Highlights
-
•
LABA+ICS, LAMA+ICS and LABA+LAMA improved FEV1 % predicted
-
•
The three therapeutic combinations showed statistically similar safety profiles and efficacy
-
•
The results of this pilot study suggest that TNF-α, fibrinogen and IL-6 can be used to monitor disease progression or guide therapeutic decisions.
Key words: COPD, Fibrinogen, ICS, LABA, LAMA, TNF-ɑ
Abstract
Background
There are differences of opinion about both the most effective combined therapeutic strategy and the clinical benefit of inhaled corticosteroids in nonasthmatic patients with chronic obstructive pulmonary disease. Furthermore, many inflammatory cytokines are reportedly correlated with severity of the disease.
Objectives
To compare the effectiveness of long acting β-agonist + long-acting muscarinic antagonist (LABA + LAMA) versus LABA + inhaled corticosteroid and LAMA + inhaled corticosteroid in nonasthmatic patients with moderate-to-severe chronic obstructive pulmonary disease. To assess the changes that occurred in plasma concentrations of tumor necrosis factor α, fibrinogen, and interleukin 6, and correlate these with disease activity.
Methods
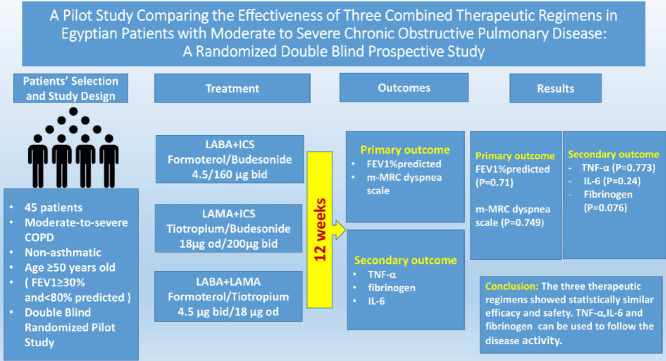
In this pilot study, 45 nonasthmatic patients with moderate to severe chronic obstructive pulmonary disease were randomized into 3 groups with 15 patients in each group. Group I (LABA + inhaled corticosteroid) received formoterol/budesonide, group II (LAMA + inhaled corticosteroid) received tiotropium/budesonide and group III (LABA + LAMA) received formoterol/tiotropium for 12 weeks. Patients were assessed initially and then at 4 and 12 weeks by measuring the changes that occurred in forced expiratory volume in 1 second as a percent of predicted and in the modified Medical Research Council dyspnea scale. Plasma concentrations of tumor necrosis factor α, fibrinogen, and interleukin 6 were simultaneously measured.
Results
The 3 study groups were statistically similar with respect to their demographic data and disease characteristics. All therapeutic options produced an improvement in forced expiratory volume in 1 second as a percent of predicted and in the modified Medical Research Council dyspnea scale as well as a reduction in plasma concentrations of the inflammatory markers. The effects produced by the three therapeutic combinations on forced expiratory volume in 1 second as a percent of predicted, plasma tumor necrosis factor α, interleukin 6, and fibrinogen concentrations were statistically similar after 4 and 12 weeks (4 weeks after treatment: P = 0.358, P = 0.284, P = 0.155, and P = 0.155, respectively, and 12 weeks after treatment: P = 0.710, P = 0.773, P = 0.240, and P = 0.076, respectively).
Conclusions
In nonasthmatic patients with moderate to severe chronic obstructive pulmonary disease, the 3 therapeutic combinations showed similar effectiveness. The results of this pilot study also suggest that inflammatory markers can be used to track disease activity. Clinicaltrials.gov identifier: NCT04520230. (Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
Graphical abstract
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with abnormal inflammatory response of the lungs toward noxious particles and gases, usually from cigarette smoke. Patients with symptomatic COPD can have their condition managed with 1 of the following inhaled medications: long-acting β-agonists (LABA), long-acting muscarinic antagonists (LAMA), or inhaled corticosteroids (ICSs).1 Administration of 2 or more medications from different classes seems beneficial when the disease cannot be controlled adequately with LAMA or LABA monotherapy.1 LAMAs dilate the airway by selectively blocking acetylcholine M3 receptors.2 LABAs are β2-agonists, which provide smooth muscle relaxation by stimulating β2-adrenergic receptors.3 Furthermore, LAMAs and LABAs were reported to exert anti-inflammatory activity.2,4,5 LAMAs and LABAs are considered ideal treatments for patients with symptomatic COPD through improving lung function, exercise capacity, and quality of life, and reducing exacerbations.6,7
Despite little evidence for their clinical benefit, and increasing evidence that the high doses currently recommended are both harmful and costly, ICSs are grossly overprescribed as a result of successful marketing.8,9 ICSs were reported to be effective in bronchial asthma, whereas eosinophils play a key role and they seem poorly effective in patients with COPD because neutrophils play a critical role.10 In addition, clinical studies have shown that ICS use may be associated with increased risk of adverse effects such as hoarseness, cataract formation, candidiasis, or pneumonia.11, 12, 13, 14
Proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL) 1β, and IL-6, appear to amplify inflammation in COPD through activation of the transcription factor, nuclear factor-κB, thereby leading to increased expression of multiple inflammatory genes. TNF-α has been reported to be involved in airway inflammation during COPD.15 Fibrinogen is an acute phase soluble plasma glycoprotein that regulates inflammation in many diseases and its plasma concentration may be a promising biomarker to indicate the disease severity.16 Additionally, it was postulated that IL-6 plays a considerable role in the systemic inflammatory response during COPD.17
There are differences of opinion about the most effective combined therapeutic strategy for patients with COPD.18 In addition, forced expiratory volume in 1 second (FEV1) as a percent of predicted has been reported to be poorly correlated with both symptoms and other measures of the disease progression; therefore, there is an urgent need for other biological biomarkers to enable tracking of the disease activity.16,19 Furthermore, current evidence suggests that an abnormal inflammatory response causes disease progression, and many inflammatory cytokines were reported to induce airway inflammation and to be correlated with severity of the disease.15,17 To provide evidence to help resolve these differences, the effectiveness of LABA + LAMA versus both LABA + ICS and LAMA + ICS were compared in patients with moderate-to-severe COPD by evaluating the changes occurring in FEV1 as a percent of predicted, and in the modified Medical Research Council (mMRC) dyspnea scale. In addition, changes that occurred in plasma concentrations of inflammatory markers (ie, TNF-α, fibrinogen, and IL-6) were compared with changes in disease activity.
Patients and Methods
Study design
The design of this pilot study was a randomized double-blind prospective parallel study that included 45 adult Egyptian patients of both sexes with moderate to severe COPD according to Global Initiative for Chronic Obstructive Lung Disease guidelines. All patients were recruited from the Chest Disease Department, Tanta University Hospital, Tanta, Egypt, between November 2016 and December 2018. The 45 patients with COPD were randomly divided into 3 groups with 15 patients in each group. Group I (LABA + ICS) received formoterol/budesonide combination 4.5/160 μg, 2 inhalations BID. Group II (LAMA + ICS) received tiotropium18 μg inhaled capsule OD plus budesonide 200 μg, 2 inhalations BID. Group III (LABA + LAMA) received formoterol 4.5 μg inhaled capsule BID plus tiotropium18 μg inhaled capsule OD. The treatment with the study medications commenced 2 weeks after all earlier drug treatments used were discontinued. The only medication allowed during the washout period was salbutamol. The treatment duration was 12 weeks for all groups. In this double-blind study, the enrolled patients were randomized in 1:1:1 ratio using a computer-generated code according to the Consolidated Standards of Reporting Trials guidelines. The double blind included the principal investigator (physician) and the patients. The physician was provided with a sealed randomization code for each treatment generated by an independent researcher to avoid bias during the carryout of the initial pulmonary function tests. The independent researcher kept the original random allocation sequences in an inaccessible place. The 3 therapeutic regimes were similar in route of administration, taste and smell. The study was approved by the National Research Ethics Committee (CP00011), Tanta University, Egypt, and was registered retroactively as a clinical trial at ClinicalTrials.gov (identifier: NCT04520230). Eligible patients gave their written informed consent. Inclusion criteria were male and female patients with COPD aged ≥50 years, had a FEV1/forced vital capacity < 0.70 and a FEV1 30% to 80% of predicted. Exclusion criteria were patients with very severe COPD (FEV1 < 30 as a percent of predicted), patients with chronic respiratory failure or recent chest infection, and patients with an exacerbation in the past 6 weeks. Patients with a history of asthma or other inflammatory diseases and patients with clinically significant conditions such as unstable ischemic heart disease, uncontrolled hypertension, and diabetes were also excluded. The primary outcome was the measure of the effectiveness of the 3 combinations through evaluating the changes that occurred in FEV1 as a percent of predicted and in the mMRC dyspnea scale. The secondary outcome resulted from the evaluation of the changes in plasma concentrations of inflammatory markers.
Demographic factors
All participants were screened for demographic factors (ie, age, sex, and smoking habits), a physical examination and the measurement of weight and height, and calculation of body mass index.
Assessment of pulmonary function and dyspnea
Pulmonary function including FEV1 as a percent of predicted was assessed by Spirometry (Chest Spirometer Model hl-101; El-Radwan Company, Cairo, Egypt). The mMRC dyspnea scale was used for the assessment of dyspnea at the beginning the study and at 12 weeks.
Sample collection and laboratory analyses
Blood samples were collected at baseline, and 4 and 12 weeks after treatment. Plasma was separated and immediately stored at –80°C until biochemical analyses of plasma TNF-α, plasma fibrinogen, and plasma IL-6 concentrations using the commercially available ELISA kits (Assaypro; LLC Biotechnology Company, Saint Charles, Missouri) using a Tecan plate reader infinite F 50 (Tecan Group Ltd, Mannedorf, Switzerland). For assessment of inflammatory markers' concentrations, plasma was separated from blood samples using one-10th volume of 0.1 M sodium citrate as an anticoagulant. All laboratory analyses were carried out at the Laboratory of Bioequivalence and Pharmaceutical Service Unit, Faculty of Pharmacy, Tanta University. The laboratory follows the international standards with ISO/IEC 17025:2005 and ISO 9001:2015 and its certificate number is IRQS/1810543.
Subjective data analysis
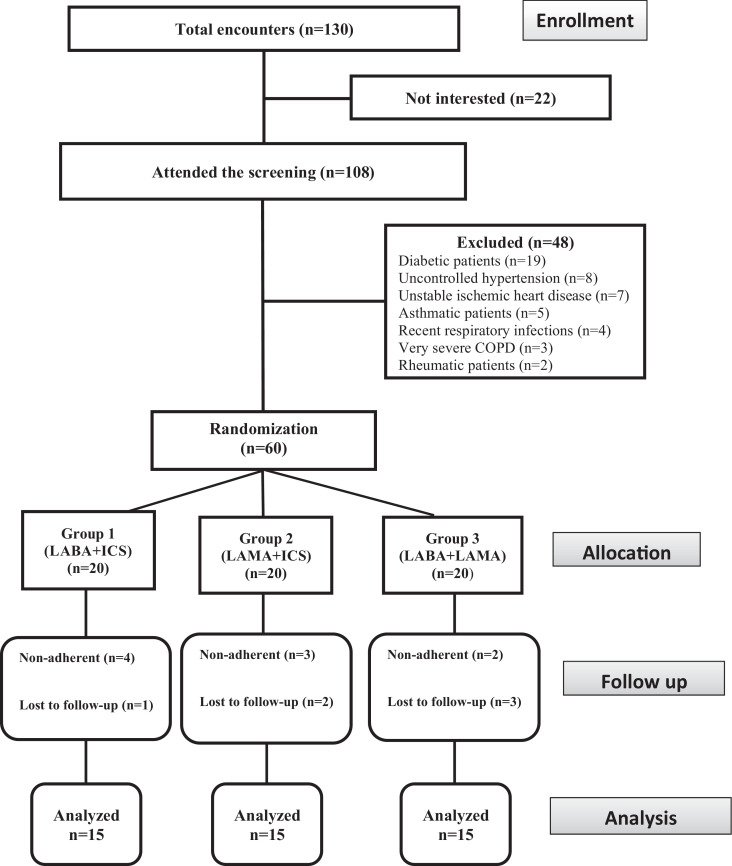
Using weekly telephone calls and biweekly direct meetings, patients were followed-up to assess their adherence, to allow any reporting of adverse effects and to ensure the correct use of inhalers. Adherence was assessed through counting the empty inhalers and by the medication refill rates. According to the study design, patients were considered nonadherent if they underused, overused, or stopped the medications for 7 consecutive days. Furthermore, patients those we were unable to follow-up were considered nonadherent. Nonadherent participants were excluded from the study as illustrated in Figure 1.
Figure 1.
Flow chart illustrating the participant screening, enrollment, and randomization. ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist.
Statistical analysis
The collected data were statistically analyzed using Statistical Package for the Social Sciences, version 16, (IBM-SPSS Inc, Chicago, Illinois). For quantitative data, the range, mean, and SD were calculated. For comparison between more than 2 means of parametric data, the F value of the ANOVA test was calculated. The Scheffe test was used to compare each 2 means if the F value was significant. Paired t test was also used. For comparison between more than 2 means of nonparametric data, Kruskal-Wallis (χ2) was used. The χ2 test was used for categorical variables. Correlation between variables was evaluated using Pearson's correlation. Significance level was set at P < .05.
Results
The total encounters, screening, randomization, and follow-up procedures of the study participants are illustrated in Figure 1. Of 130 total encounters, 22 patients were not interested in participating. One hundred eight patients attended the screening and 48 of these patients were excluded according to the study exclusion criteria (19 patients with diabetes, 8 patients with uncontrolled hypertension, 7 patients with unstable ischemic heart disease, 5 patients with asthma, 4 patients with recent respiratory tract infections, 3 patients with very severe COPD, and 2 patients with rheumatic disease). At baseline, the study groups were statistically similar with respect to demographic factors (ie, age, male sex, weight, height, and body mass index), smoking (duration of reported smoking, past smokers, current smokers, and nonsmokers), and disease characteristics (Table 1). There was no group or treatment change during the follow-up of all patients.
Table 1.
Baseline demographic characteristics, disease characteristics, pulmonary function test results, and dyspnea scale scores.
| Parameter | Group 1: LABA + ICS | Group 2: LAMA + ICS | Group 3: LABA + LAMA | P value |
|---|---|---|---|---|
| Age* (y) | 63.5 (9.19) | 62.5 (7.04) | 64.9 (9.38) | 0.746 |
| Male sex† | 10 (66.67) | 13 (86.67) | 11 (73.33) | 0.449 |
| Weight* (kg) | 78.47 (5.88) | 76.43 (7.357) | 77.57 (8.27) | 0.744 |
| Height* (cm) | 171.33 (5.31) | 170.27 (6.03) | 172.40 (4.12) | 0.541 |
| BMI* | 26.75 (2.36) | 26.64 (2.71) | 26.33 (3.03) | 0.976 |
| Past smokers† | 10 (66.7) | 12 (80) | 11 (73.3) | 0.726 |
| Current smokers† | 5 (33.3) | 7 (46.67) | 6 (40) | 0.76 |
| Never smoker† | 5 (33.33) | 3 (20) | 4 (26.67) | 0.71 |
| Duration of smoking* (y) | 23.9 (9.02) | 21.83 (7.25) | 22.27 (8.3) | 0.83 |
| Gold classification† | ||||
| B | 12 (80) | 8 (53.3) | 10 (66.7) | 0.301 |
| D | 3 (20) | 7 (46.7) | 5 (33.3) | |
| FEV1 as a % of predicted | ||||
| Range | 30.00-79.10 | 37.80-75.80 | 35.90-76.90 | |
| Mean (SD) | 61.62 (15.14) | 54.53 (11.57) | 60.24 (15.59) | 0.359 |
| mMRC dyspnea scale | ||||
| Range | 2.00-4.00 | 2.00-4.00 | 2.00-4.00 | 0.530 |
| Mean (SD) | 3.07 (0.70) | 3.33 (0.72) | 3.07 (0.80) | |
| Median | 3.00 | 3.00 | 3.00 | |
| Entry medications† | ||||
| SABA | 9 (60.00) | 7 (46.66) | 7 (46.66) | |
| SAMA | 5 (33.33) | 4 (26.66) | 7 (46.66) | |
| Mucolytic | 11 (73.33) | 8 (53.33) | 10 (66.66) | |
| Expectorant | 7 (46.66) | 5 (33.33) | 8 (53.33) | |
| Oral xanthines | 5 (33.33) | 3 (20) | 4 (26.66) | |
| LABA: Formoterol | 6 (40.00) | 8 (53.33) | 7 (46.66) | |
| ICS: Budesonide | 2 (13.33) | 4 (26.66) | 2 (13.33) |
ICS = inhaled corticosteroid; LABA: long-acting β2-agonist; LAMA: long-acting muscarinic antagonist; BMI = body mass index; mMRC: modified Medical Research Council; SABA = short-acting β2-agonist; SAMA = short-acting muscarinic antagonist.
Values are presented as mean (SD).
Values are presented as n (%).
Group II showed a significant increase in FEV1 as a percent of predicted and significant improvement in mMRC dyspnea scale 12 weeks after treatment when compared with baseline data (P = 0.002 and P = 0.0001, respectively). Group I and group III showed significant improvement in mMRC dyspnea scale 12 weeks after treatment (P = 0.0001 and P = 0.0001, respectively), which was associated with nonsignificant elevation of FEV1 as a percent of predicted 4 and 12 weeks after treatment compared with baseline data (P > 0.05) as illustrated in Tables 2 and 3.
Table 2.
Forced expiratory volume in 1 second (as a percent of predicted) of patients with moderate-to-severe chronic obstructive pulmonary disease in each of the 3 groups.
| Time of assessment | Group 1: LABA + ICS (n = 15) | Group 2: LAMA + ICS (n = 15) | Group 3: LABA + LAMA (n = 15) | F value | P value |
|---|---|---|---|---|---|
| Baseline | |||||
| Range | 30.00-79.10 | 37.80-75.80 | 35.90-76.90 | ||
| Mean (SD) | 61.62 (15.14) | 54.53 (11.57) | 60.24 (15.59) | 1.050 | 0.359 |
| 4 wk after treatment | |||||
| Range | 33.50-94.20 | 46.50-96.50 | 43.50-87.20 | 1.052 | 0.358 |
| Mean (SD) | 75.29 (21.22) | 66.10 (14.21) | 70.85 (15.89) | ||
| 12 wk after treatment | |||||
| Range | 59.80-116.40 | 53.80-102.80 | 52.30-96.50 | 0.346 | 0.710 |
| Mean (SD) | 77.61 (21.34) | 72.60 (13.31) | 73.83 (15.97) | ||
| F value | 2.962 | 7.349 | 3.060 | ||
| P value | 0.063 | 0.002* | 0.057 | ||
| Scheffe test | |||||
| Baseline vs 4 wk after treatment | Group 2: 0.064 | ||||
| Baseline vs 12 wk after treatment | Group 2: 0.002* | ||||
| 4 wk vs 12 wk after treatment | Group 2: 0.404 |
ICS = inhaled corticosteroid; LABA = long acting β2-agonist; LAMA = long-acting muscarinic antagonist.
Significant.
Table 3.
Baseline and 12-week modified Medical Research Council dyspnea scale scores.
| Time of assessment | Group 1: LABA/ICS (n = 15) | Group 2: LAMA/ICS (n = 15) | Group 3: LABA/LAMA (n = 15) | F value | P value |
|---|---|---|---|---|---|
| Baseline | |||||
| Range | 2.00-4.00 | 2.00-4.00 | 2.00-4.00 | 0.644 | 0.530 |
| Mean (SD) | 3.07 (0.70) | 3.33 (0.72) | 3.07 (0.80) | ||
| Median | 3.00 | 3.00 | 3.00 | ||
| 12 wk after treatment | |||||
| Range | 1.00-3.00 | 1.00-3.00 | 1.00-3.00 | 0.292 | 0.749 |
| Mean (SD) | 1.73 (0.80) | 1.73±0.80 | 1.93 (0.88) | ||
| Median | 2.00 | 2.00 | 2.00 | ||
| Paired t test | 6.325 | 12.220 | 8.500 | ||
| P value | 0.0001* | 0.0001* | 0.0001* |
ICS = inhaled corticosteroid; LABA = long-acting β2-agonist; LAMA = long-acting muscarinic antagonist.
Significant difference with ANOVA test.
Compared with baseline data, plasma TNF-ɑ concentration showed a significant decrease 4 and 12 weeks after treatment in group I and group III (P = 0.019 and P = 0.009, respectively, for group I and P = 0.036 and P = 0.007, respectively, for group III). For group II, plasma TNF-ɑ concentration showed a significant decrease 12 weeks after treatment with its baseline value (P = 0.001) as shown in Table 4.
Table 4.
Tumor necrosis factor alpha results by treatment group.
| Time of assessment | Group 1: LABA + ICS (n = 15) | Group 2: LAMA + ICS (n = 15) | Group 3: LABA + LAMA (n = 15) | F value | P value |
|---|---|---|---|---|---|
| Baseline | |||||
| Range | 3-44 | 18-55 | 18-76 | 0.601 | 0.553 |
| Mean (SD) | 27.53 (9.50) | 30.87 (9.29) | 31.87 (14.45) | ||
| 4 wk after treatment | |||||
| Range | 10-30 | 12-48 | 14-31 | 1.297 | 0.284 |
| Mean (SD) | 20.07 (5.59) | 24.20 (9.10) | 22.33 (5.89) | ||
| 12 wk after treatment | |||||
| Range | 13-30 | 10-35 | 11-30 | 0.259 | 0.773 |
| Mean (SD) | 19.33 (4.70) | 18.47 (7.06) | 20.07 (6.27) | ||
| F value | 6.453 | 7.914 | 6.238 | ||
| P value | 0.004* | 0.001* | 0.004* | ||
| Scheffe test | |||||
| Baseline vs 4 wk after treatment | Group 1: 0.019* Group 2: 0.114 Group 3: 0.036* |
||||
| Baseline vs 12 wk after treatment | Group 1: 0.009* Group 2: 0.001* Group 3: 0.007* |
||||
| 4 wk vs 12 wk after treatment | Group 1: 0.959 Group 2: 0.197 Group 3: 0.816 |
ICS: Inhaled corticosteroid, LABA: Long acting β2-agonist, LAMA: Long acting muscarinic antagonist.
Significant.
IL-6 plasma concentration showed a statistically significant decrease within group I at 4 and 12 weeks after treatment versus baseline data (P = 0.026 and P = 0.001, respectively). Group II showed a significant decrease in serum IL-6 concentration only 12 weeks after treatment compared with baseline data (P = 0.043). Group III showed a nonsignificant decrease in IL-6 plasma concentration 4 and 12 weeks after treatment when compared with baseline data (P > 0.05) as shown in Table 5.
Table 5.
Mean interlukin-6 values.
| Time of assessment | Group 1: LABA + ICS (n = 15) | Group 2: LAMA + ICS (n = 15) | Group 3: LABA + LAMA (n = 15) | F value | P value |
|---|---|---|---|---|---|
| Baseline | |||||
| Range | 3.50-13.10 | 0.33-19.05 | 3.60-22.50 | 1.270 | 0.291 |
| Mean (SD) | 6.11 (2.90) | 6.03 (4.17) | 8.17 (5.12) | ||
| 4 wk after treatment | |||||
| Range | 2.15-7.30 | 1.30-7.60 | 3.10-21.70 | 1.953 | 0.155 |
| Mean (SD) | 4.01 (1.47) | 4.05 (1.80) | 5.87 (4.54) | ||
| 12 wk after treatment | |||||
| Range | 1.50-6.30 | 0.50-7.10 | 2.20-13.50 | 1.477 | 0.240 |
| Mean (SD) | 3.07 (1.33) | 3.35 (1.81) | 4.29 (2.75) | ||
| F value | 8.809 | 3.636 | 3.132 | ||
| P value | 0.001* | 0.035* | 0.054 | ||
| Baseline vs 4 wk after treatment | Group 1: 0.026* Group 2: 0.172 |
||||
| Baseline vs 12 wk after treatment | Group 1: 0.001* Group 2: 0.043* |
||||
| 4 wk vs 12 wk after treatment | Group 1: 0.452 Group 2: 0.792 |
ICS = inhaled corticosteroid, LABA = long-acting β2-agonist, LAMA = long-acting muscarinic antagonist.
*Significant.
Furthermore, group I showed a significant decrease in plasma fibrinogen concentration 4 and 12 weeks after treatment compared with its baseline value (P = 0.015 and P = 0.003, respectively). On the other hand, both group II and group III showed nonsignificant decline in plasma fibrinogen concentration 4 and 12 weeks after treatment when compared with baseline concentrations (P > 0.05); as shown in Table 6.
Table 6.
Changes in fibrinogen levels over time.
| Time of assessment | Group 1: LABA + ICS (n = 15) | Group 2: LAMA + ICS (n = 15) | Group 3: LABA+ LAMA (n = 15) | F value | P value |
|---|---|---|---|---|---|
| Baseline | |||||
| Range | 2.65-16.17 | 3.23-26.16 | 3.82-14.73 | 0.637 | 0.534 |
| Mean (SD) | 7.14 (4.2) | 9.03 (6.14) | 7.61 (3.38) | ||
| 4 wk after treatment | |||||
| Range | 1.47-7.05 | 1.32-20.82 | 1.76-13.52 | 1.949 | 0.155 |
| Mean (SD) | 4.08 (1.7) | 6.94 (5.7) | 5.91 (3.59) | ||
| 12 wk after treatment | |||||
| Range | 1.94-6.47 | 2.03-19.99 | 0.88-13.82 | 2.741 | 0.076 |
| Mean (SD) | 3.44 (1.38) | 6.82 (5.32) | 5.23 (3.99) | ||
| F value | 7.771 | 0.697 | 1.692 | ||
| P value | 0.001* | 0.504 | 0.197 | ||
| Scheffe test | |||||
| Baseline vs 4 wk after treatment | Group 1: 0.015* | ||||
| Baseline vs 12 wk after treatment | Group 1: 0.003* | ||||
| 4 wk vs 12 wk after treatment | Group 1: 0.822 |
ICS = inhaled corticosteroid; LABA = long-acting β2-agonist; LAMA = long-acting muscarinic antagonist.
*Significant.
There was a nonsignificant difference between the 3 therapeutic strategies after 4 and 12 weeks of treatment with respect to FEV1 as a percent of predicted, plasma TNF-α, IL-6, and fibrinogen concentrations (4 weeks after treatment: P = 0.358, P = 0.284, P = 0.155, and P = 0.155, respectively) and (12 weeks after treatment: P = 0.710, P = 0.773, P = 0.240, and P = 0.076, respectively). In addition, after 12 weeks of treatment, there was no significant difference in mMRC dyspnea scale values between the 3 therapeutic combinations (P = 0.749). The comparisons between the 3 groups are illustrated in Table 2, Table 3, Table 4, Table 5, Table 6.
For group I, there was a significant positive correlation between plasma TNF-ɑ and IL-6 at baseline and 12 weeks after treatment (r = 0.61, P = 0.015 and r = 0.562, P = 0.029, respectively). Four weeks after treatment, TNF-α showed a significant positive correlation with plasma fibrinogen (r = 0.874, P = 0.0001). For group II, a significant positive correlation was observed between plasma TNF-α and IL-6 at baseline (r = 0.599, P = 0.018). Additionally, 4 weeks after treatment, significant negative correlation was observed between plasma TNF-α and FEV1 as a percent of predicted (r = –0.678, P = 0.006).
Only mild and manageable drug-related adverse effects were reported, whereas 2 patients in group I (13.33%) and 1 patient in group II (6.66%) showed oral thrush. One patient in group III reported mild palpitation (6.66%). There was no significant difference in the incidence of reported adverse effects between the 3 study groups (P = 0.34 for oral thrush and P = 0.36 for palpitation).
Discussion
In this randomized double-blind pilot study, the effectiveness of LABA + LAMA was compared with both (LABA + ICS) and (LAMA + ICS) in nonasthmatic patients with moderate to severe COPD by evaluating the changes that occurred in FEV1 as a percent of predicted and mMRC dyspnea scale. In addition we aimed at assessing the changes occur in plasma concentrations of inflammatory markers (ie, TNF-α, fibrinogen, and IL-6) were compared with changes in disease activity. The sample size used in the current study, although small, exceeds the sample size (12 per group) suggested for a pilot study.20 Furthermore, the sample size, follow-up period, and ages (ie, age ≥50 years) were based on previously reported studies of patients with COPD.21, 22, 23, 24, 25
Compared with baseline data, the results observed in group 1 (LABA + ICS) and group III (LABA + LAMA) after 4 and 12 weeks of treatment suggest a statistically nonsignificant but potentially clinically important improvement in FEV1 as a percent of predicted. On the other hand, the results obtained with group II (LAMA + ICS) after 4 and 12 weeks of treatment showed a statistically and clinically significant improvement in FEV1 as a percent of predicted. After 12 weeks of treatment, the three study groups all showed significant improvements in mMRC dyspnea scale as compared with their baseline data. These improvements are consistent with the fact that, LAMAs are muscarinic antagonists, which block acetylcholine-mediated bronchoconstriction by binding to M3 receptors in airway smooth muscles,26 whereas LABAs are β2 agonists, which provide smooth muscle relaxation by stimulating β2-adrenergic receptors.3 In addition, it has been demonstrated that, LAMA and LABA combined therapy show synergistic bronchodilator effects and symptom improvements in COPD patients.26,27 Finally, ICSs have been postulated to enhance the efficacy of LAMAs.28
Patients in group I showed significant decreases in the concentrations of all inflammatory markers, although group III patients had significant reductions in TNF-α concentrations after both 4 and 12 weeks of treatment compared with baseline data. On the other hand, patients in group II showed significant reductions in TNF-α and IL-6 concentrations but only after 12 weeks of treatment. These results are consistent with previous reports that, LAMAs and LABAs exert anti-inflammatory activity.2,4,5 However, although ICS monotherapy reportedly failed to reduce inflammatory markers in sputum or bronchial biopsies of patients with COPD, combining drugs with different modes of action may improve outcomes.29 Two-way synergistic activity between ICSs and LABAs has been demonstrated.30,31 Among the cellular actions of ICSs is to translocate glucocorticoid receptors from the cytoplasm to the nucleus.30 This action is enhanced in the presence of β-agonists and results in an anti-inflammatory effect greater than from either drug alone.31 In addition, ICSs activate β-receptor genes to produce more β-receptors, thereby enhancing the bronchodilating effect of LABAs.32 Therefore, the improvement in both FEV1 as a percent of predicted and mMRC dyspnea scale may reasonably be associated with the reduction in plasma concentrations of inflammatory markers through the anti-inflammatory properties of all 3 therapeutic regimens.
The 3 therapeutic combinations showed similar efficacy; there were no statistically significant differences between them after 4 and 12 weeks of treatment for all measured parameters. Previously reported lack of any significant increases in effects of combinations containing ICS over LAMA + LABA combination may be because ICS seems to be more effective in patients with asthma–COPD overlap syndrome.33 This may also be related to smoking, which increases airway inflammation and decreases corticosteroid responsiveness.34 Our results are in accordance with a former study demonstrating that, in a real-world clinical practice setting of COPD treatment, combined LABA/LAMA inhalers appear to be as effective as combined LABA/ICS inhalers in preventing COPD exacerbations.35 Additionally, these data are in agreement with a former study that reported the absence of any significant difference in transition dyspnea index focal score between LABA/LAMA and LABA/ICS treated groups.36 In contrast, the current study data appear to be incompatible with a previous study which revealed that, the lung function profile of once-daily tiotropium and olodaterol via Respimat was superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler (ENERGITO(Ⓡ) study). This study concluded that, dual bronchodilators can optimize lung function in patients with COPD who requires maintenance treatment.37 These results appear to contradict other reports that LAMA + LABA combinations have greater efficacy compared with LABA + ICS.38,39 In agreement with previously reported findings,38,40 all 3 regimens were well tolerated and have statistically similar safety profiles.
Changes in TNF-α were inversely associated with FEV1 as a percent of predicted, as previously reported.41 This suggests that changes in plasma concentrations of inflammatory markers might be useful to follow disease activity or aid in treatment selection. This suggestion is based on the current concept that an abnormal inflammatory response causes COPD disease progression, and drugs with anti-inflammatory activities can modify the mechanisms driving disease progression.42
Study limitations
The small sample size, short follow-up period, long enrollment period, lack of cotinine testing to confirm smoking status or more objective measures of medication adherence, and the dropouts during the follow-up period are all limitations of the current study. In addition, the lack of younger patients with COPD, exclusion of patients with asthmatic and very severe COPD also represent limitations. Large-scale and more detailed studies are needed to confirm the results obtained in the current study and to explore the hypothesized use of inflammatory markers to monitor or guide therapy.
Conclusions
The 3 therapeutic regimens currently used for the treatment of nonasthmatic patients with moderate-to-severe COPD showed statistically similar safety profiles and efficacy in improving FEV1 as a percent of predicted, improvements in the mMRC dyspnea scale, and in reducing plasma concentrations of inflammatory markers. Furthermore, the changes that occurred in plasma TNF-α, fibrinogen, and IL-6 concentrations during the treatment suggest the possible use of 1 or more of these markers to monitor disease progression or guide therapeutic decisions.
Declaration of Competing Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank all of the participants and are most grateful to the physicians in the Chest Department at Tanta University Hospital for their recommendations.
Tarek M. Mostafa, Gamal A. El-Azab and Noran S. Lotfy reviewed the literature and constructed the study design. Eligibility assessment, enrollment of participants and measurement of pulmonary function was done by Ghada A. Atia. Clinical data acquisition and collection of laboratory samples were done by Noran S. Lotfy. Tarek M. Mostafa and Noran S. Lotfy performed the laboratory analyses of the collected samples and data interpretation. All authors shared in performing the statistical analyses, writing, revising and approving the final Manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2021.100625.
Appendix. Supplementary materials
References
- 1.Global Initiative for Chronic Obstructive Lung Disease [GOLD]. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. (2018). Available at: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf (accessed November 20, 2018).
- 2.Alagha K, Palot A, Sofalvi T, Pahus L, Gouitaa M, Tummino C, Martinez S, Charpin D, Bourdin A, Chanez P. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases Ther Adv Chronic Dis. 2014;5(2):85-98. doi: 10.1177/2040622313518227. [DOI] [PMC free article] [PubMed]
- 3.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26(1):112–120. doi: 10.1016/j.pupt.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson R, Theron AJ, Steel HC, Durandt C, Tintinger GR, Feldman C. The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benfante A, Braido F, Scichilone N. The anti-inflammatory properties of tiotropium. Lancet Respir Med. 2018;6(8):e37. doi: 10.1016/S2213-2600(18)30190-5. [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP. Long-acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safety. Curr Opin Pulm Med. 2010;16(2):97–105. doi: 10.1097/MCP.0b013e328335df1e. [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149. doi: 10.1186/1465-9921-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes PJ. Inhaled corticosteroids are not helpful in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(2 Pt 1):342–344. doi: 10.1164/ajrccm.161.2.16125_2. [DOI] [PubMed] [Google Scholar]
- 9.Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34(1):13–16. doi: 10.1183/09031936.00190908. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration. 2010;80(2):89–95. doi: 10.1159/000315416. [DOI] [PubMed] [Google Scholar]
- 11.Burge PS, Calverley PM, Jones PW. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008:2407–2416. doi: 10.1001/jama.2008.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauwels RA, Lofdahl CG, Laitinen LA. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 14.Yang IA, Fong KM, Sim EH, Black PN, Lasserson TJ. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD002991. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNF alpha in pulmonary pathophysiology. Respir Res. 2006;7(1):125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duvoix A, Dickens J, Haq I, Mannino D, Miller B, Tal-Singer R, Lomas DA. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. doi: 10.1136/thoraxjnl-2012-201871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, Man SF, DeMeo DL, Litonjua AA. Associations of IL-6 polymorphisms with lung function decline and COPD. Thorax. 2009;64(8):698–704. doi: 10.1136/thx.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, Kaneko T. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2(2):CD012066. doi: 10.1002/14651858.CD012066.pub2. [DOI] [PMC free article] [PubMed]
- 19.Vestbo J, Rennard S. Chronic obstructive pulmonary disease biomarker(s) for disease activity needed—urgently. Am J Respir Crit Care Med. 2010;182:863–864. doi: 10.1164/rccm.201004-0602ED. [DOI] [PubMed] [Google Scholar]
- 20.Julious Steven A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist. 2005;4(4) doi: 10.1002/pst.185. 287 - 291. [DOI] [Google Scholar]
- 21.Cazzola M, Matera MG, Santangelo G, Vinciguerra A, Rossi F, D'Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89(5):357–362. doi: 10.1016/0954-6111(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 22.Banerji D, Patalano F. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2016;110:79–80. doi: 10.1016/j.rmed.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63:402–407. doi: 10.1136/thx.2007.085456. [DOI] [PubMed] [Google Scholar]
- 24.Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT) Respir Med. 2010;104(11):1659–1667. doi: 10.1016/j.rmed.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Martinez CH, Diaz AA, Parulekar AD, Rennard SI, Kanner RE, Hansel NN, Couper D, Holm KE, Hoth KF, Curtis JL, Martinez FJ, Hanania NA, Regan EA, 3rd Paine R, Cigolle CT, Han MK, Gene COPD, Investigators SPIROMICS. Age-Related Differences in Health-Related Quality of Life in COPD: An Analysis of the COPDGene and SPIROMICS Cohorts. Chest. 2016;149(4):927–935. doi: 10.1016/j.chest.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swinney DC. Biochemical mechanisms of drug action: what does it take for success? Nat Rev Drug Discov. 2004;3(9):801–808. doi: 10.1038/nrd1500. [DOI] [PubMed] [Google Scholar]
- 27.Cazzola M, Calzetta L, Ora J. Searching for the synergistic effect between aclidinium and formoterol: from bench to bedside. Respir Med. 2015;109(10):1305–1311. doi: 10.1016/j.rmed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Um SW, Yoo CG, Kim YW, Han SK, Shim YS. The combination of tiotropium and budesonide in the treatment of chronic obstructive pulmonary disease. J Korean Med Sci. 2007;22(5):839–845. doi: 10.3346/jkms.2007.22.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loppow D, Schleiss MB, Kanniess F, Taube C, Jörres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respir Med. 2001;95(2):115–121. doi: 10.1053/rmed.2000.0960. [DOI] [PubMed] [Google Scholar]
- 30.Haque R, Hakim A, Moodley T, Torrego A, Essilfie-Quaye S, Jazrawi E. Inhaled long-acting β2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. J Allergy Clin Immunol. 2013;132(5):1166–1173. doi: 10.1016/j.jaci.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 31.Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, Adcock IM. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172(6):704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19(1):182–191. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 33.Miravitlles M, Soler-Cataluña JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J. 2013;41(6):1252–1256. doi: 10.1183/09031936.00118912. [DOI] [PubMed] [Google Scholar]
- 34.Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med. 2012;106(3):319–328. doi: 10.1016/j.rmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Suissa S, Dell'Aniello S, Ernst P. Comparative Effectiveness and Safety of LABA-LAMA vs LABA-ICS Treatment of COPD in Real-World Clinical Practice. Chest. 2019;155(6):1158–1165. doi: 10.1016/j.chest.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Mahler DA, Witek TJ., Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi: 10.1081/COPD-200050666. [DOI] [PubMed] [Google Scholar]
- 37.Beeh KM, Derom E, Echave-Sustaeta J, Grönke L, Hamilton A, Zhai D. The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study) Int J Chron Obstruct Pulmon Dis. 2016;11:193–205. doi: 10.2147/COPD.S95055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigo GJ, Price D, Anzueto A, Singh D, Altman P, Bader G, Patalano F, Fogel R, Kostikas K. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis Int J Chron Obstruct Pulmon Dis. 2017;12:907-922. doi: 10.2147/COPD.S130482. eCollection 2017 [DOI] [PMC free article] [PubMed]
- 39.Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25. doi: 10.1136/thoraxjnl-2014-206732. [DOI] [PubMed] [Google Scholar]
- 40.Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5) doi: 10.2165/00003495-200969050-00004. 549-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang AX, Lu LW, Liu WJ, Huang M. Plasma Inflammatory Cytokine IL-4, IL-8, IL-10, and TNF-α Levels Correlate with Pulmonary Function in Patients with Asthma-Chronic Obstructive Pulmonary Disease (COPD) Overlap Syndrome. Med Sci Monit. 2016;22:2800–2808. doi: 10.12659/msm.896458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes PJ. Emerging pharmacotherapies for COPD. Chest. 2008;134(6):1278–1286. doi: 10.1378/chest.08-1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.