Abstract
Chemokines play a crucial role in combating microbial infection by recruiting blood neutrophils to infected tissue. In mice, the chemokines Cxcl1/KC and Cxcl2/MIP2 fulfill this role. Cxcl1 and Cxcl2 exist as monomers and dimers, and exert their function by activating the Cxcr2 receptor and binding glycosaminoglycans (GAGs). Here, we characterized Cxcr2 G protein and β-arrestin activities, and GAG heparan sulfate (HS) interactions of Cxcl1 and Cxcl2 and of the trapped dimeric variants. To understand how Cxcr2 and GAG interactions impact in vivo function, we characterized their neutrophil recruitment activity to the peritoneum, Cxcr2 and CD11b levels on peritoneal and blood neutrophils, and transport profiles out of the peritoneum. Cxcl2 variants compared with Cxcl1 variants were more potent for Cxcr2 activity. Native Cxcl1 compared with native Cxcl2 and dimers compared with native proteins bound HS with higher affinity. Interestingly, recruitment activity between native Cxcl1 and Cxcl2, between dimers, and between the native protein and the dimer could be similar or very different depending on the dose or the time point. These data indicate that peritoneal neutrophil recruitment cannot be solely attributed to Cxcr2 or GAG interactions, and that the relationship between recruited neutrophils, Cxcr2 activation, GAG interactions, and chemokine levels is complex and highly context dependent. We propose that the ability of Cxcl1 and Cxcl2 to reversibly exist as monomers and dimers and differences in their Cxcr2 activity and GAG interactions coordinate neutrophil recruitment and activation, which play a critical role for successful resolution of inflammation.
Keywords: GPCR, proteoglycan, inflammation, monomer–dimer, leukocyte
1 |. INTRODUCTION
A hallmark of acute microbial infection and injury is robust neutrophil recruitment to the insult site.1–3 Chemokines, expressed by resident cells, guide neutrophils to the target tissue by forming concentration gradients across the endothelium, extracellular matrix (ECM), and epithelium.4,5 Chemokine levels and their recruitment activity are coupled to the flux and duration of neutrophil trafficking, which, in turn, are dependent on the nature and severity of infection and injury and the location of the infection. Impaired or uncontrolled recruitment can result in incomplete resolution or collateral tissue damage and exacerbate the disease process.
Several inflammatory diseases are characterized by elevated levels of multiple neutrophil-activating chemokines.6–8 Mouse inflammation models show that Cxcl1/keratinocyte-derived chemokine (KC) and Cxcl2/MIP2 are differentially induced, and that their levels can vary substantially during the course of the disease.9–17 In humans, CXCL1 is also known as MGSA or Groα, and CXCL2 as Groβ. The systematic nomenclature of human and mouse chemokines (CXCL1 for human and Cxcl1 for mice) allows comparing human and rodent data.18 We will use the Cxcl1 and Cxcl2 nomenclature for murine chemokines in this manuscript. Importance of both Cxcl1 and Cxcl2 is evident from Cxcl1 null mice that show impaired neutrophil recruitment and higher susceptibility to infection.16,17 Intraperitoneal administration of LPS induces rapid neutrophil recruitment in mice.10,11 These studies indicate LPS triggers release of Cxcl1 and Cxcl2 by macrophages and mast cells, the source of the chemokines are from both prestored granules and new protein synthesis via activation of multiple transcription mechanisms, and that Cxcl1 and Cxcl2 play nonredundant roles. Why 2 chemokines allow better control of neutrophil trafficking requires knowledge of structural and functional properties of the individual chemokines and how these properties determine neutrophil function.
Cxcl1 and Cxcl2 share 64% sequence identity and 88% sequence similarity. The structure of Cxcl2 and a structural model of Cxcl1 show similar tertiary and quaternary structures,19,20 indicating that differences in their function must arise from differences in the amino acid sequence. Cxcl1 and Cxcl2 exist as monomers and dimers (dimer dissociation constants of ~35 and 15 μM, respectively), and orchestrate neutrophil trafficking by activating the Cxcr2 receptor on neutrophils and interacting with tissue glycosaminoglycans (GAGs). Activation of Cxcr2-coupled G protein and β-arrestin signaling pathways plays important roles by triggering, among other things, cytoskeletal changes and integrin activation that facilitates adhesion, transendothelial migration, and crossing the ECM to reach the insult site.21,22 G protein signaling activates a variety of effectors including adenyl cyclase and Ca2+ channels. β-Arrestin serves as a multifunctional adaptor and recruits an array of molecules, including those that signal via the MAPK and tyrosine kinase pathways.
GAGs are a family of linear sulfated polysaccharides covalently attached to core proteins, and are the glycan part of proteoglycans.23–28 Chemokines predominantly bind heparan sulfate (HS) and chondroitin sulfate (CS) on the epithelium, endothelium, ECM, and glycocalyx during their transport from the injured tissue to the vasculature. GAG interactions impact chemokine function in several ways including in the presentation for Cxcr2 binding, endothelial activation, transport to the vasculature, chemokine lifetime, protection from proteolysis, and makeup of haptotactic and chemotactic gradients.29–35 We have previously shown that Cxcl1 and Cxcl2 dimerization and GAG binding are coupled, and that their residue-specific interactions and binding geometry for GAGs are different.36
In this study, we characterized Cxcr2 signaling and HS binding activity of native Cxcl1 and Cxcl2 and of the trapped dimeric variants. To understand how Cxcr2 signaling and GAG interactions orchestrate in vivo function, we characterized their neutrophil recruitment activity to the peritoneum, Cxcr2 and CD11b levels on peritoneal and blood neutrophils, and transport kinetics out of the peritoneum. Native Cxcl2 and Cxcl2 dimers compared with Cxcl1 variants were more potent and more efficacious for Cxcr2 activities. Native Cxcl1 compared with native Cxcl2 and dimers compared with native proteins bound HS with higher affinity. Neutrophil recruitment varied substantially, with dimers showing the highest recruitment. Further, comparing recruitment between native Cxcl1 and Cxcl2, between dimers, and between the native protein and the dimer indicates that they could be the same or significantly different depending on the dose or time point. The process of recruitment also defines the killing potency of neutrophils at the injured site. We conclude that distinct properties and coordinated action of Cxcl1 and Cxcl2 orchestrate recruitment and define the neutrophil phenotype for successful resolution of inflammation and restoring homeostasis.
2 |. METHODS
2.1 |. Mice
Female, 7–8 weeks old, BALB/c mice (Harlan, Houston, TX) were maintained in pathogen-free conditions in the animal research facility of UTMB, in accordance with NIH and UTMB institutional guidelines for animal care. Cages, bedding, food, and water were sterilized before use. All animal work was approved by the Institutional Animal Care and Use Committee.
2.2 |. Cxcl1 and Cxcl2 expression
Cxcl1 and Cxcl2 wild-type (WT) and dimer variants were cloned, expressed, and purified as described previously.36 Disulfide-trapped dimeric Cxcl1 and Cxcl2 were constructed by introducing K28C and T27C mutations on WT Cxcl1 and WT Cxcl2 backgrounds, respectively. Cloning of Cxcl1 resulted in an extra glycine at the N-terminus. The extra glycine is unlikely to have any impact on neutrophil recruitment or Cxcr2 activities. Relative activity of our Cxcl1 variant and Cxcl2 are comparable to those reported in the literature,37 and previous N-terminal deletion studies have also shown minimal changes in receptor activity.38
2.3 |. Functional assays
Calcium release activity of Cxcl1 and Cxcl2 variants was determined using mouse bone marrow neutrophils.39 Neutrophils from bone marrow cells harvested from mice were purified using Histopaque density gradients-1077 and -1119 (Sigma, St. Louis, MO, USA).40 Briefly, bone marrow cells were pipetted over the Histopaque solutions, and centrifuged at 805 g for 25 min at room temperature (RT) using no brake. The middle layer consisting of neutrophils was removed, washed using cold HBSS, and the cells were counted using a hemocytometer. Purified neutrophils, cultured in a 96-well plate at 2 × 105 cells/well, were allowed to settle at RT for 1 h and then incubated with the dye from Calcium assay 6 kit (Molecular Devices, San Jose, CA, USA) for 90 min. Cxcl1 and Cxcl2 variants over a range of concentrations were then added and changes in fluorescence were monitored (λex 485 nm, λem 525 nm) every 5 s for 240–500 s. The agonist response was determined by expressing the maximum change in fluorescence in arbitrary units over base line.
β-Arrestin recruitment activity of Cxcl1 and Cxcl2 variants was measured using mCxcr2 PathHunter kit (DiscoveRx, Fremont, CA, USA). The assay was carried out according to manufacturer’s instruc tions. The mouse Cxcr2 CHO.K1 cells were thawed and cultured using the Cell Plating reagent in 96-well white walled, clear bottom plates for 48 h. Chemokine variants over a range of concentrations were added to the cells and incubated for 90 min at 37 °C. The detection reagents were then added and incubated for 1 h in the dark. β-Galactosidase-induced luminescence upon β-arrestin–Cxcr2 interaction was then measured using BMG Optima. The data were analyzed using GraphPad prism.
2.4 |. GAG binding measurements
Surface plasmon resonance (SPR) experiments were performed using Biacore T100 (GE Healthcare, Chicago, IL, USA) as described.39 Heparin (MW ~15 kDa from Calbiochem, San Diego, CA, USA) and HS (~9 kDa from Iduron, UK) were biotinylated using the EZ-Link Biotin LC Hydrazide (Pierce, Waltham, MA, USA). To create the heparin immobilized chip, a new Sensor Chip SA (Biacore, Marlborough, MA, USA) was conditioned with several injections of 1 M NaCl in 50 mM NaOH, and then equilibrated with HBS buffer (10 mM HEPES, 300 mM NaCl, pH 7.4). The biotinylated heparin was dissolved in the same buffer to a final concentration of 200 ng/ml and injected onto the surface at 10 μL/min to immobilize heparin (81 RU). The low-density (78 RU) and high-density (248 RU) HS chips were prepared as described for heparin. The flow rate was chosen to minimize mass transfer effects. The chemokines (2 nM to 20 μM) in HBS–EP buffer (10 mM HEPES, 150 mM NaCl, 2 mM EDTA, 0.005% surfactant P20) were injected onto the GAG surface at 30 μl/min for 3 min at 25 °C, and then dissociated by flowing HBS–EP buffer for 6–7 min. Any residual binding was removed by washing with the buffer containing 1.5 M NaCl for 6 min. The binding curves were analyzed using the Biacore T100 evaluation software. All measurements were repeated at least twice, and the binding parameters were essentially the same for both runs.
2.5 |. Peritoneal neutrophil recruitment
Chemokine-mediated peritoneal neutrophil recruitment was carried out as described.39 Briefly, mice were injected intraperitoneally with Cxcl1 or Cxcl2 variants in 100 μl DPBS buffer. Mice were euthanized at various time points by intraperitoneal injection of a mixture of ketamine (200 mg/kg) and xylazine (70 mg/kg). The peritoneal cells were harvested by flushing the peritoneal cavity twice with 3 ml of cold PBS. The cells were centrifuged and the pellet resuspended in PBS for cytospin slides. Slides were fixed, stained with Wright-Giemsa stain, and a differential leukocyte count was performed. Total leukocytes were counted using a hemocytometer after staining with Turk’s solution.
2.6 |. FACS analysis
Peritoneal cells or peripheral blood leukocytes (1 × 106) were first incubated with Fc block (BD Biosciences, San Jose, CA, USA) for 20 min. For detection of Cxcr2 on neutrophils, cells were washed with PBS containing EDTA and 1% FBS, stained with Ly6G FITC (Clone: 1A8), CD11b PerCPCy5.5 (Clone: M1/70) (both from BD Biosciences), and Cxcr2 PE (Clone: 242216) (R&D Systems) and incubated in the dark for 30 min at 4 °C. The corresponding isotype antibodies were used as controls. The cells were then washed twice and fixed using 1% paraformalde-hyde. Cells were acquired with BD FACS Canto II (BD Biosciences) and analyzed using FlowJo software. Leukocytes were isolated from blood drawn from hearts of chemokine-treated mice immediately after euthanasia and transferred to heparinized microcentrifuge tubes. The samples were centrifuged for 3 min at 10 °C and RBCs were lysed using ACK lysing buffer (Gibco) at RT. The cells were then washed 3 times with PBS, and resuspended for FACS analysis.
2.7 |. Protein estimation
Levels of Cxcl1 and Cxcl2 in the peritoneal supernatants from chemokine-treated mice were determined using Cxcl1 or Cxcl2 ELISA kit (R&D systems, Minneapolis, MN, USA) according to manufacturer’s instructions.
2.8 |. Statistical analysis
Statistical significance of the variants against PBS was determined using Student’s 2-tailed t-test. ANOVA was used to determine the statistical significance between the variants using Tukey’s post hoc analysis. The statistical tests were performed using Graph pad Prism 5 software. P < 0.05 was considered to be statistically significant.
3 |. RESULTS
3.1 |. Cxcr2 activity
Cxcl1 and Cxcl2 bind Cxcr2 with nanomolar (nM) affinity,37,41 indicating that the WT exists as a monomer at the concentrations used in these assays. Trapped dimers were created by introducing a disulfide bond across the dimer interface.36 Knowledge of both the potency and efficacy of G protein and β-arrestin-coupled cellular responses is necessary to fully describe Cxcr2 activity. Potency is defined by EC50, which is the concentration that corresponds to 50% of the maximum activity. Efficacy can be defined as the maximum effect of the agonist at a saturating dose. We measured Ca2+ release as a readout for G protein activity (Fig. 1A). Cxcl2 was more potent (EC50 8.9 nM) compared with Cxcl1 (EC50 57 nM), and was also more efficacious. Previous studies also indicate that Cxcl2 is ~10-fold more potent than Cxcl1.37,42 Between the dimers, Cxcl2 dimer was more potent (EC50 176 nM), whereas Cxcl1 dimer was only marginally active. The Cxcl2 dimer was more efficacious, but not as efficacious as native Cxcl2. We measured β-arrestin recruitment as a readout for β-arrestin function (Fig. 1B). Cxcl2 was more potent (EC50 1.3 nM) compared with Cxcl1 (EC50 6.1 nM). The dimers were less active, with Cxcl2 dimer showing higher activity (EC50 41 nM) and Cxcl1 dimer showing negligible activity. The efficacies of the variants paralleled their potencies. These data collectively indicate that Cxcl2 is more potent and more efficacious compared with Cxcl1, and that the Cxcl2 dimer is also more active compared to Cxcl1 dimer.
FIGURE 1. Cxcr2 activity of Cxcl1 and Cxcl2 variants.

(A) Calcium release activity of Cxcl1 and Cxcl2 variants using FLIPR assay. Measurements were carried out using mouse bone marrow neutrophils. Chemokine variants at different doses were added to the dye-loaded neutrophils and the changes in fluorescence was measured using Flexstation 3 and expressed as relative fluorescence units. (B) β-Arrestin recruitment activity of Cxcl1 and Cxcl2 variants using DiscoveRx mCxcr2 PathHunter kit. Chemokine variants were added at different doses to the mCxcr2 CHO.K1 cells and β-galactosidase-induced luminescence upon β-arrestin–Cxcr2 interaction was measured. Data were collected in triplicate, and the results are expressed as mean ± SD, and are representative of 2 independent experiments
3.2 |. GAG interactions
We characterized the binding interactions of Cxcl1 and Cxcl2 variants to HS at low and high densities and heparin using SPR. SPR provides insights into both kinetic (kon and koff) and dissociation constants (KD). Our reasons for using different density HS chips are as follows: distribution of GAGs on cell surface is not homogeneous; GAG densities can vary as a function of location and time due to cleavage of GAG chains by metalloproteases and heparanases secreted as a component of immune response; and previous studies have shown that the binding profiles can vary with GAG densities and that these differences can provide insights into binding mechanisms.43–45 Heparin functions as a surrogate for the highly sulfated regions in the HS chain. The kinetic and dissociation constants are shown in Table 1 and the binding profiles are shown in Fig. 2. The KD values were determined from kinetic constants and at steady state. Curves for calculating steady-state KD and protein accumulation are shown in Fig. 3.
TABLE 1.
Kinetic and binding constants of Cxcl1 and Cxcl2 variants for HS and heparina
| kon (M−1s−1) | koff (s−1) | KD (nM) | KD (SS) (nM) | Rmax (RU) | nb | |
|---|---|---|---|---|---|---|
| LD_HS | ||||||
| WT Cxcl1 | 7.1 × 105 | 1.6 × 10−1 | 225 | 3,300 | 170 | 2.4 (1.2) |
| WT Cxcl2 | 3.5 × 105 | 1.5 × 10−1 | 429 | 12,300 | 116 | 1.6 (0.8) |
| Cxcl1 dimer | 20 × 104 | 0.6 × 10−3 | 3 | 2,300 | 153 | 1.1 |
| Cxcl2 dimer | 7.5 × 104 | 3.2 × 10−3 | 43 | 4,700 | 78 | 0.55 |
| HD_HS | ||||||
| WT Cxcl1 | 3.8 × 105 | 6.1 × 10−2 | 161 | 2,200 | 1836 | 8.3 (4.1) |
| WT Cxcl2 | 3.8 × 105 | 28 × 10−2 | 785 | 5,600 | 1402 | 6.3 (3.1) |
| Cxcl1 dimer | 8.1 × 104 | 1.6 × 10−3 | 19 | 1,300 | 893 | 2.0 |
| Cxcl2 dimer | 1.6 × 104 | 1.3 × 10−3 | 77 | 2,900 | 621 | 1.4 |
| Heparin | ||||||
| WT Cxcl1 | 66 × 104 | 14 × 10−2 | 212 | 1,800 | 179 | 4.1 (2.0) |
| WT Cxcl2 | 0.5 × 104 | 0.1 × 10−2 | 255 | 2,300 | 146 | 3.4 (1.7) |
| Cxcl1 dimer | 3.3 × 104 | 0.4 × 10−3 | 11 | 900 | 190 | 2.1 |
| Cxcl2 dimer | 1.4 × 104 | 1.1 × 10−3 | 78 | 1,200 | 152 | 1.8 |
Data are representative of 2 independent experiments.
Stoichiometry (n) for the trapped dimers is defined as number of bound dimers per GAG chain, and for native proteins as number of bound monomers (and dimers in brackets) per GAG chain.
FIGURE 2. SPR profiles of Cxcl1 and Cxcl2 variants.

SPR sensorgrams of WT Cxcl1, WT Cxcl2, Cxcl1 dimer, and Cxcl2 dimer for immobilized LD-HS, HD-HS, and heparin on a BIAcore SA chip. The curves represent the signal (RU) minus the reference signal (no GAG)
FIGURE 3. Binding curves for steady-state KD measurements of Cxcl1 and Cxcl2 variants.

Binding profiles of Cxcl1 WT, Cxcl2 WT, Cxcl1 dimer, and Cxcl2 dimer on LD-HS (A), HD-HS (B), and heparin (C) chips. The response units (RU) were plotted against concentration for direct comparison between different chemokines
The KD values from kinetic measurements compared with values from the steady state were higher for all chemokine variants, but the differences in affinities among variants for a given GAG were more or less similar. Cxcl1 and Cxcl2 dimer variants, compared with the WT, bound GAGs with higher affinity. Accumulation corresponds to the highest RU (Rmax) value that can be related to stoichiometry. For both low-density HS (LD-HS) and high-density HS (HD-HS), WT Cxcl1 showed the highest and Cxcl2 dimer the lowest accumulation, and both dimers showed lower accumulation compared with the WT (Fig. 3). Stoichiometry varied between LD-HS and HD-HS that was especially evident for the native proteins (Table 1). For LD-HS, stoichiometry corresponded to 1 dimer (assuming native proteins also bind as dimers) per GAG chain except for Cxcl2 dimer. For Cxcl2 dimer, the stoichiometry corresponded to 1 dimer binding 2 GAG chains. High stoichiometry of native proteins for HD-HS is strikingly suggesting pivotal roles for monomer–dimer equilibrium and GAG densities. The stoichiometry for Cxcl2 dimer binding HD-HS was once again different, and a value of 1.4 suggests a mixture of dimer-bound GAG chains. For heparin, dimers compared with native proteins bound with higher affinity but the accumulation profiles of WT and dimers were similar. Stoichiometry for all proteins was 1 dimer per heparin chain. Trapped CXCL12/SDF-1 dimer was shown to bind heparin and HS with higher affinity and higher accumulation compared with the WT.43 These data indicate that it is very likely that all chemokine dimers bind GAG with higher affinity but not necessarily show higher accumulation.
3.3 |. Peritoneal neutrophil recruitment
Recruitment activity is dependent on local chemokine concentration and their Cxcr2 and GAG interactions, and so measuring recruitment at a single dose or time will not be sufficient to describe how chemokines recruit neutrophils. Therefore, we measured peritoneal neutrophil recruitment activity of Cxcl1 and Cxcl2 variants at various doses and times (Fig. 4). These data also allowed describing how differences between native Cxcl1 and Cxcl2, between dimers, and between native and dimer variants determine neutrophil recruitment at a given dose and time.
FIGURE 4. Neutrophil recruitment activity of Cxcl1 and Cxcl2 variants.
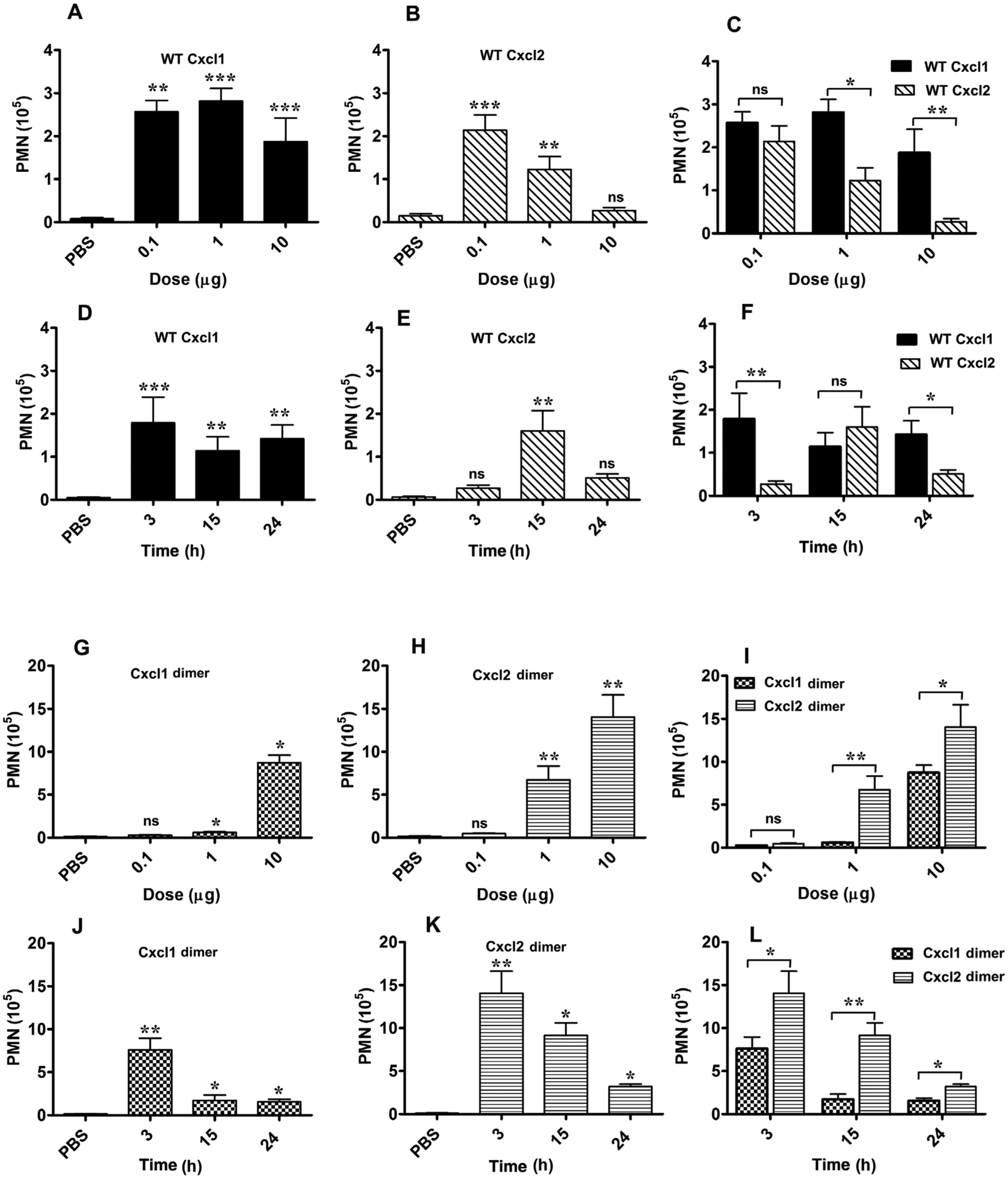
Neutrophil recruitment activity of native Cxcl1 (A and D) and native Cxcl2 (B and E) as a function of dose at 3 h postinjection and as a function of time for the 10 μg dose. Comparison of recruitment activity of Cxcl1 vs. Cxcl2 as a function of dose and time (C and F). Neutrophil recruitment activity of dimeric variants of Cxcl1 (G and J) and Cxcl2 (H and K) as a function of dose and time. Comparison of recruitment activity of Cxcl1 dimer vs. Cxcl2 dimer as a function of dose and time (I and L). Peritoneal neutrophils are expressed as mean ± SE from 4 to 19 mice for each variant from 1 to 5 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
To understand how dosage impact recruitment, we intraperitoneally administered Cxcl1 and Cxcl2 at 3 different doses (0.1, 1, and 10 μg), and measured neutrophil numbers in peritoneal lavage after 3 h using cytospin and differential cell counts (Fig. 4). Compared with the PBS control, Cxcl1 and Cxcl2 showed robust recruitment at all doses except for Cxcl2 at the 10 μg dose. It is notable that recruitment activity of Cxcl1 is essentially the same despite a 100-fold difference in dosage (Fig. 4A). However, Cxcl2, at the lowest 0.1 μg dose, showed robust recruitment comparable to Cxcl1, but essentially no recruitment at the 10 μg dose (Figs. 4B and 4C). To understand how recruitment levels vary with time, we also characterized recruitment at 15 and 24 h for the 10 μg dose. Cxcl1 showed robust recruitment at all time points (Fig. 4D). Interestingly, Cxcl2 showed elevated recruitment at 15 h, comparable to levels observed for the 1 μg at 3 h, resulting in a bell-shaped profile (Fig. 4E). The recruitment activities indicate Cxcl1 was significantly more active at 3 and 24 h and shared similar activity for the 15 h timepoint (Fig. 4F). High recruitment activity of native Cxcl2 at the lowest 0.1 μg dose and similar recruitment activity of Cxcl1 at all doses indicate local Cxcl1/Cxcl2 concentrations are sufficiently high and not limiting for maximal Cxcr2 activity. The inability of native Cxcl2 at the highest dose to recruit neutrophils suggest GAG interactions are unable to regulate Cxcr2 function.
To understand how dimerization impacts neutrophil recruitment, we characterized the recruitment activity of Cxcl1 and Cxcl2 trapped dimers for the same 3 doses (0.1, 1, and 10 μg) at 3 h, and also at 15 and 24 h for the 10 μg dose. The recruitment profiles of Cxcl1 and Cxcl2 dimers were distinct. The Cxcl1 dimer showed negligible recruitment for the 0.1 and 1 μg doses, but significant recruitment for the 10 μg dose (Fig. 4G). The Cxcl2 dimer showed minimal recruitment for the 0.1 μg dose and significantly higher recruitment for the 1 and 10 μg doses (Fig. 4H). Recruitment profiles of Cxcl1 and Cxcl2 dimers as a function of time show recruitment for Cxcl1 dimer falling steeply at 15 h and for Cxcl2 dimer more gradually with time. Cxcl1 dimer showed significant recruitment only for the 3 h, and Cxcl2 dimer for both 3 and 15 h times (Figs. 4J and 4K). The recruitment activity of Cxcl2 dimer was higher at all doses and time points except for the 0.1 μg dose (Figs. 4I and 4L).
Comparison of recruitment activities between native and dimeric variants provided several notable insights (Fig. 5). In particular, differences in recruitment between native Cxcl2 and trapped dimer were striking (Figs. 5C and 5D). Native Cxcl2 was more active than the dimer only for the 0.1 μg dose at 3 h, with Cxcl2 dimer showing higher recruitment at other doses and time points. However, native Cxcl1 was more active than the dimer at 0.1 and 1 μg doses and Cxcl1 dimer was more active for the 10 μg dose (Figs. 5A and 5B).
FIGURE 5. Comparison of recruitment activity between native proteins and dimers.

Recruitment profiles of Cxcl1 WT and dimer as a function of dose at 3 h postinjection (A) and as a function of time for the 10 μg dose (C). Recruitment profiles of Cxcl2 WT and dimer as a function of dose at 3 h postinjection (B) and as a function of time for the 10 μg dose (D). *P < 0.05, ***P < 0.001
3.4 |. Mobilization of bone marrow neutrophils
Clinical data and animal disease models have shown that chemokines not only direct blood neutrophils to the insult site but can also mobilize neutrophil reservoirs from the bone marrow and organs such as lung.15,46 Cxcl1 and Cxcl2 released in response to thioglycolate-induced peritonitis mobilize bone marrow neutrophils, which was also confirmed by demonstrating that intravenous chemokine administration resulted in elevated blood and reduced bone marrow neutrophils.15 We characterized blood neutrophil levels to determine if peritoneal administration of chemokines also results in elevated blood neutrophils at 3 h (Fig. 6). Blood neutrophil levels for native Cxcl1 and Cxcl2 at the 0.1 μg dose were similar to the PBS control. However, blood neutrophil levels for the 10 μg dose were higher, indicating both Cxcl1 and Cxcl2 mobilize neutrophils from the bone marrow. However, blood neutrophil levels for the trapped dimers at the 10 μg dose were no different from the PBS control, which could be attributed to lower dimer levels in the blood and/or lower Cxcr2 activity of the dimer. Despite a ~3-fold increase in blood neutrophil levels, impaired peritoneal recruitment for Cxcl2 at the 10 μg dose indicates a breakdown in molecular mechanisms that orchestrate neutrophil trafficking to the tissue. It is likely Cxcr2 function is impaired due to receptor internalization and/or desensitization due to prolonged exposure to elevated chemokine levels.47,48 Therefore, we characterized Cxcr2 levels on blood and peritoneal neutrophils, and transport kinetics of the Cxcl1 and Cxcl2 variants out of the peritoneum.
FIGURE 6. Mobilization of bone marrow neutrophils.

Mobilization of bone marrow neutrophils in response to exogenous chemokines were determined from blood neutrophil levels at 3 h using flow cytometry. Neutrophil fraction of the blood leukocytes is expressed as mean ± SE from 4 mice for each variant. Compared with the PBS control, blood neutrophils were elevated for Cxcl1 and Cxcl2 at the 10 μg dose. *P < 0.05
3.5 |. Cxcr2 and CD11b levels in neutrophils
We used flow cytometry to measure surface Cxcr2 levels on neutrophils isolated from the blood and peritoneal cavity at the 3 h time point. The peritoneal cavity does not contain resident neutrophils. Therefore, neutrophil Cxcr2 levels were compared with those from the Cxcl1 0.1 μg dose (Fig. 7A), as any receptor internalization will be the least for the lowest chemokine dose. Cxcr2 levels were comparable between Cxcl1 and Cxcl2 for the 0.1 μg dose; Cxcr2 levels for Cxcl1 at the 10 μg dose were significantly lower compared with the 0.1 μg dose, and data are not available for Cxcl2 at the 10 μg due to lack of recruitment. However, Cxcr2 levels on any recruited neutrophils exposed to the 10 μg dose of Cxcl2 are likely to be low, comparable or lower than what was observed for Cxcl1. Reduced cell surface Cxcr2 is also observed in blood neutrophils at the 10 μg dose, and the extent of reduction was higher for Cxcl2 though statistically not significant. Cell surface Cxcr2 on blood neutrophils exposed to Cxcl1 and Cxcl2 at the 0.1 μg dose was comparable to the PBS control. Considering Cxcr2 on peritoneal neutrophils at the 0.1 μg dose was similar, recruitment at this dose can be attributed to Cxcr2 activity and GAG interactions and also that it is not influenced by Cxcr2 internalization. Cell surface Cxcr2 on dimer-recruited peritoneal neutrophils for the 10 μg dose was lower compared with the native chemokines at the 0.1 μg dose, and were also lower for Cxcl2 dimer compared with the Cxcl1 dimer. However, Cxcr2 on blood neutrophils for the trapped dimers at the 10 μg dose was no different from the PBS control (Fig. 7B), indicating neutrophils in the peritoneum must have encountered high dimer concentrations during their transit across the endothelium, epithelium, and connective tissue. These observations also indicate Cxcr2 signaling activities and GAG interactions override any negative impact of Cxcr2 internalization in dimer-mediated recruitment.
FIGURE 7. CXCR2 and CD11b levels.
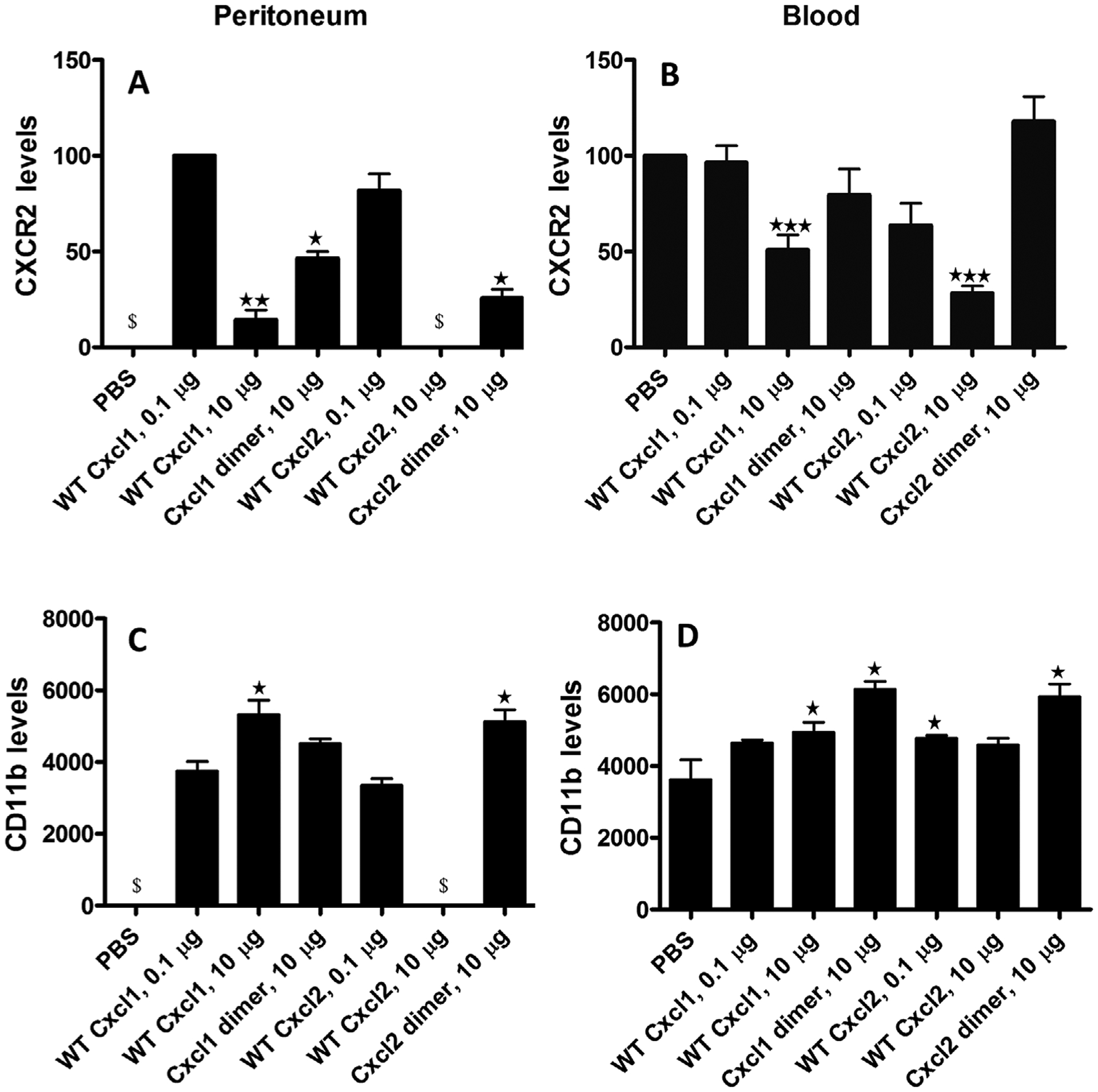
The peritoneal (A and C) and blood (B and D) neutrophils harvested at 3 h were stained with antibodies specific for identification of neutrophils, Cxcr2, and Cd11b using flow cytometry. FACS analysis was done by excluding the doublets (FSC-A/FSC-H), gating for Ly6G+ /Cd11b+ cells (neutrophils) in the FSC-A/SSC-A subset and then measuring Cxcr2 (A and B) or CD11b (C and D) levels. Cxcr2 and CD11b levels are expressed as means ± SE from 4 to 8 mice for each variant from 1 or 2 independent experiments. Cxcr2 levels in the peritoneum for PBS and Cxcl2 at 10 μg could not be reported due to absence of neutrophils ($). Significance of the Cxcr2 levels in the peritoneum are compared with the Cxcl1 0.1 μg data and in the blood to the PBS control. CD11b levels in the peritoneum for PBS and for Cxcl2 at 10 μg data are not shown due to absence of neutrophils ($). Significance of the CD11b levels in the peritoneum are compared with the Cxcl1 0.1 μg data and in the blood to the PBS control. *P < 0.05, **P < 0.01, ***P < 0.001
Engagement of integrins plays a crucial role in neutrophil trafficking, and their levels change dynamically during different phases of neutrophil recruitment.49,50 As Cxcr2 signaling is coupled to mobilization of intracellular integrin to the cell surface, we used flow cytometry to measure cell surface integrin CD11b (also known as αMβ2) on peritoneal and blood neutrophils harvested at the 3 h time point. In peritoneal neutrophils, CD11b was similar for native Cxcl1 and Cxcl2 at the 0.1 μg dose, and higher for native Cxcl1 for the 10 μg compared with the 0.1 μg dose (Fig. 7C). However, CD11b was not correlated to peritoneal neutrophil numbers, considering native Cxcl1 at 0.1 and 10 μg doses show similar neutrophil recruitment (Fig. 4). In blood neutrophils, CD11b was higher for all chemokine variants at all doses though statistical significance was not achieved for Cxcl1 at 0.1 μg and Cxcl2 at 10 μg doses (Fig. 7D). For both dimers at the 10 μg dose, Cxcr2 on blood neutrophils was unaltered but CD11b was elevated, suggesting CD11b is recruited at lower and Cxcr2 is internalized at higher chemokine concentrations.
3.6 |. Transport of Cxcl1 and Cxcl2 variants
Chemokines instilled in the peritoneal cavity cross the epithelium, connective tissue, and the endothelium to enter the blood stream. In order to determine whether chemokine transport rates and levels impact neutrophil trafficking, we measured peritoneal (Fig. 8, top) and blood (Fig. 8, bottom) chemokine levels using ELISA at 3 and 15 h for the 10 μg dose. We chose these times to best understand how chemokines influence neutrophil recruitment over 24 h. At 3 h, chemokine values could be accurately determined for the 10 μg dose but not for the 0.1 μg dose. Our previous studies with chemokine CXCL8 have shown that chemokine transport follows first-order kinetics, and so the relative change for different chemokine doses at different times should be the same.51 Comparison of peritoneal chemokine levels shows significant amount of Cxcl2 was still present at 15 h, indicating Cxcl1 transport from the peritoneal cavity was more rapid. In rats, Cxcl1/CINC, compared with Cxcl2, was also observed to move more rapidly out of alveolar space.52,53 The transport profiles of Cxcl1 and Cxcl2 dimers paralleled native proteins. Chemokine levels in the blood indicate significant levels of Cxcl1 and Cxcl2 at 3 h and lower but significant levels of Cxcl1 but not Cxcl2 at 15 h. Blood levels of the dimer were also different, with significantly lower Cxcl1 dimer compared with Cxcl2 dimer at 3 h, and similar dimer levels for the 15 h time point. For Cxcl2 dimer alone, levels at 3 and 15 h were similar in the blood and peritoneum.
FIGURE 8. Trafficking of Cxcl1 and Cxcl2 variants from the peritoneum.

Levels of Cxcl1 (A and E), Cxcl2 (C and G), Cxcl1 dimer (B and F), and Cxcl2 dimer (D and H) in the peritoneum (top row, A–D) and blood (bottom row, E–H) at 3 and 15 h postinjection determined using ELISA. Chemokine levels are expressed in absorbance (O.D.) units from the ELISA. The values plotted are mean ± SE from 4 to 8 mice for each variant from 1 or 2 independent experiments
Chemokine levels and the recruitment activity for native Cxcl2 can be reconciled, with high chemokine values both in the blood and peritoneum at 3 h resulting in no recruitment, and relatively higher chemokine levels in the peritoneum compared with blood at 15 h resulting in recruitment. A similar argument does not apply for native Cxcl1. High and comparable Cxcl1 levels both in the blood and peritoneum at 3 h is inconsistent with significant recruitment. Cxcl1 levels in the blood are higher compared with peritoneum at 15 h, which if anything, should result in reverse migration and are also not consistent with significant recruitment. In the vasculature, chemokines can also bind to endothelial glycocalyx and to atypical chemokine receptor Ackr1 (also known as DARC) expressed on endothelial cells and erythrocytes. It is possible that a significant fraction of secreted chemokines exists in the bound form and also that different amounts of Cxcl1 and Cxcl2 could be bound to Ackr1 and glycocalyx GAGs. Therefore, knowledge of the bound fraction of the chemokines are needed for better understanding of how chemokine transport determines gradients and local chemokine concentrations, and hence recruitment.
3.7 |. Peritoneal tissue integrity
Excess neutrophil recruitment in response to infection or an injury could compromise tissue integrity and exacerbate the disease process. We characterized tissue integrity by fixing sections of the peritoneal mesentery tissue at 24 h for the 10 μg dose of all chemokine variants (Fig. 9). The H&E sections of the peritoneal mesentery tissue for the chemokine-treated mice showed no evidence of tissue damage and were no different from the control mice, indicating that the mechanics of recruitment are coordinated, and that differences in neutrophil recruitment and Cxcr2 activities do not trigger neutrophil activation for releasing their cytotoxic proteins and reactive oxygen species.
FIGURE 9. Histochemistry of mesentery tissue.

Representative sections of peritoneal mesentery from mice treated with Cxcl1, Cxcl1 dimer, Cxcl2, Cxcl2 dimer, and PBS at the 24 h time point. Peritoneal mesenteries were fixed and tissue sections were stained with H&E. Data show no evidence of tissue damage compared to the PBS control
4 |. DISCUSSION
In response to infection and injury, multiple chemokines released by resident cells coordinate neutrophil transport from initial arrest and adhesion to directed migration across the endothelium and ECM to the insult site. Neutrophils, as first responders, set in motion a series of events including eliminating invading pathogens and initiating the tissue repair process. Neutrophils combat infection by releasing proteases, superoxide, and NETs. Clinical and animal model studies show neutrophils are “good” and “bad,” with the latter attributed to excess neutrophil activation resulting in collateral tissue damage. Clinical data, obtained during active inflammation, cannot provide insights on early events following an infection or injury. Such information can only be obtained from animal models.
LPS peritoneal inflammation, a well-studied mouse model, shows that the production and release of Cxcl1 and Cxcl2 are highly orchestrated, and that both chemokines are released rapidly and are essential for a successful response. However, disease models cannot provide the answer to why and how multiple chemokines allow better control of neutrophil recruitment or the molecular mechanisms by which chemokines mediate recruitment. Recruitment activity of individual chemokines and structural features such as the role of dimerization can be characterized by exogenous administration of a chemokine and determining neutrophil recruitment as a function of dose and time.39,54–57 These studies show that a single chemokine is able to trigger the cytoskeletal structural changes and signaling events that are essential for trafficking blood neutrophils to the target tissue. Cxcl1 and Cxcl2 reversibly exist as monomers and dimers, and mediate neutrophil recruitment by activating Cxcr2 on neutrophils and interacting with tissue GAGs. Therefore, knowledge of Cxcr2 and GAG interactions is essential to describe how Cxcl1 and Cxcl2 orchestrate in vivo neutrophil trafficking to the target tissue.
CXCR2-activating chemokines share similar tertiary and quaternary structures. Structure–function studies show that receptor interactions are mediated by N-terminal and N-loop residues.21 The N-terminal “ELR” residues are conserved, therefore differences in N-loop residues must be responsible for differences in CXCR2 activity (Fig. 10). Cxcl2 compared with Cxcl1, native proteins compared with dimer, and Cxcl2 dimer compared with Cxcl1 dimer were more active for both G protein and β-arrestin activities. Native Cxcl2 and Cxcl2 dimer showed the lowest and highest neutrophil recruitment activity, respectively, highlighting the importance of monomer–dimer equilibrium in regulating recruitment. It has also been shown for human chemokines that the monomer is a potent agonist but that receptor activity of the dimer can vary, and that the recruitment activity of the monomers and dimers differ.50,56–62 Cxcl1 and Cxcl2 dimerization do not occlude receptor binding N-terminal and N-loop residues and so dimers can bind their receptors. In contrast, CC dimerization occludes N-terminal and N-loop residues and so CC dimers are inactive. Reduced activity of the dimers arises due to reduced conformational dynamics of the receptor-binding residues compared with the monomer, as flexibility of the binding residue has been shown to be critical for optimal receptor interactions.
FIGURE 10. Sequences of CXCR2-activating chemokines.

Conserved N-terminal “ELR” and N-loop residues that mediate CXCR2 and GAG interactions are highlighted. A GAG-binding residue is defined as conserved if present in 5 or more of the 7 human sequences.31,68 Conserved GAG-binding residues are shaded and those specific to a chemokine are in bold
Our previous studies for Cxcl1 and Cxcl2 show that the GAG binding site lies within the monomer, binding geometries are different, thermodynamic signatures are different, and that there are striking differences in binding interactions at a residue-specific level.36 Our previous studies for Cxcl1 and Cxcl2 and human chemokines CXCL1, CXCL5, CXCL7, and CXCL8 show that conserved residues and unique residues in a given chemokine mediate GAG-binding interactions (Fig. 10). These studies also show that dimerization and GAG binding are coupled, the dimer binds GAG with higher affinity, and GAG-binding geometries significantly vary that also impacts how chemokines are presented to CXCR2 for neutrophil activation.36,63–68
Our current studies show that binding and kinetic constants and protein accumulation for HS vary for Cxcl1 and Cxcl2 variants. The higher GAG affinity of Cxcl1 dimer compared with Cxcl2 dimer and lower receptor activity of Cxcl1 dimer compared with Cxcl2 dimer are in agreement with the higher recruitment activity of Cxcl2 dimer. The greater HS accumulation of native chemokines compared with dimer suggests that they bind with higher efficiency, and that the ability of the native chemokine to reversibly exist as monomers and dimers must play a role in optimal binding. However, higher accumulation did not translate to higher recruitment, suggesting other aspects of GAG interactions drive recruitment. Native Cxcl2 compared with native Cxcl1 bound HS with lower affinity, which results in higher free Cxcl2 levels. This could be responsible for the dysregulated Cxcr2 activation and lower neutrophil recruitment activity. Stronger binding and slow dissociation observed for the dimers should promote stable gradients and are consistent with increased recruitment with increased dose. These observations also suggest that dissociation of the native dimer to monomers and dysregulated receptor activity of the monomer at high concentrations negatively regulate recruitment, which could be critical to minimize collateral tissue damage in the context of disease. These observations also raise the question whether/how alterations in sulfation on HS in inflamed tissues alter chemokine binding, and we will address the relevance of these changes in our future studies.
Significant neutrophil recruitment for the dimers is notable considering their low Cxcr2 activity and also because their local concentrations are expected to be low due to their higher GAG binding affinities. Nevertheless, increased recruitment with increasing dosage and higher recruitment activity of Cxcl2 dimer compared with Cxcl1 dimer indicate that recruitment is correlated to Cxcr2 activity. Significant recruitment also indicates that neutrophils must encounter sustained high local dimer concentrations to elicit sufficient Cxcr2 signaling required for neutrophil migration. In addition, the ability of the Cxcl2 dimer to promote neutrophil migration for a longer duration suggests that the higher GAG affinity of the dimer prolongs its lifetime, which results in more effective and durable gradients. Recruitment profiles indicate that it is possible to establish a causal relationship of how GAG and receptor interactions determine recruitment activity for the dimer variants but not for the native proteins. The complex recruitment profiles for the native proteins arise due to their ability to reversibly exist as monomers and dimers and differences in their GAG and receptor interactions that cannot be linearly correlated to chemokine dose. It is very likely that neutrophils encounter local chemokine monomer and dimer concentrations that vary significantly as a function of space and time during their migration to injured tissue. Chemokine binding not only leads to signaling, but prolonged exposure and high chemokine levels can also lead to receptor internalization and desensitization. Desensitization disables the receptor from responding to further chemokine stimulation. Transgenic mice that overexpress Cxcl1 show elevated blood Cxcl1 levels and these mice show impaired neutrophil migration in a disease model.47 Therefore, it is likely that receptor internalization and/or desensitization is responsible for lack of recruitment for Cxcl2 at the highest dose.
Cxcl2 is more potent than Cxcl1 in eliciting chemotaxis of murine neutrophils in vitro, consistent with Cxcl2’s higher Cxcr2 activity.37 Cxcl2 is substantially more active than Cxcl1 in recruiting neutrophils in the air pouch,69 indicating Cxcr2 activity plays a prominent role in directing neutrophils in this model. In the lung, Cxcl1 was more active than Cxcl2 in recruiting neutrophils, indicating GAG, and not Cxcr2, interactions play a pivotal role in driving recruitment.54 Our current studies indicate that Cxcl1 was also more active than Cxcl2 in the peritoneum. However, Cxcl1 recruitment activity remained the same at different doses, and Cxcl2 recruitment fell steadily with increasing dose and was essentially inactive at the highest dose. Further, whereas Cxcl1 recruitment activity was essentially unaltered with time, Cxcl2 showed a bell-shaped recruitment profile. These data collectively indicate that tissue architecture determines recruitment activity of a given chemokine and differences in recruitment between chemokines. Although the lung is highly vascularized, the air pouch is poorly vascularized, and the peritoneal lining provides a larger and less-convoluted surface area compared with the alveoli.54,57 Differences in tissue architecture most likely impact local chemokine concentrations and how GAG interactions and Cxcr2 activity determine recruitment. This is best illustrated for Cxcl2, where increasing dosage functions as an off-switch in the peritoneum but results in higher neutrophil recruitment in the air pouch. These observations also make a case that the phenotype of neutrophils recruited by Cxcl1 and Cxcl2 in different tissues are likely to be different, and that these differences play important roles for successful resolution in a tissue-dependent manner. Future studies focused on the phenotype of recruited neutrophils will answer these questions.
Peritoneal recruitment activity of human chemokines has been characterized in mice. For both CXCL8 and CXCL1, native proteins are more active than the dimer. For CXCL8, recruitment levels at all doses were similar,57 a trend that was also observed for Cxcl1. For WT CXCL1, neutrophil levels increased with increasing dose similar to that observed for Cxcl1 and Cxcl2 dimers.39 Recruitment profiles of CXCL1 and CXCL8 dimers were similar to Cxcl1 dimer.39,57 The recruitment profiles of CXCL1 and CXCL8 in the lung differ from that in the peritoneum,51,56 and also differ from the profiles observed for Cxcl1 and Cxcl2. Further, although CXCL1 and CXCL8 mutants of GAG-binding residues (GAG mutants) show impaired peritoneal recruitment but higher or similar alveolar recruitment,39,54,56 Cxcl2 GAG mutants show significantly reduced alveolar recruitment but similar peritoneal recruitment.55 These observations for human chemokines also indicate a prominent role for tissue architecture in determining recruitment profiles.
Ackr1 expressed on endothelial cells and erythrocytes also regulates neutrophil migration by trafficking chemokines across the endothelium, and functioning as a reservoir and regulating blood chemokine levels.70 Ackr1 binds Cxcl1 and Cxcl2 with nM affinity, and internalizes chemokines in a β-arrestin dependent manner.71,72 A recent intravital microscopy study in a cremaster muscle model shows distinct compartmentalization of Cxcl1 and Cxcl2 determines discrete stages of neutrophil transmigration.73 In summary, we conclude that Cxcl1 and Cxcl2’s ability to reversibly exist as monomers and dimers, and their composite Cxcr2/GAG/Ackr1 interactions and activity determine tissue-specific neutrophil recruitment. We further propose that differences in GAG, Cxcr2, and Ackr1 interactions finetune chemokine-specific neutrophil recruitment, and that coordinated recruitment by 2 chemokines in response to an insult, provides better control for optimal recruitment and successful resolution of inflammation.
ACKNOWLEDGMENTS
This work was supported in part by the NIH grants R21AI124681 and R21AI135606 and a pilot grant from the Institute for Human Infections and Immunity at UTMB to K.R. and by the NIH grant R01AI1364681 to C.F. We thank Drs. Ralf Richter (University of Leeds) and Sudarshan Rajagopalan (Duke University) for valuable discussions, Dr. Alex Peniche (UTMB) for assistance with FACS, and Dr. Heather Lander for editorial assistance.
Abbreviations:
- Cxc11
CXC ligand 1
- Cxcl2
CXC ligand 2
- Cxcr2
CXC receptor 2
- ECM
extracellular matrix
- GAG
glycosaminoglycan
- KC
keratinocyte-derived chemokine
- MIP2
macrophage inflammatory protein 2
- SPR
surface plasmon resonance
- WT
wild-type
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019;40:635–647. [DOI] [PubMed] [Google Scholar]
- 2.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. [DOI] [PubMed] [Google Scholar]
- 4.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 5.Colditz IG, Schneider MA, Pruenster M, Rot A. Chemokines at large: in-vivo mechanisms of their transport, presentation and clearance. Thromb Haemost. 2007;97:688–693. [PubMed] [Google Scholar]
- 6.Imada A, Ina K, Shimada M, et al. Coordinate upregulation of interleukin-8 and growth-related gene product-alpha is present in the colonic mucosa of inflammatory bowel. Scand J Gastronenterol. 2001;36:854–864. [DOI] [PubMed] [Google Scholar]
- 7.Leaker BR, Barnes PJ, O’Connor B. Inhibition of LPS-induced airway neutrophilic inflammation in healthy volunteers with an oral CXCR2 antagonist. Respir Res. 2013;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gijsbers K, Van Assche G, Joossens S, et al. CXCR1-binding chemokines in inflammatory bowel diseases: down-regulated IL-8/CXCL8 production by leukocytes in Crohn’s disease and selective GCP-2/CXCL6 expression in inflamed intestinal tissue. Eur J Immunol. 2004;34: 1992–2000. [DOI] [PubMed] [Google Scholar]
- 9.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185:6930–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;80:4308–4315. [DOI] [PubMed] [Google Scholar]
- 11.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. [DOI] [PubMed] [Google Scholar]
- 12.Call DR, Nemzek JA, Ebong SJ, et al. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock. 2001;15: 278–284. [DOI] [PubMed] [Google Scholar]
- 13.Call DR, Nemzek JA, Ebong SJ, et al. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am J Pathol. 2001;158:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metkar S, Kim KS, Silver J, Goyert SM. Differential expression of CD14-dependent and independent pathways for chemokine induction regulates neutrophil trafficking in infection. J Leukoc Biol. 2012;92:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Batra S, Douda DN, Palaniyar N, Jeyaseelan S. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J Immunol. 2014;193:3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea-Donohue T, Thomas K, Cody MJ, et al. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao W, Jerva LF, West J, Lolis E, Schweitzer BI. Solution structure of murine macrophage inflammatory protein-2. Biochemistry. 1998;37:8303–8313. [DOI] [PubMed] [Google Scholar]
- 20.Poluri KM, Joseph PR, Sawant KV, Rajarathnam K. Structural basis of glycosaminoglycan binding to chemokine CXCL1 dimer. J Biol Chem. 2013;288:25143–25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: dissecting the signaling pathways. Cell Signal. 2019;54:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molteni R, Crespo CL, Feigelson S, et al. β-Arrestin 2 is required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation. Blood. 2009;114: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 23.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karamanos NK, Piperigkou Z, Theocharis AD, et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem Rev. 2018;118:9152–9232. [DOI] [PubMed] [Google Scholar]
- 25.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. [DOI] [PubMed] [Google Scholar]
- 26.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9: 121–167. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–246. [DOI] [PubMed] [Google Scholar]
- 28.Pomin VH, Mulloy B. Glycosaminoglycans and proteoglycans. Pharmaceuticals. 2018;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monneau Y, Arenzana-Seisdedos F, Lortat-Jacob H. The sweet spot: how GAGS help chemokines guide migrating cells. J Leukoc Biol. 2016;99:935–953. [DOI] [PubMed] [Google Scholar]
- 30.Proudfoot AEI, Johnson Z, Bonvin P, Handel TM. Glycosaminoglycan interactions with chemokines add complexity to a complex system. Pharmaceuticals. 2017;10:E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajarathnam K, Sepuru KM, Joseph PB, Sawant K, Brown AJ. Glycosaminoglycan interactions finetune chemokine-mediated neutrophil trafficking: structural insights and molecular mechanisms. J Histochem Cytochem. 2018;66:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massena S, Christoffersson G, Hjertstrom E, et al. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood. 2010;116: 1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279:43854–43860. [DOI] [PubMed] [Google Scholar]
- 34.Kurupati P, Turner CE, Tziona I, et al. Chemokine-cleaving Streptococcus pyogenes protease SpyCEP is necessary and sufficient for bacterial dissemination within soft tissues and the respiratory tract. Mol Micro-biol. 2010;76:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frevert CW, Kinsella MG, Vathanaprida C, et al. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol. 2003;28:464–472. [DOI] [PubMed] [Google Scholar]
- 36.Sepuru KM, Nagarajan B, Desai UR, Rajarathnam K. Structural basis, stoichiometry, and thermodynamics of chemokines KC/mCXCL1 and MIP2/mCXCL2 binding to glycosaminoglycan heparin. J Biol Chem. 2018;293:17817–17828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 38.Clark-Lewis I, Schumacher C, Baggiolini M, Moser B. Structure-activity relationship of interleukin-8 determined using chemically synthesized analogs. J Biol Chem. 1991;266:23128–23134. [PubMed] [Google Scholar]
- 39.Sawant KV, Poluri KM, Dutta A, et al. Chemokine CXCL1 mediated neutrophil recruitment: role of glycosaminoglycan interactions. Sci Reports. 2016;6:33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swamydas M, Lionakis MS. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp. 2013;77:e50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozic CR, Kolakowski LF Jr, Gerard NP, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 42.Fan X, Patera AC, Pong-Kennedy A, et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;282:11658–11666. [DOI] [PubMed] [Google Scholar]
- 43.Dyer DP, Salanga CL, Volkman BF, Kawamura T, Handel TM. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology. 2016;26:312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christoffersson G, Phillipson M. The neutrophil: one cell on many missions or many cells with different agendas? Cell Tissue Res. 2018;37:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiekowski MT, Chen SC, Zalamea P, et al. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol. 2001;167:7102–7110. [DOI] [PubMed] [Google Scholar]
- 48.Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J Biol Chem. 2004;279:24372–24386. [DOI] [PubMed] [Google Scholar]
- 49.Lefort CT, Ley K. Neutrophil arrest by LFA-1 activation. Front Immunol. 2012;3:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengeløv H, Kjeldsen L, Diamond MS, et al. Subcellular localization and dynamics of Mac-1 (αmβ2) in human neutrophils. J Clin Invest. 1993;92:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das ST, Rajagopalan L, Guerrero-Plata A, et al. Monomeric and dimeric CXCL8 are both essential for in vivo neutrophil recruitment. PLoS One. 2010;5:e11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinton LJ, Nelson S, Zhang P, et al. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. Am J Physiol Lung Cell Mol Physiol. 2004;286:L465–472. [DOI] [PubMed] [Google Scholar]
- 53.Zamjahn JB, Quinton LJ, Mack JC, et al. Differential flux of macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant from the lung after intrapulmonary delivery. Am J Physiol Lung Cell Mol Physiol. 2011;301:L568–L574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanino Y, Coombe DR, Gill SE, et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol. 2010;184:2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajasekaran D, Keeler C, Syed MA, et al. A model of GAG/MIP-2/CXCR2 interfaces and its functional effects. Biochemistry. 2012;51:5642–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawant K, Xu R, Cox R, et al. Chemokine CXCL1 mediated neutrophil recruitment in the mouse lung – Role CXCR2 activation. J Innate Immun. 2015;9:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gangavarapu P, Rajagopalan L, Kohli D, et al. The monomerdimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J Leuk Biol. 2011;91:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasser MW, Raghuwanshi SK, Grant DJ, et al. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183:3425–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravindran A, Sawant K, Sarmiento JM, et al. Chemokine CXCL1 dimer is a potent agonist for the CXCR2 receptor. J Biol Chem. 2013;288:12244–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajarathnam K, Kay CM, Dewald B, et al. Neutrophil activating peptide-2 (NAP-2) and melanoma growth stimulatory activity (MGSA) are functional as monomers for neutrophil activation. J Biol Chem. 1997;272:1725–1729. [DOI] [PubMed] [Google Scholar]
- 61.Rajarathnam K, Prado GN, Fernando H, et al. Probing receptor binding activity of interleukin-8 dimer using a disulfide trap. Biochemistry. 2006;45:7882–7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lowman HB, Fairbrother WJ, Slagle PH, et al. Monomeric variants of IL-8: effects of side chain substitutions and solution conditions upon dimer formation. Protein Sci. 1997;6:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sepuru KM, Rajarathnam K. Structural basis of chemokine interactions with heparan sulfate, chondroitin sulfate, and dermatan sulfate. J Biol Chem. 2019;294:15650–15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sepuru KM, Nagarajan B, Desai UR, Rajarathnam K. Molecular basis of chemokine CXCL5-Glycosaminoglycan interactions. J Biol Chem. 2016;291:20539–20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sepuru KM, Rajarathnam K. CXCL1/MGSA is a novel glycosaminoglycan (GAG)-binding chemokine: structural evidence for two distinct non-overlapping binding domains. J Biol Chem. 2016;291: 4247–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown AJ, Sepuru KM, Sawant KV, Rajarathnam K. Platelet-derived Chemokine CXCL7 dimer preferentially exists in the glycosaminoglycan-bound form: implications for Neutrophil-Platelet crosstalk. Front Immunol. 2017;8:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sepuru KM, Iwahara J, Rajarathnam K. Direct detection and characterization of lysine side chain NH3+ in protein-heparin complexes using NMR spectroscopy. Analyst. 2018;143:635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajarathnam K, Desai UR. Structural insights into how proteoglycans determine chemokine-CXCR1/CXCR2 interactions: progress and challenges. Front Immunol. 2020;11:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- 70.Mei J, Liu Y, Dai N, et al. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity. 2010;33: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonecchi R, Graham GJ. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol. 2016;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee JS, Frevert CW, Wurfel MM, et al. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol. 2003;170: 5244–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Girbl T, Lenn T, Perez L, et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity. 2018;49:1062–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]


