Abstract
Background: Breast cancer is the most common cancer among women. The Korean Genome and Epidemiology Study (KoGES) is a large cohort study that is available to the public. Using this large cohort study, we aimed to unravel the relationship between breast cancer development and a family history of breast cancer in Korea. Methods: This cohort study relied on data from the KoGES from 2001 through 2013. A total of 211,725 participants were screened. Of these, 129,374 women were evaluated. They were divided into two groups, including participants with and without breast cancer. A logistic regression model was used to retrospectively analyze the odds ratio of breast cancer history in families of women with and without breast cancer. Results: Of 129,374 women, 981 had breast cancer. The breast cancer group had more mothers and siblings with histories of breast cancer (p < 0.001). A history of breast cancer in the participant’s mother resulted in an odds ratio of 3.12 (1.75–5.59), and a history of breast cancer in the participant’s sibling resulted in an odds ratio of 2.63 (1.85–3.74). There was no interaction between the history of maternal breast cancer and the history of sibling breast cancer. Based on the subgroup analysis, family history was a stronger factor in premenopausal women than in menopausal and postmenopausal women. Conclusions: A family history of breast cancer is a significant risk factor for breast cancer in Korea. Premenopausal women with a maternal history of breast cancer are of particular concern. Intensive screening and risk-reducing strategies should be considered for this vulnerable subpopulation.
Keywords: breast cancer, family history, epidemiology, genetic predisposition, environment
1. Introduction
Breast cancer is the most common cancer among women [1,2]. The incidence of breast cancer in Korea was at 5848 cases in 2000 [3]. The incidence rate of breast cancer in Korea is steadily increasing even though it is still lower than the rates in Western countries [3,4,5]. Since 2016, over 20,000 individuals per year have been diagnosed with breast cancer in Korea. The number of breast cancer cases in the United States rises with increasing patient age [1,2]. In Korea, the peak is observed at 40–49 years of age and decreases after the age of 50 [3].
The well-known risk factors for breast cancer include environmental exposure, factors related to reproduction or pregnancy, and lifestyle factors such as diet, smoking, or drinking [6,7]. Approximately 30–50% of cases are caused by these factors [8,9]. Genetic predisposition accounts for 5–10% of breast cancer [10]. However, the remaining 40–65% of cases are caused by unknown factors related to emerging areas, such as gene-gene associations and gene-environment interactions [11].
Familial breast cancer accounts for approximately 20–30% of breast cancer [8]. Family history collected during preventive care visits is defined as first- and second-degree family history [12]. First-degree family history includes parents, siblings, and children. Second-degree family history includes grandparents, aunts, uncles, grandchildren, nieces, nephews, and half siblings. Family history can identify individuals who should be referred for genetic counseling and testing, which causes considerable anxiety in women [13,14,15]. Women with a family history of breast cancer can overcome psychological distress by receiving appropriate supportive counselling [16].
The risk of breast cancer is increased up to 5.7 times in individuals with first-degree relatives who have a history of breast cancer and approximately two times in individuals with any first-degree or second-degree relatives with a history of breast cancer [17,18,19,20,21,22]. Because family history involves both genetic predisposition and environment, only a part of familial breast cancer is due to inherited genetic alterations. Inherited breast cancer is generally considered to be caused by high-penetrance BRCA 1/2 mutations [16]. Although the frequency of BRCA 1/2 mutations in the population is low, the presence of mutations in these genes can cause breast cancer with high penetrance [23]. Therefore, these mutations are frequently observed in patients with familial breast cancer.
Researchers reported a 6–9% incidence of familial breast cancer in a case-control study with approximately 3000 pairs [24]. However, the sample size was still too small to determine the association of breast cancer with family history. Recently, big data have been made available to the public, which has made large-scale research possible. The Korean government (National Research Institute of Health, Centers for Disease Control and Prevention, and the Ministry of Health and Welfare) initiated a large cohort study, which was called the Korean Genome and Epidemiology Study (KoGES) [25]. These data completed the quality control process [25]. Researchers can access these epidemiological data.
In this study, we aimed to determine the relationship between breast cancer and family history in Korea using data from the KoGES. We could perform in-depth analyses because KoGES, as an umbrella trial, has six cohorts. To the best of our knowledge, this is the first investigation of a large cohort of participants with a family history of breast cancer in Korea.
2. Materials and Methods
2.1. Study Design
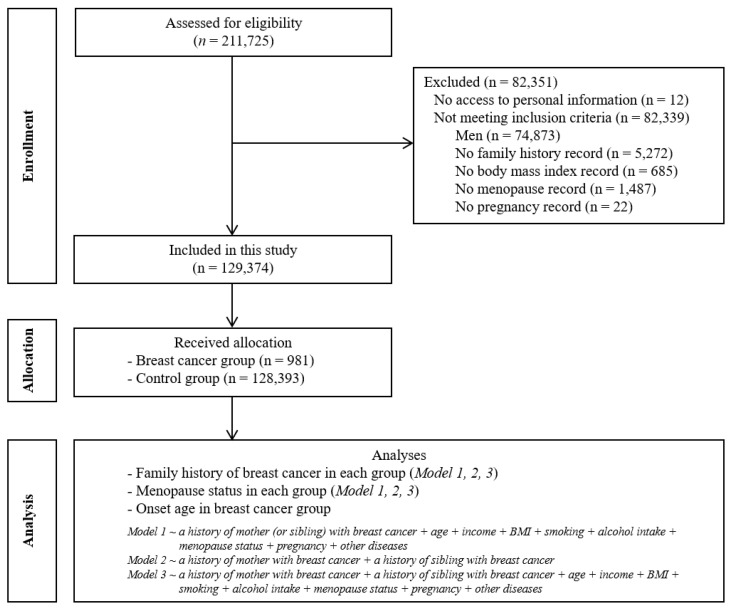
This cohort study relied on data from the KoGES from 2001 through 2013. A detailed description of these data is provided in a previous study [20]. A total of 211,725 participants were screened. Of these, we excluded men (n = 74,873) and participants who had no family history records (n = 5272) or records of body mass index (BMI) (n = 685), menopause (n = 1487), or pregnancy (n = 22) (Figure 1). Then, a total of 129,374 women were evaluated. Survey participants ranged from 40 to 91 years of age. Cancer incidence was identified by the Korea Central Cancer Registry. They were divided into two groups of participants, those with and without breast cancer.
Figure 1.
Flow diagram depicting the study design.
2.2. Data Survey
Trained interviewers asked participants about their disease history of breast cancer and their age at the time of diagnosis. Participants were also asked about their family history of breast cancer and family members’ ages at the time of diagnosis. Anthropometric and clinical measurements were obtained from the KoGES consortium. In the present study, we categorized family histories of breast cancer into groups of mothers and siblings (sisters or brothers). Monthly household incomes were categorized into four groups, including no information, lowest (less than $1500), middle ($1500–$3000), and highest (more than $3000). Each participant had descriptive records of menopause status, pregnancy, and other disease history such as hypertension, diabetes mellitus, and hyperlipidemia. Obesity was measured by BMI (kg/m2), using height and weight as continuous variables. Smoking duration was calculated as pack-years and the consumption of alcohol was measured as the mean consumption (g/day), using the frequency and the type of alcohol.
2.3. Statistics
The chi-square test was used to compare the rates of sex, income, other disease history, menopause, pregnancy, and family history of breast cancer between the breast cancer group and the control group. Independent t-tests were used to compare age, BMI, smoking duration, and alcohol consumption. A logistic regression model was used to analyze the odds ratio of a family history of breast cancer based on breast cancer occurrence as a dependent variable. In the crude model, only family history of breast cancer was used as an independent variable. Model 1 was adjusted for age, income, BMI, smoking, alcohol intake, menopause status, pregnancy, and other diseases. Model 2 was adjusted for family histories of mothers and family histories of siblings with breast cancer. Model 3 was adjusted for the variables from models 2 and 3. Additionally, we analyzed the interaction in model 4, which was adjusted for a history of maternal breast cancer, history of sibling breast cancer, and history of maternal and sibling breast cancer. The adjusted odds ratios and 95% confidence intervals were calculated from the final model. Two-tailed analyses were conducted, and p values less than 0.05 indicated significance. The results were statistically analyzed using SPSS 22.0 (IBM, Armonk, NY, USA).
3. Results
From a total of 129,374 women, 981 women had experienced breast cancer. They were categorized as the breast cancer group. Therefore, 128,393 women without breast cancer were categorized as the control group. The average age in the breast cancer and control groups was 54.2 ± 7.5 and 53.4 ± 8.5 years, respectively (Table 1). There were no significant differences in other diseases (hypertension, diabetes, and dyslipidemia) between the two groups. More women in the breast cancer group had family histories of mothers and siblings with breast cancer (p < 0.001).
Table 1.
General characteristics of participants in the present study.
| Variable | Total Participants | p | |
|---|---|---|---|
| Breast Cancer | Control | ||
| Total Number (n, %) | 981 (100.0) | 128,393 (100.0) | |
| Age (year) | 54.2 ± 7.5 | 53.4 ± 8.5 | n.s |
| Income (n, %) | <0.001 † | ||
| No information | 145 (14.8) | 26,826 (20.9) | |
| Lowest | 227 (23.1) | 28,348 (22.1) | |
| Middle | 289 (29.5) | 32,927 (25.6) | |
| Highest | 320 (32.6) | 40,292 (31.4) | |
| Hypertension (n, %) | n.s | ||
| Yes | 165 (16.8) | 24,417 (19.0) | |
| No | 816 (83.2) | 103,976 (81.0) | |
| Diabetes (n, %) | n.s | ||
| Yes | 67 (6.8) | 7379 (5.7) | |
| No | 914 (93.2) | 121,014 (94.3) | |
| Dyslipidemia (n, %) | n.s | ||
| Yes | 100 (10.2) | 11,011 (8.6) | |
| No | 881 (89.8) | 117,382 (91.4) | |
| Pregnancy (n, %) | 0.001 † | ||
| Yes | 934 (95.2) | 124,613 (97.1) | |
| No | 47 (4.8) | 3780 (2.9) | |
| Menopause (n, %) | <0.001 † | ||
| Yes | 835 (85.1) | 79,748 (62.1) | |
| No | 146 (14.9) | 48,645 (37.9) | |
| BMI (kg/m2) | 23.5 ± 2.9 | 23.8 ± 3.0 | n.s |
| Smoking (pack-year) | 0.22 ± 2.12 | 0.56 ± 3.57 | <0.001 * |
| Alcohol (g/day) | 0.66 ± 3.71 | 1.81 ± 7.75 | <0.001 * |
| Family history of mother (n, %) | <0.001 † | ||
| Breast cancer | 12 (1.2) | 546 (0.4) | |
| No breast cancer | 969 (98.8) | 127,847 (99.6) | |
| Family history of sibling (n, %) | <0.001 † | ||
| Breast cancer | 33 (3.4) | 1519 (1.2) | |
| No breast cancer | 948 (96.6) | 126,874 (98.8) | |
n.s = not significant. * Independent t-test. † Chi-square test.
In model 1, a family history of a mother with breast cancer resulted in an odds ratio of 3.21 (1.80–5.74), and a family history of a sibling with breast cancer resulted in an odds ratio of 2.66 (1.87–3.78). In model 2, a family history of a mother with breast cancer resulted in an odds ratio of 2.78 (1.56–4.94), and a family history of a sibling with breast cancer resulted in an odds ratio of 2.86 (2.02–4.07). In model 3, a family history of a mother with breast cancer resulted in an odds ratio of 3.12 (1.75–5.59), and a family history of a sibling with breast cancer resulted in an odds ratio of 2.63 (1.85–3.74) (Table 2). There was no interaction between a family history of a mother with breast cancer and a family history of a sibling with breast cancer (Table 3).
Table 2.
Association of family history with the development of breast cancer.
| Variable | Odds Ratio of Breast Cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude | p | Model 1 | p | Model 2 | p | Model 3 | p | |
| Family History of a Mother with Breast Cancer | ||||||||
| Breast cancer | 2.90 (1.63–5.16) | <0.001 * | 3.21 (1.80–5.74) | <0.001 * | 2.78 (1.56–4.94) | 0.001 * | 3.12 (1.75–5.59) | <0.001 * |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Family history of a sibling with breast cancer | ||||||||
| Breast cancer | 2.91 (2.05–4.13) | <0.001 * | 2.66 (1.87–3.78) | <0.001 * | 2.86 (2.02–4.07) | <0.001 * | 2.63 (1.85–3.74) | <0.001 * |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
Model 1: adjusted for age, income, body mass index, smoking, alcohol consumption, menopause status, pregnancy, and other diseases including hypertension, diabetes mellitus, and dyslipidemia. Model 2: adjusted for history of a mother with breast cancer and history of a sibling with breast cancer. Model 3: adjusted for model 1 and model 2. * Logistic regression analysis.
Table 3.
Analysis of interaction between a family history of a mother with breast cancer and a family history of a sibling with breast cancer.
| Variable | Odds Ratio of Breast Cancer | p |
|---|---|---|
| Model 4 | ||
| Family history of maternal breast cancer | 2.56 (1.36–4.79) | 0.003 * |
| Family history of sibling breast cancer | 2.78 (1.94–4.00) | <0.001 * |
| Family history of maternal and sibling breast cancer | 1.99 (0.39–10.13) | 0.406 |
Model 4: adjusted for history of maternal breast cancer, history of sibling breast cancer, and history of maternal and sibling breast cancer. * Logistic regression analysis.
In the subgroup analysis, participants were divided into groups of 80,583 women who had experienced menopause and 48,791 women who had not experienced menopause (Table 4). In women who had experienced menopause, a family history of a mother with breast cancer resulted in an odds ratio of 2.50 (1.17–5.35), and a family history of a sibling with breast cancer resulted in an odds ratio of 2.53 (1.72–3.71). In women who had not experienced menopause, a family history of a mother with breast cancer resulted in an odds ratio of 4.93 (1.99–12.20), and a family history of a sibling with breast cancer resulted in an odds ratio of 3.13 (1.27–7.72). Based on the subgroup analysis, family history was a stronger factor in women who had not experienced menopause than in those who had experienced menopause.
Table 4.
Subgroup analysis of the association between breast cancer and family history according to menopause status.
| Variable | Odds Ratio of Breast Cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude | p | Model 1 | p | Model 2 | p | Model 3 | p | |
| Menopause (n = 80,583) | ||||||||
| Family history of a mother with breast cancer | ||||||||
| Breast cancer | 2.86 (1.35–6.09) | 0.006 * | 2.56 (1.20–5.47) | 0.015 * | 2.78 (1.30–5.91) | 0.008 * | 2.50 (1.17–5.35) | 0.018 * |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Family history of a sibling with breast cancer | ||||||||
| Breast cancer | 2.68 (1.83–3.93) | <0.001 * | 2.54 (1.73–3.73) | <0.001 * | 2.66 (1.82–3.90) | <0.001 * | 2.53 (1.72–3.71) | <0.001 * |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| No menopause (n = 48,791) | ||||||||
| Family history of a mother with breast cancer | ||||||||
| Breast cancer | 5.51 (2.24–13.54) | <0.001 * | 5.23 (2.12–12.89) | <0.001 * | 5.18 (2.10–12.78) | <0.001 * | 4.93 (1.99–12.20) | 0.001 * |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Family history of a sibling with breast cancer | ||||||||
| Breast cancer | 3.42 (1.39–8.37) | 0.007 * | 3.36 (1.37–8.23) | 0.008 * | 3.17 (1.29–7.81) | 0.012 * | 3.13 (1.27–7.72) | 0.013 * |
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
Model 1: adjusted for age, income, body mass index, smoking, alcohol intake, menopause status, pregnancy, and other diseases including hypertension, diabetes mellitus, and dyslipidemia. Model 2: adjusted for history of a mother with breast cancer and history of a sibling with breast cancer. Model 3: adjusted for model 1 and model 2. * Logistic regression analysis.
Of 981 women, nine participants were excluded from the subgroup analysis due to missing values for age. Thus, in 972 women with breast cancer, family history and age were re-evaluated with a cut-off of 50 years (Table 5). A greater number of women with a family history of relatives with breast cancer were younger than 50 years old, although the difference was not significant.
Table 5.
Risk calculation for family history of breast cancer according to the onset of breast cancer.
| Variable | Onset of Breast Cancer | p | |
|---|---|---|---|
| <50 years | ≥50 years | ||
| Family history of a mother with breast cancer (n, %) | |||
| Yes | 8 (1.4) | 4 (1.0) | 0.771 * |
| No | 573 (98.6) | 387 (99.0) | |
| Family history of a sibling with breast cancer (n, %) | |||
| Yes | 19 (3.3) | 14 (3.6) | 0.793 † |
| No | 562 (96.7) | 377 (96.4) | |
* Fisher’s exact test. † Chi-square test.
4. Discussion
In the present study, we performed a risk calculation of family history based on a Korean nationwide public registry database. By analyzing this public database, meaningful family histories were identified in patients with breast cancer. The odds ratios were 3.12 for family history of a mother with breast cancer and 2.63 for family history of a sibling with breast cancer. There was no interaction between history of maternal breast cancer and history of sibling breast cancer. Furthermore, these family histories were more influential in premenopausal women. Only among women with breast cancer were more family histories observed in the group with onset ages less than 50 years, although the results were not statistically significant.
To the best of our knowledge, this is the first investigation of a large cohort of participants with a family history of breast cancer in Korea. After adjustment of multiple environmental risk factors, our study showed that family history was a strong risk factor for breast cancer in a large cohort of Asian women. Particularly, premenopausal women with a maternal history of breast cancer were the most vulnerable group, of which the odds ratio was almost five times higher than the control group. Age, BMI, pregnancy, menopause, other disease, smoking, alcohol consumption, or income could be related to the risk of breast cancer, according to previous studies [2,26,27]. However, some studies did not consider these factors when calculating the risk of family history [19,21,28]. Large-scale data from epidemiologic studies are essential for determining the association between cancer and family history because familial breast cancer is relatively rare. Therefore, our calculations were statistically adjusted by these variables using big data from breast cancer and control groups. Furthermore, the findings were supported by in-depth analyses because KoGES, as an umbrella trial, has six cohorts. Various data in KoGES as an open access resource can be legitimately presented to investigators who have interests in further research derived from the current study.
Inherited breast cancer is different from familial breast cancer, which is driven not only by genes but also by interactions with the environment. However, there is a strong possibility of inherited breast cancer among familial breast cancers [29,30]. BRCA 1/2 are the representative genes assessed in inherited breast cancer, although a risk assessment should be performed for all women [18,31]. Therefore, adult women with a family history of breast cancer are eligible for BRCA 1/2 testing, although the analysis of BRCA 1/2 mutations was not available in our study due to the lack of BRCA information in the KoGES database. BRCA, as a tumor suppressor gene, repairs DNA damage and inhibits neoplasms [32]. This examination is performed using peripheral blood after genetic counseling based on the individual’s pedigree. Imaging studies and clinical data are usually needed for breast cancer screening [33]. Women with BRCA 1/2 mutations should undergo annual magnetic resonance imaging (MRI) scans between the ages of 25 and 29 years, and annual mammograms and MRI scans between the ages of 30 and 75 years [34,35]. Women harboring these mutations have a lifetime risk of 46–87% for breast cancer and 39–63% for ovarian cancer [36]. However, they can take tamoxifen or raloxifene to reduce their risk [34]. For women who have a lifetime risk ≥ 20%, for breast cancer, annual MRI scans and mammograms starting at 10 years prior to the age at which the youngest family member developed breast cancer, but not prior to the age of 25 and 30 years, respectively, are recommended even in the absence of BRCA 1/2 mutations [37]. As women with family members diagnosed with breast cancer diagnosed after 50 years of age have an average risk, an annual mammogram is recommended for these women [2,33].
The records regarding a family history of cancer have not been standardized in a structured manner [38]. However, these records should include cancer type, lineage, degree, age at diagnosis, and ethnicity [12,39]. It is recommended to update an individual’s family history of cancer every 5–10 years between 30 and 60 years of age [38,40]. The American Cancer Society (ACS) guidelines also recommend annual MRI scans plus mammograms for women with family members harboring breast cancer-associated mutations, and these women should also undergo a genetic assessment [32,41]. In the current study, the KoGES provided only the family history as pertains to an individual having a mother or sibling with breast cancer, which does not satisfy the recommended minimum record regarding a family history of cancer. Participants included in the present cohort should update their family history for future research.
The present study has some limitations. First, this study was based on the limited data provided, which is a general limitation of public data analysis. Therefore, our study could not consider covariates such as age at menarche, age at first birth, previous biopsies, or occupational exposures, which might be related to breast cancer. Moreover, we could not conduct a comprehensive investigation of first- and second-degree family histories. Second, there could be recall bias given the use of a survey to collect data, which could lead to less reliable results. Last, the present study has the usual limitations of observational studies such as detection bias and selection bias.
5. Conclusions
In conclusion, a family history of breast cancer is a significant risk factor for breast cancer in Korea. The identification of a family history of breast cancer can contribute to early detection in populations at risk. In particular, premenopausal women with a maternal history of breast cancer are at the highest risk. Intensive screening and risk-reducing strategies should be considered for this vulnerable subpopulation.
Acknowledgments
Data in this study were from the Korean Genome and Epidemiology Study (KoGES; 4851-302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea. The manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by the highly qualified native English-speaking editors at American Journal Experts (e 3FAC-6DAB-4AE2-F6A1-4FCP).
Author Contributions
Conceptualization, H.G.C. and Y.J.S.; methodology, H.G.C., J.H.P., Y.J.C. and Y.J.S.; formal analysis, H.G.C. and Y.J.S.; investigation, H.G.C., J.H.P., Y.J.C. and Y.J.S.; resources, H.G.C.; data curation, H.G.C.; writing—original draft preparation, J.H.P., Y.J.C. and Y.J.S.; writing—review and editing, H.G.C., J.H.P., Y.J.C. and Y.J.S.; supervision, Y.J.S.; project administration, J.H.P., Y.J.C. and Y.J.S.; funding acquisition, H.G.C. and Y.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hallym University Research Fund (HURF-2020-03). This work was supported by the National Research Foundation (NRF) of Korea (NRF-2018-R1D1A1A0-2085328 and NRF-2019-R1G1A1004679).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Hallym University (20 February 2019).
Informed Consent Statement
The requirement for written informed consent was waived.
Data Availability Statement
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Warner E. Breast-Cancer Screening. N. Engl. J. Med. 2011;365:1025–1032. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 3.Hong S., Won Y.-J., Park Y.R., Jung K.-W., Kong H.-J., Lee E.S., Community of Population-Based Regional Cancer Registries Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2017. Cancer Res. Treat. 2020;52:335–350. doi: 10.4143/crt.2020.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Mph K.D.M., Sauer A.G., Jemal A., Siegel R.L. Breast cancer statistics, 2019. CA A Cancer J. Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 5.Dafni U., Tsourti Z., Alatsathianos I. Breast Cancer Statistics in the European Union: Incidence and Survival across Euro-pean Countries. Breast Care. 2019;14:344–353. doi: 10.1159/000503219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islami F., Sauer A.G., Miller K.D., Siegel R.L., Fedewa S.A., Jacobs E.J., McCullough M.L., Patel A.V., Ma J., Soerjomataram I., et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA A Cancer J. Clin. 2017;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 7.Tamimi R.M., Spiegelman D., Smith-Warner S.A., Wang M., Pazaris M., Willett W.C., Eliassen A.H., Hunter D.J. Popu-lation Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am. J. Epidemiol. 2016;184:884–893. doi: 10.1093/aje/kww145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engmann N.J., Golmakani M.K., Miglioretti D.L., Sprague B.L., Kerlikowske K. For the Breast Cancer Surveillance Consortium Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017;3:1228–1236. doi: 10.1001/jamaoncol.2016.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteman D.C., Webb P.M., Green A.C., Neale R.E., Fritschi L., Bain C.J., Parkin D.M., Wilson L.F., Olsen C.M., Nagle C.M., et al. Cancers in Australia in 2010 attributable to modifiable factors: Summary and conclusions. Aust. N. Z. J. Public Heal. 2015;39:477–484. doi: 10.1111/1753-6405.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalloo F., Evans D.G. Familial Breast Cancer. Clin. Genet. 2012;82:105–114. doi: 10.1111/j.1399-0004.2012.01859.x. [DOI] [PubMed] [Google Scholar]
- 11.Pharoah P.D., Antoniou A.C., Easton D.F., Ponder B.A. Polygenes, risk prediction, and targeted prevention of breast cancer. N. Engl. J. Med. 2008;358:2796–2803. doi: 10.1056/NEJMsa0708739. [DOI] [PubMed] [Google Scholar]
- 12.Lu K.H., Wood M.E., Daniels M., Burke C., Ford J., Kauff N.D., Kohlmann W., Lindor N.M., Mulvey T.M., Robinson L., et al. American society of clinical oncology expert statement: Collection and use of a cancer family history for oncology providers. J. Clin. Oncol. 2014;32:833–840. doi: 10.1200/JCO.2013.50.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgart L.A., Postula K.J.V., Knaus W.A. Initial clinical validation of Health Heritage, a patient-facing tool for personal and family history collection and cancer risk assessment. Fam. Cancer. 2015;15:331–339. doi: 10.1007/s10689-015-9863-3. [DOI] [PubMed] [Google Scholar]
- 14.Daly M.B., Pilarski R., Yurgelun M.B., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Garber J.E., et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020;18:380–391. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 15.Scott D., Friedman S., Telli M.L., Kurian A.W. Decision Making About Genetic Testing Among Women With a Personal and Family History of Breast Cancer. JCO Oncol. Pract. 2020;16:e37–e55. doi: 10.1200/JOP.19.00221. [DOI] [PubMed] [Google Scholar]
- 16.Owens D.K., Davidson K.W., Krist A.H., Barry M.J., Cabana M., Caughey A.B., Doubeni C.A., Epling J.W., Jr., Kubik M., Landefeld C.S., et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US preventive ser-vices task force recommendation statement. JAMA. 2019;322:652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 17.Gail M.H., Brinton L.A., Byar D.P., Corle D.K., Green S.B., Schairer C., Mulvihill J.J. Projecting individualized probabili-ties of developing breast cancer for white females who are being examined annually. J. Natl. Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 18.Wood M.E., Rehman H.T., Bedrosian I. Importance of family history and indications for genetic testing. Breast J. 2020;26:100–104. doi: 10.1111/tbj.13722. [DOI] [PubMed] [Google Scholar]
- 19.Mukama T., Kharazmi E., Sundquist K., Sundquist J., Brenner H., Fallah M. Familial risk of breast cancer by dynamic, accumulative, and static definitions of family history. Cancer. 2020;126:2837–2848. doi: 10.1002/cncr.32815. [DOI] [PubMed] [Google Scholar]
- 20.Liaw Y.Y., Loong F.S., Tan S., On S.Y., Khaw E., Chiew Y., Nordin R., Mat T.N., Arulanantham S., Gandhi A. A retrospective study on breast cancer presentation, risk factors, and protective factors in patients with a positive family history of breast cancer. Breast J. 2019;26:469–473. doi: 10.1111/tbj.13520. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey S.D., Yoon P., Moonesinghe R., Khoury M.J. Population-based study of the prevalence of family history of cancer: Implications for cancer screening and prevention. Genet. Med. 2006;8:571–575. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Parmigiani G. Meta-Analysis of BRCA1 and BRCA2 Penetrance. J. Clin. Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J.-Y., Lee K.-M., Park S.K., Noh D.-Y., Ahn S.-H., Yoo K.-Y., Kang D. Association of paternal age at birth and the risk of breast cancer in offspring: A case control study. BMC Cancer. 2005;5:143. doi: 10.1186/1471-2407-5-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y., Han B.-G., KoGES Group Cohort profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017;46:e20. doi: 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewer H.R., Jones M.E., Schoemaker M.J., Ashworth A., Swerdlow A.J. Family history and risk of breast cancer: An anal-ysis accounting for family structure. Breast Cancer Res. Treat. 2017;165:193–200. doi: 10.1007/s10549-017-4325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah M., Zhu K., Palmer R.C., Jatoi I., Shriver C., Wu H. Breast, colorectal, and skin cancer screening practices and family history of cancer in U.S. women. J. Womens Health. 2007;16:526–534. doi: 10.1089/jwh.2006.0108. [DOI] [PubMed] [Google Scholar]
- 28.Braithwaite D., Miglioretti D.L., Zhu W., Demb J., Trentham-Dietz A., Sprague B., Tice J., Onega T., Henderson L.M., Buist D.S.M., et al. Family History and Breast Cancer Risk Among Older Women in the Breast Cancer Surveillance Consortium Cohort. JAMA Intern. Med. 2018;178:494–501. doi: 10.1001/jamainternmed.2017.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang E., Seong M.-W., Park S.K., Lee J.W., Lee J., Kim L.S., Lee J.E., Kim S.Y., Jeong J., Han S.A., et al. The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: Recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res. Treat. 2015;151:157–168. doi: 10.1007/s10549-015-3377-4. [DOI] [PubMed] [Google Scholar]
- 30.Han S.-A., Kim S.-W., Kang E., Park S.K., Ahn S.-H., Lee M.H., Nam S.-J., Han W., Bae Y.T., Kim H.-A., et al. The prevalence of BRCA mutations among familial breast cancer patients in Korea: Results of the Korean Hereditary Breast Cancer study. Fam. Cancer. 2013;12:75–81. doi: 10.1007/s10689-012-9578-7. [DOI] [PubMed] [Google Scholar]
- 31.Claus E.B., Risch N., Thompson W.D. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643–651. doi: 10.1002/1097-0142(19940201)73:3<643::AID-CNCR2820730323>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Lord C.J., Ashworth A. The DNA damage response and cancer therapy. Nat. Cell Biol. 2012;481:287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- 33.Oeffinger K.C., Fontham E.T., Etzioni R., Herzig A., Michaelson J.S., Shih Y.-C.T., Water L.C., Church T.R., Flowers C.R., LaMonte S.J., et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bevers T.B., Ward J.H., Arun B.K., Colditz G.A., Cowan K.H., Daly M.B., Garber J.E., Gemignani M.L., Gradishar W.J., Jordan J.A., et al. Breast Cancer Risk Reduction, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015;13:880–915. doi: 10.6004/jnccn.2015.0105. [DOI] [PubMed] [Google Scholar]
- 35.White M.C., Soman A., Weinberg C.R., Rodriguez J.L., Sabatino S.A., Peipins L.A., DeRoo L., Nichols H.B., Hodgson M.E., Sandler D.P. Factors associated with breast MRI use among women with a family history of breast cancer. Breast J. 2018;24:764–771. doi: 10.1111/tbj.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccinin C., Panchal S., Watkins N., Kim R.H. An update on genetic risk assessment and prevention: The role of genetic testing panels in breast cancer. Expert Rev. Anticancer. Ther. 2019;19:787–801. doi: 10.1080/14737140.2019.1659730. [DOI] [PubMed] [Google Scholar]
- 37.Bevers T.B., Helvie M., Bonaccio E., Calhoun K.E., Camp M., Daly M.B., Lehman C.D., Farrar W.B., Garber J.E., Gray R., et al. Breast Cancer Screening and Diagnosis, Version 1. [(accessed on 6 April 2020)];2019 Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- 38.Acheson L.S. Recording, interpreting, and updating the family history of cancer: Implications for cancer prevention. JAMA. 2011;306:208–210. doi: 10.1001/jama.2011.980. [DOI] [PubMed] [Google Scholar]
- 39.Nindrea R.D., Aryandono T., Lazuardi L., Dwiprahasto I. Family History of Breast Cancer and Breast Cancer Risk between Malays Ethnicity in Malaysia and Indonesia: A Meta-Analysis. Iran. J. Public Heal. 2019;48:198–205. doi: 10.18502/ijph.v48i2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziogas A., Horick N.K., Kinney A., Lowery J.T., Domchek S.M., Isaacs C., Griffin C.A., Moorman P.G., Edwards K.L., Hill D.A., et al. Clinically Relevant Changes in Family History of Cancer Over Time. JAMA. 2011;306:172–178. doi: 10.1001/jama.2011.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saslow D., Boetes C., Burke W., Harms S., Leach M.O., Lehman C.D., Morris E., Pisano E., Schnall M., Sener S., et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA A Cancer J. Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.