Abstract
Individuals with Neurofibromatosis I may develop plexiform neurofibromas throughout the body, however they are rarely seen in the pelvis. We present a 15-year-old patient with NF1 with a large unresectable neurofibroma located between the prostate and bladder discovered incidentally on renal/bladder ultrasound during the evaluation of bowel/bladder dysfunction. Despite the extensive nature of the mass, the patient presented with minimal symptoms and the lesion was thought to be stool or enlarged prostate on subsequent evaluations. Urologists should be aware of NF1 related pelvic masses in the evaluation of bowel bladder dysfunction and specify NF1 on radiologic requisitions.
Keywords: Neurofibromatosis, NF1, Pediatric, Pelvic
Introduction
Neurofibromatosis Type I is an autosomal dominant disorder wherein the genetic mutation results in an increased risk of developing nerve sheath tumors which may undergo malignant transformation. Although patients with NF1 may have masses throughout multiple body systems, pediatric pelvic tumors are uncommon. In a study of 368 pediatric peripheral nerve tumors in NF1 patients, 6% were found in either the pelvis or abdomen with 33% of these patients needing surgical intervention.1 In a literature review of 79 cases of neurofibromas with genitourinary involvement, the most common presentations were irritative lower urinary tract symptoms and a newly discovered abdominal mass but, many are asymptomatic.2
Case description
A 15-year-old male with a history of neurofibromatosis I was referred to the pediatric urology clinic for further evaluation of dark and strong-smelling urine and microscopic hematuria. An outside ultrasound from a year prior showed left hydronephrosis, posterior bladder mass versus rectal stool, and diffuse serosal nodularity along the anterior bladder wall. His review of systems included holding his urine for prolonged periods of time, poor fluid intake and irregular bowel movements with straining. He denied urinary incontinence, bed wetting, nocturia, dysuria or frequency. Pertinent surgical history includes right eyelid neurofibroma excision. Pertinent medical history includes one previous urinary tract infection. Physical examination including genital examination was normal. A rectal examination was deferred.
A behavioral therapy regimen including timed voiding every 2–3 hours, hydration, high-fiber diet, and polyethylene glycol was instituted. Urinalysis demonstrated 50 red blood cells per high power field. A follow-up renal/bladder ultrasound demonstrated bilateral pelvicaliectasis with no ureteral dilation and normal bladder. Abdominal radiograph displayed moderate amount of non-obstructed stool. A uroflow/EMG demonstrated a bell-shaped flow curve with Qmax of 38.9 ml/sec, average flow rate of 17.3 ml/sec, and mild-to-moderate pelvic floor muscle activity throughout voiding. Volume voided was 192.6 ml with post-void residual of 3 ml. Behavioral therapy regimen was continued. A follow-up renal/bladder ultrasound obtained 5 months later demonstrated a heterogeneous 170 ml pelvic mass, initially thought to be a prostatic mass (Fig. 1). The bladder volume was 710 ml and post-void residual was 100 ml. The bladder wall was not thickened. Mild-to-moderate left pelvicaliectasis was noted.
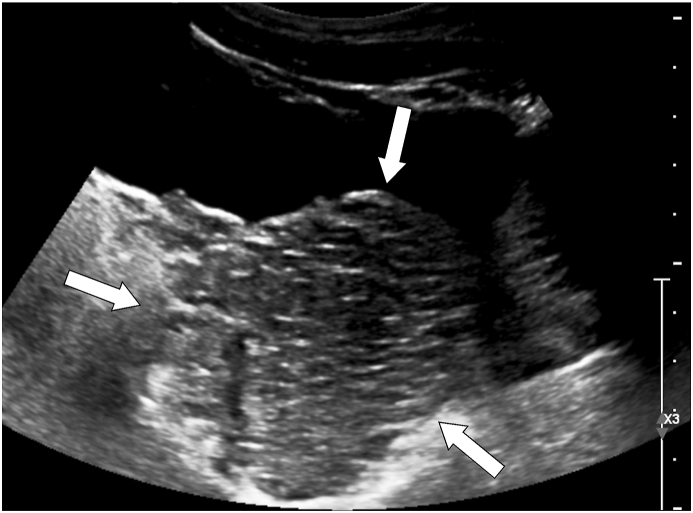
Fig. 1.
Longitudinal ultrasound image of the pelvis shows a large, predominantly hypoechoic mass (arrows) posterior to the bladder initially thought to represent prostatic enlargement, although other space occupying lesions could not be excluded, which prompted the subsequent pelvic MRI.
Subsequent MRI demonstrated a large focal T2 hyperintense, multicystic mass in the pelvis with its epicenter between the urinary bladder and prostate. The central component of the mass measured approximately 8.4 × 6.7 X 9.2 however, the mass extended anteriorly around the urinary bladder, posteriorly into the rectal space, and inferiorly to the prostatic and membranous urethra (Fig. 2A/2B). Another mass was discovered in the left groin. The characteristics of the masses were consistent with plexiform neurofibromas without suggestion of malignant transformation.
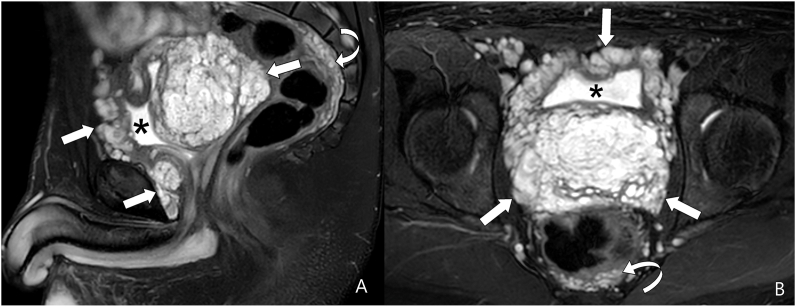
Fig. 2.
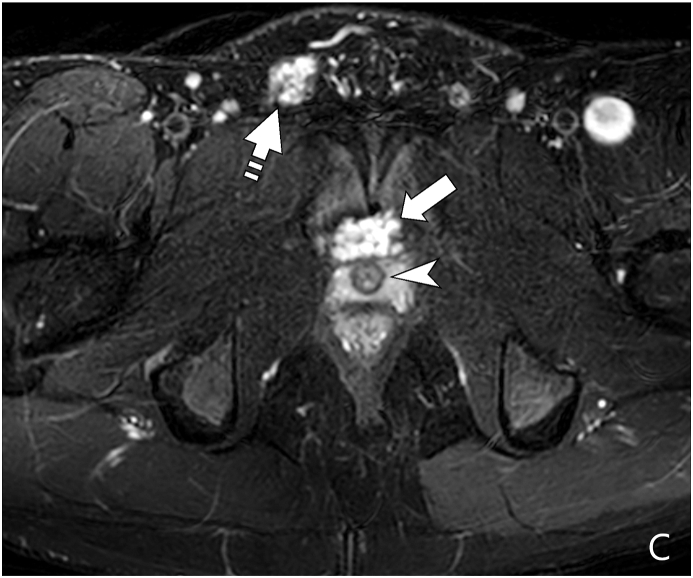
Sagittal (A) and axial (B) T2-weighted fat-suppressed MR images show a large, heterogeneous, predominantly hyperintense pelvic plexiform neurofibroma (arrows) displacing the bladder (asterisk) anteriorly and superiorly. The bladder is encircled by the lesion. A portion of the lesion is also seen posterior to the rectum (curved arrow). Figure 2C: Axial T2-weighted fat suppressed MR image at a lower level than B shows a portion of the plexiform neurofibroma (arrow), which is anterior to the urethra (arrowhead in C). In addition, the lesion also extends along the right spermatic cord (dash arrow in C). Plexiform neurofibroma is also seen posterior to the rectum (curved arrow). The mass shows multiple rounded areas of central low and peripheral high signal intensity. This finding is referred to as the “target sign” and is highly suggestive of plexiform neurofibroma.
Cystourethroscopy demonstrated a mass effect on the posterior aspect of the bladder wall. There was no evidence of intramural or mucosal involvement of the lesion. Clear urine was noted to efflux from each ureteral orifice. A percutaneous biopsy of the pelvic mass was not obtained at the discretion of hematology-oncology and radiology. Given tumor extent, hematology/oncology began Koselugo (selumetinib) with no change after one month.
Discussion
We present a case of a patient with Neurofibromatosis type I referred for microscopic hematuria, bowel/bladder dysfunction with a large pelvic mass initially thought to be stool or prostatic mass. The limited impact of the tumor on lower urinary tract symptoms is unusual given the extent of the tumor which wrapped around and pushed into the bladder.
Bowel bladder dysfunction is commonly seen in the pediatric population representing close to 40% of pediatric urology consults. First line treatment includes treatment of underlying cause and behavioral therapy. Urodynamics may be indicated for refractory cases.
Ultrasound and abdominal radiograph are often obtained in the evaluation of bowel/bladder dysfunction. In this patient, the absence of known history of neurofibromatosis and a clinical diagnosis of bowel/bladder dysfunction and pelvicaliectasis on the ultrasound requisition, the pelvic mass was not initially identified. On subsequent ultrasound, with significant enlargement of the mass, it was initially thought to be prostatic enlargement. Given this finding and recognition of underlying neurofibromatosis, MRI was obtained.
MRI is the imaging study of choice if there is a suspicion of abdominopelvic involvement of neurofibromatosis due to the ability to discriminate characteristic findings and between benign and malignant lesions in comparison to CT or US, thus it may obviate the need for biopsy. On T2-weighted MR imaging, the neurofibromas are hyperintense with a characteristic “target sign”, consistent of a hyperintense rim with a central area of low signal (Fig. 2C). On T1-weighted MR imaging, the center has a higher signal intensity than the border.3 If inconclusive or if there is concern for malignancy, biopsy is recommended. Rhabdomyosarcoma should be included on the differential in NF1 patients with pelvic masses.
In general, NF1 patients undergo annual surveillance for complications and tumors through history and physical exam as well as regular ophthalmologic examinations to monitor for optic gliomas. A whole body MRI is recommended to assess for internal tumors between the ages of 16–20.4 A multidisciplinary team including hematology/oncology, radiology, and other surgical/medical specialties is recommended for patients with NF1.
Although surgical intervention is the treatment of choice, unresectable tumors require medical management. Previously, limited options were available for management of unresectable masses. However, surgical intervention for palliation may be indicated. Radiation therapy is not recommended due to the risk of inducing malignancy in NF1 patients. Several drugs have been studied in treatment of neurofibromatosis, including trametinib and sirolimus. Koselugo, a MEK inhibitor, has been FDA-approved for symptomatic, inoperable plexiform neurofibromas. In a trial that enrolled pediatric patients, all treated children had a partial response and 82% of responders had sustained responses lasting at least 12 months.5 Although Koselugo is not curative, these partial and sustained responses may represent a decrease in morbidity from the invasion and mass effect of the surrounding structures.
In the evaluation of children with NF1 presenting with bowel/bladder dysfunction, urologists should have an awareness of the possibility of pelvic neurofibromas and ensure that NF1 is included on radiologic requisition.
Funding statement
Financially supporting body of publication is Nemours Children's Hospital and the University of Central Florida College of Medicine.
Declaration of competing interest
Authors declare no conflicts of interest.
Contributor Information
Ricci Allen, Email: ricciallen@knights.ucf.edu.
Monica Epelman, Email: monica.epelman@nemours.org.
Omar Cruz-Diaz, Email: omar.cruz-diaz@nemours.org.
Pamela Ellsworth, Email: pamella.ellsworth@nemours.org.
References
- 1.Prada C.E., Rangwala F.A., Martin L.J. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160(3):461–467. doi: 10.1016/j.jpeds.2011.08.051. 2012/03/01/ [DOI] [PubMed] [Google Scholar]
- 2.Gao B., DeCotiis K., Bobrowski A., Koyle M., O'Kelly F. The association of Neurofibromatosis Type 1 and lower urinary tract dysfunction in the paediatric population - a critical review of literature. J Pediatr Urol. 2020 Jun;16(3):357–365. doi: 10.1016/j.jpurol.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson L.M., Manson D., Smith C.R. Best cases from the AFIP. Radiographics. 2004;24(suppl_1):S237–S242. doi: 10.1148/rg.24si035170. 2004/10/01. [DOI] [PubMed] [Google Scholar]
- 4.Evans D.G.R., Salvador H., Chang V.Y. Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Canc Res. 2017 Jun 15;23(12):e46–e53. doi: 10.1158/1078-0432.CCR-17-0589. [DOI] [PubMed] [Google Scholar]
- 5.Gross A.M., Wolters P.L., Dombi E. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020 Apr 9;382(15):1430–1442. doi: 10.1056/NEJMoa1912735. [DOI] [PMC free article] [PubMed] [Google Scholar]