Abstract
Background
Aerosolized drug therapy administered to mechanically ventilated patients is a standard part of pulmonary critical care medicine. Aerosol particle size and distribution are important factors in the optimal delivery of aerosolized drugs to ventilated patients.
Objective
The objective of this study was to characterize aerosol droplet size and distribution with laser diffraction for nebulized 3% sodium chloride, albuterol, and epoprostenol sodium (containing glycine) delivered via Aeroneb Solo Mesh Nebulizers (Aerogen, Mountain View, California).
Methods
A series of functional flow tests were run on each of 8 Solo mesh nebulizers before the study to verify accuracy of flow rates in milliliters per minute. Aerosolized droplets exiting the nebulizer heads were then measured using a phase Doppler particle analyzer. Data collected during delivery of 3% sodium chloride, albuterol, and epoprostenol sodium included aerosol droplet size distribution, mass median aerodynamic diameter (MMAD), and geometric standard deviation. For each Solo nebulizer, droplet size measurements were taken 2 cm away from the nebulizer head and 2 cm away from the wye of a heated, humidified adult ventilator circuit. For measurements taken at the wye, 4 distinct, continuous flow rates (2, 10, 20, and 40 L/min) were generated by an air pump to simulate inspiratory flows delivered with mechanical ventilation. The expiratory limb was capped, and the nebulizer head was inserted into the breathing circuit upstream of the humidifier.
Results
Each Solo nebulizer met Aerogen's recommended minimum flow rate of 0.2 mL/min, ranging from 0.23 to 0.31 mL/min. The MMAD of the 3 tested aerosols was several times smaller when measured at the wye outlet of the heated/humidified breathing circuit (0.82–2.73 µm) compared with droplets measured directly at the nebulizer outlet (MMAD, 4.6–7.3 µm). There was also significant variability across Solo heads with some ventilator flow rates. The mean MMAD at the wye for the 3% sodium chloride solution, albuterol, and epoprostenol test solutions was 1.62 µm, 1.09 µm, and 1.18 µm, respectively. The mean MMAD at the nebulizer for the 3% sodium chloride solution, albuterol, and epoprostenol test solutions was 5.37 µm, 5.73 µm, and 6.73 µm, respectively.
Conclusions
Results from this study suggest that particle size of aerosolized drugs administered via a commonly used setup for delivery of in-line aerosols to mechanically ventilated patients may be several times smaller than expected and may result in less drug being delivered to the patient than previously realized.
(Curr Ther Res Clin Exp. 2021; 82:XXX–XXX)
© 2021 Elsevier HS Journals, Inc.
Keywords: Epoprostenol, Flolan, Inhalation devices, Nebulizer, Particle size
Introduction
Aerosolized drug therapy administered to mechanically ventilated patients is a routine part of pulmonary critical care medicine.1 The chemical and physical properties of the aerosolized drug, the type of nebulizer, and patient-specific factors (eg, extent of lung damage or disease and changes in lung compliance) can influence the success of delivering aerosol therapy to the lungs.1, 2, 3, 4 For example, the size of aerosolized drug particles, as well as temperature and humidity within the inhalation ventilatory circuit, contributes to the pattern of distribution in the lungs.1 Large particles (>6 µm) tend to be deposited in the upper airways, small particles (<2 µm) mainly penetrate deep in the lung and alveoli, and particles >2 to <6 µm in size are typically deposited in the central and smaller airways.4,5
Particle size can be influenced by the formulation of the drug(s) and the type of aerosol device used.1,5,6 For example, epoprostenol, a pulmonary vasodilator, is commonly administered off-label using the Aeroneb Solo Mesh Nebulizer (Aerogen, Mountain View, California) in the acute care setting as a continuously nebulized medication.7 Uninterrupted delivery of inhaled pulmonary vasodilators is critical to avoiding complications, including rebound pulmonary hypertension.8, 9, 10, 11 It has been reported that formulations of inhaled epoprostenol containing glycine can clog ventilator filters and impair ventilation to the patient12, 13, 14 and potentially cause abrupt interruption of therapy.
The type of nebulizer used also influences drug deposition.1,6 For example, residual volume, the volume of drug remaining in the nebulizer after aerosol therapy, can vary across different types of nebulizer.1 Jet nebulizers have greater residual volume (ie, less efficient aerosol delivery) compared with mesh nebulizers (0.8–2.0 mL vs <0.2 mL).1,3 Also, placement of the nebulizer within the inhalation ventilatory circuit can influence drug deposition.6
The objective of this study was to characterize aerosol droplet size and distribution with laser diffraction for nebulized 3% sodium chloride, albuterol, and epoprostenol sodium (Flolan [GlaxoSmithKline, Research Triangle Park, North Carolina] reconstituted with pH 12 sterile diluent) using Aeroneb Solo Mesh Nebulizers.
Methods
Experimental setup
A simulated inspiratory limb system was designed to evaluate aerosol droplet size distribution, the mass median aerodynamic diameter (MMAD) (defined as the diameter above and below which lies 50% of the mass of the particles), and the geometric standard deviation using 8 Aeroneb Solo vibrating mesh nebulizers. The mist formed by the Aeroneb Solo nebulizer is created by the pumping action of a plate containing 1000 apertures vibrating at a high frequency (128 kHz), forcing the liquid through the apertures in the plate creating a low-velocity, fine-particle aerosol.15, 16, 17 Mesh nebulizers are characterized as being very efficient (ie, low residual volume) and are associated with short-treatment times.3,16
As illustrated in Fig. 1, the experimental setup consisted of an air pump connected by a piece of 22-mm diameter tubing to a high-efficiency particulate air filter, which was connected to a digital flow meter (TSI Alnor 4000 Series, TSI Incorporated, Shoreview, Minnesota). An Aeroneb Solo nebulizer with Pro-X controller (Aerogen) was inserted upstream of a Fisher & Paykel heated humidifier (Fisher & Paykel Healthcare, East Tamaki, Auckland, New Zealand) and inspiratory limb of a heated-wire adult ventilator circuit.
Fig. 1.
Experimental setup. HEPA = high-efficiency particulate air; IV = intravenous; PDPA = phase Doppler particle analyzer.
Before the start of the study, functional testing was performed on each Aeroneb Solo nebulizer to ensure minimal flow rates and proper function in accordance with manufacturer's guidelines. This procedure involved placing 0.6 mL saline in the medication cup of the nebulizer, turning on the nebulizer, and recording the time it took for the saline to completely nebulize. The minimum flow rate for the Aeroneb Solo nebulizer is 0.2 mL/min.18
Doppler testing procedure
Three testing solutions were used for this study: continuous 3% sodium chloride infusion delivered to the Aeroneb Solo nebulizer at 0.2 mL/min (12 mL/h); a 3-mL ampoule of albuterol in the nebulizer; a 1.5-mg vial of epoprostenol mixed with 50 mL pH 12 sterile diluent for a concentration of 30 µg/mL. The epoprostenol solution was covered in foil due to its light sensitivity, and delivered to the Aeroneb Solo nebulizer at 0.2 mL/min (12 mL/h). Aerogen's recommended input rate of medication into the Aeroneb Solo nebulizer during continuous nebulization is up to a maximum of 12 mL per hour.18 The same 8 nebulizer heads were used for each study solution. The nebulizer was rinsed by nebulizing 0.5 mL sterile water. The testing of each solution was done on different days to allow for adequate drying time between trials.
For each nebulizer head, droplet size measurements were taken at 2 locations: 2 cm away from the nebulizer head (Fig. 2A) and 2 cm away from the wye of a heated, humidified adult ventilator circuit to mitigate droplet evaporation (Fig. 2B). For measurements taken at the wye, 4 distinct, continuous flow rates (2, 10, 20, and 40 L/min) were generated by an air pump to simulate inspiratory flows delivered with mechanical ventilation. Flows had to be within ±0.2 L/min and heated to within ±2ᵒC of 37ᵒC after flow changes before data recording commenced. The expiratory limb was capped for the duration of testing, and the nebulizer head was inserted into the breathing circuit upstream of the humidifier for collection of droplet size measurements. Aerosolized droplets from the nebulizer head and at the wye were measured using a phase Doppler particle analyzer (receiver model RV2100, analyzer model FSA4000-1P; TSI Incorporated).
Fig. 2.
(A) Aerosol droplet size measurement setup at nebulizer. (B) Aerosol droplet size measurement setup at wye.
MMAD and geometric standard deviation were calculated using MATLAB software (MathWorks, Natick, Massachusetts) and the methodology of Lefebvre.19 Geometric standard deviation (sg) was obtained from a lognormal distribution fit to the data:
where D is diameter, µ is ln(Dng), and Dng is geometric number mean drop size.
MMAD is the drop diameter such that 50% of total liquid volume is in drops of smaller diameter. This was calculated both directly from the measured diameters and by fitting the data to the Rosin-Rammler distribution. The Rosin-Rammler volume-based probability distribution function was fit to the data:
where V is volume, X is size parameter, and q is spread parameter (large q indicates monodisperse drops). From this distribution, MMAD was calculated using parameters:
and error estimate of MMAD was calculated using error propagation:
Results
Functional test results showed that each Aeroneb Solo nebulizer met Aerogen's recommended minimum flow rate of 0.2 mL/min, ranging from 0.23 to 0.31 mL/min.
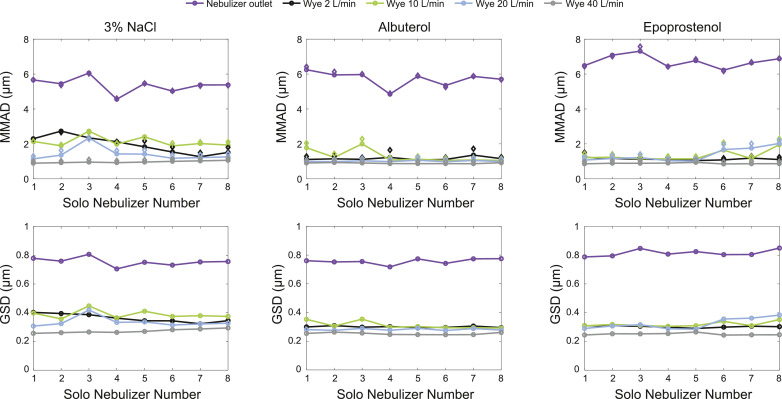
The MMAD of the 3 tested aerosols was several times smaller when measured at the wye outlet of the heated/humidified breathing circuit (0.82–2.73 µm) compared with droplets measured directly at the nebulizer cup outlet (MMAD, 4.6–7.3 µm). There was also significant variability across Solo heads with some ventilator flow rates (Fig. 3). The mean MMAD at the wye for the 3% sodium chloride solution, albuterol, and epoprostenol was 1.62 µm, 1.09 µm, and 1.18 µm, respectively. The mean MMAD at the nebulizer for the 3% sodium chloride solution, albuterol, and epoprostenol was 5.37 µm, 5.73 µm, and 6.73 µm, respectively. The MMAD for each Solo nebulizer by solution are summarized in Table 1 and illustrated in Fig. 4. These distributions show a general trend favoring decreases in particle size measured at the wye as the inspiratory flow from the air pump increases. In all cases, the MMAD measured at the wye was several times smaller than those measured at the nebulizer, with significant variability in droplet size across the different nebulizer heads.
Fig. 3.
Mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) for 3% sodium chloride (NaCl), albuterol, and epoprostenol.a aFor the MMAD plots, circle markers show measured data and diamond markers show fit data. Republished with permission from Daedalus Enterprises Inc., for Respiratory Care: the official science journal of the American Association for Respiratory Care, Droplet size and distribution of nebulized 3% NaCl, Albuterol, and Epoprostenol by Phase Doppler Particle Analyzer, Kelly McDermott, Jason G. Oakley, American Association for Respiratory Care, American Association for Respiratory Therapy, American Association for Inhalation Therapy, volume 64, supplement 10, © 2019; permission conveyed through Copyright Clearance Center, Inc.
Table 1.
Mass median aerodynamic diameter for (MMAD) each Aeroneb Solo Mesh nebulizer (Aerogen, Mountain View, California) by solution.
| Solo Nebulizer | MMAD Measurement at nebulizer (µm) | MMAD Measurement at the Wye (µm) |
|||
|---|---|---|---|---|---|
| 2 L/min | 10 L/min | 20 L/min | 40 L/min | ||
| 3% Sodium chloride | |||||
| 1 | 5.66 | 2.29 | 2.14 | 1.14 | 0.90 |
| 2 | 5.44 | 2.73 | 1.89 | 1.36 | 0.92 |
| 3 | 6.06 | 2.35 | 2.73 | 2.34 | 0.96 |
| 4 | 4.56 | 2.12 | 1.98 | 1.42 | 0.93 |
| 5 | 5.46 | 1.83 | 2.41 | 1.42 | 0.96 |
| 6 | 5.02 | 1.54 | 1.88 | 1.18 | 1.00 |
| 7 | 5.38 | 1.27 | 2.02 | 1.22 | 1.03 |
| 8 | 5.38 | 1.51 | 1.93 | 1.26 | 1.06 |
| Albuterol | |||||
| 1 | 6.25 | 1.11 | 1.78 | 1.00 | 0.91 |
| 2 | 5.95 | 1.14 | 1.23 | 0.98 | 0.93 |
| 3 | 5.97 | 1.12 | 2.00 | 1.03 | 0.91 |
| 4 | 4.85 | 1.22 | 1.07 | 0.98 | 0.87 |
| 5 | 5.88 | 1.08 | 1.12 | 1.06 | 0.87 |
| 6 | 5.35 | 1.11 | 1.06 | 0.98 | 0.87 |
| 7 | 5.87 | 1.36 | 1.08 | 1.05 | 0.87 |
| 8 | 5.70 | 1.15 | 1.05 | 0.99 | 0.92 |
| Epoprostenol | |||||
| 1 | 6.48 | 1.24 | 1.21 | 1.08 | 0.86 |
| 2 | 7.08 | 1.17 | 1.24 | 1.16 | 0.89 |
| 3 | 7.31 | 1.12 | 1.14 | 1.21 | 0.89 |
| 4 | 6.44 | 1.11 | 1.14 | 1.04 | 0.90 |
| 5 | 6.77 | 1.04 | 1.14 | 1.02 | 0.94 |
| 6 | 6.22 | 1.08 | 1.64 | 1.68 | 0.85 |
| 7 | 6.64 | 1.19 | 1.16 | 1.76 | 0.87 |
| 8 | 6.88 | 1.10 | 1.95 | 2.03 | 0.87 |
Fig. 4.
Summary of nebulizers 1–8 for: (A) 3% Sodium chloride (NaCl) (all Aeroneb Solo nebulizer data [Aerogen, Mountain View, California] 1–8 combined); (B) Albuterol (all Aeroneb Solo nebulizer data, 1–8 combined); and (C) Epoprostenol (all Aeroneb Solo nebulizer data, 1–8 combined). D = diameter; dV/dD = volume-based probability density function; f (D) = lognormal distribution fit; GSD = geometric standard deviation; lpm = liters per minute; MMAD = mass median aerodynamic diameter; PDF = probability density function; RR = Rosin-Rammler distribution.
Discussion
The objective of this study was to describe aerosol droplet size and distribution with laser diffraction for nebulized solutions of 3% sodium chloride, albuterol, and epoprostenol using the vibrating mesh Aeroneb Solo nebulizer. Phase Doppler particle analysis from this simulation study showed that aerosol size at the wye was several times smaller than expected, with significant variability across Solo heads with some ventilator flow rates. We observed that most particles ≥3 µm in diameter appeared to be trapped in the inspiratory circuit before they could be theoretically delivered to the patient. This observation, along with the fact that aerosol droplets that are up to 1 µm may be exhaled before diffusion takes place,20 suggests there may actually be less drug delivered to the patient than previously realized. However, other studies have shown that extra-fine particles of inhaled corticosteroids (eg, hydrofluoroalkane-flunisolide and bec-lomethasone), with an MMAD of 1.0 to 1.2 µm, reduced measures of peripheral and small airway inflammation (bronchial and alveolar exhaled nitric oxide, eosinophilic and interleukin-5–mediated inflammation).21,22 If small particles were simply removed with exhalation, no change in inflammatory mediators would be expected. In our study, the average MMAD at the wye was <2 µm (1.09 µm and 1.18 µm for albuterol and epoprostenol, respectively). Based on our study, the actual fate of large particles and very fine aerosol particles remains unclear as does the potential influence of the reduced deliverable mass on outcomes in the clinical setting.23
Estimates of particle size vary based on the equipment used to measure it. Previous studies using cascade impactors to evaluate MMAD indicated that vibrating mesh nebulizers should be expected to generate fine-particle aerosol droplets <3.3 µm in diameter.1,3 Evaluation of Aeroneb Solo nebulizers in high-flow nasal cannulas, using low-pressure cascade impactors, shows that more than 80% of the inhalable mass were fine particles between 0.4 and 4.4 µm.24 Another in vitro study evaluated 4 different nebulizers, including the Aeroneb Solo nebulizer, by comparing aerosol characteristics of albuterol as assessed by laser diffraction.25 That study showed that the mean (SD) mass median diameter of albuterol using the Aeroneb Solo was smaller than with jet and ultrasonic nebulizers (4.60 [0.54] µm, 5.00 [0.36] µm, and 5.80 [0.07] µm, respectively). Also, the percentage of particles <5 µm and respirable fraction of aerosol and albuterol were higher with the Aeroneb Solo than with either ultrasonic or jet nebulizers. The current study indicates that aerosols delivered by in-line nebulizers to mechanically ventilated patients may reflect a narrower range (only very fine particles), especially at higher inspiratory flows.
The results of our study are supported by the strengths of the test setup used for this analysis. Specifically, the use of an inspiratory limb allowed for objective assessments across the simulated ventilator inspiratory flow rate range tested. Laser Doppler studies such as ours allow for assessment of particle size in real time rather than approximation as with estimates using cascade impactors. Also, laser diffraction is less dependent on flow rate than cascade impaction and is not affected by water condensation (eg, water condensation inside a cascade impactor may lead to erroneously measured larger droplets). Unlike cascade impactors that can only measure droplet size categories, laser diffraction analyzers measure actual droplets. The use of vibration mesh nebulizers also has several advantages in that mesh nebulizers have a very low residual volume, high respiratory fraction, do not change medication temperature during nebulization, have rapid treatment times, and are considered more convenient to use than jet nebulizers.1,3,26 Our study had several limitations. An inspiratory limb with simulated inspiratory flow rates across a range of potential inspiratory flows was used for testing rather than dynamically changing ventilator flow rates or an upper airway model. Also, our study model did not account for anatomical considerations that may affect amounts of drug delivered in the clinical setting; it is unclear exactly what happens to the larger particles that are measured directly out of the Solo head as they are transported to the wye.
Conclusions
Phase Doppler particle analysis from this simulation study showed that aerosol size at the wye was several times smaller than expected, with significant variability across Solo heads with some ventilator flow rates. Our results significantly differ from those observed in studies using cascade impactors, and suggest that particle sizes of aerosolized drugs administered via a commonly used setup for delivery of in-line aerosols to intubated patients may be several times smaller than expected, which may result in less drug being delivered to the patient than previously realized. These observations are relevant to the clinicians who deliver therapeutic aerosols and the researchers who study them, but our findings must be validated in other adult ventilator models before changes in nebulizer placement and/or dose adjustments can be recommended.
Conflicts of Interest
This analysis was funded by Mallinckrodt Pharmaceuticals. The sponsor participated in the study design and in collection, assembly, analysis, and interpretation of data and participated in manuscript preparation, review, revisions, and final approval. K. McDermott received honoraria in 2017 as a participant for Mallinckrodt user manual validation testing. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
Medical writing and editorial support, which was conducted in accordance with Good Publication Practice and the International Committee of Medical Journal Editors guidelines, was provided by Michael D. Morren, RPh, MBA, of Peloton Advantage, LLC, an OPEN Health company, and funded by Mallinckrodt Pharmaceuticals.
K. McDermott and J. Oakley were the study investigators and were responsible for study design, collection, and assembly of data; data analysis; data interpretation; manuscript review and revisions; and final approval of the manuscript. K. McDermott was responsible for manuscript preparation.
References
- 1.Ari A. Aerosol therapy in pulmonary critical care. Respir Care. 2015;60:858–874. doi: 10.4187/respcare.03790. discussion 874-879. [DOI] [PubMed] [Google Scholar]
- 2.Burgess D.J., Duffy E., Etzler F., Hickey A.J. Particle size analysis: AAPS workshop report, cosponsored by the Food and Drug Administration and the United States Pharmacopeia. AAPS J. 2004;6:e20. doi: 10.1208/aapsj060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhand R. How should aerosols be delivered during invasive mechanical ventilation? Respir Care. 2017;62:1343–1367. doi: 10.4187/respcare.05803. [DOI] [PubMed] [Google Scholar]
- 4.Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–147. doi: 10.1089/jamp.2011.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patton J.S., Byron P.R. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 6.Cosa N., Costa E., Jr Inhaled pulmonary vasodilators for persistent pulmonary hypertension of the newborn: safety issues relating to drug administration and delivery devices. Med Devices. 2016;9:45–51. doi: 10.2147/MDER.S99601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill N.S., Preston I.R., Roberts K.E. Inhaled therapies for pulmonary hypertension. Respir Care. 2015;60:794–802. doi: 10.4187/respcare.03927. discussion 802-795. [DOI] [PubMed] [Google Scholar]
- 8.Augoustides J.G., Culp K., Smith S. Rebound pulmonary hypertension and cardiogenic shock after withdrawal of inhaled prostacyclin. Anesthesiology. 2004;100:1023–1025. doi: 10.1097/00000542-200404000-00040. doi: [DOI] [PubMed] [Google Scholar]
- 9.Lavoie A., Hall J.B., Olson D.M., Wylam M.E. Life-threatening effects of discontinuing inhaled nitric oxide in severe respiratory failure. Am J Respir Crit Care Med. 1996;153:1985–1987. doi: 10.1164/ajrccm.153.6.8665066. [DOI] [PubMed] [Google Scholar]
- 10.Miller O.I., Tang S.F., Keech A., Celermajer D.S. Rebound pulmonary hypertension on withdrawal from inhaled nitric oxide. Lancet. 1995;346:51–52. doi: 10.1016/s0140-6736(95)92681-x. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. MAUDE adverse event report: Aerogen LTD Aeroneb Solo, Convenience, 10/pk. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=2577645. Accessed February 19, 2020.
- 12.Flolan [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2018.
- 13.De Wet C.J., Affleck D.G., Jacobsohn E. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004;127:1058–1067. doi: 10.1016/j.jtcvs.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt A.M., Stein E.J. Clinical complications with the delivery of inhaled epoprostenol in the operating room. Anesthesiology. 2017;127:383. doi: 10.1097/aln.0000000000001611. [DOI] [PubMed] [Google Scholar]
- 15.Ari A., Atalay O.T., Harwood R., Sheard M.M., Aljamhan E.A., Fink J.B. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55:845–851. [PubMed] [Google Scholar]
- 16.Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16:1–7. doi: 10.5152/ejp.2014.00087. [DOI] [Google Scholar]
- 17.Pritchard J.N., Hatley R.H.M., Denyer J., von Hollen D. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther Deliv. 2018;9:121–136. doi: 10.4155/tde-2017-0102. [DOI] [PubMed] [Google Scholar]
- 18.Aeroneb Solo System Instruction Manual . Aerogen Ltd; Dangan, Galway, Ireland: 2014. [Google Scholar]
- 19.Lefebvre A.H. 1st ed. CRC Press; Boca Raton, FL: 1988. Atomization and Sprays (Combustion) [Google Scholar]
- 20.Dolovich M. Physical principles underlying aerosol therapy. J Aerosol Med. 1989;2:171–186. [Google Scholar]
- 21.Nicolini G., Chetta A., Simonazzi A., Tzani P., Aiello M., Olivieri D. Both bronchial and alveolar exhaled nitric oxide are reduced with extrafine beclomethasone dipropionate in asthma. Allergy Asthma Proc. 2010;31:e85–e90. doi: 10.2500/aap.2010.31.3367. [DOI] [PubMed] [Google Scholar]
- 22.Hauber H.P., Gotfried M., Newman K. Effect of HFA-flunisolide on peripheral lung inflammation in asthma. J Allergy Clin Immunol. 2003;112:58–63. doi: 10.1067/mai.2003.1612. [DOI] [PubMed] [Google Scholar]
- 23.Nichols S.C., Mitchell J.P., Shelton C.M., Roberts D.L. Good Cascade Impactor Practice (GCIP) and considerations for ``in-use'' specifications. AAPS PharmSciTech. 2013;14:375–390. doi: 10.1208/s12249-012-9905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reminiac F., Vecellio L., Heuze-Vourc'h N. Aerosol therapy in adults receiving high flow nasal cannula oxygen therapy. J Aerosol Med Pulm Drug Deliv. 2016;29:134–141. doi: 10.1089/jamp.2015.1219. [DOI] [PubMed] [Google Scholar]
- 25.Sidler-Moix A.L., Di Paolo E.R., Dolci U., Berger-Gryllaki M., Cotting J., Pannatier A. Physicochemical aspects and efficiency of albuterol nebulization: comparison of three aerosol types in an in vitro pediatric model. Respir Care. 2015;60:38–46. doi: 10.4187/respcare.02490. [DOI] [PubMed] [Google Scholar]
- 26.Ehrmann S., Chastre J., Diot P., Lu Q. Nebulized antibiotics in mechanically ventilated patients: a challenge for translational research from technology to clinical care. Ann Intensive Care. 2017;7:78. doi: 10.1186/s13613-017-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]