Abstract
Background
The first treatment option for major depressive disorder (MDD) is antidepressants, however, there is substantial demand for alternative therapies due to its low compliance and remission rates. This study was aimed to explore the effectiveness, safety, and feasibility of electroacupuncture plus moxibustion therapy for MDD.
Methods
Thirty adults with MDD were randomly assigned to the treatment group (TG) or control group (CG). The TG was treated with electroacupuncture plus moxibustion, and the CG received sham interventions at non-acupoints for 8 weeks. The primary outcome measure was the intergroup difference of the mean change of total score of the Hamilton rating scale for depression (HRSD) between baseline and week 9. Secondary outcome measures were Beck's depression inventory, insomnia severity index, the state-trait anxiety inventory, the EuroQol-5 dimension index, the measure yourself medical outcome profile version 2, and frontal alpha asymmetry measured by electroencephalography. Adverse events (AEs) were monitored for safety assessment.
Results
The primary outcome measure was not significantly different between the two groups (p=0.2641), although the scores of HRSD in both groups improved significantly after treatment. No significant difference was identified between groups in secondary outcome measures. The incidence of AE was not significantly different between the two groups (p=0.1067).
Conclusion
A clinical trial using electroacupuncture plus moxibustion for MDD seems feasible. However, further studies with the larger size, adopting ideal controls are warranted to provide a confirmative conclusion to the efficacy and safety of electroacupuncture plus moxibustion for MDD.
Keywords: Major depressive disorder, Electroacupuncture, Moxibustion, Randomized controlled trial
1. Introduction
Major depressive disorder (MDD) is expected to be a major cause of disability worldwide by 2030.1 .Antidepressant is a routinely accepted therapeutic choice for MDD, but the low compliance, row remission rate, and adverse drug reactions in long term use often lead to discontinuation of medication.2
These limitations of antidepressants increase the demand for non-pharmacological treatment.3 Many patients with MDD use a variety of therapeutic approaches including complementary and alternative approaches such as traditional medicine,3 and simultaneous performance of acupuncture or electroacupuncture plus moxibustion is one of the commonly used therapies for the treatment of depression in traditional medicine.4,5 Acupuncture plus moxibustion therapy has been shown to have greater benefits and fewer adverse events.
Previous studies demonstrated that this treatment had better antidepressant effects than no treatment6,7 or minimal acupuncture at non-acupoint intervention 8,9 and the finding from these studies have shown the therapeutic potential of acupuncture, or electroacupuncture and moxibustion, as alternative options for MDD. However, systematic reviews of these studies did not draw definitive conclusions because the methodological shortcomings of the primary clinical trials.10, 11, 12 Therefore, this study aimed to explore the effectiveness, safety and feasibility of electroacupuncture plus moxibustion therapy for MDD compared to sham control through rigorously designed randomized controlled trial (RCT).
2. Methods
2.1. Study design
This is a multicenter, randomized, gender-stratified, patient- and assessor-blinded, sham-controlled, two-armed preliminary clinical trial. This RCT was conducted at two hospitals in South Korea (Daejeon Oriental Hospital of Daejeon University, Kyung Hee University Oriental Hospital at Gangdong) from December 2015 to November 2016. The protocol of the trial, approved from the institute of review board (IRB) of each hospital, complied with the consolidated standards of reporting trials (CONSORT) and standards for reporting interventions in clinical trials of acupuncture (STRICTA) checklists (Additional file 1 &2). The protocol was registered in the clinical research information service (CRIS-KCT0001810, Korean Clinical Trial Registry) and has already been published.13
Candidate participants were recruited using flyers, local newspapers, and online/offline billboards of the hospitals. Written informed consent was obtained from all participants who voluntarily visited the clinical trial centers to take part in this trial.
2.2. Study participants
This study is for adults (19–65 years) with MDD which meets the diagnostic and statistical manual of mental disorders version 4 (DSM-IV) criteria. Only patients whose total score between 7 and 24 on the Hamilton rating scale for depression (HRSD) were included to select mild to moderate depression. Patients at high risk of suicidal attempts, those with impaired communication due to severely unstable mental disorders, and those with severely unstable medical conditions were excluded from the study. Pregnant women, nursing mothers, and women with pregnancy plans were not included. Those with hormonal disorders that could affect mood and who have experienced major stressful life events within one year were also excluded from the study. Persons who have received any therapeutic interventions using traditional Korean medicine or non-psychopharmacological drugs with psychotropic activities within 14 days prior to the screening date were excluded. Persons who received any type of psychotropic drugs, psychotherapy, electroshock therapy, or transcranial magnetic stimulation within 30 days before screening date were not included in this study. Patients who have inflammation at the site to be treated, those with hemorrhagic diseases, those taking anticoagulants, and those with a history of severe head injury or other serious physical disorders were not allowed to be the participants of this study.
2.3. Randomization and allocation concealment
Thirty participants who met the eligibility criteria were randomly assigned to two groups, treatment group (TG) and control group (CG) in an equal ratio. Random sequence was generated by an independent statistician using the SAS package (version 9.4, SAS Institute, INC., Cary, NC, USA). Stratification was assigned according to gender and clinical trial center. Group assignments were sealed in opaque envelopes numbered sequentially. When the subject passed the screening, the practitioner opened the envelope to confirm the group assignment. Opened envelopes were stored in a separate double-lock cabinet to make them inaccessible to anyone except practitioners.
2.4. Blinding
This study was designed to blind assessors and participants. Because of the unique nature of the intervention, electroacupuncture and moxibustion, the practitioner could not be blinded. Therefore, the outcome assessment was done by researchers who did not involve in the intervention process. To blind participants, sham devices were adopted for control intervention (See interventions part and pre-published protocol13). To confirm the patient blinding, a survey using a questionnaire was conducted. After the first intervention, participants were asked to choose one out of the three: whether they guess they received a real treatment, sham treatment, or do not know. To evaluate the success of the patient blinding, the blinding index was calculated according to the Bang's method.14
2.5. Interventions
Subject of TG received the electroacupuncture plus moxibustion therapy according to a treatment regimen made through reviewing relevant textbooks and academic achievements and discussion between clinicians and researchers. Acupuncture was done using disposable stainless steel acupuncture needles (Dong-Bang Acupuncture Inc., Seoul, Korea, diameter 0.25 mm, length 40 mm) at following acupoints: GV20, EX-HN3, GV24, CV17, and bilateral LI4 and PC6. Up to 2 more acupoints could be added at the practitioner's discretion. After inserting the acupuncture and acquiring deqi, electrical stimulation (10 Hz, using ES-160, Ito Co. Ltd., Tokyo, Japan) between GV20 and EX-HN3 was delivered for 20 min. For moxibustion, Mox-A JookYoum device (GuoKu Industrial, Gimpo, Korea, diameter 40 mm, height 50 mm) was applied at acupoints, CV12 and CV4, for 20 min.
Participants in CG received electroacupuncture plus moxibustion therapy with sham devices. For electroacupuncture, sham acupuncture using Park sham placebo device (Dong-Bang AcuPrime Ltd., Exeter, UK) with mock electrical stimulation at non-acupoints. For moxibustion, a sham device with a thermal barrier buffer installed on the base of the same moxibustion device was used at non-acupoints. The detailed description of the developed sham moxibustion device and the location of the non-acupoints are presented in the pre-published protocol13 and Additional file 2.
All participants received 20 sessions for 8 weeks. For treatment, participants were asked to visit the clinical trial center three times a week for the first four weeks and then twice a week for the remaining four weeks. All participants received a brochure with information about MDD.
The electroacupuncture plus moxibustion treatment was performed by licensed doctors of Korean medicine. The practitioners and assessors were instructed to allow only minimal contact with the patient and to avoid unnecessary interaction. To standardize the whole process of the trial, all researchers were given a one-day course orientation. To assess the investigator's compliance with the protocol, trial monitors regularly visited the clinical trial centers to check the study procedures and documentation.
2.6. Drop-out criteria
Participants were discontinued from the study: if they withdrew their consent to participate in the study, if they were found to have violation of the eligibility criteria, if they could not remain in the study due to serious adverse reactions, if they missed five or more sessions of planned treatment, if they lose contact, or if they received any treatment to alleviate symptoms of MDD other than the interventions provided in this study during the 8-week treatment period.
2.7. Outcome measures
The primary outcome measure of this study was the intergroup difference of the mean change of total score of 17-item HRDS between baseline and week 9 (after completion of the 8-week treatment). Secondary outcome measures included the mean change of HRDS measured at week 5 and 13 compared to baseline. The changes of the Beck's depression inventory (BDI), the insomnia severity index (ISI), the state-trait anxiety inventory (STAI), the EuroQol-5 dimension index (EQ-5D) and the measure yourself medical outcome profile version 2(MYMOP2) were also evaluated. To assess frontal alpha asymmetry, alpha power of the left and right frontal lobe was measured by computerized electroencephalography (EEG) (Model: QEEG-8, LEXJ108, LAXTHA Inc., Korea) and then the values were substituted into the formula by Rosenfeld et al: (R-L)/(R+L).15
A subgroup analysis was performed to identify the gender differences in treatment response. Pre- and post-treatment changes in HRDS, BDI, and ISI values obtained from female participants only were evaluated.
We used validated version of Korean translation for all questionnaires. A detailed description of each rating scale was presented in the pre-published protocol.13
2.8. Adverse events
For safety assessment, all adverse events (AEs) that occurred during the study period were recorded at every visit. The trial monitors who regularly visit the trial centers checked the procedures and documentation to confirm the safety status of the participants. The severity of AEs was classified into mild, moderate and severe. The causal relationship between the AEs and the interventions were also assessed. The incidence of intervention-related AEs per visit was calculated and compared by group.
2.9. Statistical analysis
Sample size of this study was calculated based on the results of similar previous study (mean difference 4.34, standard deviation 3.75).16 Assuming a significance level of 0.05, power of 90%, and dropout rate of 20%, a total of 30 participants were required for this trial.13
Statistical analysis was performed using SAS® Version 9.4 (SAS institute. Inc, Cary, NC) by an independent statistician. To realize the ideals of intention-to-treat (ITT) analysis, statistical analysis was done using a full analysis set (FAS). Missing values were processed by the multiple imputation method. A two-sided test was done with a significance level of 0.05.
The mean or frequency of demographic characteristics, including gender, age, body mass index, occupation, duration since onset was presented along with their 95% confidence interval (CI). The difference in outcomes measured before and after the initiation of the treatment was also presented by mean along with their 95% CI.
Analysis of covariance (ANCOVA) was performed to evaluate the primary outcome measure, with the post-treatment value of HRSD as a dependent variable, the group as a fixed factor, and the baseline measure as a covariate. Changes of secondary outcome measures before and after treatment were analyzed using the same methods as those of the primary outcome measure. Differences in the pre- and post-treatment values within the groups were assessed using the paired t-test. The difference between the two groups of the blinding index was analyzed using the new blind index method proposed by Bang.14
3. Results
3.1. Flow of participants
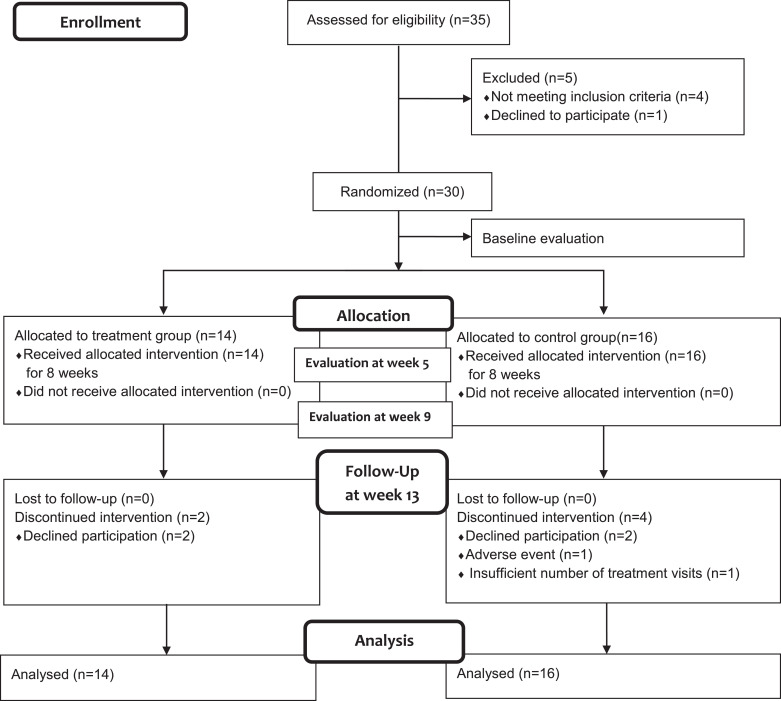
Thirty five participants signed the consent form. Four of them did not meet the eligibility criteria and one withdrew consent from the study. A total of 30 people from two centers were enrolled in this study. Fourteen were assigned to TG and sixteen to CG. After randomization, two in the TG and four in the CG dropped out during the study period. Two of the TG withdrew their consent. Two of the CG withdrew with their consent, one missed the scheduled treatment more than five times, and the other gave up receiving treatment due to AE (Fig. 1).
Fig. 1.
Flow chart.
3.2. Baseline characteristics
Demographic characteristics of the TG and CG in the baseline were summarized in Table 1. Although the duration since onset was about 15 years for TG and 22 years for CG, none of the demographic characteristics showed statistically significant difference between groups (Table 1).
Table 1.
Baseline characteristics of study participants.
| Treatment group (n = 14) | Control group (n = 16) | p-value | |
|---|---|---|---|
| Characteristics | Mean (95% CI) or Freq (%) | Mean (95% CI) or Freq (%) | |
| Gender (Male / Female)† | 3 (21.43%) / 11 (78.57%) | 4 (25.00%) / 12 (75.00%) | 0.9999 |
| Age‡ | 48.36 (40.62, 56.09) | 51.94 (45.69, 58.18) | 0.4414 |
| BMI(kg/m2)‡ | 22.28 (20.06, 24.50) | 23.41 (21.22, 25.60) | 0.4465 |
| Job (Yes / No)† | 8 (57.14%) / 6 (42.86) | 5 (31.25%) / 11 (68.75) | 0.2685 |
| Duration of symptom (mn)‡ | 177.10 (69.27, 285.00) | 259.80 (111.70, 407.80) | 0.1585 |
| HRSD‡ | 19.21 (18.22, 20.21) | 16.75 (15.20, 18.30) | 0.0098 |
| BDI‡ | 30.29 (25.28, 35.29) | 27.69 (21.49, 33.88) | 0.4987 |
| ISI‡ | 18.57 (16.23, 20.91) | 16.19 (14.49, 17.89) | 0.0823 |
| STAI (state)‡ | 39.36 (34.18, 44.53) | 39.38 (36.60, 42.15) | 0.9946 |
| STAI (trait)‡ | 49.71 (44.85, 54.58) | 46.13 (42.93, 49.32) | 0.1853 |
| EQ-5D‡ | 0.766 (0.680, 0.853) | 0.775 (0.701, 0.850) | 0.8627 |
| MYMOP2 (symptom 1)‡ | 4.86 (4.36, 5.36) | 4.25 (3.94, 4.56) | 0.0299 |
| MYMOP2 (symptom 2)‡ | 4.23 (3.73, 4.73) | 4.33 (3.77, 4.90) | 0.7682 |
| MYMOP2 (activity)‡ | 4.25 (3.70, 4.80) | 4.38 (3.95, 4.80) | 0.6972 |
| MYMOP2 (well-being)‡ | 4.07 (3.50, 4.65) | 3.75 (3.29, 4.21) | 0.3502 |
| MYMOP2 (profile)‡ | 4.13 (3.74, 4.51) | 3.91 (3.56, 4.25) | 0.3705 |
| Frontal alpha asymmetry‡ | -1.23(-8.61, 6.15) | 2.77(-4.01, 9.54) | 0.3965 |
†Fisher`s exact test; ‡ Student`s independent t-test
CI, confidence interval; Freq, frequency; BMI, body mass index; mn, months; HRSD, Hamilton rating scale for depression; BDI, Beck's depression inventory; ISI, insomnia severity index; STAI, state-trait anxiety inventory; EQ-5D, EuroQol-5 dimension index; MYMOP2, measure yourself medical outcome profile version 2
3.3. Primary outcome
The primary outcome measure, the mean change of HRSD from baseline to week 9, was not significantly different between TG and CG (MD3.42, 95% CI -2.60 to 9.45, P = 0.2641) (Table 2). However, the mean HRSD score of the TG was significantly higher than that of CG (Table 1). Within-group improvement in HRSD from baseline to week 9 was statistically significant in both groups (Table 3).
Table 2.
Primary and secondary outcome measures (post-treatment values).
| Outcome | Group | week 5 | week 9 | week 13 |
|---|---|---|---|---|
| HRSD | TG-CG | -1.53 (-5.79, 2.69) | 3.42 (-2.60, 9.45) | 0.34 (-5.50, 6.19) |
| p-value | 0.4753 | 0.2641 | 0.9079 | |
| BDI | TG-CG | 2.75 (-3.53, 9.03) | 4.65 (-2.56, 11.85) | -0.54 (-8.18, 7.11) |
| p-value | 0.3892 | 0.2053 | 0.8902 | |
| ISI | TG-CG | -0.06 (-4.36, 4.24) | -1.04 (-6.48, 4.39) | -1.98 (-6.80, 2.84) |
| p-value | 0.9770 | 0.7053 | 0.4177 | |
| STAI (state) | TG-CG | 0.73 (-3.13, 4.59) | -1.77 (-6.00, 2.47) | -1.20 (-5.29, 2.90) |
| p-value | 0.7090 | 0.4130 | 0.5660 | |
| STAI(trait) | TG-CG | 3.97 (-0.66, 8.61) | 1.66 (-2.84, 6.17) | 0.72 (-4.72, 6.15) |
| p-value | 0.0916 | 0.4688 | 0.7954 | |
| EQ-5D | TG-CG | -0.050 (-0.132, 0.031) | -0.011 (-0.100, 0.077) | 0.007 (-0.096, 0.111) |
| p-value | 0.2271 | 0.7990 | 0.8902 | |
| MYMOP2 (symptom1) | TG-CG | -0.53 (-1.54, 0.49) | -0.69 (-1.91, 0.52) | -0.25 (-1.71, 1.20) |
| p-value | 0.2952 | 0.2481 | 0.7201 | |
| MYMOP2 (symptom2) | TG-CG | -0.32 (-1.49, 0.86) | 0.39 (-0.97, 1.75) | 0.56 (-0.75, 1.86) |
| p-value | 0.5777 | 0.5491 | 0.3769 | |
| MYMOP2 (activity) | TG-CG | -0.76 (-1.69, 0.17) | -0.60 (-1.94, 0.73) | 0.60 (-1.00, 2.20) |
| p-value | 0.1019 | 0.3522 | 0.4370 | |
| MYMOP2 (well-being) | TG-CG | -1.02 (-2.11, 0.07) | -0.92 (-1.88, 0.03) | -0.33 (-1.49, 0.83) |
| p-value | 0.0648 | 0.0569 | 0.5616 | |
| MYMOP2 (profile) | TG-CG | -0.50 (-1.43, 0.43) | -0.51 (-1.45, 0.42) | 0.09 (-0.80, 0.98) |
| p-value | 0.2723 | 0.2667 | 0.8361 | |
| Frontal alpha asymmetry | TG-CG | 3.17 (-13.62, 19.97) | -3.11 (-11.78, 5.55) | -1.69 (-12.66, 9.28) |
| p-value | 0.6960 | 0.4601 | 0.7508 |
P-values are for the difference of least square mean by analysis of covariance (between-group comparison).
N=14 for TG, and 16 for CG.
HRSD, Hamilton rating scale for depression; BDI, Beck's depression inventory; ISI, insomnia severity index; STAI, state-trait anxiety inventory; EQ-5D, EuroQol-5 dimension index; MYMOP2, measure yourself medical outcome profile version 2; TG, treatment group; CG, control group
Table 3.
Primary and secondary outcome measures (changes from baseline).
| Outcome | group | Baseline to week 5 | p-value | Baseline to week 9 | p-value | Baseline to week 13 | P-value |
|---|---|---|---|---|---|---|---|
| HRSD | TG | -8.25 (-11.69, -4.81) | 0.0003 | -8.00 (-12.65, -3.35) | 0.003 | -9.00 (-13.68, -4.32) | 0.0014 |
| CG | -6.38 (-8.94, -3.83) | 0.0002 | -10.38 (-13.78, -6.99) | <.0001 | -9.42 (-12.70, -6.14) | <.0001 | |
| BDI | TG | -11.17 (-16.10, -6.23) | 0.0004 | -12.25 (-18.27, -6.23) | 0.0009 | -15.33 (-22.23, -8.44) | 0.0005 |
| CG | -10.08 (-16.30, -3.86) | 0.0041 | -13.46 (-19.91, -7.01) | 0.0007 | -12.33 (-18.67, -6.00) | 0.0013 | |
| ISI | TG | -4.17 (-7.35, -0.99) | 0.0149 | -7.33 (-11.01, -3.66) | 0.0011 | -8.08 (-11.60, -4.57) | 0.0004 |
| CG | -3.15 (-6.74, 0.43) | 0.0792 | -6.77 (-10.74, -2.80) | 0.003 | -7.00 (-10.16, -3.84) | 0.0005 | |
| STAI (state) | TG | -2.17 (-8.03, 3.69) | 0.433 | -3.25 (-8.86, 2.36) | 0.2282 | -2.58 (-7.47, 2.30) | 0.2693 |
| CG | -1.92 (-6.68, 2.83) | 0.3955 | -0.85 (-5.33, 3.63) | 0.688 | -1.25 (-6.37, 3.87) | 0.6017 | |
| STAI (trait) | TG | -1.50 (-5.90, 2.90) | 0.4686 | -3.75 (-6.99, -0.51) | 0.0272 | -5.42 (-9.58, -1.25) | 0.0155 |
| CG | -2.62 (-6.03, 0.80) | 0.121 | -2.69 (-7.15, 1.76) | 0.2127 | -3.00 (-8.06, 2.06) | 0.2187 | |
| EQ-5D | TG | 0.013 (-0.091, 0.116) | 0.7937 | 0.032 (-0.041, 0.106) | 0.3508 | 0.056 (-0.018, 0.129) | 0.1239 |
| CG | 0.046 (-0.016, 0.106) | 0.1301 | 0.053 (-0.037, 0.143) | 0.221 | 0.059 (-0.056, 0.173) | 0.2857 | |
| MYMOP2 (symptom1) | TG | -1.50 (-2.01, -0.99) | <0.0001 | -1.42 (-2.25, -0.58) | 0.0033 | -2.17 (-3.21, -1.12) | 0.0008 |
| CG | -1.75 (-2.61, -0.89) | 0.0010 | -1.77 (-2.59, -0.95) | 0.0005 | -2.17 (-3.10, -1.23) | 0.0003 | |
| MYMOP2 (symptom2) | TG | -0.58 (-1.22, 0.05) | 0.0674 | -1.00 (-1.85, -0.15) | 0.0255 | -1.27 (-2.32, -0.23) | 0.0218 |
| CG | -0.89 (-2.01, 0.23) | 0.1038 | -0.67 (-1.82, 0.48) | 0.2191 | -0.63 (-1.51, 0.26) | 0.1395 | |
| MYMOP2 (activity) | TG | -0.67 (-1.44, 0.10) | 0.0805 | -1.11 (-2.09, -0.14) | 0.0304 | -1.89 (-3.13, -0.65) | 0.0080 |
| CG | -1.58 (-2.50, -0.67) | 0.0029 | -2.08 (-3.05, -1.11) | 0.0005 | -1.83 (-2.91, -0.76) | 0.0032 | |
| MYMOP2 (wellbeing) | TG | -1.18 (-2.17, -0.19) | 0.0237 | -0.75 (-1.80, 0.30) | 0.1455 | -1.18 (-2.26, -0.11) | 0.0344 |
| CG | -1.36 (-2.42, -0.31) | 0.0162 | -1.25 (-2.23, -0.27) | 0.0172 | -1.33 (-2.32, -0.34) | 0.0128 | |
| MYMOP2 (profile) | TG | -0.98 (-1.62, -0.33) | 0.0065 | -1.00 (-1.68, -0.32) | 0.0082 | -1.50 (-2.17, -0.83) | 0.0004 |
| CG | -1.40 (-2.08, -0.71) | 0.0009 | -1.46 (-2.12, -0.80) | 0.0004 | -1.44 (-2.07, -0.80) | 0.0004 | |
| Frontal alpha asymmetry | TG | -14.76 (-27.15, -2.37) | 0.0252 | -4.95 (-15.29, 5.39) | 0.3016 | -6.10 (-4.28, 14.89) | 0.2882 |
| CG | 1.54 (-8.73, 11.81) | 0.7423 | -4.68 (-15.80, 6.44) | 0.3701 | -6.98 (-18.83, 4.88) | 0.2159 |
P-values are for the paired t-test (within-group comparison).
N=14 for TG, and 16 for CG.
HRSD, Hamilton rating scale for depression; BDI, Beck's depression inventory; ISI, insomnia severity index; STAI, state-trait anxiety inventory; EQ-5D, EuroQol-5 dimension index; MYMOP2, measure yourself medical outcome profile version 2; TG, treatment group; CG, control group
3.4. Secondary outcomes
The mean HRSD total scores measured at week 5 and week 13 were significantly improved in both groups compared to baseline. However, the mean difference between the two groups were not statistically significant (Tables 2 and 3).
There was no significant difference in ISI values between two groups at baseline (Table 1). The TG began to show significant improvement from week 5 and CG from week 9 (Table 3). However, no significant differences were found between the two groups after intervention (Table 2).
Anxiety scores of TG and CG assessed by STAI were not significantly different at baseline (Table 1). In the state anxiety scores, neither groups showed significant changes after treatment (Table 3) and no significant intergroup differences were observed (Table 2). Trait anxiety scores of the two groups were also not statistically different at baseline (Table 1). After treatment, only TG showed significant decrease from week 9 compared to baseline, however, no significant differences were found between groups in trait anxiety scores (Tables 2 and 3).
There was no significant difference between the two groups of the total scores of EQ-5D at baseline (Table 1). No significant difference between the groups, and no significant longitudinal changes within the groups were observed after intervention (Tables 2 and 3).
In case of MYMOP2, no significant differences were observed between groups at baseline except for the domain symptom 1 (Table 1). After the intervention, significant changes were observed in most of the domains of the MYMOP2 (Table 3), however, any significant differences were found in the intergroup comparisons (Table 2).
In frontal alpha asymmetry measured by EEG, no significant differences were observed between groups either at baseline or after the intervention (Table 1 & 2), although a significant change was found temporarily in TG only in week 5 (Table 3).
3.5. Subgroup analysis
A subgroup analysis was performed on female participants alone (11 in TG and 12 in CG). The results from the female subgroup were generally similar to those from the whole group analysis. There was no difference between the two groups in the mean change of HRSD, BDI and ISI. However, similar to the whole group analysis, all post-treatment outcomes showed a significant improvement over baseline (Additional file 3).
3.6. Adverse events
Of the 30 participants, 14 experienced AEs during the trial. Screening out cases with clearly no association with the study intervention, 10 AEs were detected in 8 participants. For each group, there were 8 AEs per 299 visits (2.78%) in the TG and 2 AEs per 291 visits (0.69%) in the CG, with no significant difference in the AE incidences per visit between the two groups (P-value 0.1067).
Six participants experienced 3 types of AEs (5 mild burns, 2 rash, 1 pain) in TG and two participants reported only burn (2 moderate burns) in CG. All AEs in the TG were mild which disappeared without leaving any sequelae without further measures. One of the two participants who experienced AEs in CG had to discontinue the planned intervention of this trial to receive additional treatment for moderate burn, and another one was cured without sequelae without further measures.
3.7. Blinding index
Eleven participants from TG and thirteen from CG took a survey to calculate the blinding index. About 73% of the TG had an accurate estimate of the intervention they received, while 85% of the CG had an opposite estimate. The responses of each group to the blinding questionnaire and the calculated values of the blinding index were summarized in table 4.
Table 4.
Blinding index.
| Response | Assignment |
|
|---|---|---|
| Treatment group (n = 11) | Control group (n = 13) | |
| Treatment group | 8 (72.7%) | 11 (84.6%) |
| Control group | 1 (9.1%) | 1 (7.7%) |
| Do not know | 2 (18.2%) | 1 (7.7%) |
| Blinding index | 0.636 (0.256, 1.016) | -0.769 (-1.082, -0.456) |
4. Discussion
The dropout rate (20%) of this study was consistent with the value assumed at the study design stage. Considering that this value is lower than the average dropout rate of antidepressants trials (23%) and no serious adverse drug reactions, such as suicide attempts or mania, which are the main reasons for dropout in many antidepressant clinical trials, were not occurred in this study,17 RCTs conducting this type of intervention in the participants with MDD of mild to moderate level are considered feasible.
Primary outcome measure of this study was the change of the score of HRSD, a scale to assess the severity of depression, however, the mean scores of HRSD were 19.21 in TG and 16.75 in CG, which was significantly different in statistics. The scores correspond to moderate depression and mild depression in TG and CG, respectively,18 which means that they were statistically and also clinically different groups at baseline in terms of HRSD. Although the study was designed based on rigorous methodologies, involving various steps to minimize the risk of biases, selection bias occurred. This bias is presumed to be due to the inherent limitations of the small scale of this preliminary trial. We expect that this type of bias could be adjusted increasing the sample size in a full-sized RCT in the future. However, we used ANCOVA with baseline values set as covariates. In other words, the results of the comparison between two groups of post-treatment values were corrected for differences in the baseline, so we believe that the results of this study are statistically reliable despite the difference in the baseline.
Despite the significant differences in the severity of depression measured by HRDS, the mean changes detected at week 5, 9, and 13 were not significantly different between groups. Unlike previous RCTs, where acupuncture plus moxibustion therapy significantly improved HRDS,7,9,19 studies conducting systematic review and meta-analysis20,21 dealing with this topic reported no significant difference between groups as shown in this study. Although this study could not demonstrate a substantial difference between TG and CG, both groups showed statistically significant improvement after the intervention compared to at baseline and this difference was also clinically significant, too. The minimal clinically important difference (MCID) of HRDS is 27.1 ± 25.7%22 and both groups have achieved further improvement already at week 5 (RA 42%, SA 38% change compared to baseline).
Similar to the pattern of HRSD, post-treatment scores of BDI, another tool for assessing the severity of depression, also did not differ between groups. However, both groups showed statistically significant improvement over baseline, and this difference was also clinically significant. The MCID of BDI is known as 5 points or 30%.22 At week 5, both groups showed a more than 10-points reduction compared to baseline, showing a substantial change over the MCID .In addition, after the planned treatment was completed, both groups maintained mild depression levels.
The mechanism of acupuncture to relieve depression is reported to be related to the multi-target involvement in the neuroendocrine and immune system.23 Moxibustion is also known to be involved in the regulation of the hypothalamic-pituitary-adrenal axis, hippocampal upregulation and neurotransmitter production.24,25 The causes and mechanisms of depression have not yet been elucidated, but previous studies suggest that a variety of factors, including social and genetic factors, contribute to the dysfunction of the central nervous system.26 Acupuncture and moxibustion may also contribute to relieving depression by acting on associated multi-targets.
Depression is not the only problem in MDD patients. The relationship between sleep disturbance, anxiety and depression is well known. Post-treatment scores of ISI, a scale to assess the quality of sleep, showed no significant difference between two groups in this study. However, starting from week 5, clinical severity of insomnia decreased to subthreshold level in both groups27 In addition, the improvement in ISI scores of the two groups was statistically significant, larger than the MCID of ISI, 6-points, from week 9.28 No substantial change was identified in terms of anxiety and quality of life.
Burns, allergic reactions, and infections of the treatment site are well-known AEs of moxibustion.29 Previous studies reported the incidence of moxibustion-related AEs were only 1%.30,31 This study, however, detected more frequent, especially moderate level of burns. Possible reasons for the difference from the previous studies are considered that, first, this study collected AEs very actively and recorded every AEs completely. Second, the inconsistency and instability of the temperature control of the sham moxibustion device used in this study could be causative to the frequent occurrence of burns. Third, we need to consider the possibility of dull heat sensitivity in MDD patients, the participants of this study. However, given the nature of patients with depression, it is more likely that the participants of this study were hesitant to actively inform the practitioner even if they had felt aches from excessive heat stimulation. When considering moxibustion treatment for patients with MDD in future clinical trials or clinical settings, special consideration should be given to the high likelihood of burns.
This study was rigorously designed to complement the limitations of existing similar studies. First, most of them were from RCTs in China,32 so it was difficult to apply the findings from the previous studies to the general population outside China. The target population of this study, adults with MDD in South Korea, expands the scope of existing trials. Second, compared with previous studies that did not include various outcome measures,10,32 this study used more diverse variables. Third, unlike previous studies that used rating scales that are not commonly accepted internationally,10,32 this study adopted only widely used and validated assessment scales in the clinical and research field. Fourth, unlike previous similar studies that lacked safety reports,32 this study collected AEs at every visit and reported them without exception. Fifth, some of the previous studies were assessed for lack of follow-up monitoring,10,32 but this study monitored the participants for four more weeks even after completion of the intervention. Finally, the study was designed to minimize the possible risk of biases at whole procedures of the study. In particular, it is the strength of this study that blinding of participants and assessors were rigorously performed.
This study, nevertheless, did not show any significant differences between the TG and CG. Regarding the results of the safety profile of this study, which includes the frequent occurrence of burns in both groups, sham devices, especially sham moxibustion devices, did not seem to work well. Despite the heat buffer installed on the bottom of the sham moxibustion device, it seems that the temperature of the base of the sham device that is in contact with the skin was not controlled stably, which delivered a certain thermal stimulus to the skin of the participants. As a result, contrary to our intentions, it seems to have been a substantial thermal stimulus, and some of them cannot be ruled out as to whether they lead to therapeutic effects, whether specific or nonspecific. Proper development of sham devices for acupuncture, or electroacupuncture and moxibustion is urgent.
Despite the limitations described above, this study found the possibility that electroacupuncture plus moxibustion therapy might improve clinical symptoms in MDD, might be a relatively safe treatment, and this type of RCT could be feasible in patients with MDD. However, this is a pilot study involving a small number of participants, and there is a limit to applying an incomplete sham control as described above. Therefore, further studies with larger-sized RCTs with ideal controls are required to provide confirmative answers to the efficacy and safety of electroacupuncture plus moxibustion therapy for MDD.
Funding
This study was funded by Korea Institute of Oriental Medicine (K16122 and KSN1522120).
Ethical statement
This study was approved by the institutional review boards of the participating centers including Daejeon Oriental Hospital of Daejeon University (djomc-132-1) and Kyung Hee University Oriental Hospital at Gangdong (KHNMCOH 2015-09-001-003). Written informed consent was obtained from all participants before study participation.
Data availability
The datasets generated during the current study will be made available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Mikyung Kim: Writing – original draft, Writing – review & editing. Eun-Ji Choi: Conceptualization, Writing – review & editing. O-Jin Kwon: Methodology, Formal analysis, Writing – review & editing. Hyo-Ju Park: Conceptualization, Writing – review & editing. Ae-Ran Kim: Conceptualization, Writing – review & editing. Bok-Nam Seo: Conceptualization, Writing – review & editing. Sun-Yong Chung: Conceptualization, Writing – review & editing. Jun-Hwan Lee: Conceptualization, Writing – review & editing. Joo-Hee Kim: Conceptualization, Writing – review & editing.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We appreciate all the participants who volunteered for this trial.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2021.100727.
Contributor Information
Jun-Hwan Lee, Email: omdjun@kiom.re.kr.
Joo-Hee Kim, Email: jhkim714v@gmail.com.
Appendix. Supplementary materials
References
- 1.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. 2019 doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Ho SC, Chong HY, Chaiyakunapruk N, Tangiisuran B, Jacob SA. Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: a systematic review. J Affecti Disord. 2016;193:1–10. doi: 10.1016/j.jad.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 3.MacPherson H, Richmond S, Bland M, Brealey S, Gabe R, Hopton A. Acupuncture and counselling for depression in primary care: a randomised controlled trial. PLoS Med. 2013;10(9) doi: 10.1371/journal.pmed.1001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo JA, Nam YJ, Park YJ, Kwon YK. Review of recent clinical trials for depression in traditional Chinese medicine-based on randomized controlled trials and systematic reviews. J Physiol Pathol Korean Med. 2015;29(6):458–466. [Google Scholar]
- 5.Kim YJ, Park DS, Lee YH. A study of depression with acupuncture and moxibustion treatment in Chinese medical literature. J Acupunct Res. 2005;22(1):223–234. [Google Scholar]
- 6.Wang C, Liu M, Lv J, Li N. Effect of acupuncture and moxibustion on depressive states of stroke patients’ spouses. Zhongguo zhen jiu. 2015;35(3):223–226. [PubMed] [Google Scholar]
- 7.Jiang JF, Xu L, Lin YH, Lu JH, Chen LZ, Sun YN. Anti-depression effect of acupunctrue and moxibustion based on SSRIs medication. Zhongguo zhen jiu. 2012;32(3):219–223. [PubMed] [Google Scholar]
- 8.Fan L, Fu W-B, Xu N-G, Liu J-H, Fan L, Ou A-H. Impacts of acupuncture and moxibustion on outcome indeices of depression patients’ subjective reports. Zhongguo zhen jiu. 2012;32(5):385–389. [PubMed] [Google Scholar]
- 9.Fan L, Gong J, Fu W, Chen Z, Xu N, Liu J. Gender-related differences in outcomes on acupuncture and moxibustion treatment among depression patients. J Altern Complement Med. 2015;21(11):673–680. doi: 10.1089/acm.2015.0068. [DOI] [PubMed] [Google Scholar]
- 10.Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3 doi: 10.1002/14651858.CD004046.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartlehner G, Gaynes BN, Amick HR, Asher G, Morgan LC, Coker-Schwimmer E. Agency for Healthcare Research and Quality (US); Rockville (MD): 2015. AHRQ Comparative Effectiveness Reviews. Nonpharmacological Versus Pharmacological Treatments for Adult Patients With Major Depressive Disorder. [PubMed] [Google Scholar]
- 12.Gartlehner G, Wagner G, Matyas N, Titscher V, Greimel J, Lux L. Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open. 2017;7(6):e014912–e014e12. doi: 10.1136/bmjopen-2016-014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Choi E-J, Kim S-P, Kim J-E, Park H-J, Kim A-R. Electroacupuncture plus moxibustion therapy for patients with major depressive disorder: study protocol for a randomized controlled trial. Trials. 2017;18(1):16. doi: 10.1186/s13063-016-1741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Controlled Clin Trials. 2004;25(2):143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld JP, Baehr E, Baehr R, Gotlib IH, Ranganath C. Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. Int J Psychophysiol. 1996;23(1):137–141. doi: 10.1016/0167-8760(96)00037-2. [DOI] [PubMed] [Google Scholar]
- 16.Dong J-P, Sun W-Y, Wang S, Wu Z-Q, Liu F. Clinical observation on head point-through-point electroacupuncture for treatment of poststroke depression. Zhongguo zhen jiu. 2007;27(4):241–244. [PubMed] [Google Scholar]
- 17.Rohden AI, Benchaya MC, Camargo RS, Moreira TC, Barros HMT, Ferigolo M. Dropout prevalence and associated factors in randomized clinical trials of adolescents treated for depression: systematic review and meta-analysis. Clin Ther. 2017;39(5):971–992. doi: 10.1016/j.clinthera.2017.03.017. e4. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150(2):384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Ran L-h, Yuan X-i. Clinical observation on acupuncture and moxibustion in treating post-traumatic stress disorder after earthquake. J Chenghu Univ TCM. 2010;33(1):21–24. [Google Scholar]
- 20.Li S, Zhong W, Peng W, Jiang G. Effectiveness of acupuncture in postpartum depression: a systematic review and meta-analysis. Acupuncture Med. 2018;36(5):295–301. doi: 10.1136/acupmed-2017-011530. [DOI] [PubMed] [Google Scholar]
- 21.Dong B, Chen Z, Yin X, Li D, Ma J, Yin P, et al. The efficacy of acupuncture for treating depression-related insomnia compared with a control group: a systematic review and meta-analysis. 2017;2017:9614810. [DOI] [PMC free article] [PubMed]
- 22.Masson SC, Tejani AM. Minimum clinically important differences identified for commonly used depression rating scales. J Clin Epidemiol. 2013;66(7):805–807. doi: 10.1016/j.jclinepi.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 23.R-z Kou, H Chen, M-l Yu, T-c Xu, S-p Fu, S-f Lu. Acupuncture for behavioral changes of experimental depressive disorder: a systematic review and meta-analysis. Sci Rep. 2017;7(1):9669. doi: 10.1038/s41598-017-09712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Sang L, Xia X, Zhao R, Wang M, Hou X. Therapeutic duration and extent affect the effect of moxibustion on depression-like behaviour in rats via regulating the brain tryptophan transport and metabolism. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/7592124. 7592124-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi T, Qi L, Li J, Le J-J, Shao L, Du X. Moxibustion upregulates hippocampal progranulin expression. Neural Regen Res. 2016;11(4):610–616. doi: 10.4103/1673-5374.180746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4(1):7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Seo CW, Back YH, Lee JD, Choi DY. Review of papers on adverse events in the course of oriental medical treatment in the JKAMS and JCTKAM. J Acupunct Res. 2011;28(1):45–63. [Google Scholar]
- 30.Zhao JC, Shi K, Xue Y, Hong L, Yu JA. Moxibustion induced burns in a burn unit in northeast China: an 8 year retrospective analysis. J Burn Care Res. 2019 doi: 10.1093/jbcr/irz151. [DOI] [PubMed] [Google Scholar]
- 31.A multicenter prospective survey of adverse events associated with acupuncture and moxibustion in Japan. Med Acupunct. 2017;29(3):155–162. doi: 10.1089/acu.2017.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armour M, Smith CA, Wang LQ, Naidoo D, Yang GY, MacPherson H. Acupuncture Depress. 2019;8(8) doi: 10.3390/jcm8081140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study will be made available from the corresponding author on reasonable request.