.
Results
8-NICD exerted significant antitumor activity against several tumor cell lines with IC50 between 8.0 and 142.8 µM and was not toxic to healthy fibroblasts and epithelial cells at concentrations up to 100 µM. Moreover, 8-NICD strongly inhibited the proliferation of MGC803 cells without causing toxicity to human umbilical vein endothelial cells with a selectivity index of 19.2 and arrested MGC803 cells in the S phase. Further, the percentages of apoptotic MGC-803 and BGC823 cells increased in a concentration-dependent manner, and the expression of apoptosis regulator Bax increased, whereas that of Bcl-2 decreased in response to 8-NICD treatment. Further, 8-NICD significantly suppressed MGC-803 tumor growth in nude mice. In addition, 8-NICD was a potent scavenger of radicles in a 1,1-diphenyl-2-picrylhydrazyl (IC50 = 11.12 µM) antioxidant assay.
Conclusions
These results suggest that 8-NICD exerts significant antitumor effects on GC cells by inducing apoptosis and cell cycle arrest and is a promising candidate anti-GC drug. The particularly high sensitivity of MGC803 cells suggest that the potential of 8-NICD to treat GC should be further explored. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
Key words: 8-amino isocorydine, antioxidant, apoptosis, aporphine alkaloid, flow cytometry, gastric carcinoma
Introduction
Gastric carcinoma (GC) is the fourth most common cancer worldwide and is the third and fifth most frequent cause of cancer mortality in men and women, respectively.1 Although some patients experience remission through surgical resection alone, the most common treatments for GC include a combination of surgery, perioperative chemotherapy for resectable cases, and adjuvant chemoradiotherapy for unresectable cases.2 Evidence to date indicates a good prognosis for patients who undergo chemotherapy.3 However, despite advances in conventional treatments and surgical interventions, improved survival of patients still relying on the research and development of different strategies such as neoadjuvant chemotherapy, adjuvant chemeradiotherapy, and development of targeted therapies.4 Thus, improvements in long-term survival require a better understanding of GC and more effective treatments.
Dicranostigma leptopodum (Maxim) Fedde (DLF) has been used in traditional Chinese medicine for the treatment of tonsillitis, hepatitis, and inflammation.5,6 DLF belongs to the poppy family (Papaveraceae), which is widely distributed in highland areas, especially in Western China. Whole DLF extracts exhibit antitumor properties.7 The main ingredients of DLF extracts are aporphine, protoberberine, and protopine alkaloids, and these display a wide range of biological activities.8, 9, 10
Isocorydine (ICD) isolated from DLF11 and its derivatives have recently attracted considerable attention because of their diverse biological activities12 and therapeutic potential. ICD exhibits important therapeutic properties, including antibacterial,13 antiulcer,14 antiarrhythmic,15 anticancer,16 and antioxidant17 activities. Previous studies have shown that ICD has multiple inhibitory effects on hepatocellular carcinoma cells (HCC), for example, it induces G2/M phase arrest and apoptosis,18 targets drug-resistant HCC subpopulations via programmed cell death 4-related apoptosis,16 and when coadministered with doxorubicin has a synergistic inhibitory effect on HCC cell lines.19 The results suggests that ICD might be a promising lead compound for the development of novel chemotherapeutic drugs. Zhong et al20 reported that ICD (200 mM) exerts a potent antitumor effect on HCC cells, but this is a cumbersome dose for clinical treatment. Further, the pharmacokinetics (PK) and tissue distribution data of ICD in rats indicate that ICD is quickly absorbed and eliminated, with Tmax in plasma <20 minutes, t1/2 in plasma <1 hour, and the absolute bioavailability a mere 25.8% to 33.4% following oral administration.21,22 Therefore, to enhance antitumor activity and PK of ICD there have been a series of studies on its derivatives, including the semisynthetic ICD derivate 8-amino-isocorydine (8-NICD).
This study explored the cytotoxic and antioxidant activities of 8-NICD both in vitro and in vivo. First, studies were done to identify which tumor cells were most sensitive to 8-NICD as the basis for subsequent experiments. Second, experiments were conducted to verify the tumor-inhibiting effects in vitro and in vivo. Finally, the effects of 8-NICD on the proliferation and apoptosis of tumor cells were evaluated to explore its potential application in clinical treatment.
Materials and Methods
Synthesis of 8-NICD
The 8-NICD was synthesized from ICD as previously reported20 and its synthetic route is shown below. Reagents and conditions: nitric acid (HNO3)/sulphuric acid (H2SO4)/trichloromethane (CHCl3)/methylene dichloride (CH2Cl2), –30°C, stir, 1.5 hours; 0.3 Milli Pascal (MPa) hydrogen (H2), palladium on carbon, Ethanol (EtOH), 25°C, stir, 2.5 hours. Synthesized samples were dissolved and diluted in phosphate-buffered saline (PBS) at 20 mM and stored at 4°C in the dark.
![]()
Cell lines and cell culture
Human lung carcinoma (A549), breast adenocarcinoma (MCF-7), GC (MGC803, BGC823), renal carcinoma (786-O), HCC (SMMC-7721, HepG2), colon carcinoma (SW620), murine renal carcinoma (Renca), human fibroblast (MRC-5), as well as human umbilical vein endothelial cells (HUVEC), were obtained from the Shanghai Cell Bank (Chinese Academy of Sciences, Shanghai, China). The cells were cultured in a humidified atmosphere at 37°C in 5% carbon dioxide. Roswell Park Memorial Institute 1640 medium (Invitrogen, Eugene, Oregon) was used to culture MCF-7, A549, MGC803, BGC823, 786-O, Renca, SMMC-7721, HepG2, and SW620 cells. Minima essential medium (MEM) and Dulbecco's modification of Eagle's medium (DMEM) (Invitrogen) were used to culture MRC-5 cells and HUVECs, respectively. All media were supplemented with 10% fetal calf serum (Gibco, Life Technologies, Carlsbad, California), 2 mM L-glutamine (Sigma-Aldrich, St Louis, Missouri), and 1% (v/v) penicillin/streptomycin (Gibco, Grand Island, New York).
Screening of anticancer activity
The effects of 8-NICD on cell viability was measured using the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.23 Briefly, 96-well plates were seeded separately with 100 µL cell complete medium containing the following number of cells: 2000 for HUVEC, MRC-5, 3000 for MCF-7; 4000 for MGC803, SMMC-7721,786-O, and HepG2; and 5000 for BGC823, A549, and SW620. The cells were allowed to adhere for 24 hours. 8-NICD was diluted in the same complete medium to 6.25 to 180 µM (6.25, 12.5, 25, 50, and 100 for tumor cells; 20, 40, 60, 80, 100, 120, 140, 160, and 180 for nonmalignant cells), and 100-µL aliquots of medium included 8-NICD were added to the wells in quadruplicate. In control wells, the corresponding culture medium for each cell type was added alone. Vincristine (VCR) and paclitaxel (Taxol) (both Sigma-Aldrich) were used as positive controls, diluted in medium to 0.1 to 100 µM (0.1, 1, 10, 20, 40, 60, 80, and 100) and 0.1 to 12.8 µM (0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8), respectively. After incubation for 48 hours, 20 µL 5 mg/mL 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (Sigma-Aldrich) was added to each well, and viable cells were visualized under light microscopy by the development of purple coloration. The plates were incubated for 4 hours at 37°C. After the addition of dimethyl sulphoxide (150 µL), plates were incubated for an additional 10 minutes. The absorbance of each sample was measured at 570 nm on a Bio-Rad 560 Microplate Reader (Bio-Rad, Hercules, California).
IC50 values were obtained from the sigmoidal curves generated by plotting the cell viability percentage against the 8-NICD concentration. The anticancer activity of 8-NICD against tumor cells was defined as the estimate of the degree of selectivity that 8-NICD targeted cancer cells, expressed by selectivity index (SI) value.24 A high SI value (> 2) for 8-NICD indicates selective toxicity in cancer cells (SI = IC50 for normal cells/IC50 for cancer cells).
Acute toxicity test
The LD50 of 8-NICD was determined according to Mohamed et al.25 Doses were determined by the minimum tolerated dose (0% mortality) and maximum tolerated dose (100% mortality); therefore, 90 adult mice were randomly divided into 9 groups of 10 and each group intraperitoneally injected with 750, 1500, 2000, 2500, 3000, 3500, 4000, 4500, or 5000 mg/kg body weight of 8-NICD in PBS solution. In the process of operator injection and subsequent toxicity observation, doses of 8-NICD were hidden by personnel blinded to avoid subjective judgment. The percentage of mice that died and/or displayed signs of toxicity throughout 24 hours was recorded. The median LD50 was calculated by plotting the logarithmic dose against the probit value. Experiments were conducted with the ethical approval (No. XBMU-YX-2019008) of our institution's Animal Care and Use Committee.
Flow cytometric analysis of the cell cycle and apoptosis
MGC803 cells (5 × 105 cells/mL) were plated in 100-mm dishes and incubated with 10, 20, or 40 µM 8-NICD at 37°C for 24 hours, and untreated cells served as controls. The cells were trypsinized, collected, and washed twice with cold PBS, then fixed in 70% ethanol for at least 12 hours at 4°C. Next, MGC803 cells were washed twice with PBS and incubated with 1 mL 50 µg/mL RNAse A (Sigma-Aldrich). Propidium iodide (PI) (Sigma-Aldrich) was added at a concentration of 100 µg/mL and the cells were further incubated in the dark for 1 hour. Finally, samples were analyzed by a BD Accuri C6 instrument (BD Biosciences, Franklin Lakes, New Jersey). Ten thousand cells were acquired and analyzed per sample, and 3 replicates were performed for each treatment. Cell cycle analysis was performed using ModFit LT 3.0 DNA analysis software (Verity Software House, Inc, Topsham, Maine).
MGC803 cells (5 × 105 cells/mL) were plated in 60-mm dishes and incubated at 37°C for 24 hours. After removing the medium, 2 mL Roswell Park Memorial Institute 1640 medium containing 10, 20, or 40 µM 8-NICD was added to each dish, and cells were incubated for a further 24 hours, with untreated cells serving as controls. The cells were collected after treatment with 0.25% trypsin, washed twice with PBS, and suspended in 100 µL binding buffer (10 mM N-2-hydroxyethylpiperazine-N-ethane-sulphonicacid (HEPES)/sodium hydroxide (NaOH) pH7.4, 140 mM sodium chloride (NaCl), 2.5 mM calcium chloride (CaCl2)). Cell suspensions were stained with annexin V-fluoresceine isothiocyanate (annexin V-FITC)/Propidium iodide (PI) apoptosis detection kits (Nanjing Vazyme Biotech Co, Ltd, Nanjing, China), each coverslip was incubated with 5 µL annexin V-FITC and 5 µL PI for 20 minutes in the dark, at room temperature to detect apoptosis according to the manufacturer's instructions, and the results are presented as the mean percentage of annexin V+ cells (SD).
Western blot analysis
MGC803 cells were treated with 20 µM 8-NICD at 37°C for 24 hours, after which the proteins were extracted using an ice-cold RIPA lysis buffer (50 mM trihydroxymethyl aminomethane, Tris-hydrochloric acid (Tris-HCl), pH 7.5, 150 mM sodium chloride (NaCl), 1% octylphenoxypolyethoxyethanol (NP-40), 0.5% sodium deoxycholate (DOC), and 0.1% Sodium dodecyl sulfate (SDS)) and the protein concentration was measured using a Bradford protein assay kit (BioTek China, Beijing, China). The lysates were resolved on precast 10% sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, Massachusetts), which were blocked for 1 hour in blocking buffer (5% nonfat dry milk) at 37°C. Subsequently, the membranes were incubated with the following primary antibodies overnight at 4°C, per the manufacturers’ recommended dilutions: rabbit polyclonal anti-BCL2 apoptosis regulator (BCL2; 1:1,000; catalog No. YT0471, ImmunoWay Biotechnology Company, Plano, Texas), rabbit monoclonal anti-BCL2 associated X, apoptosis regulator (BAX; 1:1,000; catalog No. YT0455; ImmunoWay Biotechnology Company), rabbit monoclonal anti-β actin (1:1,000; catalog No. 4970; Cell Signaling Technology, Inc, Danvers, Massachusetts).
After washing with Tris-buffered saline containing 0.1% Tween 20 (Sigma-Aldrich) 3 or 4 times, the membranes were incubated with goat anti-rabbit polyclonal horseradish peroxidase-conjugated secondary antibody (10,000; catalog No. ab6721; Abcam, Cambridge, Massachusetts) for 1 hour at room temperature. After washing 3 times in Tris-buffered saline containing 0.1% Tween 20, proteins were visualized using 3,3′-diaminobenzidine. Relative protein levels were normalized to β-actin and quantified using Image J software (National Institutes of Health, Bethesda, Maryland).
In vivo tumorigenicity assay
Female BALB/c nude mice (n = 20; 4–6 weeks old; 18–20 g) were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Science (Shanghai, China). All mice were housed in a specific pathogen-free laboratory. Experimental protocols were performed according to the guidelines provided by the EC Directive 86/609/EEC for animal experiments and were conducted with the approval (No. XBMU-YX-2019009) of our institution's Animal Care and Use Committee.
Tumor xenograft models were established by subcutaneously injecting 1 × 106 MGC803 cells into the right flanks of the mice. Next, the mice were randomly assigned to control, high-dose 8-NICD, low-dose 8-NICD, and 5-fluorouracil (5-FU) groups (n = 5 mice per group) on the eighth day and received even intraperitoneal injections of 250 mg/kg 8-NICD, 100 mg/kg 8-NICD, or 40 mg/kg 5-FU, respectively, which was administered at 3-day intervals, 7 injections in total; control mice received 150 µL PBS. Tumor length and width were measured using a Vernier caliper every 3 days, and the bodyweights of the mice were also recorded. The tumor size was calculated according to the formula volume (cm3) = 1/2 length × width2. Tumor weight was measured after mice were sacrificed, and tumors were fixed in 4% formalin and embedded in paraffin for histology staining.
Free radical scavenging activity assays
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activities of purified 8-NICD and standard agents were tested based on the method described by Shimada et al.26 Briefly, 40 µL 8-NICD (0, 1, 2, 4, 8, 16, and 32 µM) was added to 100 µL DPPH (200 µM in methanol) in triplicate wells of a 96-well plate. Ascorbic acid, α-tocopherol, and 2,6-di-tert-butyl-4-methylphenol (butylated hydroxytoluene [BHT]) at the same concentration series as 8-NICD were used as standards, and methanol (MeOH) was used as a negative control. All chemicals used in the study were purchased from Sigma-Aldrich. After incubation in the dark for 30 minutes, the absorbance of each sample was read at 517 nm with the Infinite M200 Pro (TECAN, Mannedorf, Switzerland) Multimode Microplate Reader. The DPPH free radical scavenging activity was expressed as the percentage of DPPH inhibition, which was calculated as follows:
Inhibition of DPPH (%) = [(Absorbance of control – Absorbance of sample) / Absorbance of control] × 100.
The IC50, indicated by the concentration of sample required to scavenge 50% of the DPPH free radical, was determined by plotting the percent inhibition against the 8-NICD concentration.
Statistical analysis
The in vitro experiments were performed in triplicates and the data are expressed as mean (SD). Grouped data were statistically analyzed using 1-way ANOVA and the Tukey-Kramer multiple comparisons post hoc test in SPSS version 16.0 (IBM, Armonk, New York). Results of P < 0.05 were considered statistically significant. IC50 values were calculated in Sigma Plot version 12.0 (Systat Software, Inc, San Jose, California).
Results
Cytotoxicity of 8-NICD
The in vitro cytotoxicity of 8-NICD was tested against 8 human cancer cell lines (A549, MCF-7, MGC803, BGC823, 786-O, SMMC-7721, HepG2, and SW620) and murine renal carcinoma (Renca) cells. Cytotoxicity was also assessed using the MRC-5 cell and HUVEC lines to examine the effects of 8-NICD on nonmalignant cells. IC50 values were calculated based on the survival of cells after 48 hours of exposure to 8-NICD (Figure 1); the concentrations of 8-NICD treatments resulting in half-maximal proliferation after 48 hours are listed in Table 1. IC50 values of 8-NICD were compared with the clinically relevant anticancer drugs VCR and Taxol. 8-NICD displayed significant cytotoxicity toward most of the human carcinoma lines and the murine carcinoma cells tested, with IC50 values ranging from 8.0 (1.1) to 142.8 (4.4) µM. However, 8-NICD was not cytotoxic to MRC-5 cells and HUVECs (both with IC50 >100 µM), especially the MRC-5 cells, which were unaffected at the highest tested concentration (180 µM).
Figure 1.
Effects of 8-amino-isocorydine (8-NICD) on the growth of malignant and nonmalignant cell lines. (A) Growth inhibition rates of 8-NICD in 9 cancer cell lines. (B) Growth inhibition rate of 8-NICD in human fetal lung fibroblasts (MRC-5 cells) and human umbilical vein endothelial cells.
Table 1.
Cytotoxicity of 8-amino-isocorydine (8-NICD) on 9 cancer cell lines and a normal fibroblast cell.
| Cell | IC50* (µM)† |
SI‡ | ||
|---|---|---|---|---|
| 8-NICD | Paclitaxel | vincristine | ||
| MGC803 | 11.8 (1.3) h§ | 0.1 (0.0) | 0.9 (0.0) | 19.2 |
| SMMC7721 | 25.3 (1.3) g | 3.0 (0.2) | 45.0 (1.9) | 9.0 |
| SW620 | 36.4 (1.9) f | 0.2 (0.0) | 0.8 (0.0) | 6.2 |
| BGC823 | 36.9 (2.5) f | 0.2 (0.0) | 6.8 (0.6) | 6.2 |
| 786-O | 45.2 (2.5) e | 1.5 (0.1) | 34.6 (0.2) | 5.0 |
| Renca | 47.7 (4.2) e | 4.5 (0.5) | 15.0 (0.4) | 4.8 |
| MCF-7 | 48.3 (1.6) e | 12.4 (0.6) | 59.4 (1.2) | 4.7 |
| A549 | 97.5 (4.6) d | 1.4 (0.1) | 14.3 (0.4) | 2.3 |
| HepG2 | 142.8 (4.4) c | 1.1 (0.1) | 84.2 (1.5) | 1.6 |
| HUVEC | 227.1 (9.1) b | n.t. | n.t. | – |
| MRC-5 | 420.4 (13.1) a | n.t. | n.t. | – |
n.t. = not tested. SI = selectivity index.
IC50 values were the 50% inhibition concentration and calculated from regression lines using 5 different concentrations in replicate experiments 3 times.
Results are expressed as mean (SD); paclitaxel and vincristine were used as positive controls.
Values were calculated by IC50 for HUVEC cells / IC50 for cancer cells.
One-way ANOVA followed by Dunnett's multiple comparison tests suggested values at the column with different letters are statistically different (P < 0.05).
The tumor cells tested in these experiments were roughly made up of 4 categories according to their sensitivity to the 8-NICD (IC50 values) and their SI values compared to nontumor cells. The tumor-suppressing effects of 8-NICD treatment on GC lines were observed to occur in a dose-dependent manner, with IC50 of 11.8 (1.3) µM on MGC803, 25.3 (1.3), 36.4 (1.9), and 36.9 (2.5 µM) on SMMC7721, SW620, and BGC823, respectively, which suggests that gastrointestinal tumors such as the ones studied were generally more sensitive to 8-NICD, especially the MGC803 cells. 8-NICD displayed moderate tumor-suppressing effects for 786-O, Renca, and MCF-7 cells, with IC50 values of 45.2 (2.5), 47.7 (4.2), and 48.3 (1.6) µM, respectively. Finally, 8-NICD showed weak tumor-suppressing effects on A549 and HepG2 cells, with IC50 values of 97.5 (4.6) and 142.8 (4.4) µM, respectively. Moreover, almost all the SI values (Table 1) calculated with HUVEC as a reference are > 2, indicating that 8-NICD is selective for tumor cells, the most noteworthy of these is the MGC803, which had an SI value of 19.2. The particularly high sensitivity of MGC803 cells suggest that the potential of 8-NICD to treat GC should be further explored.
Acute toxicity of 8-NICD
Intraperitoneal administration of 8-NICD solution at concentrations from 750 up to 5000 mg/kg to mice caused signs of toxicity and death at doses of 1500 mg/kg and upward. The calculated intraperitoneal median LD50 of 8-NICD in mice was estimated to be 2588 mg/kg (range = 1623–4127 mg/kg). However, there was no toxicity observed in mice injected with 8-NICD at 750 mg/kg body weight.
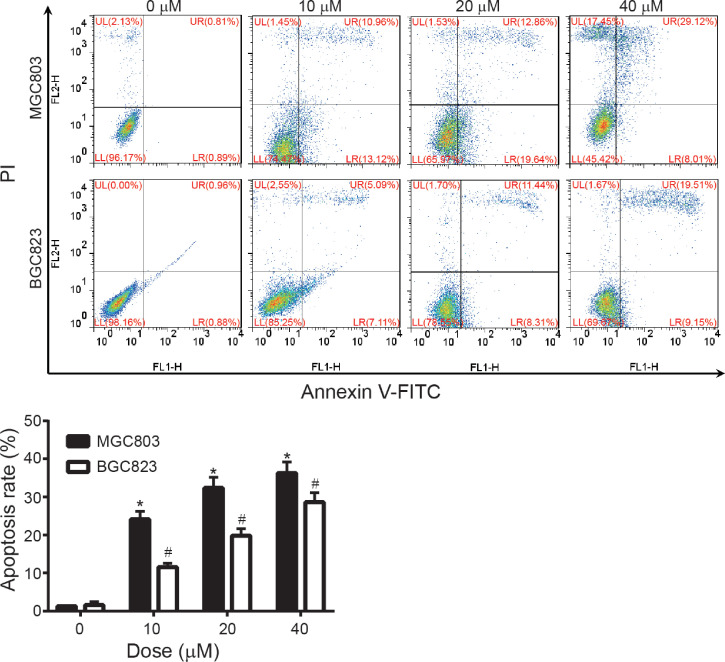
8-NICD induced apoptosis in MGC803 and BGC823 cells
The major differences noted between the inhibition of cell viability by 8-NICD in the 2 GC cell lines (MGC803 and BGC823) prompted studies to determine the type of cell death induced by 8-NICD in these 2 cell lines. The percentage of apoptotic increased significantly and dose-dependently after 24 hours treatment with 8-NICD (Figure 2), from 24.1% (2.1%) in the 10 µM dose condition to 36.3% (2.9%) in the 40 µM dose condition for MGC803 cells, and from 11.6% (1.0%) to 28.6% (2.5%) at same 10 and 40 µM concentrations used for BGC823 cells. In MGC803 cells, the proapoptotic protein Bax was significantly upregulated throughout 8-NICD treatment, whereas the antiapoptotic protein Bcl-2 was significantly downregulated (Figure 3) (both P values < 0.05).
Figure 2.
Effects of 8-amino-isocorydine (8-NICD) on apoptosis in MGC803 and BGC823 cells. Cells were treated with 8-NICD (0, 10, 20, and 40 µM) for 24 hours before harvest, stained with annexin annexin V-fluoresceine isothiocyanate (V-FITC) and Propidium iodide (PI), and analyzed by flow cytometry. Data are expressed as mean (SD) of 3 separate experiments. *P < 0.05 and #P < 0.05 compared with no treatment control group.
Figure 3.
Effect of 8-amino-isocorydine (8-NICD) on the expression of apoptosis-related proteins in MGC803 cells. Cells were treated with 20 µM 8-NICD for 0, 4, 8, 12, and 24 hours, and the protein levels of BAX, BCL2, and β-actin were analyzed by western blotting. Data are expressed as mean (SD) of 3 separate experiments. *P < 0.05 compared with no treatment.
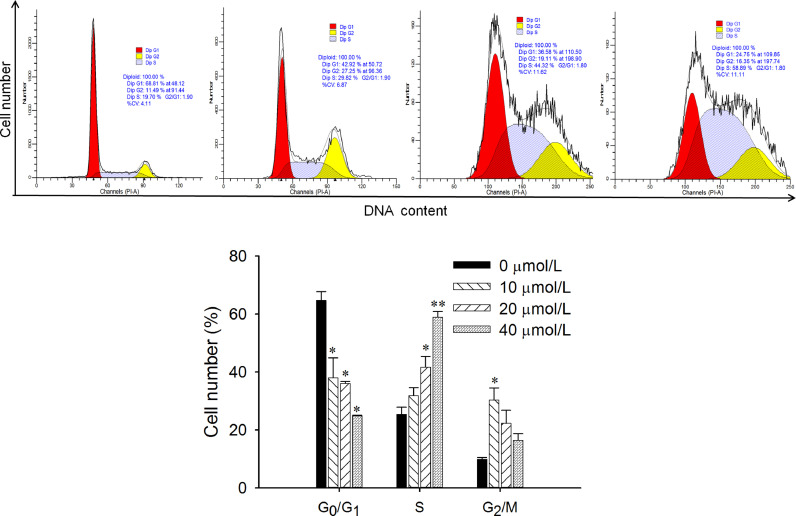
8-NICD induced cell cycle arrest in MGC803 cells
Cell cycle analysis indicated that 8-NICD treatment changed the cell cycle distribution in MGC803 cells (Figure 4). After treatment with different concentrations of 8-NICD for 24 hours, a significant increase was observed in the number of cells in the S phase, with concurrent decreases in the number of cells in the G0/G1 and G2/M phases. This indicated that the cell cycle was arrested in the S phase after treatment with 8-NICD.
Figure 4.
Influence of 8-amino-isocorydine (8-NICD) on the cell cycle progression of MGC-803 cells. Cells were treated with different concentrations of 8-NICD (0, 10, 20, and 40 µM) for 24 hours, stained with PI, and analyzed by flow cytometry. Data are expressed as mean (SD) of triplicate samples. Dip = diploid. *P < 0.05. **P < 0.01.
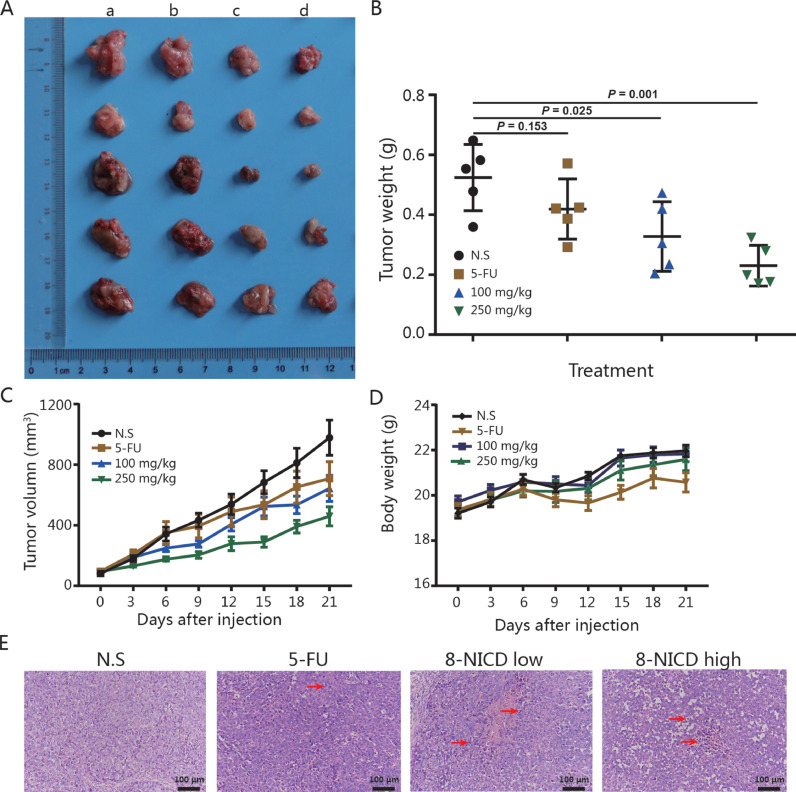
8-NICD inhibition of the tumorigenic properties of GC cells
Validation of the antitumor activity of 8-NICD against GC was performed by measuring its in vivo effects using an MGC803 xenograft tumor model with 10% of the LD50 dose (2588 mg/kg) as the maximum experimental dose of 8-NICD. As shown in Figures 5A-C, and 5D, both 100 mg/kg and 250 mg/kg intraperitoneal injections of 8-NICD significantly (P = 0.025 and P = 0.001, respectively) inhibited MGC803 xenograft tumor growth in mice compared with the control group. Notably, the tumor volume in the high-dose 8-NICD group was even smaller than in the 5-FU treatment group (Figures 5B, 5C). Further more, mice body weight in 8-NICD treatment groups was also having no difference compared with that of in control group (Figure 5D). The pro-apoptotic effect of 8-NICD has also been observed in xenograft tumor tissues (Figrue 5E). These results demonstrate the ability of 8-NICD to inhibit GC growth in vivo.
Figure 5.
Antitumor effects of 8-amino-isocorydine (8-NICD) in vivo. (A) MGC-803 tumor xenografts excised from female BALB/c nude mice after 3 weeks of treatment with 8-NICD or 5-FU. Images in each column show (a) the control group (administered an equal volume of physiological saline vehicle), (b) the 40 mg/kg 5-FU group, (c) the 100 mg/kg 8-NICD group, and (d) the 250 mg/kg 8-NICD group. All therapies were administered by intraperitoneal injection every 3 days. (B) Statistical analysis (Student t test) of the tumor weight among different treatment groups. 5-Data points represent the mean tumor volume for each group at each time point. (C) Tumor growth curves. (D) The bodyweight of mice during different treatments. (E) Representative microphotographs showing the histological morphology (hematoxylin and eosin staining) of tumors from animals receiving either with normal saline, 5-fluorouracil, 8-NICD (100 mg/kg), or 8-NICD (250 mg/kg), arrows indicate apoptotic bodies and pyknotic nucleus undergoing cell death. Magnification, × 200; scale bar, 100 µm. Error bars indicate the mean (SD) (n = 5). 5-FU = 5-fluorouracil; 8-NICD = 8-amino isocorydine.
DPPH scavenging ability of 8-NICD
The antioxidant activity of 8-NICD was evaluated by measuring its ability to scavenge DPPH free radicals compared to ascorbic acid, α-tocopherol, and BHT. The radical scavenging activities of these reagents, in decreasing order, were 3.92 (0.28) µM for ascorbic acid (P < 0.05 compared with the others), 11.12 (0.64) µM for 8-NICD (P < 0.05 compared with ascorbic acid and BHT), 13.03 (4.23) µM for α-tocopherol (P < 0.05 compared with ascorbic acid and BHT), and 23.24 (4.11) µM for BHT (P < 0.05 compared with the others). These results demonstrate the ability of low concentrations (11.12 [0.64] µM) of 8-NICD to exert antioxidant effects that were comparable to α-tocopherol (13.03 [4.23] µM) and significantly (P < 0.05) higher activity than BHT (23.24 [4.11] µM).
Discussion
GC is among the deadliest digestive system malignancies; with an estimated 952,000 new cases and 723,000 deaths per annum worldwide. Patients with advanced disease have an especially poor prognosis.1 Despite recent advances in surgery and chemotherapy, more effective drugs for GC are sorely needed. These results suggest, for the first time in GC cells, that 8-NICD, a chemically produced derivative of ICD, enhances apoptosis and inhibits proliferation both in vivo and in vitro tumor models.
ICD is isolated from D leptopodum11 and previous authors have reported that it had multiple inhibitory effects on HCC cells including the induction of G2/M phase arrest and apoptosis.18 It appears that altering the chemical structure of ICD results in a molecule that can cause S-phase arrest in GC cells. Many ICD derivatives have potential antitumor activity.27 For example, Li et al28 reported that a derivative of ICD may inhibit HCC cells by decreasing the expression of insulin-like growth factor 2 mRNA binding protein 3, rendering HCC cells more sensitive to chemotherapy. Also, the derivative of ICD inhibits HCC cell migration and invasion, in part by regulating E2F transcription factor 1 and integrin subunit alpha 1 expression.29 8-NICD was found to exhibit significant, cell-type–specific toxicity against a series of tumor cell lines in vitro, particularly human GC cell lines, whilst no toxic effects were detected against non-malignant fibroblasts (MRC-5 cells) and HUVECs. VCR and Taxol were used as positive controls as is commonly done in experiments evaluating GC chemoprevention.30,31 The IC50 of 8-NICD satisfied the criteria for cytotoxic activity with a similar dosage threshold to that of the established positive control agents.
The many detrimental effects of cancer chemotherapies on healthy cells have prompted attempts to identify drugs that more selectively act on tumor cells relative to healthy cells. Nonmalignant cells are extensively used in cytotoxicity studies to test the selective effects of drugs; for instance, curcumin has been reported to induce cell death in nonmalignant cells such as rat thymocytes and human T cells.32 Results of these experiments showed that 8-NICD had low (IC50 = 420.4 [13.1] µM) cytotoxicity against MRC-5 fibroblast cell lines and relatively low (IC50 = 227.1 [9.1] µM) cytotoxicity against HUVECs (Table 1). It is worth noting that although 8-NICD display nontoxic to MRC-5 and HUVEC cells at concentration up to 100 µM, yet the IC50 of HepG2 (142.8 [4.4] µM) and A549 (97.5 [4.6] µM) also exceeds or approaches this concentration, showing signs of resistance by HCC and lung carcinoma; whereas by use of the up-limit concentration of 180 µM, 8-NICD also showed toxicity to nonmalignant cells, especially for HUVEC cells. Because the SI reflects the selectiveness of 8-NICD in targeting cancer cells, the greater the SI value is, the greater the selectivity for target cells. Acute in vivo toxicity tests performed in mice revealed that a relatively high dose (LD50 >2,500 mg/kg) of 8-NICD was required to induce toxicity. Although this needs confirmation in actual patients, the results suggest that 8-NICD might be well tolerated. The selective cytotoxicity of 8-NICD toward tumor cells and its low toxicity against nonmalignant cells suggests it has potential for development as a therapy for GC.
Zhong et al20 reported that 200 µM ICD exerts potent antitumor effects in HCC cells; however, this is a relatively high dose to be used for clinical treatment. To improve the anticancer activity of ICD, its chemical structure has been modified, and the synthetic derivatives of ICD have been studied in recent years. The derivative of ICD is isocorydione, was evaluated for antitumor activity using in vivo and in vitro assays, and whilst its inhibitory effects on tumor cells have been confirmed, its IC50 values range from 186.97 to 212.46 µM, indicating a relatively weak anticancer activity against 3 tested cell lines.20 The PK and bioavailability of 8-acetamino-isocorydine (AICD), another derivative of ICD, have been reported.33 The absolute bioavailability of orally administered AICD reached 76.5%, and its Tmax and t1/2 were 0.8 hour and 2.0 hours, respectively. The PK properties of 8-NICD in vivo were not studied here. This decision was based on the assumption that 8-NICD is produced by deacetylation of AICD in the liver, based on the pro-drug theory. 8-NICD possesses superior anti-cancer activity compared with ICD and its administration by injection avoids the first elimination that oral administration must overcome. It was found that 8-NICD was effective at suppressing the growth of a wide range of tumor cells and exerted its effect on GC via the induction of apoptosis. The positive in vivo and in vitro anticancer effects prompted an in-depth evaluation of its mechanism of action of 8-NICD.
The DPPH radical scavenging model is widely used to study the antioxidant activities of the compounds found in plant extract and their derivatives.34,35 Results showed that 8-NICD may have superior antioxidative abilities to its precursor ICD, which also exhibits free radical DPPH scavenging activity (IC50 = 229.85 [7.51] µM). Uncontrolled production of free radicals leads to the development of inflammation and various diseases, such as cardiovascular diseases, degenerative aging processes, and cancer.36 In cancer cells, the accumulation of reactive oxygen species due to defective mitochondria and increased metabolic rate may promote tumor growth.37 To prevent the dangerous effect of excessive reactive oxygen species, cancer cells induce cytoprotective mechanisms, overexpression of reactive oxygen species and increased expression of antioxidant enzymes facilitates multidrug resistance of cancer cells, through multiple pathways (eg, nuclear factor-kappa B (NF-κβ), mitogen-activated protein kinase (MAPK), Janus Kinase - signal transducer and activator of transcription (JNK-STAT) and Phosphatidylinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR)).37,38 Satheesh and colleagues39 found ascorbic acid (vitamin C) kills tumor cells and targets cancer stem cells due to its ability to affect the redox status of the cell, influence epigenetic modification, and promote HIF-1α signaling.
Curcumin is a well-known hermetic antioxidant with potent anticancer activities that can be deployed against many human malignancies because curcumin exerts antioxidant activities at low concentrations, and pro-oxidant activities at high concentrations.40 At low concentration, curcumin protects the body against radicals present in the blood,41 and at high concentrations, curcumin exerts pro-oxidant effects via intracellular reactive oxygen species production, inhibiting the production of factors that induce tumorigenesis. Hence, modulation of reactive oxygen species scavengers by antioxidant treatments in transformed cells have to be considered as an anticancer strategy. Despite the radical-scavenging ability of 8-NICD that we found in this study, a molecular link between it and antitumor effects was not observed, so further experiments should be designed to explain the mechanisms of relationship between radical-scavenging and antiproliferation of 8-NICD in GC.
Conclusions
8-NICD exhibited strong anticancer and antioxidant properties against several cell lines. 8-NICD was selectively cytotoxic against cancer cells by inducing apoptosis and cell cycle arrest, with no cytotoxic effects on normal fibroblasts, and its antioxidant activity was comparable to that of α-tocopherol. The particularly high sensitivity of MGC803 cells suggest that the potential of 8-NICD to treat GC should be further explored. These results suggest that 8-NICD exerts significant antitumor effects on GC cells by inducing apoptosis and cell cycle arrest and is a promising anti-GC candidate drug.
CRediT authorship contribution statement
Junxi Liu: Conception and design.
Fei Zhao: Implementation of experiment.
Xiaonong Guo: Collection and assembly of data.
Yong Liu: Data analysis and interpretation.
Chengli Wu: Literature search.
Lei Song: Manuscript writing.
Acknowledgments
Acknowledgments
The authors thank 2 anonymous referees for their helpful comments and critical review of the manuscript. The authors also thank Mr Vikas Narang for his assistance with English language editing.
This research was supported by the grants from the Characteristic Discipline of Bioengineering Construction for The Special Guide Project of The “world-class universities and world-class disciplines” of Northwest Minzu University (11080306(2019)), the National Science and Technology Major Project for “Significant New Drugs Development,” the Ministry of Science and Technology of China (2015ZX09102016), the National Natural Science Foundation of China (Nos. 21672225 and 31700720), and the Fundamental Research Funds for the Central Universities (Nos. 31920190095 and 31920190210).
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
- 1.Goetze O.T., Al-Batran S.E., Chevallay M., Monig S.P. Multimodal treatment in locally advanced gastric cancer. Updates Surg. 2018;70:173–179. doi: 10.1007/s13304-018-0539-z. [DOI] [PubMed] [Google Scholar]
- 2.Quero L., Guimbaud R. [Chemotherapy and chemoradiotherapy indications in the treatment of locally advanced gastric carcinoma] Cancer Radiother. 2018;22:546–551. doi: 10.1016/j.canrad.2018.07.134. [DOI] [PubMed] [Google Scholar]
- 3.Schernberg A., Rivin Del Campo E., Rousseau B., Matzinger O., Loi M., Maingon P. Adjuvant chemoradiation for gastric carcinoma: State of the art and perspectives. Clin Transl Radiat Oncol. 2018;10:13–22. doi: 10.1016/j.ctro.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo A., Strong V. Gastric Cancer Etiology and Management in Asia and the West. Annual review of medicine. 2019;70:353–367. doi: 10.1146/annurev-med-081117-043436. [DOI] [PubMed] [Google Scholar]
- 5.Wang F., Li Y.M. New hopane triterpene from Dicranostigma leptopodum (Maxim) Fedde. J Asian Nat Prod Res. 2010;12:94–97. doi: 10.1080/10286020903443028. [DOI] [PubMed] [Google Scholar]
- 6.Dang Y., Gong H.F., Liu J.X. Alkaloid from Dicranostigma leptopodum (Maxim)Fedde. Chin Chem Lett. 2009;20:1218–1220. [Google Scholar]
- 7.Zhang W., Lv M., Hai J., Wang Q., Wang Q. Dicranostigma leptopodum (maxim) fedde induced apoptosis in SMMC-7721 human hepatoma cells and inhibited tumor growth in mice. Natural Science. 2010;2:457–463. [Google Scholar]
- 8.Liu Y., Liu J., Di D., Li M., Fen Y. Structural and mechanistic bases of the anticancer activity of natural aporphinoid alkaloids. Curr Top Med Chem. 2013;13:2116–2126. doi: 10.2174/15680266113139990147. [DOI] [PubMed] [Google Scholar]
- 9.Wright C.W., Marshall S.J., Russell P.F., Anderson M.M., Phillipson J.D., Kirby G.C. In vitro antiplasmodial, antiamoebic, and cytotoxic activities of some monomeric isoquinoline alkaloids. J Nat Prod. 2000;63:1638–1640. doi: 10.1021/np000144r. [DOI] [PubMed] [Google Scholar]
- 10.Sun R., Jiang H., Zhang W., Yang K., Wang C., Fan L. Cytotoxicity of Aporphine, Protoberberine, and Protopine Alkaloids from Dicranostigma leptopodum (Maxim.) Fedde. Evid Based. Complement Alternat Med. 2014;2014 doi: 10.1155/2014/580483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Y., Yang A.M., Liu J.X., Di D.L., Liu Y.J., Li M. [Extraction and purification of isocorydine from Dicranostigma leptopodum] Zhong Yao Cai. 2013;36:807–809. [PubMed] [Google Scholar]
- 12.Zhong M., Ma Y.X., Liu J.X., Di D.L. A new quaternary protoberberine alkaloid isolated from Dicranostigma leptopodum (Maxim) Fedde. Nat Prod Res. 2014;28:507–510. doi: 10.1080/14786419.2013.879586. [DOI] [PubMed] [Google Scholar]
- 13.Cosar G., Bilgehan H., Gozler T. [The antibacterial effects of some alkaloids isolated from Glaucium flavum Crantz] Mikrobiyol Bul. 1981;15:105–109. [PubMed] [Google Scholar]
- 14.Yadav D.K., Singh N., Dev K., Sharma R., Sahai M., Palit G. Anti-ulcer constituents of Annona squamosa twigs. Fitoterapia. 2011;82:666–675. doi: 10.1016/j.fitote.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D.H., Yang X.M., Sheng B.H. [Anti-arrhythmic action of d-isocorydine hydrochloride] Zhongguo yao li xue bao = Acta pharmacologica Sinica. 1986;7:131–134. [PubMed] [Google Scholar]
- 16.Lu P., Sun H., Zhang L., Hou H., Zhang L., Zhao F. Isocorydine Targets the Drug-Resistant Cellular Side Population through PDCD4-Related Apoptosis in Hepatocellular Carcinoma. Molecular Medicine. 2012;18:1136–1146. doi: 10.2119/molmed.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahari A., Ablat A., Omer N., Nafiah M.A., Sivasothy Y., Mohamad J. Ultraviolet-visible study on acid-base equilibria of aporphine alkaloids with antiplasmodial and antioxidant activities from Alseodaphne corneri and Dehaasia longipedicellata. Sci Rep. 2016;6:21517. doi: 10.1038/srep21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H., Hou H., Lu P., Zhang L., Zhao F., Ge C. Isocorydine Inhibits Cell Proliferation in Hepatocellular Carcinoma Cell Lines by Inducing G2/M Cell Cycle Arrest and Apoptosis. Plos One. 2012;7 doi: 10.1371/journal.pone.0036808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J.-X., Chen G., Li J.-J., Zhu Q.-D., Li J.-J., Chen Z.-J. Isocorydine suppresses doxorubicin-induced epithelial-mesenchymal transition via inhibition of ERK signaling pathways in hepatocellular carcinoma. American Journal of Cancer Research. 2018;8:154–164. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong M., Liu Y., Liu J., Di D., Xu M., Yang Y. Isocorydine derivatives and their anticancer activities. Molecules. 2014;19:12099–12115. doi: 10.3390/molecules190812099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo C., Yu C., Li L., Wang Y., Wang S., Wang W. Rapid determination of isocorydine in rat plasma and tissues using liquid chromatography–tandem mass spectrometry and its applications to pharmacokinetics and tissue distribution. Xenobiotica; the fate of foreign compounds in biological systems. 2012;42:466–476. doi: 10.3109/00498254.2011.640965. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Li H., He J., Feng E., Rao G., Xu G. Development and validation of a high-performance liquid chromatography coupled with ultraviolet detection method for the determination of isocorydine in rat plasma and its application in pharmacokinetics. Drug research. 2013;63:558–563. doi: 10.1055/s-0033-1347256. [DOI] [PubMed] [Google Scholar]
- 23.Jin J., Wang F.P., Wei H., Liu G. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. Cancer Chemother Pharmacol. 2005;55:179–188. doi: 10.1007/s00280-004-0868-0. [DOI] [PubMed] [Google Scholar]
- 24.Badisa R., Darling-Reed S., Joseph P., Cooperwood J., Latinwo L., Goodman C. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer research. 2009;29:2993–2996. [PMC free article] [PubMed] [Google Scholar]
- 25.Syed M., Gnanakkan A., Pitchiah S. Exploration of acute toxicity, analgesic, anti-inflammatory, and anti-pyretic activities of the black tunicate, Phallusia nigra (Savigny, 1816) using mice model. Environmental science and pollution research international. 2020 doi: 10.1007/s11356-020-10938-2. [DOI] [PubMed] [Google Scholar]
- 26.Kazuko Shimada K.F., Yahara Keiko, Nakamura Takashi. Antioxidative properties of xanthan on the auto xidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. [Google Scholar]
- 27.Yan Q., Li R., Xin A., Han Y., Zhang Y., Liu J. Design, synthesis, and anticancer properties of isocorydine derivatives. Bioorganic & Medicinal Chemistry. 2017;25:6542–6553. doi: 10.1016/j.bmc.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Li M., Zhang L., Ge C., Chen L., Fang T., Li H. An isocorydine derivative (d-ICD) inhibits drug resistance by downregulating IGF2BP3 expression in hepatocellular carcinoma. Oncotarget. 2015;6:25149–25160. doi: 10.18632/oncotarget.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X., Tian H., Li H., Ge C., Zhao F., Yao M. Derivate Isocorydine (d-ICD) Suppresses Migration and Invasion of Hepatocellular Carcinoma Cell by Downregulating ITGA1 Expression. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mothana R.A., Al-Said M.S., Al-Musayeib N.M., El Gamal A.A., Al-Massarani S.M., Al-Rehaily A.J. In vitro antiprotozoal activity of abietane diterpenoids isolated from Plectranthus barbatus Andr. Int J Mol Sci. 2014;15:8360–8371. doi: 10.3390/ijms15058360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bent M.J. Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro Oncol. 2014;16:1570–1574. doi: 10.1093/neuonc/nou297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielak-Zmijewska A., Koronkiewicz M., Skierski J., Piwocka K., Radziszewska E., Sikora E. Effect of curcumin on the apoptosis of rodent and human nonproliferating and proliferating lymphoid cells. Nutrition and cancer. 2000;38:131–138. doi: 10.1207/S15327914NC381_18. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Yan Q., Zhong M., Zhao Q., Liu J., Di D. Study on pharmacokinetics and tissue distribution of the isocorydine derivative (AICD) in rats by HPLC-DAD method. Acta Pharmaceutica Sinica B. 2015;5:238–245. doi: 10.1016/j.apsb.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehi S., Mirzaie A., Sadat Shandiz S., Noorbazargan H., Rahimi A., Yarmohammadi S. Chemical composition, antioxidant, antibacterial and cytotoxic effects of Artemisia marschalliana Sprengel extract. Natural product research. 2017;31:469–472. doi: 10.1080/14786419.2016.1174234. [DOI] [PubMed] [Google Scholar]
- 35.Blagojević B., Agić D., Serra A., Matić S., Matovina M., Bijelić S. An in vitro and in silico evaluation of bioactive potential of cornelian cherry (Cornus mas L.) extracts rich in polyphenols and iridoids. Food chemistry. 2021;335 doi: 10.1016/j.foodchem.2020.127619. [DOI] [PubMed] [Google Scholar]
- 36.Vona R., Gambardella L., Cittadini C., Straface E., Pietraforte D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxidative Medicine and Cellular Longevity. 2019;2019 doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirtonia A., Sethi G., Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cellular and molecular life sciences: CMLS. 2020;77:4459–4483. doi: 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Q., Wang J., Assaraf Y., Ren L., Gupta P., Wei L. Modulating ROS to overcome multidrug resistance in cancer. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Satheesh N., Samuel S., Büsselberg D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules. 2020:10. doi: 10.3390/biom10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanmugam M., Rane G., Kanchi M., Arfuso F., Chinnathambi A., Zayed M. The multifaceted role of curcumin in cancer prevention and treatment. Molecules (Basel, Switzerland) 2015;20:2728–2769. doi: 10.3390/molecules20022728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J., Wanming D., Zhang D., Liu Q., Kang J. Water-soluble antioxidants improve the antioxidant and anticancer activity of low concentrations of curcumin in human leukemia cells. Die Pharmazie. 2005;60:57–61. [PubMed] [Google Scholar]