Abstract
Recent preclinical and clinical studies suggest that lorcaserin, a preferential serotonin 2C receptor (5-HT2CR) agonist that was approved for the treatment of obesity, possesses antiepileptic properties. Here, we tested whether lorcaserin (1, 3, 5.6, 10 mg/kg) is prophylactic against audiogenic seizures (AGSs) in juvenile Fmr1 knockout mice, a mouse model of fragile X syndrome (FXS). MPEP (30 mg/kg), a non-competitive mGluR5 receptor antagonist, was used as a positive control. As lorcaserin likely engages 5-HT2ARs at therapeutic doses, we pretreated one group of mice with the selective 5-HT2AR antagonist/inverse agonist, M100907 (0.03 mg/kg), alone or before administering lorcaserin (5.6 mg/kg), to discern putative contributions of 5-HT2ARs to AGSs. We also assessed lorcaserin’s in vitro pharmacology at human (h) and mouse (m) 5-HT2CRs and 5-HT2ARs and its in vivo interactions at m5-HT2CRs and m5-HT2ARs. MPEP significantly decreased AGS prevalence (P=0.011) and lethality (P=0.038). Lorcaserin, 3 mg/kg, attenuated AGS prevalence and lethality by 14% and 32%, respectively, however, results were not statistically significant (P=0.5 and P=0.06); other doses and M100907 alone or with lorcaserin did not significantly affect AGSs. Lorcaserin exhibited full efficacy agonist activity at h5-HT2CRs and m5-HT2CRs, and near full efficacy agonist activity at h5-HT2ARs and m5-HT2ARs; selectivity for activation of 5-HT2CRs over 5-HT2ARs was greater for human (38-fold) compared to mouse (13-fold) receptors. Lorcaserin displayed relatively low affinities at antagonist-labeled 5-HT2CRs and 5-HT2ARs, regardless of species. Lorcaserin (3 and 5.6 mg/kg) increased the 5-HT2AR-dependent head-twitch response (HTR) elicited by (±)-2,5-dimethoxy-4-iodoamphetamine (DOI) in mice (P=0.03 and P=0.02). At 3 mg/kg, lorcaserin alone did not elicit an HTR. If mice were treated with the selective 5-HT2CR antagonist SB242084 (0.5 and 1 mg/kg) plus lorcaserin (3 mg/kg), a significantly increased HTR was observed, relative to vehicle (P=0.01 and P=0.03), however, the HTR was much lower than what was elicited by DOI or DOI plus lorcaserin. Lorcaserin, 3 mg/kg, significantly reduced locomotor activity on its own, an effect reversed by SB242084, and lorcaserin also dose-dependently reduced locomotor activity when administered prior to DOI (Ps<0.002). These data suggest that lorcaserin engages 5-HT2CRs as well as 5-HT2ARs at doses as low as 3 mg/kg in mice. The similar activity at m5-HT2CRs and m5-HT2ARs suggests careful dosing of lorcaserin is necessary to selectively engage 5-HT2CRs in vivo. In conclusion, lorcaserin was ineffective at preventing AGSs in Fmr1 knockout mice. Lorcaserin may not be a suitable pharmacotherapy for seizures in FXS.
Keywords: Fmr1 knockout mouse, audiogenic seizures, lorcaserin, head-twitch response, 5-HT2A, 5-HT2C
1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) elicits intracellular signaling through at least 14 genetically-encoded 5-HT receptors (5-HTRs) expressed in unique patterns and densities in the brain, spinal cord, and peripheral tissues. Distinct proteins of the peripheral and central 5-HT systems have been targeted successfully to treat numerous disorders such as migraine, depression, anxiety, psychosis, obesity, constipation, and emesis (Barnes et al., 2021), and drug discovery targeting 5-HTRs remains vibrant. For example, scientists are currently testing small molecule 5-HT2R agonists for the treatment of substance use disorders, treatment-resistant depression, asthma, and epilepsy, and results are promising (Davis et al., 2020; Flanagan et al., 2019; Griffin et al., 2017; Nichols et al., 2017; Nutt et al., 2020; Palacios et al., 2017; Patel et al., 2018).
Lorcaserin was the first FDA approved, selective 5-HT2R–Gαq agonist, and exhibits preferential activity at 5-HT2CRs over the other 5-HT2R subtypes, 5-HT2ARs and 5-HT2BRs (Higgins et al., 2020; Thomsen et al., 2008). It was approved to treat obesity, and was marketed under the brand name Belviq® and Belviq XR® for almost 8 years, until recently, when it was withdrawn due to potential cancer risk; the mechanism(s) for this adverse event are unclear (Di Giovanni and De Deurwaerdère, 2020) but might involve alterations in the activity of the intracellular Gαq heterotrimeric complex that are known to be associated with some cancers (Annala et al., 2019). Lorcaserin’s anorectic effects are thought to be due to activation of 5-HT2CRs in the arcuate nucleus of the hypothalamus and in the nucleus of the solitary tract (D’Agostino et al., 2018), however, after steady-state clinical dosing of 10 mg twice daily in monkeys, brain concentrations reach ~1.7μM (see FDA briefing document NDA22529), which is above lorcaserin’s Ki—radiolabeled with the agonist [125I]2,5-dimethoxy-4-iodoamphetamine (DOI)—and EC50 values at 5-HT2A and 5-HT2BRs (Thomsen et al., 2008). These data imply that lorcaserin activates each of the 5-HT2R subtypes at therapeutic doses. Early reports showed that lorcaserin is a full agonist at human (h) 5-HT2CRs and h5-HT2BRs but a partial agonist at h5-HT2ARs (Thomsen et al., 2008). Interestingly, despite its h5-HT2AR agonist activity—which is understood to be the essential pharmacological mechanism of action of serotonergic psychedelics (Preller et al., 2017)—at the canonical h5-HT2AR-Gαq signaling pathway, lorcaserin does not produce LSD-like psychedelic effects at therapeutic doses. Even at supratherapeutic doses, lorcaserin’s psychoactive effects are less prominent than the sleep aid, zolpidem, and the psychedelic anesthetic and antidepressant, ketamine (Shram et al., 2011), suggesting lorcaserin has unique pharmacology relative to serotonergic psychedelics, such as DOI, despite their structural similarity.
The observation that 5-HT2CR knockout mice are susceptible to seizures elicited by auditory stimuli, i.e., audiogenic seizures (AGSs), (Brennan et al., 1997) inspired studies evaluating 5-HT2CR agonists as antiepileptics. Preclinical studies with rats demonstrated lorcaserin has efficacy to attenuate genetic absence seizures and temporal lobe epilepsy, which is the most frequent type of intractable epilepsy (Orban et al., 2014; Venzi et al., 2016). Lorcaserin also reduces the frequency of electrographic seizure events in scn1Lab mutant zebrafish larvae, a model of Dravet syndrome (DS) (Griffin et al., 2017). A recent, small clinical trial and a retrospective study of Belviq® supported the preclinical observations, showing that Belviq® reduced seizure frequency in children with DS, Lennox-Gastaut syndrome and other treatment-resistant focal and generalized epilepsies (Griffin et al., 2017; Tolete et al., 2018). Conversely, another recent study evaluating several 5-HT2CR agonists in numerous, induced-seizure models in wild-type rats and mice showed that lorcaserin was ineffective in preventing or attenuating seizures, but the authors surmised that 5-HT2CR-elicited antiepileptic effects may be confined to genetic disorders that include an epilepsy phenotype (Silenieks et al., 2019). Indeed, few studies have systematically assessed lorcaserin’s efficacy in genetic mouse models of neurodevelopmental disorders—apart from DS—that present with a seizure phenotype.
Intriguingly, the onset of AGS susceptibility in 5-HT2CR knockout mice coincides with the development of social deficits (Sejourne et al., 2015), and social deficits and epilepsy are often diagnosed in fragile X syndrome (FXS)—a neurodevelopmental disorder, caused by mutation and inactivation of the FMR1 gene, that is the leading monogenic cause of autism spectrum disorder (ASD) and is characterized by intellectual disabilities, anxiety, attention deficit hyperactivity disorder, and other neuropsychiatric symptoms (see https://www.fraxa.org/). Approximately 25% of children with FXS experience seizures, some of which are intractable to anticonvulsant treatment and progress into adulthood (Bailey et al., 2008; Berry-Kravis, 2002; Berry-Kravis et al., 2010; Heard et al., 2014; Incorpora et al., 2003; Musumeci et al., 1999); the seizure risk increases by three times in individuals with comorbid ASD (Kaufmann et al., 2017). Seizure prevalence in clinical studies of FXS ranges from 4–45%, and seizures include focal, generalized tonic-clonic seizure (TCS), benign focal (Rolandic epilepsy), febrile seizures, and status-epilepticus, in rare cases (Berry-Kravis, 2002; Berry-Kravis et al., 2010; Cowley et al., 2016; Gauthey et al., 2010; Hagerman and Stafstrom, 2009; Heard et al., 2014; Incorpora et al., 2003; Musumeci et al., 1999). Status epilepticus early in development can contribute to long-term autistic-like behaviors in Fmr1 knockout mice (Hodges et al., 2019). Thus, seizures and ASD appear to be interrelated and may share similar biological mechanisms. Existing anticonvulsants, however, cause adverse events such as cognitive impairment, aggression and hyperactivity during treatment or upon withdrawal, which can worsen FXS symptoms (Ijff and Aldenkamp, 2013; Loring and Meador, 2004). Therefore, treating seizures early in development with medications that have few adverse side effects may improve clinical outcomes for individuals with FXS. Like 5-HT2CR knockout mice, the Fmr1 knockout mouse model of FXS also is susceptible to AGSs (Armstrong et al., 2020; Musumeci et al., 2007), and this is arguably the most robust and reproducible phenotype in the FXS mouse model (Hagerman et al., 2017).
These observations led us to test the hypothesis that treating juvenile Fmr1 knockout mice with lorcaserin would attenuate or prevent AGSs. To validate lorcaserin’s interaction with 5-HT2Rs in mice, we assessed its effects on the mouse 5-HT2AR-dependent head-twitch response (HTR), an in vivo proxy for central 5-HT2AR activation; the HTR in mice is also modulated by 5-HT2CRs (Canal et al., 2013a; Canal and Morgan, 2012; Fantegrossi et al., 2010). We also assessed lorcaserin’s effects on locomotor activity, which is sensitive to 5-HT2AR and to 5-HT2CR activation, in adult, male, wild-type C57BL/6J mice: 5-HT2AR activation enhances locomotion, conversely, 5-HT2CR activation decreases locomotion (Halberstadt et al., 2009). C57BL/6J mice were chosen for these experiments, as they exhibit a robust HTR relative to sighted FVB mice (wild-type of our Fmr1 knockout mice), allowing observations of subtle, but significant effects of drugs on the HTR (Canal and Morgan, 2012; Canal et al., 2010; Chen et al., 2019; Halberstadt et al., 2020). We also report, for the first time to our knowledge, lorcaserin’s affinity at antagonist-labeled human (h) and C57BL/6J mouse (m) 5-HT2CRs and 5-HT2ARs, in addition to reporting its function at them.
2. Methods
2.1. Subjects
All experimental protocols involving FVB.129P2-Pde6b+ Tyrc-ch Fmr1tm1Cgr/J (Fmr1 knockout, stock #004624, Jackson Laboratory) and FVB.129P2-Pde6b+ Tyrc-ch/AntJ (sighted FVB or wild-type, stock #004828, Jackson Laboratory) were approved by the Mercer University Institutional Animal Care and Use Committee, and all experimental protocols involving C57BL/6J (Stock #000664, Jackson Laboratory) mice were approved by Northeastern University’s Institutional Animal Care and Use Committee. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition. Adult (Postnatal day 60 (P60) – P100), male C57BL/6J mice—used for in vivo 5-HT2R assessments—were procured from the Jackson Laboratory, housed in groups of four, and were acclimated to the vivarium for at least one week prior to testing. All AGS tests were performed using juvenile (P23 – P25), male and female, FVB wild-type or Fmr1 knockout mice bred and raised in the vivarium at Mercer University College of Pharmacy. Breeding pairs and trios of Fmr1 knockout and wild-type mice were procured from the Jackson Laboratory to develop a colony. Mice were bred, housed and genotyped as previously described (Armstrong et al., 2020). Briefly, 1–2 homozygous Fmr1 knockout females (P60–P240) were paired with an adult, hemizygous Fmr1 knockout male for breeding Fmr1 knockout mice, and 1–2 wild-type females (P60–P240) were paired with an adult, wild-type male for breeding wild-type mice. Homozygous mice on the FVB background were chosen due to their increased susceptibility to seizures over other strains (Yan et al., 2005).
The breeding scheme was employed to avoid the possibility of cannibalization and unequal nurturing of littermates with mixed genotypes by the dam, i.e., to preclude poor nurturing of Fmr1 knockout pups by heterozygote dams (Goel et al., 2018). This breeding scheme also maintains phenotypes in Fmr1 knockout mice (Wen et al., 2019). The pups were raised by either a single parent (dam) or 2 parents (both dam and sire or dam and another adult female). Pups were weaned at P21±1, at which time an ~0.5 cm tail snip or ear punch tissue sample was collected for quality control, genotyping. All mice were group housed (N = 2–4) in home cages (polycarbonate cages (15 cm × 25 cm × 13 cm) with open-air, stainless steel wire lids and nesting sheets (Bio-Serv)) and maintained on a 12h/12h light/dark cycle at a temperature of 70–75°F and relative humidity of ~50%, with food and water available ad libitum. Cage mates were matched for sex and genotype and identified by ear punch. All tests were conducted during the light cycle (7:00–19:00).
2.2. Compounds
All compounds were dissolved in Milli-Q water (MilliporeSigma), which served as the vehicle control, unless noted. Lorcaserin hydrochloride was purchased from ChemScene and Adooq Biosciences. 5-HT hydrochloride was procured from Alfa Aesar. (±)-DOI hydrochloride (DOI) was purchased from MilliPore Sigma. M100907 hydrochloride was generously provided by Professor Kevin Murnane, who originally obtained the compound from Dr. Kenner Rice. M100907 was first dissolved in 40% DMSO/60% Milli-Q water to prepare a 1 mg/ml stock and then serially diluted with Milli-Q water to obtain the tested concentration. Ketanserin tartrate was purchased from MP Biomedical. SB242084, mesulergine hydrochloride, and mianserin hydrochloride were purchased from Tocris Biosciences. SB242084 was dissolved in Milli-Q water with 5% DMSO. MPEP (2-methyl-6-(phenylethynyl)pyridine) hydrochloride was purchased from MedChemExpress. [3H]Mesulergine, [3H]Ketanserin, [3H]5-CT, and [3H]myo-inositol were purchased from PerkinElmer. All compounds were ≥98% pure according to the manufacturers. Vehicle and all compounds for in vivo studies were administered systemically—intraperitoneally (i.p.) to Fmr1 knockout and wild-type mice and subcutaneously (s.c.) to C57BL/6J mice—at a volume of 10 ml/kg. All doses were selected based on previous studies showing their in vivo efficacy (Canal et al., 2013a; Canal et al., 2015; Canal and Morgan, 2012; Fantegrossi et al., 2010; Serafine et al., 2015; Yan et al., 2005).
2.3. Audiogenic Seizures
AGS testing in Fmr1 knockout and wild-type mice was conducted as previously described (Armstrong et al., 2020). Convulsive behaviors elicited by auditory stimuli are documented as AGSs, and reflect seizure activity measured by electroencephalography (Garcia-Cairasco, 2002; Iida et al., 1998). Experimentally-naïve, juvenile mice were acclimated to a procedure room with dimmed lighting, relative to the vivarium, in their home cages for ≥ 30 minutes prior to injections. Mice were weighed and then vehicle, lorcaserin (1, 3, 5.6, or 10 mg/kg; solutions from 431 μM (0.1 mg/mL) to 4.31 mM (1 mg/mL) were made, and assuming a mouse weight of 25 g and 15 mL of total body fluid (RMI Pharmacokinetics), then in vivo concentrations were ~7.18 to 71.8 μM), or MPEP (30 mg/kg or an in vivo concentration of ~218.3 μM), a non-competitive mGluR5 antagonist used as a positive control antiepileptic (Yan et al., 2005), was administered to Fmr1 knockout mice; wild-type mice were treated with vehicle only. To assess a potential contribution of 5-HT2AR activation to the effects of lorcaserin, a separate group of mice was pretreated with the selective, high-potency 5-HT2AR antagonist/inverse agonist, M100907 (0.03 mg/kg; or an in vivo concentration of ~0.122 μM) (Canal et al., 2013a), alone or 10 minutes prior to treatment with lorcaserin (5.6 mg/kg). After injection(s), mice were placed back in their cages, and then were tested 30 minutes later. Pretreatment periods for all compounds were based on prior studies in rodents (Fantegrossi et al., 2010; Gleason and Shannon, 1997; Serafine et al., 2016; Silenieks et al., 2019; Yan et al., 2005).
Mice were placed in a clear, polycarbonate box (46 cm × 20 cm × 20 cm) covered with a plastic screen and allowed to acclimate for ≤8 minutes before being exposed to an alarm (RadioShack Kit #49-1010, doorstop alarm) for five minutes, held by hand ~20 cm away from the mice. A sound-level meter/data logger (REED Model SD-4023) with a frequency range of 31.5–8000 Hz and a decibel range of 30–130 dB was placed ~20 cm from the alarm to ensure that the sound pressure levels were approximately the same during each experiment. The sound meter was pre-programmed to frequency weighting A (human ear listening), time weighting of 200 ms and sampling rate of 1 s. Tests were video-recorded using a high-definition camcorder (Vixia HF R800, Canon). A maximum of 8 mice (4 per box) were tested simultaneously in the AGS assay. The average (± standard deviation (SD)) baseline sound pressure in the testing room was 52 ± 4 dB, and the average alarm sound pressure was 109 ± 2 dB (N=29 trials).
Behavioral responses, including “normal responses,” wild running and jumping (WRJ), TCS, and lethality caused by respiratory arrest, were documented during the test. Seizure prevalence was defined as the presence of TCS. “Normal responses” included sniffing, walking, rearing, sitting, urinating, defecating, grooming, socializing, squinting of eyes, episodes of initial hyperlocomotion, running without jumping, and jumping without running. In general, an AGS followed a sequence that began with a startle response upon first sounding the alarm, followed by freezing and squinting of eyes, one or multiple WRJ phase(s), brief opisthotonos with an open mouth, a clonic phase with the mouse lying on one side (left or right) of its body with head, neck, trunk and limbs ventro-flexed (muscle jerking and twitching with rigidity), a brief (~5 s) tonic seizure phase with full extension of extremities (muscle stiffening) and respiratory arrest (Iida et al., 1998; Ross and Coleman, 2000). Mice that recovered from the TCS phase typically exhibited Straub tail, tremors and a full body vibrating shudder that transitioned to normal behaviors or freezing until the end of alarm exposure. Latency to and duration of each response for each mouse were documented by visual observation of video recordings.
2.4. Cell Growth and Transfection of Plasmids Encoding 5-HTRs
Plasmids encoding h5-HT2A and h5-HT2C-INIRs were obtained from the cDNA Resource Center. Plasmids encoding m5-HT2A and m5-HT2C-VNVRs were obtained from Origene. Fetal Bovine Serum (FBS) and dialyzed FBS (dFBS) were obtained from Atlanta Biological, Corning Life Sciences, and Gibco. Dulbecco’s modified Eagle’s medium (DMEM) and Inositol-free DMEM were purchased from Corning Life Sciences, and UltraMEM was purchased from Lonza. Human embryonic kidney cells (HEK293, ATCC CRL-1573) were used for all binding and functional assays. Cells were grown in an incubator at 37°C, 5% CO2, and 95% humidity in 10 cm dishes with DMEM medium containing 10% FBS and 1% penicillin-streptomycin. Cells were transfected at ~80% confluency, with 15 μg of h5-HT2A, h5-HT2C, m5-HT2A or m5-HT2CR cDNA and 30 μL TurboFect transfection reagent (ThermoFisher Scientific) in 2 mL UltraMEM and 8 mL DMEM with 10% dFBS.
Mouse receptor plasmids used for in vitro receptor pharmacology experiments encode for C57BL/6J mouse 5-HT2ARs (Genome Reference Consortium Mouse Build 39) and unidentified “house mouse” (typically associated with the C57BL/6J mouse strain) 5-HT2CRs (MGC_Program_Team, 2002). To maintain consistency with in vitro pharmacology experiments, we used C57BL/6J mice for in vivo pharmacology experiments
2.5. Radioligand Competition Binding Assays
Approximately 48 hours after transfection, cells were collected and homogenized in ice-cold 50 mM Tris, 10 mM MgCl2-6H2O, and 0.1 mM EDTA, pH 7.4 (assay buffer). Homogenate was spun at 4500g for 10 minutes at 4°C using a Sorvall Legend X1R tabletop centrifuge (ThermoFisher Scientific) three successive times, discarding the supernatant after each time, and then the pellet was used immediately for testing or stored at −80°C for later testing. Competition binding assays with lorcaserin and control ligands were performed in 96 well plates, using ~2 nM [3H]Ketanserin to radiolabel 5-HT2AR, and ~2 nM [3H]Mesulergine to radiolabel 5-HT2CR orthosteric binding sites. Non-specific binding was determined in the presence of 10 μM mianserin for all 5-HT2Rs. Plates containing test ligands, radioligand, and cell membranes in assay buffer were covered and incubated on a shaker for 90 minutes at room temperature. Plate contents were rapidly filtered through Whatman GF/B filter mats using a 96-well cell harvester (Brandel), which were then washed, by filtration, three to five times with ~200 mL of ice-cold 50 mM Tris-HCl to remove unbound radioligand. Filter mats containing bound radioligand were dried, saturated with scintillation cocktail (ScintiVerse Cocktail, Fisher Scientific), and then counted for [3H]-induced scintillations using a PerkinElmer Tri-Carb 2190TR liquid scintillation counter.
2.6. 5-HT2CR- and 5-HT2AR-Mediated Phosphoinositide Hydrolysis
Growth medium was discarded 24-hr after transfection, and replaced with 14 mL of inositol-free DMEM containing 5% dialyzed FBS. Cells were detached and collected in a sterile 16 mL centrifuge tube. [3H]myo-inositol (1 μCi/mL) was added to the tube, and contents were vortexed thoroughly. Cells (300 μL per well) were seeded into 48-well CellBind plates (Corning Life Sciences), and placed in a cell incubator overnight. On the day of the assay, medium was carefully discarded to minimally disrupt the attached cells, and replaced with 450 μL of inositol-free DMEM. After a 1-hour incubation, 10 μM (final concentration) pargyline, 10 mM (final concentration) LiCl, and test ligands (50 μL) were added to the plate wells, and plates were placed again in the cell incubator for 45 minutes. Medium was then discarded, and 400 μL of 50 mM formic acid was added to lyse the cells and terminate reactions at room temperature. After 1 hour, 200 μL of 150 mM ammonium formate was added to neutralize the solution, and plates were frozen at −80°C. The next day, the plates were thawed and added to anion exchange columns (Bio-Rad Labratories, Hercules, CA) to bind and isolate [3H]inositol phosphates (Canal et al., 2013b). One mL of isolated [3H]inositol phosphates was added to 10 mL of scintillation cocktail, vortexed vigorously, then scintillations were counted using a PerkinElmer Tri-Carb 2190TR liquid scintillation counter.
2.7. Lorcaserin 5-HT2CR and 5-HT2AR In Vivo Pharmacology: Effects of Lorcaserin and SB242084 plus Lorcaserin on the HTR and on Locomotor Behavior
C57BL/6J mice were acclimated to a procedure room ≥60 minutes before administration of test compounds, and then were injected s.c. with Milli-Q water containing 5% DMSO (vehicle) or the selective, high potency 5-HT2CR antagonist, SB242084 (0.5, 1 mg/kg or an in vivo concentration of ~2.11 to 4.22 μM, respectively) (Canal et al., 2013a). Milli-Q water (vehicle) or lorcaserin (3.0 mg/kg) was injected s.c. 10 minutes later. After 10 minutes, mice were placed in a clear open-field plexiglass chamber (43 × 43 cm; Med Associates, St Albans, VT), and locomotor activity (distance traveled in cm) was video recorded and calculated by Ethovision software (Noldus Information Technology). HTRs, defined as rapid, discrete, jerks of the head—akin to myoclonus—were counted using a hand-held tally counter by a trained observer who was blind to drug treatment.
2.8. Lorcaserin 5-HT2CR and 5-HT2AR In Vivo Pharmacology: Effects of Lorcaserin on the DOI-elicited HTR and on Locomotor Activity
C57BL/6J mice were acclimated to the testing room ≥ 60 minutes before administration of test compounds, and then were injected s.c. with Milli-Q water (vehicle) or lorcaserin (1, 3, or 5.6 mg/kg) followed 10 minutes later by injection of the psychedelic 5-HT2R agonist, DOI (1.0 mg/kg or an in vivo concentration of ~ 4.67 μM). Ten minutes later, mice were placed into the open-field for a 10-minute observation session. HTRs were counted, using a tally counter, by a trained observer who was blind to treatment. Simultaneously, locomotor activity was video recorded and calculated by Ethovision software.
C57BL/6J mice were chosen for in vivo experiments evaluating 5-HT2R activity based on our observations that C57BL/6J mice exhibit a robust HTR relative to sighted FVB mice (wild-type of our Fmr1 knockout mice)(Canal et al., 2010a; Chen et al., 2019); the robust HTR in C57BL/6J mice permits observations of subtle, but significant effects of drugs on the HTR. Here we reproduced the findings of (Canal and Morgan, 2012; Canal et al., 2010; Chen et al., 2019; Halberstadt et al., 2020) that show the relatively low number of HTRs exhibited by wild-type, sighted FVB mice, 10 min after treatment with 1 mg/kg DOI (Figure S1). Furthermore, we did not observe significant differences in the HTR between wild-type, sighted FVB mice and Fmr1 knockout mice on the same background (Figure S1), suggesting no differences in in vivo 5-HT2R function caused by the loss of FMRP.
2.9. Statistical Analyses
Statistical tests were performed using GraphPad Prism (Version 9). AGS—WRJ, TCS, and lethality—frequencies were analyzed using Fisher’s exact test (two-sided, α=0.05). Fisher’s exact test was also used to evaluate sex effects on AGSs. Compound effects on latency to and duration of each response were analyzed by one-way ANOVA with Dunnett’s post-hoc test comparing all Fmr1 knockout treatment groups with vehicle-treated Fmr1 knockout mice. A one-way ANOVA with Tukey’s post-hoc test was used to make statistical comparisons of the HTR and locomotor data, except for effects of lorcaserin and lorcaserin co-treatment with SB24084 on locomotor activity, wherein Tukey’s multiple comparisons post-hoc test was used. All in vivo data were subjected to ROUT tests (Q=5%) to exclude likely outliers.
Radioligand competition binding data were analyzed using the “one site fit-Ki” model compared to the “two site fit-Ki” model. Kd values were set to 1.57 nM for [3H]Ketanserin at 5-HT2ARs and 2.00 nM for [3H]Mesulergine at 5-HT2CRs, based on (Canal et al., 2013b; Liu et al., 2017; Olaghere da Silva et al., 2010; Roth, 2013). Results from 5-HT2R-mediated phosphoinositide hydrolysis assays were analyzed using the “log(agonist) vs. response (three parameters)” model to obtain EC50 and Emax values of individual compounds. Kd and Ki values report ligand affinities, or how tight a ligand binds to a target, but they are obtained through different experimental methods; though Kd and Ki values are similar, they can be significantly different from one another. We refer readers unfamiliar with these concepts to (Canal, 2018).
3. Results
3.1. Audiogenic Seizures
As shown in Figure 1, consistent with previous findings, vehicle-treated juvenile Fmr1 knockout mice exhibited significantly higher seizure prevalence and lethality compared to vehicle-treated juvenile wild-type mice (70% vs 10%, P<0.0001, and 60% vs. 5%, P=0.0002, respectively), and the positive control, MPEP (30 mg/kg), significantly reduced seizure prevalence (12%, P=0.011) and lethality (12%, P=0.038) in Fmr1 knockout mice to wild-type levels (Yan et al., 2005).
Figure 1.
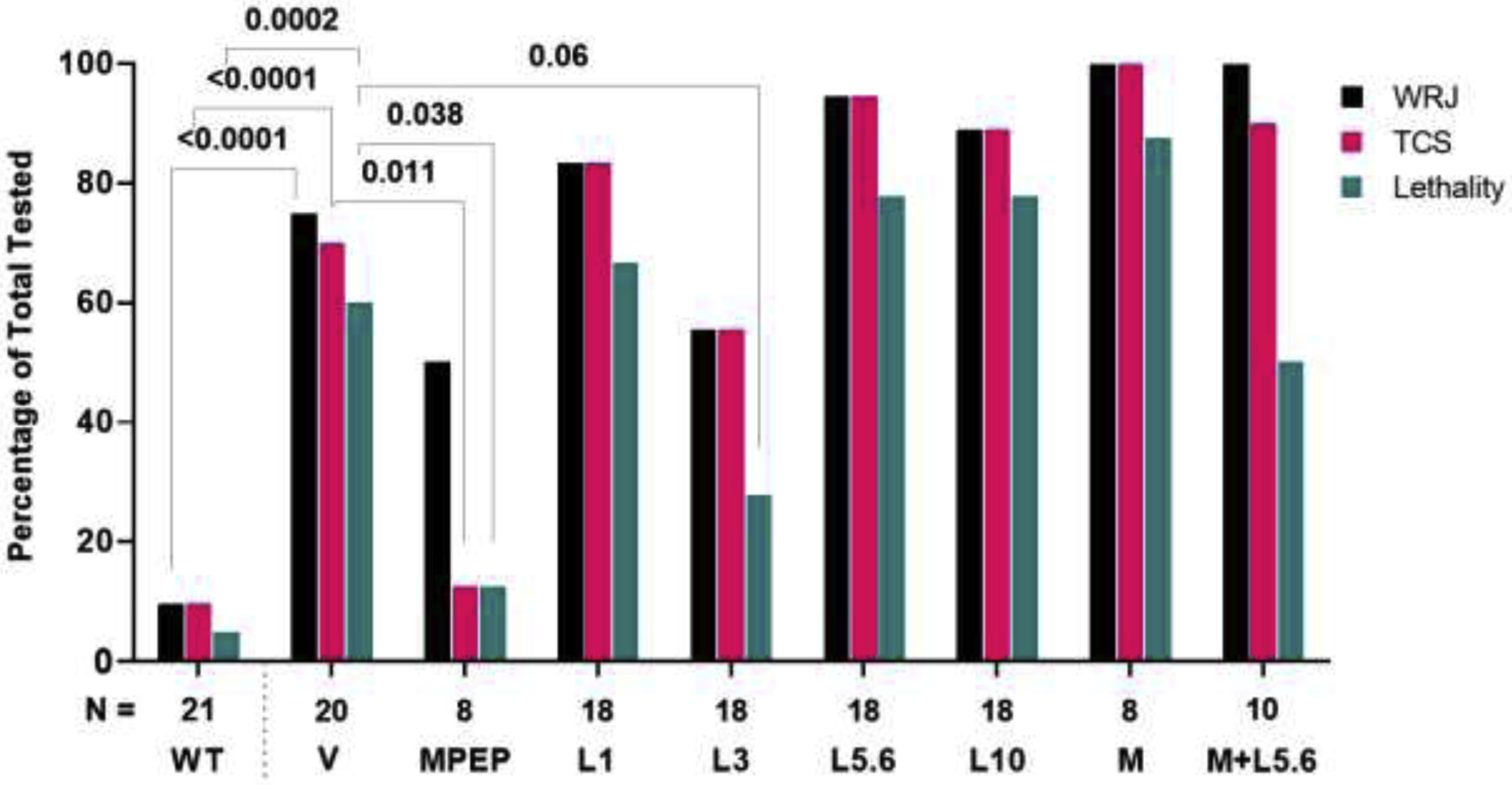
Effects of lorcaserin (L) on AGSs in juvenile, Fmr1 knockout mice. Wild running and jumping (WRJ), tonic-clonic seizures (TCS), and lethality were significantly higher in Fmr1 knockout mice that received vehicle (V) as compared to vehicle-treated wild-type (WT) mice. The positive control, MPEP 30 mg/kg (MPEP), significantly reduced TCS prevalence and lethality in Fmr1 knockout mice to WT levels. Lorcaserin 3 mg/kg (L3) tended to reduce lethality caused by AGSs, though, effects did not reach statistical significance. M, M100907 0.03 mg/kg. N, number of mice tested per group.
The main observation from this set of experiments is that lorcaserin was ineffective at preventing AGSs in Fmr1 knockout mice. However, relative to vehicle-treated Fmr1 knockout mice, lorcaserin, at 3 mg/kg, showed a trend to decrease AGS-elicited lethality (60% vs. 28%, respectively, P=0.0585). At 5.6 mg/kg, it showed a trend to increase AGS prevalence (70% vs. 94%, respectively, P=0.093). At other doses, lorcaserin did not affect lethality (67%, P=0.74 at 1 mg/kg and 78%, P=0.31 at 5.6 mg/kg and 10 mg/kg) or prevalence (1 mg/kg =83%, P=0.45; 3 mg/kg =56%, P=0.5; 10 mg/kg =89%, P=0.24) relative to vehicle (Figure 1). Similarly, treatment with M100907 (0.03 mg/kg) alone or with lorcaserin, 5.6 mg/kg had no effect on seizure prevalence (100%, P=0.14 alone and 90%, P=0.37 with lorcaserin) or lethality (88%, P=0.21 alone and 50%, P=0.71 with lorcaserin), relative to vehicle. There was no statistically significant difference in seizure prevalence between vehicle-treated male and female Fmr1 knockout mice (P=0.63), though there was a trend of decreased lethality in female Fmr1 knockout mice (P=0.17).
We also assessed whether lorcaserin affected other AGS parameters including response latency and duration (Figure 2). Mean latency in seconds (± SD) to the first episode of WRJ, TCS, and lethality of vehicle-treated Fmr1 knockout mice was 36 (±11), 41 (±8), and 54 (±7), respectively. A significant difference was observed between mean WRJ latencies (F (7, 82) = 71.91, P<0.0001, one-way ANOVA). Post-hoc test revealed that the latency to WRJ following MPEP treatment (160 (±19)) was significantly longer than the latency in vehicle-treated Fmr1 knockout mice (P<0.0001), and this was similar to wild-type mice that exhibited WRJ (144 (±93)). Lorcaserin did not affect WRJ latency. Mean TCS onset latencies were also significantly different among treatment groups (F (6, 75) = 4.042, P=0.0015, one-way ANOVA). Post-hoc test showed that lorcaserin 5.6 mg/kg increased TCS onset latency to 58 (±21) compared to vehicle (41 (±8), P=0.0082) (Figure 2A). Latency to lethality was similar among all treatment groups (F (6, 55) = 2.209, P=0.056, one-way ANOVA).
Figure 2.
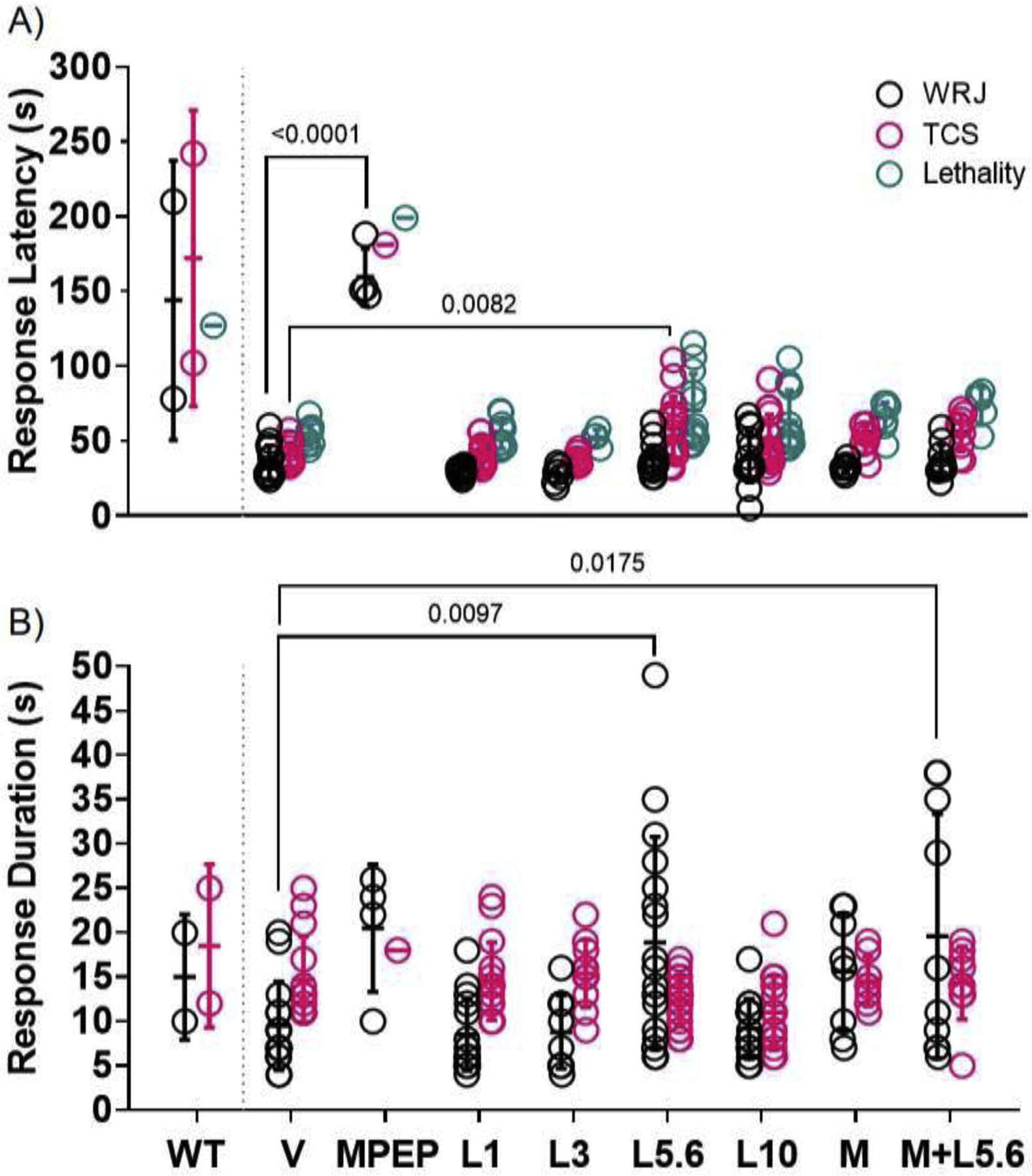
Latencies to wild running and jumping (WRJ), tonic-clonic seizures (TCS), and lethality (A) and duration of WRJ and TCS (B) across treatment groups. All treatments were administered to juvenile Fmr1 knockout mice; data from vehicle-treated wild-type mice (WT) are shown for comparison. In Fmr1 knockout mice, MPEP 30 mg/kg (MPEP) increased WRJ onset latency relative to vehicle (V). Lorcaserin 5.6 mg/kg (L5.6) increased TCS onset latency compared to V. Latency to lethality was not affected by any treatment. L5.6 also increased WRJ duration relative to V. Co-treatment with M100907 (0.03 mg/kg) and L5.6 (M+L5.6) increased WRJ duration relative to V. TCS duration was not affected by any treatment. Too few mice in the MPEP group displayed TCS and lethality to perform statistical analyses; similarly, too few WT mice displayed AGSs. Lines through data points represent means and SDs.
As shown in Figure 2B, the mean duration in seconds (± SD) of WRJ and TCS for vehicle-treated Fmr1 knockout mice was 10 (±5) and 15 (±5), respectively. A main effect of treatment was observed in mean WRJ durations (F (7, 84) = 4.886, P=0.0001, one-way ANOVA), with most of the variance attributed to the lorcaserin 5.6 mg/kg alone (19 (±12), P=0.0097) and in combination with M100907 (20 (±14), P=0.018) increasing WRJ durations compared to vehicle (10 (±5)). Initial comparisons in TCS durations showed a statistically significant difference between groups (F (6, 82) = 2.245, P=0.047, one-way ANOVA), however, post-hoc test did not reveal any differences.
Some female, but no male Fmr1 knockout mice that received lorcaserin alone (≥3 mg/kg) displayed WRJ episodes after recovery from TCS. This response could be a prolonged motor after-excitation, distinct from WRJ or a part of the seizure as described previously in spontaneously epileptic rats (Asano et al., 1990). This peculiar behavior was not observed in mice that received vehicle or M100907 alone. One Fmr1 knockout male that received 1 mg/kg lorcaserin displayed two full seizure sequences (separated by an extra WRJ/ motor excitation phase) and died 15 s after the alarm was stopped. Lastly, purposeless oral movements and “mild hiccups” were also observed in some animals that received lorcaserin. This is consistent with previous in vivo studies with 5-HT2CR agonists (Beyeler et al., 2010; Navailles et al., 2013; Silenieks et al., 2019).
3.2. Lorcaserin: In Vitro Pharmacology at Human and Mouse 5-HT2CRs and 5-HT2ARs
In vitro affinities (Ki) and functional (EC50) activities at h5-HT2CRs, m5-HT2CRs, h5-HT2ARs, and m5-HT2ARs are reported in Table 1 and shown in Figures 3 and 4. In vitro affinity was assessed using antagonist radioligand competition binding assays. The rank order of binding potencies at h5-HT2ARs was ketanserin>DOI>5-HT>lorcaserin, which was the same as the rank order of binding potencies at m5-HT2ARs. Though, the agonists exhibited lower affinity at m5-HT2ARs compared to h5-HT2ARs. The rank order of binding potencies at h5-HT2CRs was mesulergine>5-HT=DOI>lorcaserin. The rank order of binding potencies at m5-HT2CRs was mesulergine>DOI>5-HT>lorcaserin, owing to a lower affinity of 5-HT at m5-HT2CRs compared to h5-HT2CRs. The affinities of 5-HT and DOI at h5-HT2ARs and m5-HT2ARs and h5-HT2CRs and m5-HT2CRs that we observed here were modestly lower than our previous observations (Canal et al., 2013a).
Table 1.
Affinities, obtained from a “one-site – fit Ki” model of antagonist radioligand competition binding data, and EC50 values, obtained from phosphoinositide hydrolysis data, at h and m5-HT2A and 5-HT2CRs expressed in HEK293 cells. [3H]Ketanserin was used to label 5-HT2ARs; [3H]Mesulergine was used to label 5-HT2CRs. Note, for those compounds with affinities that fit better to a “two-sites – fit Ki” model*, the Ki’s at the “low affinity” and “high affinity” receptor populations are reported in the Results section. In most cases, the R2 values from both the “one-site – fit Ki” and “two-sites – fit Ki” models were >0.95. Ki and EC50 values are means, and pKi and pEC50 values are means (95% confidence intervals). NT is an abbreviation for “not tested.”
| h5-HT2A | m5-HT2A | h5-HT2C-INI | m5-HT2C-VNV | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Ki (nM)pKi (95% CI) | EC50 (nM)pEC50 (95% CI) | Ki (nM)pKi (95% CI) | EC50 (nM)pEC50 (95% CI) | Ki (nM)pKi (95% CI) | EC50 (nM)pEC50 (95% CI) | Ki (nM)pKi (95% CI) | EC50 (nM)pEC50 (95% CI) | ||||||||
| Lorcaserin | 1536 | 5.85 (5.89 to 5.74) | 124 | 6.91 (7.05 to 6.77) | 4464 | 5.35 (5.43 to 5.28) | 212 | 6.68 (6.82 to 6.53) | 661 | 6.18 (6.23 to 6.13) | 3.3 | 8.48 (8.70 to 8.26) | 741 | 6.13 (6.21 to 6.05) | 16 | 7.80 (8.04 to 7.56) |
| DOI | 14* | 7.85 (7.93 to 7.78) | 1.5 | 8.84 (9.00 to 8.67) | 33* | 7.48 (7.54 to 7.42) | 1.0 | 9.00 (9.44 to 8.57) | 96 | 7.02 (7.11 to 6.93) | 4.1 | 8.39 (8.76 to 8.04) | 65 | 7.19 (7.28 to 7.10) | 8 | 8.10 (8.41 to 7.80) |
| 5-HT | 146* | 6.83 (6.95 to 6.72) | 23 | 7.64 (7.82 to 7.45) | 348* | 6.46 (6.62 to 6.31) | 34 | 7.47 (7.58 to 7.36) | 86* | 7.06 (7.17 to 6.96) | 4.6 | 8.34 (8.54 to 8.14) | 148* | 6.83 (6.94 to 6.72) | 16 | 7.79 (8.00 to 7.57) |
| Ketanserin | 2.8 | 8.55 (8.61 to 8.50) | NT | NT | 2.7 | 8.57 (8.63 to 8.50) | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| Mesulergine | NT | NT | NT | NT | NT | NT | NT | NT | 3.8 | 8.42 (8.47 to 8.36) | NT | NT | 3.4 | 8.47 (8.52 to 8.41) | NT | NT |
Figure 3.
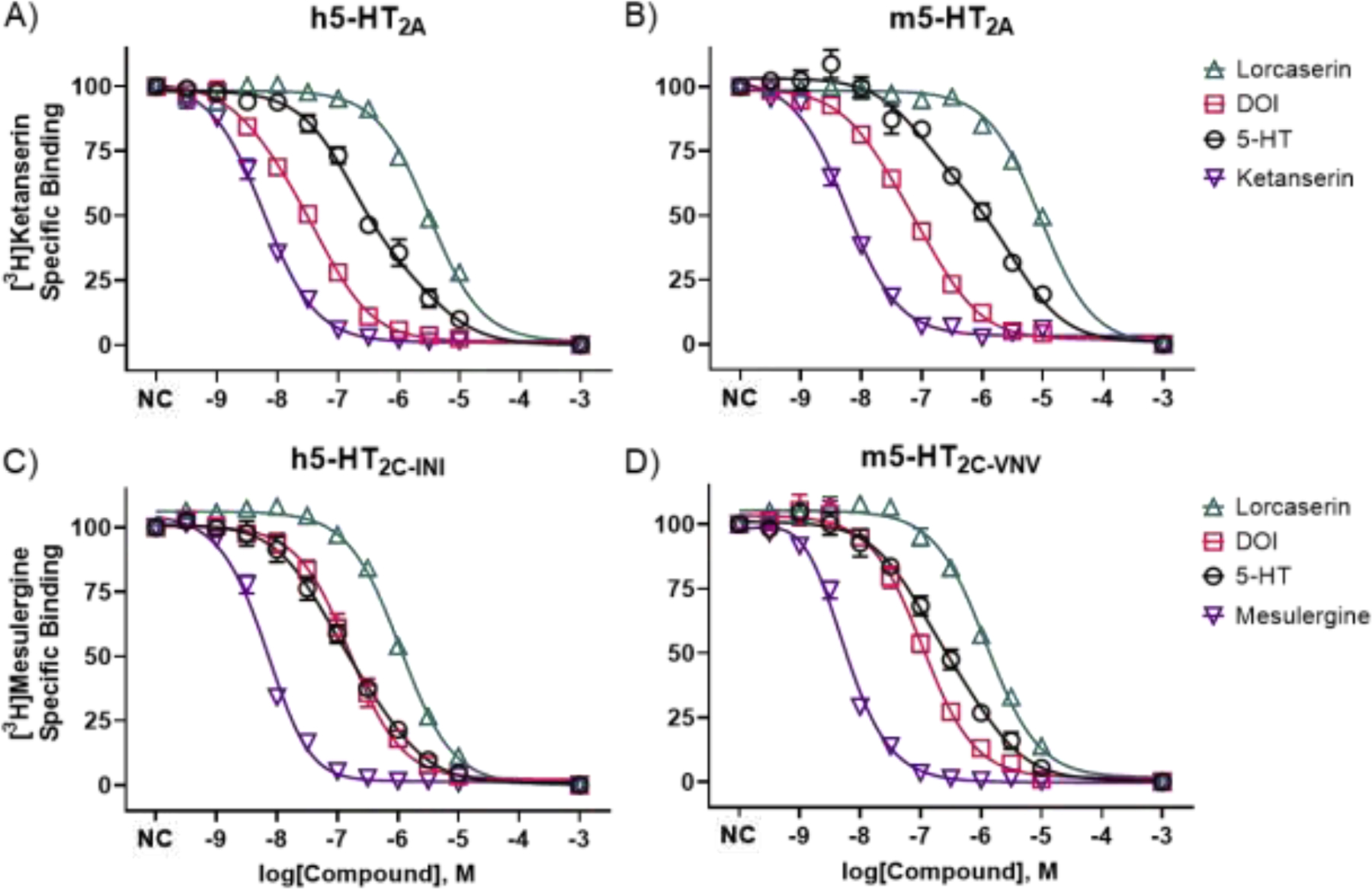
In vitro, radioligand competition binding of lorcaserin, DOI, 5-HT, and ketanserin or mesulergine at human (h) and mouse (m) 5-HT2ARs (A and B) and 5-HT2CRs (C and D). Data are concentration–response curves showing means and SEMs, compiled from at least three independent experiments, wherein each concentration was tested at least in duplicate. The 1 mM data points were interpolated, so curves reached asymptote (no specific binding). At 5-HT2ARs, DOI and 5-HT data best fit to a “two-site, fit Ki” model, which is shown, whereas lorcaserin and ketanserin data best fit to a “one-site, fit Ki model,” which is shown. At 5-HT2CRs, 5-HT data best fit to a “two-site, fit Ki” model, which is shown, whereas lorcaserin, DOI and mesulergine data best fit to a “one-site, fit Ki model,” which is shown. NC is an abbreviation for “no compound,” and the associated data points represent total, specific binding.
Figure 4.
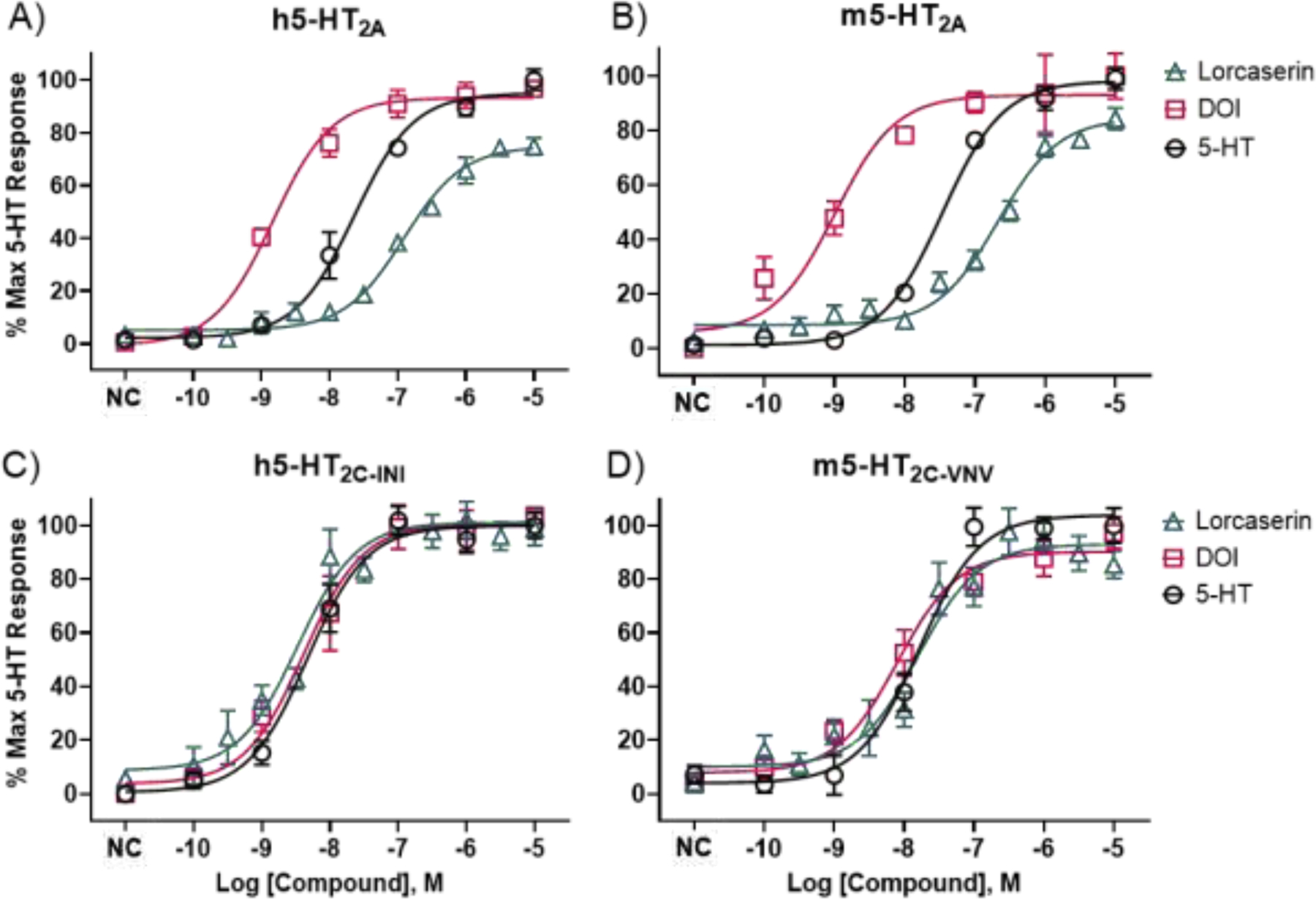
In vitro, functional activity of lorcaserin, DOI, and 5-HT at human (h) and mouse (m) 5-HT2ARs (A and B) and 5-HT2CRs (C and D). All compounds were tested in parallel measuring inositol phosphate production. Data are concentration–response curves showing means and SEMs from a minimum of three, independent experiments. Eleven concentrations of lorcaserin, in half log-increments, were tested in triplicates, and six concentrations of 5-HT and DOI (controls), in log increments, were tested in duplicates. NC is an abbreviation for “no compound,” and the associated data points represent basal receptor activity.
Intriguingly, lorcaserin exhibited relatively low affinities at all antagonist-labeled receptors, including 5-HT2CRs (i.e., >650 nM at 5-HT2CRs and >1,500 nM at 5-HT2ARs), and the lorcaserin data fit best to a one-site model, with R2≥0.96; for the two-site model, Prism was able to find best-fit values, but was unable to calculate complete confidence intervals (CI). Consistent with classic agonist binding, the DOI and 5-HT data at h5-HT2ARs and m5-HT2ARs fit best to the two-site model (P’s=0.0005 and 0.0008, respectively, compared to the one-site model).
For DOI at h5-HT2ARs, the pKi (95% CI) at the low-affinity site was 7.52 (7.74 to 7.10), and the pKi (95% CI) at the high-affinity site was 8.55 (9.17 to 8.11), with R2=0.98. For DOI at m5-HT2ARs, the pKi (95% CI) at the low-affinity site was 7.02 (7.32 to 6.30), and the pKi (95% CI) at the high-affinity site was 8.01 (8.70 to 7.64), with R2=0.98. For 5-HT at h5-HT2ARs, the pKi (95% CI) at the low-affinity site was 5.73 (6.32 to 4.81), and the pKi (95% CI) at the high-affinity site was 7.13 (7.47 to 6.91), with R2=0.97. For 5-HT at m5-HT2ARs, the pKi (95% CI) at the low-affinity site was 5.75 (6.14 to 5.13), and the pKi (95% CI) at the high-affinity site was 7.31 (7.94 to 6.81), with R2=0.90.
5-HT binding data at h5-HT2CRs and m5-HT2CRs also fit best to the two-site model (P’s=0.0015 and 0.0049, respectively). For 5-HT at h5-HT2CRs, the pKi (95% CI) at the low-affinity site was 6.34 (6.87 to 5.40), and the pKi (95% CI) at the high-affinity site was 7.50 (8.22 to 7.17), with R2=0.95. For 5-HT at m5-HT2CRs, the pKi (95% CI) at the low-affinity site was 6.15 (6.71 to 4.99), and the pKi (95% CI) at the high-affinity site was 7.30 (8.33 to 6.90), with R2=0.96. DOI binding data at h5-HT2CRs and m5-HT2CRs fit best to a one-site model, with R2=0.93 and 0.96, respectively. Ketanserin and mesulergine binding data fit best to the one-site model, with R2≥0.98, as expected.
In vitro functional activity at 5-HT2ARs and 5-HT2CRs was assessed using phosphoinositide hydrolysis assays. Lorcaserin, DOI, and 5-HT stimulated inositol phosphate production via activation of 5-HT2ARs and 5-HT2CRs. As shown in Table 1 and Figure 4, lorcaserin, DOI, and 5-HT had practically indistinguishable, low nanomolar potencies to activate h5-HT2CRs and m5-HT2CRs, and lorcaserin and DOI behaved as full agonists, relative to 5-HT. At 5-HT2ARs, however, potencies differed. The rank order of functional potencies at h5-HT2ARs was DOI>5-HT>lorcaserin (Table 1). Relative to 5-HT, DOI displayed full efficacy h5-HT2AR agonist activity, whereas lorcaserin displayed high, partial agonist activity at h5-HT2ARs (Emax=74.9%). Results at the m5-HT2AR were very similar to the h5-HT2AR. The rank order of potencies was DOI>5-HT>lorcaserin. Relative to 5-HT, DOI displayed full efficacy m5-HT2AR agonist activity, whereas lorcaserin displayed high, partial agonist activity at m5-HT2ARs (Emax=84.6%).
3.3. Lorcaserin: In Vivo 5-HT2R Pharmacology
To evaluate lorcaserin’s 5-HT2R activity in C57BL/6J mice, we first tested its ability to elicit the 5-HT2AR-dependent HTR—that can also be modulated by 5-HT2CRs—on its own and after co-administration with the selective 5-HT2CR antagonist, SB242084. There was a main effect of treatment (F (3, 23) = 4.341; P=0.01). As shown in Figure 5A, relative to vehicle treatment, lorcaserin 3 mg/kg, on its own, did not elicit an HTR (means = 2.6 and 4.1, respectively). Co-administration of the selective 5-HT2CR antagonist, SB242084 (0.5 or 1 mg/kg), with lorcaserin (3 mg/kg), however, elicited an HTR significantly greater than vehicle-treated mice (means = 7 and 6.3 compared to 2.6; P=0.01 and 0.03), suggesting 5-HT2CR activation by lorcaserin has a modest suppressive effect on the HTR elicited by 5-HT2AR activation, similar to what has been observed in rats (Serafine et al., 2015). These results provided initial in vivo evidence that lorcaserin, at 3 mg/kg, activates 5-HT2CRs and 5-HT2ARs in mice.
Figure 5.
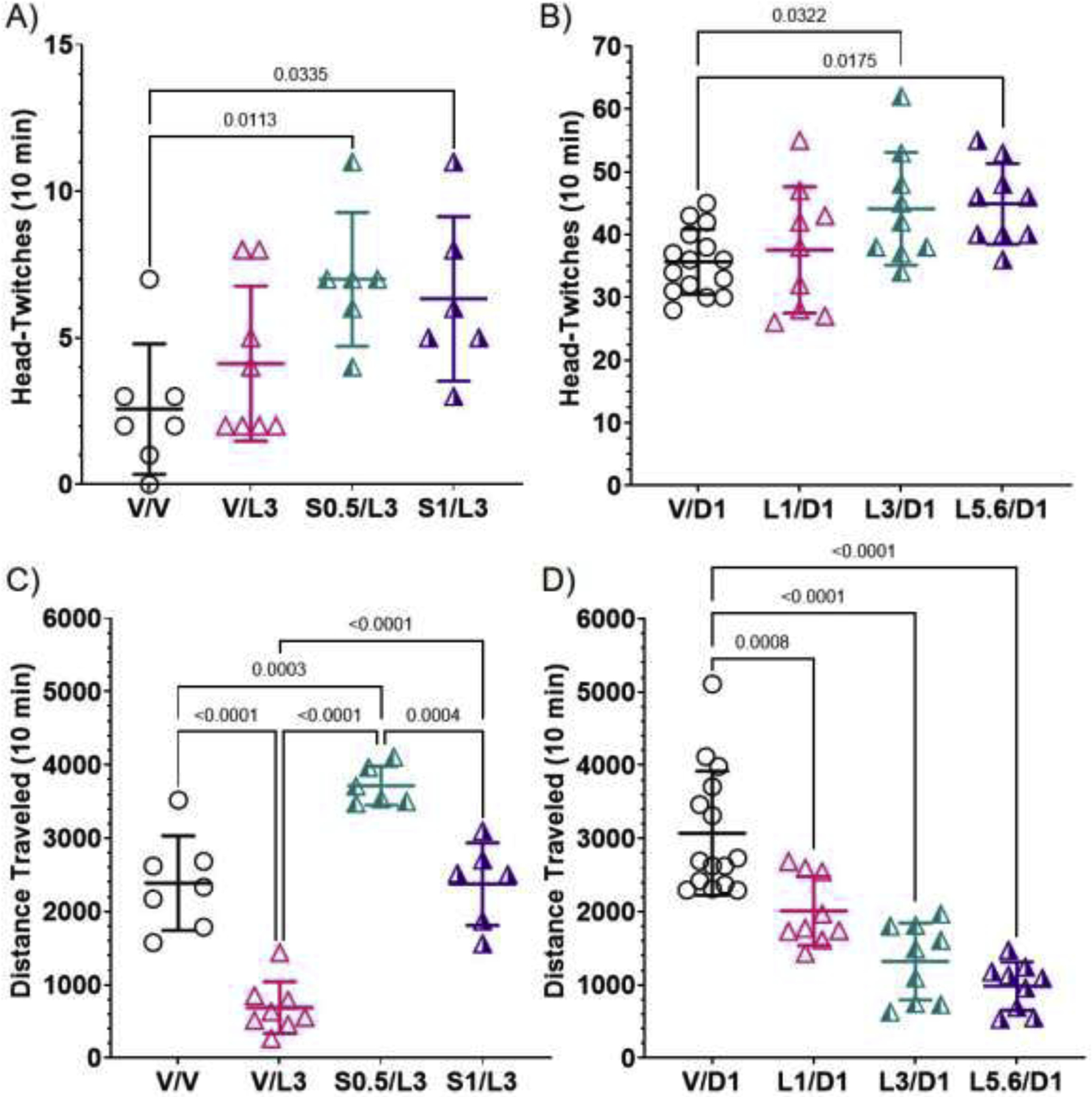
Effects of lorcaserin on the 5-HT2AR-dependent HTR (A and B) and on locomotion (C and D), sensitive to 5-HT2CR activation in C57BL/6J mice. Lines through data points represent means and SDs. A) Lorcaserin at 3 mg/kg (V/L3) did not elicit more HTRs than vehicle-treated (V/V) C57BL/6J mice, however, when co-administered with 0.5 mg/kg of the selective 5-HT2CR antagonist, SB242084 (S0.5), mice exhibited significantly more HTRs than vehicle-treated mice. B) Though the effect was small, lorcaserin dose-dependently increased HTRs elicited by the psychedelic 5-HT2 agonist, DOI. Note that co-treatment with SB242084 and lorcaserin (S0.5/L3, S1/L3) caused much fewer HTRs than DOI (V/D1) or DOI plus lorcaserin (L1/D1, L3/D1, L5.6/D1). C) Lorcaserin (3 mg/kg) caused hypolocomotion, an effect reversed by co-treatment with SB242084 (S0.5/L3, S1/L3). D) When co-administered with DOI, lorcaserin dose-dependently decreased locomotor activity.
To further investigate interactions with 5-HT2ARs in vivo, lorcaserin was co-administered with the psychedelic, modestly preferential 5-HT2AR agonist, DOI, to evaluate lorcaserin’s effects on the 5-HT2AR-dependent DOI-elicited HTR. There was a main effect of treatment (F (3, 38) = 4.109; P=0.01). Surprisingly, as shown in Figure 5B, lorcaserin, when co-administered with DOI (1 mg/kg), did not suppress HTRs, an effect that has been observed with other 5-HT2CR agonists (Canal et al., 2013a; Fantegrossi et al., 2010); rather, lorcaserin dose-dependently increased the HTR. Lorcaserin (3 and 5.6 mg/kg) plus DOI significantly increased HTRs relative to DOI alone (means = 44.1 and 44.9 compared to 37.6; P=0.03 and 0.02), suggesting lorcaserin at these doses potentiates 5-HT2AR activity in mice.
Activation of 5-HT2CRs reduces locomotion, whereas activation of 5-HT2ARs increases locomotion in C57BL/6J mice (Canal et al., 2013a; Halberstadt et al., 2009). Therefore, to further probe interactions with 5-HT2CRs and 5-HT2ARs in vivo, lorcaserin was evaluated for its effects on locomotor activity on its own, when co-administered with SB242084, and when co-administered with DOI. There was a main effect of treatment in the vehicle vs. lorcaserin (3 mg/kg) vs. lorcaserin (3 mg/kg) plus SB242084 experiment (F (3, 23) = 46.95; P<0.0001). As shown in Figure 5C, lorcaserin, 3 mg/kg, significantly reduced locomotor activity relative to vehicle (P<0.0001), an effect overturned by SB242084, 0.5 mg/kg and 1 mg/kg. In addition, the 0.5 mg/kg dose of SB242084 combined with lorcaserin 3 mg/kg, significantly enhanced locomotor activity, relative to the vehicle group (P=0.0003) and the SB242084 1 mg/kg group (P=0.0004). There was also a main effect of treatment in the DOI (1 mg/kg) vs. lorcaserin (1, 3, 5.6 mg/kg) plus DOI experiment (F (3, 38) = 26.06; P<0.0001). When co-administered with DOI, as shown in Figure 5D, lorcaserin, at each dose tested, 1, 3, and 5.6 mg/kg, significantly decreased locomotor activity relative to the DOI only group (P’s≤0.0008). Collectively, these data provide evidence that lorcaserin, at doses of 1 mg/kg or higher, stimulates 5-HT2CRs and, at doses of 3 mg/kg or higher, stimulates 5-HT2ARs in vivo in mice.
4. Discussion
This is the first study to evaluate the anticonvulsant potential of lorcaserin in juvenile male and female Fmr1 knockout mice, a model of FXS and ASD, and the first to assess lorcaserin’s pharmacology at m5-HT2CRs and m5-HT2ARs both in vitro and in vivo, in order to support conclusions about lorcaserin’s target engagement in mice. First, the mGluR5 non-competitive antagonist, MPEP, used as a positive control, prevented AGSs in juvenile, Fmr1 knockout mice, which replicates previous reports (Yan et al., 2005)—an effect also observed in another mouse model with an AGS phenotype (Kazim et al., 2017)—validating our AGS assay. Lorcaserin, at none of the four doses tested (1–10 mg/kg), was effective at preventing AGSs, however, lorcaserin at one dose, 3 mg/kg, decreased AGS-induced lethality by ~32%, which was nearly statistically significant. Lorcaserin, at the 5.6 mg/kg dose, increased the latency to TCS, while concomitantly increasing the duration of WRJ, though, these effects were not observed at any other dose. Although it did not increase latency to TCS, lorcaserin 5.6 mg/kg plus M100907 increased WRJ duration, relative to vehicle. Increased duration of WRJ may suggest either a more severe AGS response or resistance to TCS (Garcia-Cairasco, 2002; Ross and Coleman, 2000). In a temporal lobe epilepsy model in rats, lorcaserin, 3 mg/kg, decreased seizure duration without affecting onset, but in rats with absence seizures and in scn1Lab mutant zebrafish larvae, it increased seizure duration despite reducing the number of seizures (Griffin et al., 2017; Orban et al., 2014; Venzi et al., 2016). These inconsistencies do not support strong conclusions regarding our current observations that lorcaserin, at 5.6 mg/kg, increased latency to TCS and duration of WRJ.
Lorcaserin’s general lack of anticonvulsant efficacy in juvenile Fmr1 knockout mice is in line with a recent report that tested its effects on induced seizures in wild-type mice and rats (Silenieks et al., 2019). Silenieks et al. showed that lorcaserin does not protect against maximal electroshock seizure, electrical convulsive seizures, or pentylenetetrazol-induced seizures. Our results with lorcaserin, however, are distinct from reports that show lorcaserin possesses anticonvulsant effects in rat genetic absence seizures, at 3 and 10 mg/kg, in a scn1Lab mutant zebrafish model of DS, and in a clinical study of children with DS presenting with generalized TCSs (maximum 10 mg twice a day or ~0.3mg/kg/day) (Griffin et al., 2017; Sourbron et al., 2016; Venzi et al., 2016).
The reasons for the discrepancies in findings with lorcaserin may be due to pharmacodynamic and pharmacokinetic factors across species (Panczyk et al., 2015), or to different mechanisms underlying seizures in different diseases, e.g. DS—caused by non-functioning voltage-gated sodium Na(V)1.1 channels that leads to impaired GABAergic function, which can be regulated by 5-HT2CRs (Griffin et al., 2017; Tiraboschi et al., 2020)—versus FXS—caused by loss of the FMRP protein that also impairs GABAergic function (Sabanov et al., 2017), but which may not be regulated by 5-HT2CRs.
Regarding pharmacodynamics, relative to 5-HT2ARs, lorcaserin has only modest selectivity as a 5-HT2CR agonist, and thus, modest differences in dosing as well as species-dependent differences in 5-HT2Rs could impact behavioral results. Indeed, 5-HT2ARs, across various species, have been reported to modulate epileptiform activity, but whether activation or inactivation improves outcomes is unclear (Halberstadt, 2017; Hatini and Commons, 2020; Mishra and Goel, 2019; Ray et al., 2019; Sourbron et al., 2016). We replicated the original report that lorcaserin has selectivity for activating h5-HT2CRs, relative to h5-HT2ARs, 38-fold in our observations compared to 18-fold (Thomsen et al., 2008), and lorcaserin was a full agonist at h5-HT2CRs and a nearly full agonist at h5-HT2ARs. Like its activity at h5-HT2CRs and h5-HT2ARs, lorcaserin was a full agonist at m5-HT2CRs and nearly a full agonist at m5-HT2ARs. Although lorcaserin also showed selectivity for activating m5-HT2CRs, relative to m5-HT2ARs, germane to our study, its m5-HT2CR/m5-HT2AR selectivity was 13-fold. In other words, lorcaserin’s 5-HT2CR/5-HT2AR selectivity was about 3-fold less at mouse compared to human receptors, due to lower potency to activate m5-HT2CRs compared to h5-HT2CRs. These results are similar to those obtained with rat 5-HT2CRs and 5-HT2ARs, where lorcaserin has about 4-fold rat 5-HT2CR/5-HT2AR selectivity (Thomsen et al., 2008). Notably, there is at least one key amino acid in h5-HT2ARs, which contributes to binding and function of certain ligands, that is different in rat 5-HT2ARs and m5-HT2ARs (Canal et al., 2013b). Together, these results show that lorcaserin, relative to h5-HT2CRs, is a less selective rat 5-HT2CR and m5-HT2CR agonist.
To evaluate lorcaserin’s 5-HT2AR and 5-HT2CR activity in mice, we tested its effects on the 5-HT2AR-dependent HTR that can be modulated by 5-HT2CRs (Canal and Morgan, 2012; Canal et al., 2010; de la Fuente Revenga et al., 2020; Fantegrossi et al., 2010; Halberstadt and Geyer, 2013), and its effects on locomotor activity, sensitive to 5-HT2AR and 5-HT2CR activation (Canal et al., 2013a; Halberstadt et al., 2009). Lorcaserin, 3 mg/kg, caused hypolocomotion, but did not elicit an HTR. Co-treatment with SB242084, 0.5 mg/kg, however, reversed lorcaserin-induced hypolocomotion and unveiled an HTR, suggesting lorcaserin engages 5-HT2ARs and 5-HT2CRs at 3 mg/kg. This treatment, however, did not result in lorcaserin eliciting nearly as many HTRs as DOI, a highly selective psychedelic 5-HT2R agonist. Specifically, DOI elicited 5-fold more HTRs than SB242084 plus lorcaserin, suggesting that lorcaserin, 3 mg/kg, engages either additional targets that suppress the HTR, does not occupy enough 5-HT2ARs to cause a robust HTR like DOI (1 mg/kg), or that it stabilizes 5-HT2ARs in a conformation(s) that stimulates 5-HT2AR signaling that hinders the HTR (Gonzalez-Maeso et al., 2007). In support of the first possibility, 5-HT1AR activation suppresses the HTR in mice (Brandt et al., 2018; Canal et al., 2015), and lorcaserin induces forepaw treading in rats at ~3 mg/kg, an effect blocked by the 5-HT1AR antagonist, WAY-100635 (Serafine et al., 2015). These data suggest lorcaserin activates rodent 5-HT1ARs at 3 mg/kg or higher, which could explain its minimal efficacy to elicit the HTR, even when 5-HT2CRs are blocked. In support of lorcaserin 3 mg/kg not occupying a sufficient number of 5-HT2ARs to elicit a robust HTR, lorcaserin has ~200-fold lower potency to activate m5-HT2ARs relative to DOI. Thus, tests of much higher concentrations of lorcaserin are needed to evaluate this possibility.
In support of lorcaserin stabilizing unique 5-HT2AR conformations, in h5-HT2AR and m5-HT2AR competition binding assays with [3H]ketanserin, the lorcaserin concentration-response curve did not fit to a two-site model, whereas the DOI concentration-response curve did. These data suggest lorcaserin, relative to DOI, binds uniquely to 5-HT2ARs and may elicit unique 5-HT2AR signaling that does not lead to an HTR. Also, lorcaserin appears to be devoid of LSD-like psychedelic effects in humans, even at supratherapeutic doses that likely engage 5-HT2ARs (Shram et al., 2011). Interestingly, contrary to effects observed in rats with the structurally similar psychedelic 5-HT2R agonist, 2,5-dimethoxy-4-methylamphetamine (DOM) (Serafine et al., 2015), we observed that lorcaserin, up to 5.6 mg/kg, did not suppress the 5-HT2AR-dependent, DOI-elicited HTR in mice. Rather, lorcaserin potentiated the HTR, while independently causing hypolocomotion, a 5-HT2CR-sensitive response (Halberstadt et al., 2009).
It is challenging to reconcile these data with the effects of lorcaserin plus SB242084 on the HTR, i.e., we might expect that lorcaserin would dose-dependently decrease the DOI-elicited HTR if lorcaserin’s in vivo effects are predominantly mediated by activation of 5-HT2CRs and 5-HT1ARs—activation of either of these receptors can attenuate the DOI-elicited HTR in mice (Canal et al., 2013a; Canal et al., 2015; Fantegrossi et al., 2010). A recent paper, however, showed that a new 5-HT2AR agonist, with 5-HT2CR antagonist properties does not elicit the HTR in mice (Cameron et al., 2020), and 5-HT2CR agonists or antagonists can suppress the HTR in mice, suggesting there may also be unique 5-HT2CR conformations that can be stabilized by 5-HT2R agonists in vivo (Canal et al., 2013a; Canal et al., 2010). It is known that various receptors can modulate the HTR (Canal and Morgan, 2012), so uncharacterized targets of lorcaserin could also be contributing to our observations. We did take note that there appeared to be differences in the qualitative appearance of the HTR in mice treated with DOI and lorcaserin; this combination caused a “subtler” or “less intense” HTR, and we observed more head jolts forward and less side-to-side twitches (data not quantified). Future studies unraveling qualitative differences in the HTR (de la Fuente Revenga et al., 2020) may unveil unique contributions of receptors or neural circuits that could have validity for elucidating finely detailed serotonergic receptor mechanisms that produce antiepileptic effects. Collectively, our observations, at the minimum, suggest that lorcaserin, at 3 mg/kg and above, activates 5-HT2CRs and 5-HT2ARs in mice.
Because of lorcaserin’s activity at 5-HT2ARs, we tested whether blockade of 5-HT2ARs with M100907 would reveal anticonvulsant effects of lorcaserin mediated by its 5-HT2CR and/or 5-HT1AR agonist activity—like 5-HT2CR activation, 5-HT1AR activation can attenuate seizures (Armstrong et al., 2020; Lopez-Meraz et al., 2005). Co-treatment with M100907 and lorcaserin, 5.6 mg/kg, however, did not reduce AGS frequency, AGS-induced lethality, onset or duration of AGSs, relative to vehicle-treated mice. Treatment with M100907, alone, also did not impact AGSs, rather, it showed a tendency to exacerbate AGSs. These data suggest that blockade of 5-HT2ARs does not impact AGSs in juvenile Fmr1 knockout mice.
Lorcaserin is also a full agonist at h5-HT2BRs (Thomsen et al., 2008), but we did not determine lorcaserin’s pharmacology at m5-HT2BRs, owing to their very low expression in the mouse and human brain (Bonhaus et al., 1995), and to there being no well-established in vivo behavior strongly regulated by brain m5-HT2BRs. However, there are reports that deletion of m5-HT2BRs can produce neuropsychiatric-like phenotypes in mice (Pitychoutis et al., 2015), and activation of 5-HT2BRs appears to have antiepileptic effects in a zebrafish model of DS (Griffin et al., 2019). Chronic 5-HT2BR activation is linked to the development of valvular heart disease (Roth, 2007), so activation of 5-HT2BRs is a “no-go” for nearly all drug development programs.
Given that lorcaserin was generally ineffective as an anticonvulsant in our Fmr1 knockout model, these data suggest that, like its effects on the HTR, lorcaserin either has off-target activity that blunts anticonvulsant potential, or that 5-HT2Rs (and 5-HT1ARs) distinctly regulate mechanisms that can prevent seizures in models of DS, for example, but not in models of FXS. AGSs in Fmr1 knockout mice are considered generalized convulsive (motor) seizures. Previous rodent studies showed that lorcaserin attenuates focal seizures (Orban et al., 2014) and generalized but non-convulsive (non-motor) absence seizures (Venzi et al., 2016). The onset and manifestation involved in focal and absence seizures, respectively, are different from those in AGSs. Similarly, DS is characterized by spontaneous combined generalized and focal seizures (Falco-Walter et al., 2018) while seizures in juvenile Fmr1 knockout mice are, generally, provoked by sound.
Other possibilities that could explain our results include altered expression and/or function of lorcaserin’s target receptors in Fmr1 knockout mice. The inferior colliculus is an auditory system necessary for AGSs in Fmr1 knockout mice (Gonzalez et al., 2019), and a rat study showed that cochlear ablation-induced deafness increases 5-HT2CR gene expression in the inferior colliculus more than two-fold (Holt et al., 2005). These effects contrast with the auditory hypersensitivity observed in 5-HT2CR knockout mice (Brennan et al., 1997), suggesting juvenile Fmr1 knockout mice might have reduced expression and/or function of 5-HT2CRs in the inferior colliculus. We are currently investigating expression and function of various 5-HTRs in our Fmr1 knockout mice. We recently reported that a novel compound, FPT, which has potent and efficacious 5-HT1AR agonist activity and 5-HT7R antagonist/low efficacy, partial agonist activity, along with modestly potent and efficacious 5-HT2CR agonist activity, completely prevents AGSs in juvenile Fmr1 knockout mice (Armstrong et al., 2020). More experiments need to be conducted to elucidate the impact of various 5-HTRs on various types of seizures.
In conclusion, lorcaserin, across a wide dose range, was ineffective at preventing AGSs in juvenile Fmr1 knockout mice. This may due to differences in underlying mechanisms of AGSs in Fmr1 knockout mice compared to seizures present in other preclinical, genetic models and in DS, where lorcaserin has shown antiepileptic effects. We also showed that lorcaserin binds uniquely to 5-HT2ARs, compared to other 5-HT2AR agonists, including 5-HT and DOI, and is a less selective agonist at m5-HT2CRs compared to h5-HT2CRs. Lorcaserin appears to activate 5-HT2CRs and 5-HT2ARs in mice at doses as low as 3 mg/kg, so careful dose selection is necessary to conclude that effects of lorcaserin in mice are mediated by 5-HT2CRs. Activation of 5-HT2CRs and/or 5-HT2ARs by lorcaserin may not be therapeutic for seizures in FXS.
Supplementary Material
Highlights.
Lorcaserin did not prevent audiogenic seizures in juvenile Fmr1 knockout mice.
Lorcaserin’s 5-HT2C/5-HT2A selectivity was reduced in mouse compared to human.
Lorcaserin, at 3 mg/kg or lower, engages off-targets including 5-HT2A in mice.
Lorcaserin bound distinctly to antagonist-labeled 5-HT2A, compared to 5-HT and DOI.
Acknowledgements
The authors would like to thank Yiming Chen for her contribution to video recording the AGS experiments.
The project was supported by a Fellowship from the FRAXA Research Foundation (CEC), by NIH grants R15NS118352 (CEC) and R01DA047130 (RGB), and by DOD grants W81XWH-17-1 -0329 (CEC)/W81XWH-17-1 -0322 (RGB) and W81XWH-15-1-0247 (CEC, RGB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
Dr. Canal is an advisor for Adelia Therapeutics, Inc., a wholly owned subsidiary of Cybin, Inc.
References
- Annala S, Feng X, Shridhar N, Eryilmaz F, Patt J, Yang J, Pfeil EM, Cervantes-Villagrana RD, Inoue A, Haberlein F, Slodczyk T, Reher R, Kehraus S, Monteleone S, Schrage R, Heycke N, Rick U, Engel S, Pfeifer A, Kolb P, Konig G, Bunemann M, Tuting T, Vazquez-Prado J, Gutkind JS, Gaffal E, Kostenis E, 2019. Direct targeting of Galphaq and Galpha11 oncoproteins in cancer cells. Sci Signal 12. [DOI] [PubMed] [Google Scholar]
- Armstrong JL, Casey AB, Saraf TS, Mukherjee M, Booth RG, Canal CE, 2020. (S)-5-(2’-Fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine, a Serotonin Receptor Modulator, Possesses Anticonvulsant, Prosocial, and Anxiolytic-like Properties in an Fmr1 Knockout Mouse Model of Fragile X Syndrome and Autism Spectrum Disorder. ACS Pharmacol Transl Sci 3, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Okaniwa A, Serikawa T, Yamada J, 1990. Behavioural characteristics of wild jumping or running episodes in the double mutant spontaneously epileptic rat. Laboratory Animals 24, 131–136. [DOI] [PubMed] [Google Scholar]
- Bailey DB Jr., Raspa M, Olmsted M, Holiday DB, 2008. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A 146a, 2060–2069. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, Claeysen S, Cunningham KA, Fone KC, Gershon M, Di Giovanni G, Goodfellow NM, Halberstadt AL, Hartley RM, Hassaine G, Herrick-Davis K, Hovius R, Lacivita E, Lambe EK, Leopoldo M, Levy FO, Lummis SCR, Marin P, Maroteaux L, McCreary AC, Nelson DL, Neumaier JF, Newman-Tancredi A, Nury H, Roberts A, Roth BL, Roumier A, Sanger GJ, Teitler M, Sharp T, Villalón CM, Vogel H, Watts SW, Hoyer D, 2021. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol Rev 73, 310–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, 2002. Epilepsy in fragile X syndrome. Dev Med Child Neurol 44, 724–728. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB, 2010. Seizures in Fragile X Syndrome: Characteristics and Comorbid Diagnoses. American Journal on Intellectual and Developmental Disabilities 115, 461–472. [DOI] [PubMed] [Google Scholar]
- Beyeler A, Kadiri N, Navailles S, Boujema MB, Gonon F, Moine CL, Gross C, De Deurwaerdère P, 2010. Stimulation of serotonin2C receptors elicits abnormal oral movements by acting on pathways other than the sensorimotor one in the rat basal ganglia. Neuroscience 169, 158–170. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM, 1995. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol 115, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, Stratford A, Klein LM, McCorvy JD, Nichols DE, Halberstadt AL, 2018. Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test Anal 10, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH, 1997. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nature Genetics 16, 387–390. [DOI] [PubMed] [Google Scholar]
- Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, Vargas MV, McCarroll MN, Taylor JC, Myers-Turnbull D, Liu T, Yaghoobi B, Laskowski LJ, Anderson EI, Zhang G, Viswanathan J, Brown BM, Tjia M, Dunlap LE, Rabow ZT, Fiehn O, Wulff H, McCorvy JD, Lein PJ, Kokel D, Ron D, Peters J, Zuo Y, Olson DE, 2020. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D, 2013a. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 70, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Cordova-Sintjago T, Liu Y, Kim MS, Morgan D, Booth RG, 2013b. Molecular pharmacology and ligand docking studies reveal a single amino acid difference between mouse and human serotonin 5-HT2A receptors that impacts behavioral translation of novel 4-phenyl-2-dimethylaminotetralin ligands. J Pharmacol Exp Ther 347, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Felsing DE, Liu Y, Zhu W, Wood JT, Perry CK, Vemula R, Booth RG, 2015. An Orally Active Phenylaminotetralin-Chemotype Serotonin 5-HT7 and 5-HT1A Receptor Partial Agonist that Corrects Motor Stereotypy in Mouse Models. ACS Chem Neurosci 6, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D, 2012. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4, 556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC, 2010. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 209, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Blough BE, Murnane KS, Canal CE, 2019. The synthetic cathinone psychostimulant alpha-PPP antagonizes serotonin 5-HT2A receptors: In vitro and in vivo evidence. Drug Test Anal 11, 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley B, Kirjanen S, Partanen J, Castrén ML, 2016. Epileptic Electroencephalography Profile Associates with Attention Problems in Children with Fragile X Syndrome: Review and Case Series. Frontiers in Human Neuroscience 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, Lyons D, Cristiano C, Lettieri M, Olarte-Sanchez C, Burke LK, Greenwald-Yarnell M, Cansell C, Doslikova B, Georgescu T, Martinez de Morentin PB, Myers MG Jr., Rochford JJ, Heisler LK, 2018. Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake. Cell Metab 28, 619–630 e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, Griffiths RR, 2020. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Vohra HZ, Gonzalez-Maeso J, 2020. Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. J Neurosci Methods 334, 108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdère P, 2020. Serotonin research: Crossing scales and boundaries. Neuropharmacology 181, 108340. [DOI] [PubMed] [Google Scholar]
- Falco-Walter JJ, Scheffer IE, Fisher RS, 2018. The new definition and classification of seizures and epilepsy. Epilepsy Res 139, 73–79. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH, 2010. Interaction of 5-HT 2A and 5-HT 2C Receptors in R (−)-2,5-Dimethoxy-4-iodoamphetamine-Elicited Head Twitch Behavior in Mice. Journal of Pharmacology and Experimental Therapeutics 335, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan TW, Sebastian MN, Battaglia DM, Foster TP, Cormier SA, Nichols CD, 2019. 5-HT2 receptor activation alleviates airway inflammation and structural remodeling in a chronic mouse asthma model. Life Sci 236, 116790. [DOI] [PubMed] [Google Scholar]
- Garcia-Cairasco N, 2002. A critical review on the participation of inferior colliculus in acoustic-motor and acoustic-limbic networks involved in the expression of acute and kindled audiogenic seizures. Hearing Research 168, 208–222. [DOI] [PubMed] [Google Scholar]
- Gauthey M, Poloni CB, Ramelli G-P, Roulet-Perez E, Korff CM, 2010. Status epilepticus in fragile X syndrome: Status Epilepticus in Fragile X Syndrome. Epilepsia 51, 2470–2473. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE, 1997. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology 129, 79–84. [DOI] [PubMed] [Google Scholar]
- Goel A, Cantu DA, Guilfoyle J, Chaudhari GR, Newadkar A, Todisco B, de Alba D, Kourdougli N, Schmitt LM, Pedapati E, Erickson CA, Portera-Cailliau C, 2018. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nature Neuroscience 21, 1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA, 2007. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452. [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Tomasek M, Hays S, Sridhar V, Ammanuel S, Chang CW, Pawlowski K, Huber KM, Gibson JR, 2019. Audiogenic Seizures in the Fmr1 Knock-Out Mouse Are Induced by Fmr1 Deletion in Subcortical, VGlut2-Expressing Excitatory Neurons and Require Deletion in the Inferior Colliculus. J Neurosci 39, 9852–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A, Hamling KR, Knupp K, Hong S, Lee LP, Baraban SC, 2017. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 140, 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, Jaishankar P, Grandjean J-M, Olson SH, Renslo AR, Baraban SC, 2019. Zebrafish studies identify serotonin receptors mediating antiepileptic activity in Dravet syndrome. Brain Communications 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Stafstrom CE, 2009. Origins of Epilepsy in Fragile X Syndrome. Epilepsy Currents 9, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB Jr., Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, Hagerman PJ, 2017. Fragile X syndrome. Nat Rev Dis Primers 3, 17065. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, 2017. Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens. Curr Top Behav Neurosci 32, 283–311. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD, 2020. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA, 2013. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 227, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB, 2009. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34, 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini PG, Commons KG, 2020. A 5-HT1D -receptor agonist protects Dravet syndrome mice from seizure and early death. Eur J Neurosci 52, 4370–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard TT, Ramgopal S, Picker J, Lincoln SA, Rotenberg A, Kothare SV, 2014. EEG abnormalities and seizures in genetically diagnosed Fragile X syndrome. International Journal of Developmental Neuroscience 38, 155–160. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ, Shanahan WR, 2020. Lorcaserin: A review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol Ther 205, 107417. [DOI] [PubMed] [Google Scholar]
- Hodges SL, Reynolds CD, Nolan SO, Huebschman JL, Okoh JT, Binder MS, Lugo JN, 2019. A single early-life seizure results in long-term behavioral changes in the adult Fmr1 knockout mouse. Epilepsy Research 157, 106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA, 2005. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity: Gene expression in the IC following deafness. Journal of Neurochemistry 93, 1069–1086. [DOI] [PubMed] [Google Scholar]
- Iida K, Sasa M, Serikawa T, Noda A, Ishihara K, Akimitsu T, Hanaya R, Arita K, Kurisu K, 1998. Induction of convulsive seizures by acoustic priming in a new genetically defined model of epilepsy (Noda epileptic rat: NER). Epilepsy Research 30, 115–126. [DOI] [PubMed] [Google Scholar]
- Ijff DM, Aldenkamp AP, 2013. Cognitive side-effects of antiepileptic drugs in children. Handb Clin Neurol 111, 707–718. [DOI] [PubMed] [Google Scholar]
- Incorpora G, Sorge G, Sorge A, Pavone L, 2003. Epilepsy in Fragile X Syndrome. 2002 Elsevier Science B.V. All rights reserved. 24, 766–769. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, Stackhouse T, Riley C, Peacock G, Sherman SL, Brown WT, Berry-Kravis E, 2017. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 139, S194–S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim SF, Chuang S-C, Zhao W, Wong RKS, Bianchi R, Iqbal K, 2017. Early-Onset Network Hyperexcitability in Presymptomatic Alzheimer’s Disease Transgenic Mice Is Suppressed by Passive Immunization with Anti-Human APP/Aβ Antibody and by mGluR5 Blockade. Frontiers in Aging Neuroscience 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Canal CE, Cordova-Sintjago TC, Zhu W, Booth RG, 2017. Mutagenesis Analysis Reveals Distinct Amino Acids of the Human Serotonin 5-HT2C Receptor Underlying the Pharmacology of Distinct Ligands. ACS Chem Neurosci 8, 28–39. [DOI] [PubMed] [Google Scholar]
- Lopez-Meraz ML, Gonzalez-Trujano ME, Neri-Bazan L, Hong E, Rocha LL, 2005. 5-HT1A receptor agonists modify epileptic seizures in three experimental models in rats. Neuropharmacology 49, 367–375. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, 2004. Cognitive side effects of antiepileptic drugs in children. Neurology 62, 872–877. [DOI] [PubMed] [Google Scholar]
- Mishra A, Goel RK, 2019. Modulatory Effect of Serotonergic System in Pentylenetetrazole-Induced Seizures and Associated Memory Deficit: Role of 5-HT1A and 5-HT2A/2C. J Epilepsy Res 9, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci SA, Calabrese G, Bonaccorso CM, D’Antoni S, Brouwer JR, Bakker CE, Elia M, Ferri R, Nelson DL, Oostra BA, Catania MV, 2007. Audiogenic seizure susceptibility is reduced in fragile X knockout mice after introduction of FMR1 transgenes. Experimental Neurology 203, 233–240. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Bernardina BD, Tassinari CA, Sarro GB, Elia M, 1999. Epilepsy and EEG Findings in Males with Fragile X Syndrome. Epilepsia 40, 1092–1099. [DOI] [PubMed] [Google Scholar]
- Navailles S, Lagière M, Roumegous A, Polito M, Boujema MB, Cador M, Dunlop J, Chesselet M-F, Millan MJ, De Deurwaerdère P, 2013. Serotonin2C ligands exhibiting full negative and positive intrinsic activity elicit purposeless oral movements in rats: distinct effects of agonists and inverse agonists in a rat model of Parkinson’s disease. The International Journal of Neuropsychopharmacology 16, 593–606. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Johnson MW, Nichols CD, 2017. Psychedelics as Medicines: An Emerging New Paradigm. Clin Pharmacol Ther 101, 209–219. [DOI] [PubMed] [Google Scholar]
- Nutt D, Erritzoe D, Carhart-Harris R, 2020. Psychedelic Psychiatry’s Brave New World. Cell 181, 24–28. [DOI] [PubMed] [Google Scholar]
- Olaghere da Silva UB, Morabito MV, Canal CE, Airey DC, Emeson RB, Sanders-Bush E, 2010. Impact of RNA editing on functions of the serotonin 2C receptor in vivo. Front Neurosci 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban G, Bombardi C, Marino Gammazza A, Colangeli R, Pierucci M, Pomara C, Pessia M, Bucchieri F, Arcangelo B, Smolders I, De Deurwaerdère P, Di Giovanni G, 2014. Role(s) of the 5-HT2C Receptor in the Development of Maximal Dentate Activation in the Hippocampus of Anesthetized Rats. CNS Neuroscience & Therapeutics 20, 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM, Pazos A, Hoyer D, 2017. A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology (Berl) 234, 1395–1418. [DOI] [PubMed] [Google Scholar]
- Panczyk K, Golda S, Waszkielewicz A, Zelaszczyk D, Gunia-Krzyzak A, Marona H, 2015. Serotonergic system and its role in epilepsy and neuropathic pain treatment: a review based on receptor ligands. Curr Pharm Des 21, 1723–1740. [DOI] [PubMed] [Google Scholar]
- Patel N, LeWitt P, Neikrug AB, Kesslak P, Coate B, Ancoli-Israel S, 2018. Nighttime Sleep and Daytime Sleepiness Improved With Pimavanserin During Treatment of Parkinson’s Disease Psychosis. Clin Neuropharmacol 41, 210–215. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, Belmer A, Moutkine I, Adrien J, Maroteaux L, 2015. Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology 40, 2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX, 2017. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27, 451–457. [DOI] [PubMed] [Google Scholar]
- Ray A, Canal CE, Ehlen JC, Rice KC, Murnane KS, 2019. M100907 and BD 1047 attenuate the acute toxic effects of methamphetamine. Neurotoxicology 74, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KC, Coleman JR, 2000. Developmental and genetic audiogenic seizure models: behavior and biological substrates. Neuroscience & Biobehavioral Reviews 24, 639–653. [DOI] [PubMed] [Google Scholar]
- Roth BL, 2007. Drugs and valvular heart disease. N Engl J Med 356, 6–9. [DOI] [PubMed] [Google Scholar]
- Roth BL, 2013. NIMH PDSP Assay Protocol Book, Version II. [Google Scholar]
- Sabanov V, Braat S, D’Andrea L, Willemsen R, Zeidler S, Rooms L, Bagni C, Kooy RF, Balschun D, 2017. Impaired GABAergic inhibition in the hippocampus of Fmr1 knockout mice. Neuropharmacology 116, 71–81. [DOI] [PubMed] [Google Scholar]
- Sejourne J, Llaneza D, Kuti OJ, Page DT, 2015. Social Behavioral Deficits Coincide with the Onset of Seizure Susceptibility in Mice Lacking Serotonin Receptor 2c. PLoS One 10, e0136494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Rice KC, France CP, 2015. Directly Observable Behavioral Effects of Lorcaserin in Rats. Journal of Pharmacology and Experimental Therapeutics 355, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Rice KC, France CP, 2016. Characterization of the discriminative stimulus effects of lorcaserin in rats. J Exp Anal Behav 106, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM, 2011. Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther 89, 683–692. [DOI] [PubMed] [Google Scholar]
- Silenieks LB, Carroll NK, Van Niekerk A, Van Niekerk E, Taylor C, Upton N, Higgins GA, 2019. Evaluation of Selective 5-HT2C Agonists in Acute Seizure Models. ACS Chemical Neuroscience 10, 3284–3295. [DOI] [PubMed] [Google Scholar]
- Sourbron J, Schneider H, Kecskés A, Liu Y, Buening EM, Lagae L, Smolders I, de Witte P, 2016. Serotonergic Modulation as Effective Treatment for Dravet Syndrome in a Zebrafish Mutant Model. ACS Chemical Neuroscience 7, 588–598. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D, 2008. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325, 577–587. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Martina S, van der Ent W, Grzyb K, Gawel K, Cordero-Maldonado ML, Poovathingal SK, Heintz S, Satheesh SV, Brattespe J, Xu J, Suster M, Skupin A, Esguerra CV, 2020. New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome. Epilepsia 61, 549–560. [DOI] [PubMed] [Google Scholar]
- Tolete P, Knupp K, Karlovich M, DeCarlo E, Bluvstein J, Conway E, Friedman D, Dugan P, Devinsky O, 2018. Lorcaserin therapy for severe epilepsy of childhood onset: A case series. Neurology 91, 837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venzi M, David F, Bellet J, Cavaccini A, Bombardi C, Crunelli V, Di Giovanni G, 2016. Role for serotonin2A (5-HT2A) and 2C (5-HT2C) receptors in experimental absence seizures. Neuropharmacology 108, 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TH, Lovelace JW, Ethell IM, Binder DK, Razak KA, 2019. Developmental Changes in EEG Phenotypes in a Mouse Model of Fragile X Syndrome. Neuroscience 398, 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP, 2005. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49, 1053–1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


