Abstract
Background
Blood clot (BC) and platelet-rich fibrin (PRF) has been successfully used to biologically treat immature roots. It is nowadays considered the treatment of choice.
Objective
This study aimed to determine the ability of PRF and BC scaffolds to enhance regeneration of disinfected root canals and healing of apical periodontitis within experimentally enlarged canal apices of dog teeth.
Methods
Forty-eight root canals in 28 mandibular premolars from 4 healthy adult dogs were experimentally infected and developed apical periodontitis. The teeth were randomly divided into a control (untreated) group, a disinfection only group, a group that received disinfection and a BC scaffold, and a group that received disinfection and a BC + PRF scaffold. Healing of the apical radiolucency was evaluated by conventional radiography, micro-computed tomography, and histology after 3 months. The data were analyzed by χ2 test.
Results
Healing was achieved in 49% of roots as seen on radiograph and 43% as seen on micro-computed tomography. There was no significant between-group difference in the presence or absence of periapical radiolucency in the mesial roots when seen on conventional images (P = 0.255), but there was a significant difference in the distal roots (P = 0.001); similarly, on micro-computed tomography, there was no significant between-group difference in the mesial roots (P = 0.174) but there was a significant difference in the distal roots (P = 0.001). Histologically, apical closure was significantly not greater in the BC + PRF scaffold group than in the BC scaffold group (P = 0.001).
Conclusions
A mix of BC + PRF scaffold did not improve tissue regeneration in experimentally enlarged dog teeth. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX) © 2021 Elsevier HS Journals, Inc.
Key words: Apical closure, Apical enlargement, Blood clot, Periapical healing, Platelet-rich fibrin
Introduction
Long-standing necrosis of the dental pulp causes suspension of bacteria in the planktonic phase in the lumen of the root canal, as well as formation of bacterial biofilm on the walls of the canal, dentinal tubules, and lateral canals, leading to periapical disease if left untreated.1,2 This biofilm contains a variety of bacterial species and a matrix consisting of nucleic acids, proteins, polysaccharides, and salts. The complex root canal morphology acts as a shelter for the biofilm. Therefore, disinfection of the root canal during instrumentation is important.3 Furthermore, a closed apex allows better management of the canal filling.
Regeneration/revascularization of pulp tissue is typically performed for a necrotic root canal with a wide-open apex, which allows ingrowth of periradicular tissue, including blood vessels, the periodontal ligament, and bone-like tissue if a sterile environment is maintained.4 An apical diameter >1 mm facilitates regeneration. However, smaller diameters reportedly also allow for tissue formation.5
The apical foramen has been enlarged in both vital and necrotic canals in mature teeth to regenerate pulp tissue,6, 7, 8 but causes severe inflammation due to profuse hemorrhage and necrotic dentin debris being pushed far into the periradicular tissue, leading to seepage of irritants into unfilled root canals if forced beyond the apices.9
Intentional overinstrumentation of mature teeth with minimal apical irritation was believed to contribute to healing of periradicular lesions and achievement of biological hard-tissue and soft-tissue barriers in a similar way as revascularized immature teeth. A blood clot (BC) often forms during this procedure. Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) have also achieved good results.10,11 BC, PRP, and PRF act as natural scaffolds for numerous growth factors, cell proliferation, tissue regeneration, and root maturation. The importance of BC, PRP, and PRF in periapical healing and treatment of immature permanent teeth was emphasized by Ostby 6 and Murry.12 Martin et al10 used a mixture of PRP and BC and found no histological difference in the tissue that formed in the canals. However, no attempt to use a mixture of PRF and BC has been reported.
The aim of this histology, radiographic, and micro-computed tomography (micro-CT) study was to determine the ability of PRF and BC scaffolds to enhance regeneration of infected root canals and healing of apical periodontitis (AP) in experimentally enlarged apical delta of mature dog teeth.
Methods
Ethical considerations
This study was performed in line with the principles of the Declaration of Helsinki. It was approved by the Review Board at Riyadh Elm University College of Dentistry, Riyadh, Saudi Arabia (approval No. 43333002/34), and College of Dentistry, King Saud University, Riyadh, Saudi Arabia (approval No. FR-0147). Twenty-eight mature mandibular premolar teeth (4 first premolars and 24 second, third, or fourth premolars) with 52 root canals from 4 healthy mongrel dogs aged approximately 10 months were used in this study. The dogs were divided randomly into 4 (experimental [48 roots] and control [4 roots]) groups. Every effort was made to minimize the discomfort of the animals. The regulations of the Laboratory Animal Unit, Engineer Abdullah Bugshan Research Chair for Growth Factors and Bone Regeneration (No. PG0052), King Saud University College of Dentistry, were strictly observed.
Animal preparation and root canal infection
A modified version of the methodology described by Thibodeau et al4 was followed. The animals were anesthetized intramuscularly with 2 mg/kg Seton 2% (Laboratorios Calier, Barcelona, Spain) and 50 mg/kg Tekam (Hikma Pharmaceuticals PLC, Amman, Jordan) supplemented with local anesthesia with 0.5 mL 2% Xylocaine with epinephrine 1:100,000 (Novocol Pharmaceutical, Cambridge, Ontario, Canada) per quadrant for all procedures. Before starting the experiment an impression of the lower jaw was taken from each dog, a cast stone was made, and an occlusal acrylic bite guide was prepared. A slot of the same size as the radiograph film was created on the acrylic bite guide to hold and stabilize the film in position; this ensured that the radiographs were acquired consistently from the same angle and minimized distortion.
Preoperative periapical radiographs (7.0 mA, 60 kVp, 0.66 second) were taken of all teeth in each animal using a Heliodent radiograph machine (Siemens Medical Systems, Erlangen, Germany). The pulp chambers of 6 randomly selected matures mandibular (second, third, and fourth) premolar teeth per dog were exposed using a high-speed handpiece with a sterile carbide bur under nonaseptic conditions. Supragingival plaque obtained from the teeth was placed into the exposed pulp. The access opening of the teeth was sealed with intermediate restorative material (IRM) (Dentsply Caulk, Milford, Delaware). All animals received 0.2 mg/kg butorphanol tartrate (10 mg/mL Torbugesic; Zoetis Animal Health New Zealand Limited, Auckland, New Zealand) as an analgesic on completion of surgery and were monitored in the postoperative period. Forty-eight root canals were infected. The teeth were monitored radiographically until evidence of AP was detected (∼2–3 weeks postintervention).
Root canal disinfection
The animals were anesthetized (generally and locally with dental nerve blocks) for disinfection of the infected pulp spaces using the procedure devised by Thibodeau et al.4 After rubber dam isolation, the experimental tooth and operating field were disinfected with 30% hydrogen peroxide followed by 5% tincture of iodine (Alphadine; Riyadh Pharma, Riyadh, Saudi Arabia). The IRM filling was removed using a high-speed handpiece with a sterile carbide bur and a cooling water spray. The root canal apical delta of each tooth was enlarged using a size 70 (0.12/17 mm) nickel-titanium GT rotary file (Dentsply Tulsa Dental Specialties, Tulsa, Oklahoma).
The infected root canals were disinfected by irrigation with 10 mL 5.25% sodium hypochlorite for 5 minutes, flushing with 10 mL 0.9% saline to remove the sodium hypochlorite, and drying using sterile paper points. The irrigating needle was carefully placed in the canal at 1 mm short of the working length. A paste (≤100 mg/mL) containing metronidazole, ciprofloxacin, and cefuroxime (mixed with 0.9% sodium chloride) was then applied using a sterile Lentulo Spiral in a slow-speed handpiece (Dentsply, Maillefer, Ballaigues, Switzerland). The 24 teeth were sealed with IRM.
Revascularization protocol
After 4 weeks, the animals were anesthetized (generally and locally with dental nerve blocks) and intraoral radiographs of the infected teeth were acquired using the occlusal acrylic radiograph bite guide. After rubber dam isolation, the tooth and operating field were disinfected with 30% hydrogen peroxide followed by 5% tincture of iodine. The IRM temporary restoration was removed using a high-speed handpiece with a sterile carbide bur and a cooling water spray. The root canals were randomly divided into a control group, a disinfection only group, disinfection and BC scaffold group, and disinfection and BC + PRF scaffold group.
The control group consisted of 4 single rooted teeth with untreated root canals. The disinfection only group consisted of 16 root canals that were disinfected as described above and then flushed with 5.25% sodium hypochlorite (10 mL/canal) for 5 minutes followed by sterile saline. EDTA (Ultradent Products Inc, South Jordan, Utah) 17% was applied in a volume of 3 mL/canal for 10 minutes, after which the canal was flushed with sterile saline and dried using sterile paper points. The access openings were sealed with a double layer of mineral trioxide aggregate (Dentsply Tulsa Dental) and composite filling material (3M ESPE, St Paul, Minnesota). The disinfection and BC scaffold group consisted of 16 root canals that were treated in the same way as the disinfection only group. A size 30 sterile stainless steel hand K-file (Kerr, Romulus, Michigan) was then inserted past the apical foramen of the canal into the periapical tissue to induce bleeding. When the root canal space was filled with blood to approximately the level of the cemento-enamel junction, the access openings were sealed with a double layer of mineral trioxide aggregate. The disinfection and BC + PFR scaffold group consisted of 16 root canals that were treated in the same way as the disinfection and BC scaffold group, except that after the root canal space was filled with blood to approximately the level of the cemento-enamel junction, the upper half of the canal was dried using sterile paper points and filled with fragmented PRF to the level of the cemento-enamel junction after induction of clotting using an endodontic plugger. The PRF was prepared as follows. Whole venous blood was drawn from the animals into 10-mL plain-coated sterile plastic tubes without anticoagulant. The blood was immediately centrifuged (DSC-200T tabletop centrifuge; Digisystem Laboratory Instruments Inc, New Taipei City, Taiwan) at 3000 rpm for 10 minutes. This yielded a bottom layer of red blood cells, an upper layer of acellular plasma, and a clot of PRF in the middle. The clot was separated carefully and gently pressed into a dry sterile membrane to remove the fluid. The PRF was then fragmented for immediate application. The access openings were sealed with a double layer of mineral trioxide aggregate filling material. All teeth were monitored radiographically every month for 3 months.
Preparation of tissue samples
Three months after surgery, the carotid arteries were exposed and cannulated under general anaesthesia induced by intravenous injection of 30 mg/kg pentobarbital. The animals were then put to death by intravenous injection of 90 mg/kg pentobarbital. The animals were perfused with buffered formalin (10%); the jaws were then resected, and sections were cut and placed in 10% formaldehyde solution for 1 day. Each jaw was cut into right and left blocks or a total of 8 specimens.
The results of a regenerative procedure performed after experimental enlargement and infection of canal apices were evaluated by radiography, micro-CT, and histology.
Preparation of micro-CT images
Four specimens were scanned using a SkyScan 1173 micro-CT instrument, (Bruker, Kontich, Belgium) at medium resolution (1120 × 1120) and the other 4 specimens were scanned at high resolution (2240 × 2240). The following parameters were used for the high-resolution scans: 88 kV tube voltage, 90 µA current, aluminium 1.0 mm filter, 830 msec exposure time, 0.2° rotation step (360° rotation), and frame averaging of 4. For the medium-resolution scans, the parameters were as follows: 88 kV tube voltage 75 µA current, aluminium 1.0 mm filter, 250 msec exposure time, 0.4° rotation step (360° rotation), and frame averaging of 6.
Three-dimensional reconstruction was performed using NRecon software (Bruker). A 35% reduction in beam hardening and a ring artefact correction of 2 were used to produce cross-sections with a defect pixel masking value of 5.
The reconstructed set of slices was viewed using DataViewer software (Bruker). Images were displayed as a slice-by-slice movie or as 3 orthogonal sections centred at a selected point inside the reconstructed space. The viewing mode in DataViewer was used to rotate each of 3 intersecting orthogonal slices independently using the mouse.
The measurements were saved using DataViewer software. Manipulation of the x-, y-, and z-axes enabled determination of root lengths and thicknesses. A transaxial data set was created and saved using DataViewer, with the region of interest selected to allow determination of bone volume and bone mineral density. A 3-mm circular region of interest was placed at each end of the root apex. SkyScan calibration phantoms (8 mm) were scanned (using the same settings used to scan the specimens) to calibrate the bone mineral density using the bundled SkyScan software.
Histology preparation
After removing excess soft and hard tissue, the specimens were decalcified, first in Formical (Decal Chemical Corporation, Congers, New York) for 3 weeks and then in Immunocal (Decal) for 60 days; the solutions were changed 3 times per week. Next, the specimens were rinsed in running tap water for 24 hours and immersed in 70% ethyl alcohol. The specimens were dehydrated through ascending gradients of ethanol and processed using a TP1020 tissue processor (Leica, Wetzlar, Germany) for 1 hour per station as follows: 1 cycle each of 70%, 80%, and 95% ethanol, 2 cycles of 100% ethanol, 2 cycles of xylene, and 2 cycles of Paraplast paraffin (Kendall, Mansfield, Massachusetts) at 58°C. The tissue was removed from the storage cassettes, embedded in paraffin, and sectioned using a Leica RM 2145 microtome (Leica). Serial 5-µm-thick sections were cut longitudinally through the apical foramen of the roots, stained with hematoxylin and eosin, and examined by light microscopy (Leica) at up to × 20 magnification.
Evaluation criteria
Each root was considered a single unit for measurement and assessment purposes. Histology slides were evaluated for the presence of new vital tissue inside the canal space, cementum-like tissue on the internal dentinal wall of the root canal, and apical closure and inflammatory infiltration of the periapical tissue.
The conventional radiographs were placed on a viewing box (× 2 magnification) in a dark room, and micro-CT images were saved to a computer in JPG format. The JPG images were displayed at a screen resolution of 1024 × 768 pixels using a desktop computer running the Windows 8 operating system (Microsoft Corp, Redmond, Washington). Two expert endodontists independently evaluated the images obtained preoperatively on the day of disinfection with sodium hypochlorite and triple antibiotic paste, as well as those obtained postmortem. The images were assessed for radiographic presence (grade 1: Lesions present with unchanged or increased radiolucency) or absence (grade 2: No lesions or a clear reduction in radiolucency) of periapical radiolucency and closure of the root apex. The mean grade was taken as the final grade if there was no agreement. The healing outcomes were compared with those of a regular revascularization protocol without PRF performed under the same conditions.
The data were analyzed using χ2 tests. The significance level was set at P < 0.05 for the radiographic, micro-CT, and histology analyses.
Results
There were no unexpected outcomes following the experimental interventions, including no changes in behaviour or eating patterns, except in 1 animal that had total mesial root resorption (as a result of a pre-existing canal infection) and another that lost 1 crown. Tooth mobility was observed buccolingually, mesiodistally, or axially when force was applied to the experimental teeth after 1 week of infection. Purulent discharge via a sinus tract was noted in all teeth, indicating pulpal necrosis/suppuration (Fig. 1A). There was clear radiolucency at the apices of all teeth, indicating formation of a chronic apical abscess (Fig. 1B); this was also evident histologically (Fig. 1C). Table 1 and 2 show the number of roots and frequency of periapical radiolucency assessed using conventional radiography and micro-CT.
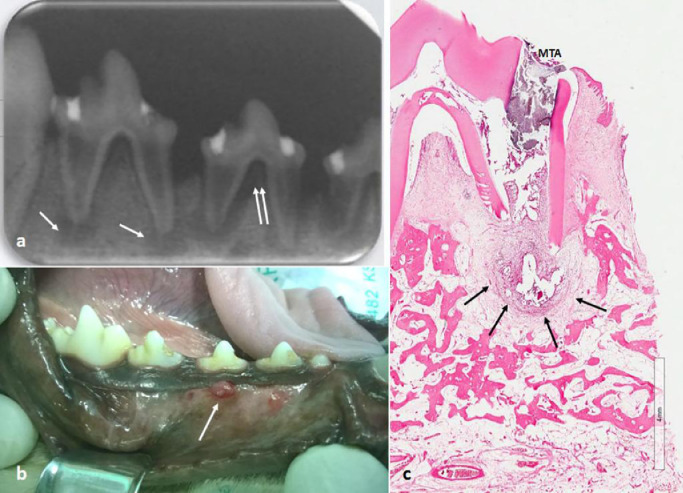
Fig. 1.
(A) Radiograph image showing periapical lucency typical of a chronic periapical abscess (white arrow) and an intense activity of bone resorption, including furcation exposure and alveolar crest lose (double white arrow). (B) Clinical photograph showing a parulis (gumboil) below the mucogingival line that indicates where a fistula from a periapical abscess is draining into the oral cavity. (C) Histologic view of the chronic apical abscess (black arrow).
MTA = mineral trioxide aggregate filling material.
Table 1.
Number of roots with presence or absence of a periapical radiolucency assessed with conventional radiograph.
| Group | Periapical radiolucency* |
Total* | |||
|---|---|---|---|---|---|
| Mesial root |
Distal root |
||||
| Present | Absent | Present | Absent | ||
| Group A: Control | 0 (0.0) | 4 (100) | Not present† | 4 (100) | |
| Group B: Disinfection | 4 (25.0) | 4 (25.0) | 5 (31.25) | 3 (18.75) | 16 (100) |
| Group C: BC | 1 (6.7) | 6 (40.0) | 4 (26.7) | 4 (26.7) | 15 (100) |
| Group D: PRF + BC | 4 (25.0) | 4 (25.0) | 5 (31.25) | 3 (18.75) | 16 (100) |
| Overall (%) | 17.6 | 29.8 | 25.5 | 19.6 | 51 |
BC = blood clot; PRF = platelet-rich fibrin.
Values are presented as n (%).
Group A: Tooth has single root.
Table 2.
Number of roots with presence or absence of a periapical radiolucency assessed with micro-computed tomography.
| Group | Periapical radiolucency* |
Total * | |||
|---|---|---|---|---|---|
| Mesial root |
Distal root |
||||
| Present | Absent | Present | Absent | ||
| Group A: Control | 0 (0.0) | 4 (100) | Not present† | 4 (100) | |
| Group B: Disinfection | 4 (25.0) | 4 (25.0) | 5 (31.25) | 3 (18.75) | 16 (100) |
| Group C: BC | 2 (13.7) | 5 (33.3) | 6 (40.0) | 2 (13.3) | 15 (100) |
| Group D: PRF + BC | 5 (31.25) | 3 (18.75) | 5 (31.25) | 3 (18.75) | 16 (100) |
| Overall (%) | 21.6 | 23.5 | 27.5 | 19.6 | 51 |
BC = blood clot; PRF = platelet-rich fibrin.
Values are presented as n (%).
Group A: Tooth has single root.
Control group
None of the 4 single-rooted teeth were lost and there was no mobility, fracture, or sinus tract. Radiography and micro-CT showed an intact periodontal ligament and lamina dura. Histologic analysis showed normal roots with closed apices, a dentin-pulp complex, and periapical tissue.
Disinfection only group
None of the teeth were lost and there was no mobility, fracture, or development of a sinus tract. The radiographic and micro-CT images showed that half of the teeth had an intact periodontal ligament and lamina dura, as well as closed apices; the other half showed periapical radiolucency. Histologically, half of the teeth showed normal periradicular tissue and the remainder had diseased tissue. Hard (cementum-like) tissue on the internal radicular dentin walls and loose connective tissue was seen in a few cases.
BC scaffold group
One mesial root was lost. There was noticeable mobility in some teeth but no fracture or sinus tract. Radiographically, more than 65% of teeth had an intact periodontal ligament and lamina dura, whereas the others had periapical radiolucency (Fig. 2A). Postmortem micro-CT showed narrowing of the root apical walls and hard tissue deposition in the apical third of the root canal space (Fig. 2B). Closure of the root apex and healing of AP was seen histologically (Figs. 2C and D). Vascularized loose connective tissue was seen inside the root canal space with filling of the internal radicular dentin wall of the root canal by mineralized (cementum-like) tissue. A few specimens contained bone-like tissue.
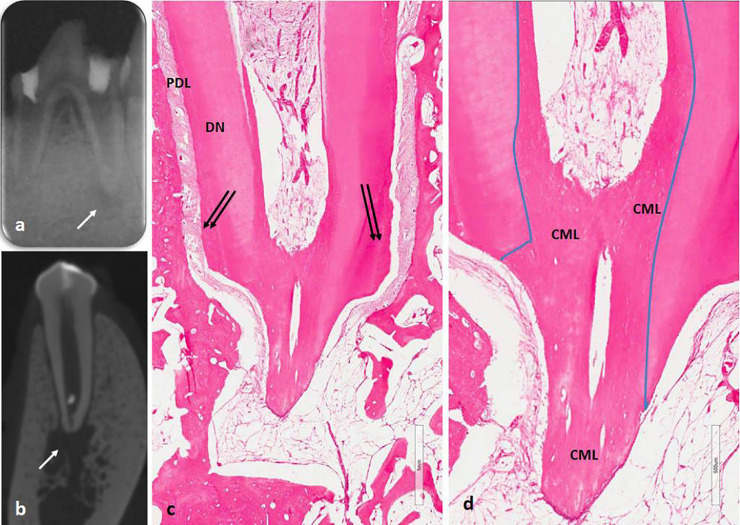
Fig. 2.
(A) Premortem periapical radiographic image and (B) micro-computed tomography postmortem showing apical radiolucency (white arrow) with closed apex. The histologic view (C and D) using hematoxylin-eosin stain shows closure of the root apex and healing of apical periodontitis. Vascularized loose connective tissue seen inside the root canal space and cementum-like (CML) deposition of mineralized tissue filled the apical third of the root canal. Double black arrow shows regular cementum. DN = dentin; PDL = periodontal ligament.
BC + PRF scaffold group
One of the crowns was fractured. Most of the teeth had mobility but none developed a sinus tract. Radiographically, less than half of the teeth had an intact periodontal ligament and lamina dura, whereas the others had periapical radiolucency (Fig. 3A). Postmortem micro-CT showed hard tissue in the canal space (Fig. 3B). There was histologic evidence of hard tissue (cementum-like) deposition on the internal root canal walls and vital dense connective tissue within the canal spaces in some of the samples (Figs. 3C and D). A few samples showed bone-like tissue. Closure of the root apex and healing of AP was seen in a few cases.
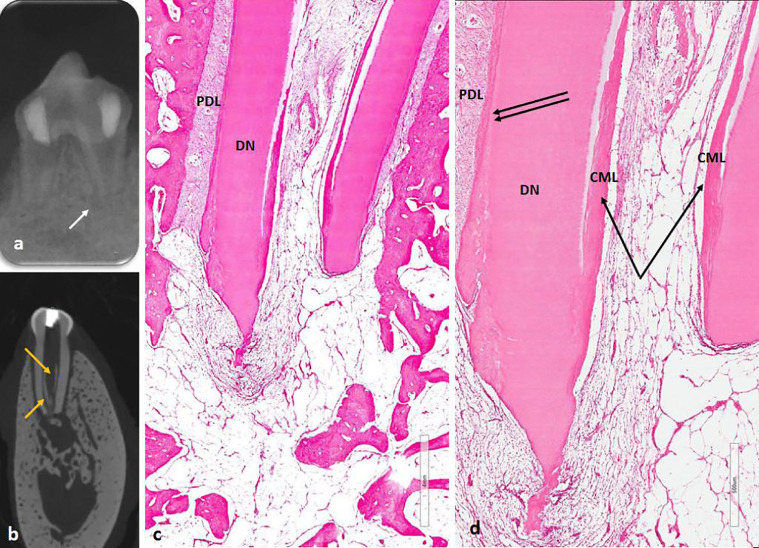
Fig. 3.
(A) Premortem periapical image showed healing of apical periodontitis of the distal root and radiolucency at mesial root (white arrow) and apical root resorption. (B) Micro-computed tomography postmortem showing deposition of radiopaque shadow (orange arrow) at the canal wall. (C) Histological section showed deposition of internal cementum-like (CML) deposition of mineralized tissue (black single arrow) and ingrowth of fibrous connective tissue. Double black arrow shows regular cementin. (D) High magnification of the root apex using hematoxylin-eosin stain. DN=dentin; PDL = periodontal ligament.
After disinfection, 49% of the roots evaluated by conventional radiographs and 43% of those evaluated by micro-CT did not show periapical radiolucency. There was no significant between-group difference in the presence or absence of periapical radiolucency in the mesial roots on conventional images (P = 0.255) but there was a significant difference in the distal roots (P = 0.001); similarly, on micro-CT, there was no significant between-group difference in the mesial roots (P = 0.174) but there was a significant difference in the distal roots (P = 0.001). Histologically, apical closure was significantly not greater in this group than in the BC scaffold group (P = 0.001).
Discussion
Controlling infection is key to successful treatment of a diseased root canal regardless of the method used. A dog model was used because of the similarity between dogs and humans with respect to apical and periapical tissue repair and the high tissue growth rate in dogs.4,13
Enlargement of the infected apical foramen was standardized using a size 70 nickel-titanium GT rotary instrument. Fuzz and Trope 14 found that extensive old perforations have a poor prognosis due to significant tissue damage and suggested that such perforations should be treated in the same manner as immature teeth.
Apical enlargement was performed to achieve an open apex for regenerative endodontic treatment. An apical foramen >1.0 mm in diameter facilitates cell migration and proliferation by inducing bleeding.7 Elimination of the clinical signs and symptoms of AP, and resolution thereof, is the primary goal of the regenerative procedure.15
The disinfection protocol was the same in all our experimental groups. The root canals were thoroughly disinfected with sodium hypochlorite and EDTA, which reportedly promotes successful revascularization of infected teeth with open apices and reimplanted avulsed teeth.16,17 Although EDTA is a weak antimicrobial agent, its use during regenerative endodontic procedures was recommended by the American Association of Endodontists.15 EDTA exposes the collagen fibers of root canal dentin after decalcification, resulting in release of growth factors that promote both odontogenic differentiation of the migrated cells and angiogenesis. Furthermore, the exposed collagen fibers reportedly improve adhesion of newly developing cells.
The canals in the experimental groups were dressed using a triple antibiotic paste, which is widely used in both experimental and clinical regenerative endodontic studies involving immature teeth with infected root canals and AP because of its ability to reach the deep dentin layers and eradicate most bacteria.15
Lin et al18 and Al-Tammami and Al-Nazhan19 reported that debridement disrupted the biofilm and infected dentin, which enhanced attachment of the newly formed tissue to the canal walls, and recommended biomechanical debridement of the infected canal in cases of regenerative endodontic failure. Debridement was not performed in our study.
We used the methods described by Thibodeau et al4 and Stambolsky et al20 in this study. Anticoagulants were not used during preparation of the PRF so that the physiological architecture of the tissue could be maintained for slow polymerization, which causes the release of cytokine molecules during remodeling of the fibrin matrix. Entrapment of these molecules within the fibrin matrix and their subsequent incorporation into the molecular architecture of the fibrin polymer is probably the mechanism underlying the healing effect of PRF.
Conventional radiographs, micro-CT, and histologic analysis were used to evaluate the teeth in this study. However, use of conventional radiographs might lead to underestimation of the lesion size and histological severity. Micro-CT provides images with excellent resolution and allows assessment of the lesion in a multiplanar view, unlike conventional radiography. Micro-CT has not been used before to evaluate regenerative endodontic treatments. According to Balto et al,21 the accuracy of micro-CT for evaluating the status of periapical pathosis is equivalent to that of histologic analysis.
Regenerative endodontics applied to immature permanent teeth with necrotic pulp and apical pathosis has been successful in both human and animal studies. The high success rate is related to the close proximity of the stem cells to the open root apices, allowing their migration into the root canal space and continuation of root development. However, this procedure has been performed only on mature teeth in a few experimental studies and case reports.6,7 A perforation in the apical third of the root is challenging because of the smaller number of apical papilla stem cells in mature teeth. It is believed that stem cells of the periodontal ligament, bone marrow, and surviving dental pulp surrounding the root apex might be involved in pulp regeneration of mature teeth.22
Several therapeutic mechanisms have been proposed for continuation of root growth, including proliferation of remnant vital pulp cells in the apical third of the root canal, which suppress apical inflammation,14 transplantation of apical papilla-derived stem cells into the root canal lumen during apical irritation,23 and proliferation of periodontal ligament cells and formation of a BC containing growth factors.24 Controlling infection in the treated canal is mandatory for a successful outcome.
In this study, the density of tissue ingrowth was identical between the groups. Cementum-like proliferated periodontal tissue was observed along the dentinal root canal walls, as was bone and connective-like tissue. Findings were similar in regenerative endodontic studies of immature 4,25 and mature 26 dog teeth. Thibodeau et al4 suggested that the histology finding of newly deposited hard tissue corresponds to the radiographic findings.
Use of a BC scaffold to promote growth of new tissue in the apical third of the unfilled disinfected root canals of mature teeth with periapical pathosis was first described by Ostby.6 The growth factors present in a BC likely stimulate the undifferentiated precursors of odontoblasts, fibroblasts, and cementoblasts to differentiate and proliferate.27
Use of other scaffolds including PRF,28 PRP,10 and soluble collagen,27 have reportedly resulted in healing of periapical pathosis and continued root development. Both PRF and BC scaffolds were used in our study. PRF constitutes a collection of immune cells and platelets on a fibrin membrane that has been found to promote wound healing and bone growth, maturation, and hemostasis.29 It is frequently used as a resorbable matrix due to its ease of preparation and handling. Moreover, PRF contains blood proteins, including growth factors, that promote wound healing without inflammation 29 in response to direction by the fibrin matrix of migration of stem cells involved in healing.
The PRF used in our study was prepared using Choukroun's technique.30 The advantage of using PRF over PRP in regenerative studies is that PRP requires addition of anticoagulant agents and bovine serum, which limits its clinical use because of the risk of transcontamination. Martin et al10 treated a case with PRP and BC in 1 root and BC only in another root after disinfection using a triple-antibiotic regimen. They found no histologic difference in the tissue formed between the 2 canals. Our findings are similar to those of 2 animal studies 10,25 that described the tissue formed as bone-like, periodontal ligament-like, and cementum-like.
In this study, healing of periapical pathosis with and without apical closure was found radiographically in 67% and 47% of teeth, respectively. Complete or partial apical closure and healed apical pathosis was seen in all experimental groups. These findings are similar to those of Thibodeau et al4 and Zhang et al.13 On radiographs, the healing rate was higher than that reported by Rodríguez-Benitez et al31 and lower than that reported by Wang et al25 using the same animal model. However, both of those studies reported low apical closure rates when evaluated histologically, which is more accurate than conventional radiographs. Healing of periapical pathosis, presence of vascularized tissue, and partial closure of the apices were all noted histologically in our study. These outcomes could reflect the short observation period and the gross damage to the periapical area sustained during the experimental procedure. Furthermore, the forces applied during packing of the PRF might have disrupted the BC by dislodging the apical papilla; a similar observation was reported by Palma et al.32 This might explain the low rate of healing determined on radiographic and micro-CT images in this study. Other reports attributed the low healing rate to persistence of bacteria or extrusion of intracanal medicaments, resulting in irritation of the apical tissue.16 Therefore, caution is needed when performing regenerative endodontics. The findings of this study highlight the importance of histological analysis when evaluating regenerative endodontics and indicate the feasibility of revascularisation of enlarged root apical delta with AP.
This study has some limitations that stem mainly from the small number of teeth included in the study and the short observation time. Further studies are needed to characterize the ingrowth tissue in more detail to understand the mechanisms of pulp revascularization and the long-term outcomes for the newly formed tissues.
Conclusions
The histologic findings of this study support the feasibility of revascularization and root enlargement of necrotic mature teeth with AP. Use of a BC + PRF scaffold in preference to a BC scaffold for regeneration of enlarged dog teeth does not appear to influence formation of mineralized tissue along the root canal walls. Therefore, inclusion of PRF in the BC scaffold is unlikely to be of any clinical benefit when performing endodontic procedures in human patients.
Acknowledgments
Acknowledgments
All authors contributed equally to the literature search, study design, data collection, and data interpretation.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
- 1.Svensäter G., Bergenholtz G. Biofilms in endodontic infections. Endod Topics. 2004;9:27–36. [Google Scholar]
- 2.Al-Nazhan S., Alsulaiman A., Alrasheed F., Alnajjar F., Al-Abdulwahab B., Al-Badah A. Microorganism Penetration in Dentinal Tubules of Instrumented and Retreated Root Canal Walls. SEM In Vitro Study. Restor Dent Endod. 2014;39:258–264. doi: 10.5395/rde.2014.39.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricucci D., Russo J., Rutberg M., Burleson J., Spångberg L. A prospective cohort study of endodontic treatments of 1,369 root canals: results after 5 years. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:825–842. doi: 10.1016/j.tripleo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Thibodeau B., Teixeira F., Yamauchi M., Caplan D.J., Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680–689. doi: 10.1016/j.joen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Laureys W.G., Cuvelier C.A., Dermaut L.R., De Pauw G.A.M. The critical apical diameter to obtain regeneration of the pulp tissue after tooth transplantation, replantation, or regenerative endodontic treatment. J Endod. 2013;39:759–763. doi: 10.1016/j.joen.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Ostby B.N. The role of the blood clot in endodontic therapy: An experimental histologic study. Acta Odontol Scand. 1961;19:324–353. [PubMed] [Google Scholar]
- 7.Paryani K., Kim S.G. Regenerative endodontic treatment of permanent teeth after completion of root development: A report of 2 cases. J Endod. 2013;39:929–934. doi: 10.1016/j.joen.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Saoud T.M., Martin G., Chen Y.H.M., Chen K.L., Chen C.A., Songtrakul K., Malek M., Sigurdsson A., Lin L.M. Treatment of mature permanent teeth with necrotic pulps and apical periodontitis using regenerative endodontic procedures: A case series. J. Endod. 2016;42:57–65. doi: 10.1016/j.joen.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Bucchi C., Gimeno-Sandig A., Manzanares-Céspedes C. Enlargement of the apical foramen of mature teeth by instrumentation and apicoectomy. A study of effectiveness and the formation of dentinal cracks. Acta Odontol Scand. 2017;75:488–495. doi: 10.1080/00016357.2017.1344877. [DOI] [PubMed] [Google Scholar]
- 10.Martin G., Ricucci D., Gibbs J.L., Lin L.M. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013;39:138–144. doi: 10.1016/j.joen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Ray H.L., Jr, Marcelino J., Braga R., Horwat R., Lisien M., Khaliq S. Long-term follow up of revascularization using platelet-rich fibrin. Dent Traumatol. 2016;32:80–84. doi: 10.1111/edt.12189. [DOI] [PubMed] [Google Scholar]
- 12.Murray P.E. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front Bioeng Biotechnol. 2018;6:139. doi: 10.3389/fbioe.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D.D., Chen X., Bao Z.F., Chen M., Ding Z.J., Zhong M. Histologic comparison between platelet-rich plasma and blood clot in regenerative endodontic treatment: An animal study. J Endod. 2014;40:1388–1393. doi: 10.1016/j.joen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Fuss Z., Trope M. Root Perforations: Classification and treatment choices based on prognostic factors. Endod Dent Traumatol. 1996;12:255–264. doi: 10.1111/j.1600-9657.1996.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 15.American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure. Available online: https://www.aae.org/uploadedfiles/publications_and_research/research/currentregenerativeendodonticconsiderations.pdf (accessed on February 2020).
- 16.Banchs F., Trope M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol. J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Cvek M., Cleaton-Jones P., Austin J., Lownie J., Kling M., Fatti P. Effect of topical application of doxycycline on pulp revascularization and periodontal healing in reimplanted monkey incisors. Endod Dent Traumatol. 1990;6:170–176. doi: 10.1111/j.1600-9657.1990.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin L.M., Shimizu E., Gibbs J.L., Loghin S., Ricucci D. Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. J Endod. 2014;40:291–295. doi: 10.1016/j.joen.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Al-Tammami M., Al-Nazhan S. Retreatment of Failed Regenerative Endodontic of Orthodontically treated Immature Permanent Maxillary Central Incisor: a Case Report. Restor Dent Endod. 2017;42:65–71. doi: 10.5395/rde.2017.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stambolsky C., Rodríguez-Benítez S., Gutiérrez-Pérez J., Torres-Lagares D., Martín-González J., Segura-Egea J.J. Histologic characterization of regenerated tissues after pulp revascularization of immature dog teeth with apical periodontitis using tri-antibiotic paste and platelet-rich plasma. Arch Oral Biol. 2016;71:122–128. doi: 10.1016/j.archoralbio.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Balto K., Muller R., Carrington D.C., Dobeck J., Stashenko P. Quantification of periapical bone destruction in mice by micro-computed tomography. J Dent Res. 2000;79:35–40. doi: 10.1177/00220345000790010401. [DOI] [PubMed] [Google Scholar]
- 22.Seo B.M., Miura M., Gronthos S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S., Mankani M., Brahim J. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:625–630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q., Lin X.J., Lin Z.Y., Liu G.X., Shan X.L. Expression of vascular endothelial growth factor in dental pulp of immature and mature permanent teeth in human. Shanghai Kou Qiang Yi Xue. 2007;16:2859. [PubMed] [Google Scholar]
- 25.Wang X., Thibodeau B., Trope M., Lin L.M., Huang G.T. Histologic characterization of regenerated tissues in canal space after the revitalization/ revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56–63. doi: 10.1016/j.joen.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X., Wang Y., Liu Y., Huang G.T., Zhang C. Immunohistochemical and histochemical analysis of newly formed tissues in root canal space transplanted with dental pulp stem cells plus platelet-rich plasma. J Endod. 2014;40:1573–1578. doi: 10.1016/j.joen.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Nagy M.M., Tawfik H.E., Hashem A.A.R., Abu-Seida A.M. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J Endod. 2014;40:192–198. doi: 10.1016/j.joen.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Ruangsawasdi N., Zehnder M., Weber F.E. Fibrin gel improves tissue ingrowth and cell differentiation in human immature premolars implanted in rats. J Endod. 2014;40:246–250. doi: 10.1016/j.joen.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate, Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Choukroun J., Adda F., Schoeffler C., Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 31.Rodríguez-Benítez S., Stambolsky C., Gutierrez Perez, Torres-Lagares D., Segura-Egea J.J. Pulp Revascularization of Immature Dog Teeth with Apical Periodontitis Using Triantibiotic Paste and Platelet-rich Plasma: A Radiographic Study. J Endod. 2015;41:1299–1304. doi: 10.1016/j.joen.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Palma P.J., Ramos J.C., Martins J.B., Diogenes A., Figueiredo M.H., Ferreira P., Viegas C., Santos J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J Endod. 2017;43:1279–1287. doi: 10.1016/j.joen.2017.03.005. [DOI] [PubMed] [Google Scholar]