Highlights
-
•
NOX66 contains idronoxil, formulated as a rectal suppository.
-
•
CEP-1 is the first study to assess NOX66 in patients with refractory solid tumors.
-
•
NOX66 was well tolerated at 400/800 mg as monotherapy and combined with carboplatin.
-
•
The safety profile justifies continuation of the NOX66 clinical research program.
-
•
Early results suggest most patients had stable disease by study end
Key words: NOX66, cytotoxic chemotherapy, drug resistance, idronoxil, immunomodulation, oncology
Abstract
Background
Although oral and intravenous forms of idronoxil have been well tolerated, the safety of NOX66, with idronoxil formulated as a rectal suppository, is not known. This Phase Ia/b clinical study (protocol No. NOX66-001A), known as Chemotherapy Enhancement Program-1, is the first to assess NOX66 in patients with refractory solid tumors.
Objective
The study aimed to determine the safety profile of NOX66 both as a monotherapy and in combination with carboplatin, and to evaluate whether or not NOX66 has a meaningful anticancer effect when combined with carboplatin in this patient population.
Methods
Chemotherapy Enhancement Program-1 was a multicenter, open-label, nonrandomized, 2-dose cohort study of NOX66 as monotherapy (Phase Ia) and in combination with carboplatin (Phase Ib). Patients with refractory solid tumors who had stopped responding to standard treatments were eligible to participate. Twenty patients were screened and 19 enrolled in the study. They were divided into 2 groups: cohort 1 (n = 8) received 1 suppository daily (400 mg) and cohort 2 (n = 11) received 2 suppositories daily (800 mg) for 14 consecutive days followed by 7 days of rest. Patients who completed Phase Ia without significant toxicity continued to Phase Ib, where NOX66 was combined with carboplatin for up to 6x 28-day treatment cycles, with low-dose carboplatin (600 mg) for cycles 1B through 3B and standard dose carboplatin (900 mg) for cycles 4B through 6B. The main outcomes assessed were safety (n = 18) and efficacy signals (n = 14).
Results
NOX66 generally was well tolerated at 400 mg and 800 mg, both as monotherapy and in combination with carboplatin in patients with refractory solid tumors. The safety profile was consistent for oncology patients, with 77.8% experiencing at least 1 treatment-emergent adverse event. The most common adverse events were blood and lymphatic system disorders (44.4%), with only anemia considered as possibly related to NOX66. Although the study was primarily designed to assess safety and tolerability, the efficacy measurements demonstrated that most patients had stable disease or better by study end.
Conclusions
The favorable safety profile of NOX66 provides reassurance to justify continuation of clinical research. The efficacy findings are encouraging in terms of the chemosensitizing potential of NOX66 in refractory solid tumors. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
Introduction
For most cancers, chemotherapy remains a standard frontline therapy. However, cancer cells can become resistant to chemotherapy and this remains among the biggest challenges facing cancer management.1 The development of drugs that restore the sensitivity of cancer cells to standard chemotherapies could offer end-stage cancer patients a viable treatment option. The main biochemical mechanisms within cancer cells responsible for treatment resistance are phosphoinositide 3-kinase and protein kinase B signaling pathways.2 However, an increasing number of studies are also directly pointing at the overexpression of sphingosine-1-phosphate (S1P) as a defense mechanism used by cancer cells to evade the immune response.3
Within the tumor microenvironment, both cancer and noncancer cells secrete S1P to recruit circulating monocytes that can differentiate into macrophages.4 S1P also increases macrophage survival, binds to S1P receptor 1 to attract additional macrophages, and stimulates tumor-associated macrophage/M2 polarization leading to the secretion of both anti-inflammatory cytokines that help the tumor evade the immune system as well as proteins that support migration and angiogenesis.4
The other S1P receptor of interest in oncology is S1P receptor 4, which is involved in immunomodulation and tumor growth. Binding of S1P to this receptor decreases interferon production, suppresses cluster of differentiation 8-positive (CD8+) T cells, produces tumor-promoting cytokines, enhances neutrophil trafficking and enriches dendritic cells in lymph nodes.5 Studies have shown that depleting S1P receptor 4 restores antitumor immunity by increasing CD8+ T cell abundance thereby enhancing the response to chemotherapy.6
Idronoxil is the first drug that selectively inhibits both the phosphoinositide 3-kinase/protein kinase B axis and S1P production in tumors by blocking the catalytic activity of ecto-NADH oxidase disulfide-thiol exchanger Type 2 (ENOX2), which is highly expressed by cancer cells.7 Because idronoxil downregulates the anti-apoptotic S1P signaling molecule upregulated in some tumors,8, 9, 10, 11 it has the capacity to make tumor cells susceptible to CD8+ T cell trafficking and immune-mediated infiltration of the tumor.12,13 In this way, idronoxil provides the capacity to overcome some of the resistance mechanisms used by tumors to evade the immune system. In contrast, idronoxil is only mildly toxic to noncancerous cells.14
In preclinical studies, idronoxil has been shown to sensitize a wide variety of human cancer phenotypes to standard chemotherapies, including cisplatin, carboplatin, doxorubicin, and gemcitabine.15, 16, 17, 18, 19, 20, 21, 22, 23, 24 The high degree of chemosensitization in cancer cells suggested that idronoxil, in addition to restoring chemosensitivity, might allow the dosage of a chemotherapeutic to be lowered to levels that are likely to be better tolerated.7
Idronoxil has been studied in clinical trials as a chemosensitizer of both carboplatin and paclitaxel in various solid cancers, but the anticancer effect was found to be variable.2,7,25 Encouraging clinical signals in a series of Phase II trials in ovarian cancer19 led to a multinational Phase III study in platinum-refractory ovarian cancer, which was abandoned in 2009 due to recruitment difficulties and a futility assessment.7,26 The variable anticancer effect of idronoxil is likely due to a mechanism of drug resistance involving inactivation by attachment of glucuronic acid during Phase II metabolism.27 NOX66 has been developed to protect idronoxil from Phase II metabolism by administering rectally, thus bypassing first pass metabolism in the liver.7
Although clinical trials of oral and intravenous forms of idronoxil have been shown to be well tolerated, the safety of NOX66 is not yet known. This Phase Ia/b study known as the Chemotherapy Enhancement Program-125 is the first clinical study to assess the safety of idronoxil formulated as a rectal suppository (ie, NOX66), in patients with chemo-refractory solid tumors. The objectives of this study were to determine the safety profile of NOX66, both as a single agent and in combination with carboplatin, and determine whether or not NOX66 is able to produce a meaningful anticancer effect when combined with carboplatin.
Materials and Methods
Study design and participants
Chemotherapy Enhancement Program-1 was a multicenter study with 4 study centers in the country of Georgia. The study design was an open-label, nonrandomized, 2-dose cohort study of NOX66 as monotherapy (Phase Ia) and in combination with carboplatin (Phase Ib), in patients with refractory solid tumors that had stopped responding to standard treatment options. The study began March 3, 2017, and was completed on May 11, 2018.
Eligible participants were patients with solid tumors (ie, prostate, breast, ovarian, lung, or head and neck) who had no standard therapeutic alternatives available; histologically confirmed locally or metastatic advanced solid tumors; at least 1 measurable lesion via computed tomography or magnetic resonance imaging scan; Eastern Cooperative Oncology Group performance status 0 or 1; adequate hematologic, hepatic, and renal function (ie, absolute neutrophil count >1.5 × 109/L; platelet count >100 × 109/L; hemoglobin >9.0 g/dL; serum bilirubin <1.5 times upper limit of normal [× ULN]; aspartate aminotransferase/alanine aminotransferase <2.5 × ULN for the reference laboratory or <5 × ULN in the presence of liver metastases; serum creatinine <1.5 × ULN); and life expectancy of 12 weeks or more. Fertile patients had to agree to the use of effective contraception during the study and for 90 days after the last dose of NOX66.
Patients were excluded in the case that they had tumors involving the central nervous system; clinically significant uncontrolled cardiac disease or myocardial infarction within the past 12 months; QTc >470 msec on their screening electrocardiogram; uncontrolled infection or systemic disease; any major surgery, radiotherapy, immunotherapy within the past 21 days (palliative radiation >2 weeks permitted); not been allowed concurrent systemic chemotherapy or biologic therapy; chemotherapy with delayed toxicity within the past 4 weeks; a history of solid organ transplant; known to be unsuitable for treatment with carboplatin or suppository; or breastfeeding or pregnant.
Withdrawal criteria, other than progressive disease, were defined as 1 or more of the following: an intercurrent illness that prevented further administration of NOX66, a dose-limiting toxicity (DLT) defined as an adverse event (AE) related to NOX66 that was intolerable, the patient withdrew consent, the patient died, and general or specific changes in the patient's condition that rendered the patient unacceptable for further treatment. In all cases, the reason for withdrawal was recorded and the patient was followed to establish whether the reason was an AE causally related to NOX66. The relatedness of an AE to either NOX66 or carboplatin was determined by judgment of the principal investigator.
Patients were considered evaluable if they completed at least 1 cycle of treatment and underwent at least 1 follow-up tumor evaluation. Patients who could not be evaluated as part of the efficacy analysis were replaced in the study, up to a total of 16 evaluable patients. Replacement of patients occurred at the end of all safety cohort assessments, and patients were enrolled at the highest tolerated dose level. Recruitment to cohort 2 commenced once all cohort 1 patients had completed 1x 21-day treatment cycle and at least 6 of those patients did not experience any toxicity greater than grade 2.
This study was conducted in accordance with the International Council for Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki and its revisions. Informed consent was obtained for each patient, and the study protocol was approved by the local Ethics Committee per site and then by the Georgian Ministry of Health.
Treatment
NOX66 consists of idronoxil formulated in a fatty base and surfactant. Patients were divided into 2 dose cohorts to receive NOX66 treatment. The 2 daily doses chosen in this study (400 mg and 800 mg) were based on doses used in the Ovarian Tumor Response (OVATURE) trial, in which 1200 mg oral idronoxil was used in combination with carboplatin.26 Given the anticipated higher bioavailability of NOX66 compared with oral idronoxil, a conservative dosing approach was taken. Patients were instructed in the drug administration procedure. NOX66 was self-administered as a rectal suppository, with each suppository containing 400 mg idronoxil.
There was no dose modification of NOX66 on the basis of body weight except where the patient exceeded 100 kg body weight, in which case the dose of NOX66 could be increased (up to double) at the discretion of the investigator. If a DLT would have occurred, it would have required half doses: cohort 1 patients to receive 1 suppository every second day and cohort 2 patients to receive 1 suppository daily. A DLT was defined when any 1 of the following occurred during the first treatment cycle:
-
•
National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) grade 3 or more neutropenia lasting 5 days or longer, grade 3 febrile neutropenia (fever ≥38.5°C), grade 4 thrombocytopenia, or grade 3 thrombocytopenia associated with bleeding;
-
•
NCI-CTCAE grade 3 or more abnormal laboratory values (except neutropenia and thrombocytopenia) assessed as clinically significant and causally associated with NOX66;
-
•
NCI-CTCAE grade 3 or more nonlaboratory toxicity assessed as causally associated with NOX66 (excluding alopecia, rash, nausea, diarrhea, and vomiting if controlled with standard supportive therapy).
Once any DLT was reported, at least 2 more patients were to be enrolled at the same dose level. Escalation continued only if a DLT was limited to 1 of 4 patients. If a DLT occurred in 2 or more patients, further dose escalation ceased and the maximum tolerable dosage was the next lower level.
Phase Ia: NOX66 as monotherapy
During the Phase Ia monotherapy study, cohort 1 received 1 suppository (400 mg) daily, whereas cohort 2 received 2 suppositories (800 mg) daily, for 14 consecutive days followed by 7 days of rest comprising a 21-day treatment cycle (Figure 1).
Figure 1.
Chemotherapy Enhancement Program-1 study design. Each patient received the following treatments consecutively: NOX66 monotherapy for 14 days with 7-day rest period (Phase Ia); NOX66 + low dose (AUC4) carboplatin 3 × 28-day cycles (NOX66 days 1–7, chemotherapy day 2); NOX66 + standard dose (AUC6) carboplatin for 3 × 28-day cycles (NOX66 days 1–7, chemotherapy day 2). AUC = area under the curve.
Phase Ib: NOX66 in combination with carboplatin
All patients who completed the NOX66 monotherapy without significant toxicity were eligible for the Phase Ib study, where NOX66 was administered in combination with carboplatin for up to 6x 28-day treatment cycles at dosages considered appropriate by the study investigators.
Cohorts 1 and 2 continued on their assigned NOX66 doses (400 and 800 mg, respectively) during this combination therapy phase (Figure 1). NOX66 was administered rectally for the first 7 days of each treatment cycle. Carboplatin was given intravenously on day 2 of each treatment cycle at 600 mg (area under the curve = 4) (low dose) for cycles 1B through 3B and 900 mg (area under the curve = 6) (standard dose) for cycles 4B through 6B, with maximum doses of 600 mg and 900 mg, respectively. Any DLT was managed by dose reduction or by withholding NOX66 and or carboplatin.
Safety outcomes
Safety outcomes were assessed by routine laboratory tests (eg, hematology, serum chemistry, and urinalysis), physical examinations, electrocardiogram analyses, vital signs, and Eastern Cooperative Oncology Group performance status. AEs were monitored and assessed using the NCI-CTCAE version 4.03 scoring system.
The Safety Steering Committee (SSC) held 2 formal safety review meetings during the study (Figure 1); the first following completion of the 21-day monotherapy treatment cycle of patient 4 to allow continued enrolment of dose cohort 1, and the second following completion of the 21-day monotherapy treatment cycle of patient 8 to allow dose escalation to cohort 2.
If any AE of grade 2 or greater toxicity per NCI-CTCAE version 4.03 occurred at any time during the study, an SSC review could be requested. Furthermore, the Study Medical Monitor reviewed all AEs on an ongoing basis and could call for an SSC review at any time. If a patient experienced an AE assessed grade 3 or greater as causally associated with NOX66 during the NOX66 monotherapy arm (Phase Ia), the patient would be withdrawn from the study. If a patient experienced grade 3 or greater AE assessed as causally associated with NOX66 during the combination therapy arm (Phase Ib), the patient could continue on NOX66 treatment at his or her assigned dose, provided the AE resolved to grade 2 or lower by the time of commencement of the next treatment cycle. A treatment break of more than 2 weeks due to an unresolved AE required discontinuation of study treatment.
Efficacy outcomes
Tumor measurements were assessed by radiologic methods (computed tomography/magnetic resonance imaging) at baseline and at subsequent intervals at the discretion of the investigator but no more than every 12 weeks. Efficacy variables included overall response, progression-free survival, and overall survival. Response and progression were to be assessed according to Response Evaluation Criteria in Solid Tumors criteria version 1.1.28 The overall response disease status was derived from both the target lesions response and nontarget lesions response, and also accounted for new lesions per Response Evaluation Criteria in Solid Tumors criteria version 1.1 criteria.
Statistical analysis
For the monotherapy and combination therapy arms of the study a total of 16 (evaluable) patients were planned to be recruited in 2 cohorts of 8. Depending on the occurrence of DLTs, the maximum number planned was 22 patients. In both parts of the study, the number of patients was chosen with the aim of obtaining adequate safety and tolerability.
Study objectives were addressed in the context of an open-label, 2-dose cohort study; therefore, statistical hypothesis testing was not performed, and analyses were primarily descriptive in nature. Continuous data were summarized by descriptive statistics, including sample size, mean, SD, median, and range. Categorical data were summarized by the number and percentage of patients. For the time-to-event end points, Kaplan-Meier curves were plotted and compared by log-rank analysis.
All patients who were enrolled in the study with the exception of 2 screened patients who did not receive study drug were included in the evaluation of safety per the statistical analysis plan.
Results
Patient disposition
Twenty patients were screened, of whom 8 were assigned to cohort 1 and 11 to cohort 2 (Figure 2). In Cohort 1, all 8 patients were included in the safety analysis. In cohort 2, 10 of 11 patients were included because 1 patient withdrew consent before the first NOX66 monotherapy dose. This resulted in a total of 18 patients for the safety population. For the efficacy analysis, only 5 patients in cohort 1 and 9 in cohort 2 were included. One patient in cohort 1 withdrew from combination therapy giving “other” as the reason and 2 patients (both from cohort 1) were nonevaluable. One patient from cohort 2 died. This resulted in a total of 14 patients for the efficacy population.
Figure 2.
Patient disposition in the Chemotherapy Enhancement Program-1 study.
All patients in both dose cohorts completed the monotherapy phase. Three patients were found to be nonevaluable at the completion of the monotherapy phase and were replaced by 3 patients who entered the combination therapy phase directly per protocol.
Patient characteristics
Patients in the 2 dose cohorts were similar with regard to age, height, weight, and gender. All patients were White. Overall, the most common location of cancer was breast (33.3%), followed by lung (27.8%), ovary (16.7%), and prostate (16.7%), distributed equally between the 2 cohorts (Table 1). All patients but one had previously received chemotherapy (Table 2). Previous treatment with hormone therapy or surgery was also common.
Table 1.
Baseline characteristics of patients enrolled in the Chemotherapy Enhancement Program-1 study.
| Characteristic | Cohort 1: NOX66 400 mg (n = 8) | Cohort 2: NOX66 800 mg (n = 10) |
|---|---|---|
| Median age, y | 61 | 64 |
| Median weight, kg | 79.3 | 75.2 |
| Female sex* | 5 (62.5) | 6 (60.0) |
| White* | 8 (100.0) | 10 (100.0) |
| Type of cancer* | ||
| Prostate | 1 (12.5) | 2 (20.0) |
| Ovarian | 1 (12.5) | 2 (20.0) |
| Lung | 3 (37.5) | 2 (20.0) |
| Breast | 3 (37.5) | 4 (40.0) |
| Disease state* | ||
| Metastatic | 8 (100.0) | 9 (90.0) |
| Locally advanced | 0 (0.0) | 1 (10.0) |
Values are presented as n (%).
Table 2.
Prior oncology treatments of patients enrolled in the Chemotherapy Enhancement Program-1 study.*
| Prior cancer treatment† | Cohort 1: 400 mg daily (n = 8) | Cohort 2: 800 mg daily (n = 10) | Overall (n = 18) |
|---|---|---|---|
| Chemotherapy | 7 (87.5) | 10 (100.0) | 17 (94.4) |
| Hormone therapy | 3 (37.5) | 5 (50.0) | 8 (44.4) |
| Radiotherapy | 0 (0.0) | 2 (20.0) | 2 (11.1) |
| Surgery | 3 (37.5) | 5 (50.0) | 8 (44.4) |
| Other | 1 (12.5) | 1 (10.0) | 2 (11.1) |
Values are presented as n (%).
Patients could have had multiple forms of treatment and multiple cycles/rounds of the same treatment type.
Safety outcomes
The safety analysis found that both doses, 400 mg and 800 mg NOX66, were generally well tolerated both as monotherapy and in combination with carboplatin (Table 3). Overall, 77.8% of patients experienced at least 1 treatment-emergent AE. The most common AEs were blood and lymphatic system disorders (44.4%) followed by gastrointestinal disorders (16.7%); metabolism and nutrition disorders (16.7%); and respiratory, thoracic, and mediastinal disorders (16.7%).
Table 3.
Treatment-emergent adverse events by Medical Dictionary for Regulatory Activities (MedDRA) preferred term within each dose cohort and in the overall safety population.*
| MedDRA | Cohort 1: 400 mg daily (n = 8) | Cohort 2: 800 mg daily (n = 10) | Overall (n = 18) |
|---|---|---|---|
| Any | 7 (87.5) | 7 (70.0) | 14 (77.8) |
| Anemia | 1 (12.5) | 3 (30.0) | 4 (22.2) |
| Iron deficiency anemia | 1 (12.5) | 1 (10.0) | 2 (11.1) |
| Neutropenia | 1 (12.5) | 2 (20.0) | 3 (16.7) |
| Pericarditis | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Abdominal pain upper | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Diarrhea | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Flatulence | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Gastrointestinal hemorrhage | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Nausea | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Asthenia | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Sudden death | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Infusion-related reaction | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Platelet count decreased | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Weight decreased | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| White blood cell count increased | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Hypoalbuminemia | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Hypocalcemia | 1 (12.5) | 2 (20.0) | 3 (16.7) |
| Back pain | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Altered state of consciousness | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Coma | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Dizziness | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Neuropathy peripheral | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Hydrothorax | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Pulmonary embolism | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Pulmonary fibrosis | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Embolism arterial | 1 (12.5) | 0 (0.0) | 1 (5.6) |
Values are presented as n (%).
Treatment-emergent AEs were equally distributed between the 2 dose cohorts (Table 3). Of these, only anemia (4 patients), neutropenia (3 patients), and hypocalcemia (3 patients) were recorded in more than 1 patient. Only 1 case was considered possibly related to NOX66. Approximately half of the patients reported AEs that were considered related to carboplatin.
Overall, 5 patients (62.5%) from the 400 mg daily cohort withdrew, 1 due to death, 2 due to disease progression, and 2 for other reasons. Four patients (40.0%) from the 800 mg daily cohort withdrew: 2 due to death, 1 due to an AE, and 1 withdrew consent.
There were 4 patients (22%) during the combination therapy phase who had serious AEs leading to premature withdrawal from the study. Three of the 4 patients who reported serious AEs were from cohort 2. With the exception of an infusion-related reaction at the injection site, which was certainly due to carboplatin, the other 3 serious AEs resulted in death (1 in cohort 1 and 2 in cohort 2).
None of the 3 deaths were considered by the principal investigator to be related to NOX66. The 2 deaths from cohort 2 were due to gastrointestinal hemorrhage and progressive disease, respectively, and causality was deemed unlikely/unrelated to either NOX66 or carboplatin, whereas the sudden death from cohort 1 was deemed unlikely/unrelated to NOX66 and possibly related to carboplatin, in the absence of an autopsy.
Efficacy
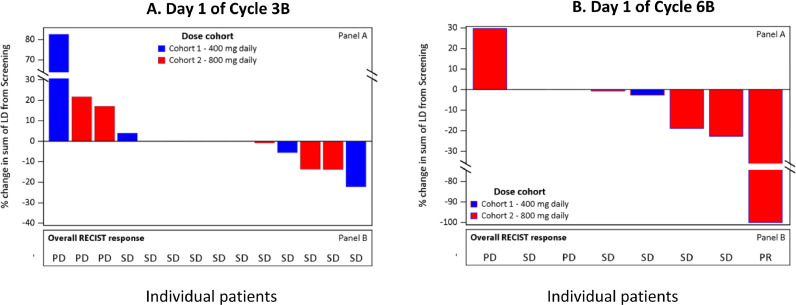
This study was not powered to measure efficacy; however, comparison of the sum of the target lesion diameters demonstrated that more patients had reductions from cycle 3B day 1 to cycle 6B day 1 than had increases (Figures 3 and 4). The majority of patients had stable disease (no tumor growth and no new tumors) throughout the treatment course and by cycle 6B, 83.3% (5 out of 6) of patients treated with NOX66 had stable disease or a partial response (Table 4). Based on progression-free and overall survival, there does not appear to be any difference between the 2 NOX66 dose cohorts (P = 0.6641).
Figure 3.
Change in target lesion diameter and overall response for the efficacy population at (A) cycle 3B and (B) cycle 6B. In cycle 3, patients 0206, 0401, 0404, 0405, and 0407 had 0% change from screening. In cycle 6, Patients 0206 and 0401 had 0% change from screening. ID = identification; LD = lesion diameter; PD = progressive disease; PR = partial response; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors v1.1; SD = stable disease.
Figure 4.
Comparison of change in target lesion diameter (LD) and overall response for cycle 3B versus cycle 6B. RECIST = Response Evaluation Criteria in Solid Tumors.
Table 4.
Summary of overall response based on the Response Evaluation Criteria in Solid Tumors v1.1 for the efficacy population.*
| Dose cohort | Assessment time point† | Count | Partial response | Stable disease‡ | Progressive disease |
|---|---|---|---|---|---|
| Cohort 1: idronoxil suppository (NOX66) 400 mg | Cycle 3B | 5 | 0 (0.0) | 4 (80.0) | 1 (20.0) |
| Cycle 6B | 2 | 0 (0.0) | 1 (50.0) | 1 (50.0) | |
| Cohort 2: NOX66 800 mg | Cycle 3B | 9 | 0 (0.0) | 7 (77.8) | 2 (22.2) |
| Cycle 6B | 6 | 1 (16.7) | 4 (66.7) | 1 (16.7) |
Values are presented as n (%) unless otherwise noted.
Assessed at the start of the cycle.
No tumor growth and no new tumors.
Discussion
This is the first clinical study to demonstrate that idronoxil delivered as a rectal suppository is well tolerated in patients with refractory solid tumors. The safety profile of NOX66, at 400 mg and 800 mg, both as monotherapy and in combination with carboplatin, was consistent with those generally observed in oncology patients. Early efficacy results from this study also appear encouraging in terms of the potential for NOX66 to improve the effectiveness of chemotherapy treatment in patients with end-stage solid tumor disease. The overall aim of the ongoing NOX66 clinical research program is to demonstrate the ability of NOX66 to sensitize cancer cells to chemotherapy so that dosages that are used in the clinic have a more potent anticancer effect. This Phase Ia/b study therefore provides important safety information to justify continuation of the NOX66 clinical research program, including the testing of higher doses.
Preclinical animal studies and a number of Phase I, II, and III studies in several hundred cancer patients have confirmed that idronoxil delivered orally or intravenously is well tolerated.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 The relatively lower dependence of nontumor cells on ENOX2 activity accounts for the low level of toxicity of idronoxil reported previously in animal toxicology studies and human clinical studies.7 Animal studies with oral or intravenous dose formulations have been unable to determine the maximum tolerated dose.7 Furthermore, no maximum tolerated dose level has been determined in human beings, and there is no observed toxicity at 40 mg/kg, the highest dose of idronoxil that can be practically administered on a repeated daily basis.27 This is far in excess of the doses (400 mg and 800 mg daily) used in this study. Moreover, in a Phase III clinical study where idronoxil was given orally on a continuous daily basis over a 28-day period (at a dose more than double the highest dose in this study) in combination with a standard dose of carboplatin, there was no toxicity above grade 2.26
Whereas NOX66 was not expected to have a different safety profile compared with the previously used oral dose form of idronoxil, the rationale of dosing as a rectal suppository to achieve a superior pharmacokinetic profile raised the prospect of a level of exposure of organs to idronoxil to a greater extent than experienced previously. Compared with the oral dose formulation, the objective of NOX66 was to avoid first-pass liver metabolism, thereby slowing down the rate of Phase II metabolism.7 Compared with the intravenous dose formulation, the objective of NOX66 was to provide a more steady-state drug exposure versus a short-lived Cmax spike with a bolus intravenous injection.7
Due to the limitations of the study design, with small sample sizes and no placebo comparator, no firm conclusions could be made about the efficacy of NOX66 as a chemosensitizer. However, these early results appear positive. Although at baseline most patients had metastatic disease and all were refractory to treatment, at least half had stable disease during the course of NOX66 and carboplatin combination therapy, and by cycle 6B, 83.3% of those treated with the higher NOX66 dose maintained stable disease or better.
These data concur with known mechanism of action of idronoxil, with the primary molecular target, the ENOX2 enzyme,29 a nicotinamide adenine dinucleotide plus hydrogen oxidase that oscillates between dual functions of maintaining the electron potential across the plasma membrane and disulphide-thiol exchange of plasma proteins.29 The anticancer and chemosensitizing actions of idronoxil are believed to follow a train of events triggered by inhibition of the electron/proton transfer function of ENOX2 across the cancer cell's plasma membrane; accumulation of protons in the membrane follows, leading to elevated levels of ubiquinol (reduced coenzyme Q10); this in turn inhibits the function of sphingosine kinase, creating an imbalance in levels of the secondary messengers, increase in ceramide (pro-apoptotic) and decrease in sphingosine-1-phosphate (pro-survival and promotion of immune evasion).9,30
The study has a number of strengths. First, it is the first-in-human study to investigate the safety and efficacy of idronoxil when delivered as NOX66. Second, the study investigated the safety and efficacy of NOX66 both as monotherapy and in combination with a commonly used chemotherapy agent; namely, carboplatin. Both treatment regimens are likely to be clinically relevant to patients with end-stage solid tumors.
The study also has a number of limitations. First, major limitations of this study are the small sample size and lack of placebo comparator. The sample size was reduced further by the 3 deaths during the course of the study. Because the patient population was restricted to those with end-stage, refractory solid tumors with no further therapy options available, the proportion of deaths was not unexpected.
Second, the dose–response was not studied in a classical way. The rationale for using a low dose of carboplatin was to explore the chemosensitizing potential of NOX66 and look for signs of efficacy with fewer AEs. There are no pharmacokinetic or pharmacodynamic data aside from that generated by the study.
Third, the types of tumors among the participants were heterogeneous, which could be viewed as diluting the implications of the study. Although this highlights the need to conduct follow-up research in patients with a single type of tumor, the intention behind the study design was to more broadly scope the response to NOX66 as monotherapy and in combination with chemotherapy, across varying indications. It is a common approach in many Phase I trials to include all solid tumors to determine which tumors respond.31, 32, 33
Furthermore, the efficacy data are still early and inconclusive because the study was not primarily designed to address efficacy. A larger group of patients is being planned for the next study; however, which will be powered to investigate efficacy in fewer tumor types.
Conclusions
Our study confirmed the safety and tolerability of NOX66 at 400 mg and 800 mg daily doses, both as monotherapy and in combination with carboplatin, in patients with refractory solid tumors. Early results are encouraging with regard to the chemosensitizing potential of NOX66, justifying further testing of its clinical response in future studies. Moreover, because idronoxil has demonstrated the ability to activate both the innate and adaptive immune system, it would be rational and interesting to test the combination of NOX66 with immunotherapy checkpoint inhibitors in so-called cold tumors; such a study is currently underway.
Acknowledgments
All authors contributed equally to this publication. Data management and analysis was conducted by Datapharm Australia Pty Ltd. Editorial assistance was provided by Yoonah Choi and Hae-Jin Song of Evidencia Medical Communications Pty Ltd, contracted by Noxopharm Limited, Sydney, Australia; and by Olivier Laczka (director of drug discovery and research, Noxopharm Limited).
Conflict of Interest
This Phase Ia/b study was entirely sponsored by Noxopharm Limited, Sydney, Australia. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- 1.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull. 2017;7(3):339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyne NJ, El Buri A, Adams DR, Pyne S. Sphingosine 1-phosphate and cancer. Adv Biol Regul. 2018;68:97–106. doi: 10.1016/j.jbior.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez YI, Campos LE, Castro MG, Aladhami A, Oskeritzian CA, Alvarez SE. Sphingosine-1 Phosphate: A New Modulator of Immune Plasticity in the Tumor Microenvironment. Front Oncol. 2016;6:218. doi: 10.3389/fonc.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olesch C, Ringel C, Brune B, Weigert A. Beyond Immune Cell Migration: The Emerging Role of the Sphingosine-1-phosphate Receptor S1PR4 as a Modulator of Innate Immune Cell Activation. Mediators Inflamm. 2017;2017:6059203. doi: 10.1155/2017/6059203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olesch C, Sirait-Fischer E, Brüne B, Weigert A. Targeting immune cell-specific sphingosine-1-phosphate receptor 4 to restore antitumor immunity resulting in improved therapy response. Abstract A209. Paper presented at: Fourth CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference; Sep 30-Oct 3, 2018; New York, NY; 2018. [Google Scholar]
- 7.Porter K, Fairlie WD, Laczka O, Delebecque F, Wilkinson J. Idronoxil as an Anticancer Agent: Activity and Mechanisms. Curr Cancer Drug Targets. 2020;20(5):341–354. doi: 10.2174/1568009620666200102122830. [DOI] [PubMed] [Google Scholar]
- 8.De Luca T, Bosneaga E, Morre DM, Morre DJ. Downstream targets of altered sphingolipid metabolism in response to inhibition of ENOX2 by phenoxodiol. Biofactors. 2008;34(3):253–260. doi: 10.3233/BIO-2009-1079. [DOI] [PubMed] [Google Scholar]
- 9.De Luca T, Morre DM, Morre DJ. Reciprocal relationship between cytosolic NADH and ENOX2 inhibition triggers sphingolipid-induced apoptosis in HeLa cells. J Cell Biochem. 2010;110(6):1504–1511. doi: 10.1002/jcb.22724. [DOI] [PubMed] [Google Scholar]
- 10.De Luca T, Morre DM, Zhao H, Morre DJ. NAD+/NADH and/or CoQ/CoQH2 ratios from plasma membrane electron transport may determine ceramide and sphingosine-1-phosphate levels accompanying G1 arrest and apoptosis. Biofactors. 2005;25(1-4):43–60. doi: 10.1002/biof.5520250106. [DOI] [PubMed] [Google Scholar]
- 11.Yao C, Wu S, Li D. Co-administration phenoxodiol with doxorubicin synergistically inhibit the activity of sphingosine kinase-1 (SphK1), a potential oncogene of osteosarcoma, to suppress osteosarcoma cell growth both in vivo and in vitro. Mol Oncol. 2012;6(4):392–404. doi: 10.1016/j.molonc.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity. 2018;48(3):417–433. doi: 10.1016/j.immuni.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 14.Brown DM, Kelly GE, Husband AJ. Flavonoid compounds in maintenance of prostate health and prevention and treatment of cancer. Mol Biotechnol. 2005;30(3):253–270. doi: 10.1385/MB:30:3:253. [DOI] [PubMed] [Google Scholar]
- 15.Choueiri TK, Mekhail T, Hutson TE, Ganapathi R, Kelly GE, Bukowski RM. Phase I trial of phenoxodiol delivered by continuous intravenous infusion in patients with solid cancer. Annals of Oncology. 2006;17(5):860–865. doi: 10.1093/annonc/mdl010. [DOI] [PubMed] [Google Scholar]
- 16.Brown DM, Heaton A, Husband AJ. Idronoxil. Drugs of the Future. 2008;33(10):844–860. [Google Scholar]
- 17.de Souza PL, Liauw W, Links M, Pirabhahar S, Kelly G, Howes LG. Phase I and pharmacokinetic study of weekly NV06 (Phenoxodiol), a novel isoflav-3-ene, in patients with advanced cancer. Cancer Chemother Pharmacol. 2006;58(4):427–433. doi: 10.1007/s00280-006-0189-6. [DOI] [PubMed] [Google Scholar]
- 18.Howes JB, de Souza PL, West L, Huang LJ, Howes LG. Pharmacokinetics of phenoxodiol, a novel isoflavone, following intravenous administration to patients with advanced cancer. BMC Clin Pharmacol. 2011;11:1. doi: 10.1186/1472-6904-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly MG, Mor G, Husband A. Phase II evaluation of phenoxodiol in combination with cisplatin or paclitaxel in women with platinum/taxane-refractory/resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers. Int J Gynecol Cancer. 2011;21(4):633–639. doi: 10.1097/IGC.0b013e3182126f05. [DOI] [PubMed] [Google Scholar]
- 20.Alvero AB, Kelly M, Rossi P. Anti-tumor activity of phenoxodiol: from bench to clinic. Future Oncol. 2008;4(4):475–482. doi: 10.2217/14796694.4.4.475. [DOI] [PubMed] [Google Scholar]
- 21.Choueiri TK, Wesolowski R, Mekhail TM. Phenoxodiol: isoflavone analog with antineoplastic activity. Curr Oncol Rep. 2006;8(2):104–107. doi: 10.1007/s11912-006-0044-2. [DOI] [PubMed] [Google Scholar]
- 22.Mor G, Fu HH, Alvero AB. Phenoxodiol, a novel approach for the treatment of ovarian cancer. Curr Opin Investig Drugs. 2006;7(6):542–548. [PubMed] [Google Scholar]
- 23.Saif MW, Tytler E, Lansigan F, Brown DM, Husband AJ. Flavonoids, phenoxodiol, and a novel agent, triphendiol, for the treatment of pancreaticobiliary cancers. Expert Opin Investig Drugs. 2009;18(4):469–479. doi: 10.1517/13543780902762835. [DOI] [PubMed] [Google Scholar]
- 24.Silasi DA, Alvero AB, Rutherford TJ, Brown D, Mor G. Phenoxodiol: pharmacology and clinical experience in cancer monotherapy and in combination with chemotherapeutic drugs. Expert Opin Pharmacother. 2009;10(6):1059–1067. doi: 10.1517/14656560902837980. [DOI] [PubMed] [Google Scholar]
- 25.Minns I, Kelly G. Chemosensitization of carboplatin by NOX66: Pharmacokinetics and safety (419TiP) Ann Oncol. 2017;28(suppl_5) [Google Scholar]
- 26.Fotopoulou C, Vergote I, Mainwaring P. Weekly AUC2 carboplatin in acquired platinum-resistant ovarian cancer with or without oral phenoxodiol, a sensitizer of platinum cytotoxicity: the phase III OVATURE multicenter randomized study. Ann Oncol. 2014;25(1):160–165. doi: 10.1093/annonc/mdt515. [DOI] [PubMed] [Google Scholar]
- 27.Tozer L, Sockler JM, Vigano GJ. April, 2019. Clinical Study Report: Phase Ia/Ib and Potential Phase IIa Study of the Safety and Pharmacokinetics of NOX66 Both as a Monotherapy and in Combination with Carboplatin in Patients with Refractory Solid Tumours (Protocol Number NOX66-001A) Noxopharm Limited, Sydney. [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Morre DJ, Chueh PJ, Yagiz K, Balicki A, Kim C, Morre DM. ECTO-NOX target for the anticancer isoflavene phenoxodiol. Oncol Res. 2007;16(7):299–312. doi: 10.3727/000000006783980973. [DOI] [PubMed] [Google Scholar]
- 30.Herst PM, Petersen T, Jerram P, Baty J, Berridge MV. The antiproliferative effects of phenoxodiol are associated with inhibition of plasma membrane electron transport in tumour cell lines and primary immune cells. Biochem Pharmacol. 2007;74(11):1587–1595. doi: 10.1016/j.bcp.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Ivy SP, Siu LL, Garrett-Mayer E, Rubinstein L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res. 2010;16(6):1726–1736. doi: 10.1158/1078-0432.CCR-09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap TA, O'Carrigan B, Penney MS. Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination With Carboplatin in Patients With Advanced Solid Tumors. J Clin Oncol. 2020;38(27):3195–3204. doi: 10.1200/JCO.19.02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naing A, Gainor JF, Gelderblom H. A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced solid tumors. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]