Abstract
Background:
While acute changes in hepatic fibrosis are recognized shortly after achieving sustained virological response (SVR) using direct-acting antiviral therapies, long-term outcomes for the growing population of successfully treated patients with HCV remain uncertain. The aim of this study is to characterize long-term changes in fibrosis following SVR in patients with and without HIV and to identify potential factors associated with progression or regression of fibrosis.
Methods:
We completed a prospective longitudinal study of 162 subjects with HCV (34% HIV-coinfected) with pre-treatment fibrosis stage determined by liver biopsy and post-SVR transient elastography. Progression of fibrosis was defined as a two-stage or greater increase in fibrosis, while regression was defined as a two-stage or greater decrease at last follow-up. The median duration of follow-up was 4.1 years.
Results:
Fibrosis progression occurred in 4% of subjects while regression occurred in 7% and 89% were stable and did not differ by HIV coinfection. Fibrosis progression was associated with increased body mass index (BMI), hepatic steatosis and smoking pack-years. In a multivariable logistic regression, HIV coinfection (P=0.009), lower steatosis score (P<0.05) and lower smoking pack-years (P=0.0007) were associated with a lower fibrosis score at last follow-up.
Conclusions:
We identify potentially important relationships between BMI, hepatic steatosis and smoking, and changes in hepatic fibrosis post-SVR in patients with and without HIV coinfection. Attention to modifiable risk factors such as body weight and smoking may reduce the risk of liver disease progression in the growing population of successfully treated chronic HCV patients.
Introduction
With the advent of highly effective direct-acting antiviral therapy (DAA) for HCV infection, there has a been a dramatic increase in sustained virological response (SVR) in patients with and without HIV. SVR is associated with significant regression of hepatic fibrosis in monoinfected and coinfected (HIV–HCV) cohorts and fibrosis regression is linked with a reduction of liver-related complications and mortality [1-4]. However, one cohort study of HCV-monoinfected patients treated with DAAs found progression of hepatic fibrosis in 17% of patients at 96 weeks despite achieving and maintaining SVR [3]. Furthermore, long-term studies of mainly interferon-treated patients have demonstrated that while SVR is associated with a decreased risk of liver-related health complications such as hepatocellular carcinoma, the risk is not eliminated and remains present in patients with advanced fibrosis for at least 8–10 years [5,6]. Less is known about the influence of HIV coinfection on long-term outcomes and progression of liver disease after HCV clearance, but previous research has linked HIV-specific factors to liver fibrosis progression due to long-term exposure to antiretroviral therapy [7]. Because liver fibrosis is a key predictor of adverse outcomes and patient mortality, it is important to identify factors that may lead to liver fibrosis progression in order to target patients that may require closer monitoring for these health complications.
The aim of this study was to characterize regression and progression of hepatic fibrosis in patients with chronic HCV following SVR and to identify potential factors associated with progression or regression of fibrosis. In addition, we evaluated the potential role of HIV infection on changes in fibrosis following HCV clearance.
Methods
Study population
Subjects participated in a prospective longitudinal study of long-term health outcomes of adults with viral hepatitis (NCT01350648). Subjects were recruited from the local Maryland/Virginia/ Washington DC area. All subjects provided written informed consent and the protocol was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board.
For the purposes of the current study, a total of 162 subjects with a history of chronic HCV (n=55 with HIV coinfection) were identified who had pre-treatment liver biopsy with fibrosis staging performed within 24 months of starting therapy, achieved SVR and had at least one follow-up visit with transient elastography. Three individuals had biopsies performed >24 months from start of therapy, however, these revealed clinical cirrhosis. SVR was defined as undetectable HCV RNA 12 weeks after the end of treatment and maintained through follow-up. Subjects were excluded if they had a history of any hepatic malignancies or chronic hepatitis B. Participants were instructed to fast after midnight for annual visits which included routine laboratory tests, HCV RNA determinations and transient elastography. Study participants reported estimates of years smoking and packs per day. Smoking pack-years was calculated by multiplying packs per day by the number of years smoking. Self-reported risk behaviours including alcohol consumption and history of intravenous drug-use were also documented. Drinks per week was calculated using self-reported drinking behaviour in the previous 12 months (drinks/week based on frequency and number of alcoholic drinks consumed). Diabetes was defined as having a medical diagnosis of diabetes and confirmed by either use of antidiabetic medication or haemoglobin A1c ≥6.5%.
Staging
Pre-treatment liver biopsies were staged using the METAVIR system [8]. At annual follow-up visits, patients underwent transient elastography (Fibroscan, Echosens, Paris, France) in order to evaluate liver stiffness (kPa) and steatosis (Controlled Attenuation Parameter [CAP]). Operators were trained and certified by manufacturer representatives of Echosens. Use of the medium (M) and extra-large (XL) probes followed manufacturer programme recommendations; 36% of subjects had fibroscan completed with the XL probe and this did not differ by HIV coinfection (39% HCV-monoinfected, 29% HIV-coinfected; P=0.2). Median kPa values of ≤7 were scored as F0–F1, kPa >7 and ≤9 as F2, kPa >9 and ≤14 as F3, and kPa >14 as F4 [9]. Hepatic steatosis grade 2 or greater was defined as a CAP score ≥311 dB/m [10]. For inclusion as valid determinations, Fibroscan data points were required to have at least 10 valid measurements and a minimum of 60% validity of total measurements taken with a kPa IQR/median value <30%. Progression of fibrosis was defined as a 2-stage increase in fibrosis or greater from pre-treatment biopsy to post-SVR transient elastography, while regression was defined as a 2-stage or greater decrease in fibrosis. Any change in fibrosis less than a 2-stage change was considered stable fibrosis. In order to account for the fact that participants no longer had active HCV at follow-up and that non-HCV characteristics may influence transient elastography performance characteristics, a sensitivity analysis was performed using fibrosis cutoff values for transient elastography validated in patients with non-alcoholic fatty liver disease (NAFLD). Median kPa values of <7 were scored as F0–F1, kPa ≥7 and <8.7 as F2, kPa ≥8.7 and <10.3 as F3, and kPa ≥10.3 as F4 [11].
Statistical analysis
All statistical analyses utilized laboratory and clinical values obtained from the latest follow-up visit excluding the pre-treatment fibrosis score. For the purpose of the pre- and post-HCV SVR analyses, pre-treatment baseline was defined as the fibrosis staging performed via liver biopsy preceding treatment. Post-SVR fibrosis staging was based on transient elastography kPa values at the latest follow-up visit. Data were analysed to determine the possible factors associated with progression or regression of fibrosis post-SVR at last follow-up and included age, transaminase levels, HIV coinfection, body mass index (BMI), change in BMI, steatosis by CAP score, smoking pack years, estimated weekly alcohol intake, CD4+ T-cell count and diabetes. Statistical analysis was performed by t-tests and χ2 likelihood ratio for group comparisons. All factors identified as significantly associated with regression/progression of fibrosis were entered into a nominal logistic regression analysis of fibrosis score at last follow-up, which also included baseline fibrosis score, age, HIV coinfection, duration of follow-up and alcohol intake. All analyses were completed using JMP statistical software (Version 13.0; SAS Institute Inc., Cary, NC, USA).
Results
Subjects had a mean age of 60 ±7 years at last follow-up, were predominantly male (65%) and Black (77%), with a mean duration of follow up of 4.1 ±1.7 years (Table 1). Median treatment length was 12 weeks (IQR 9.5–16) and the HCV treatments resulting in SVR included: 136 (84%) DAA only, 17 (10%) DAA plus ribavirin, 8 (5%) interferon and ribavirin and 1 (1%) interferon, ribavirin and DAA. The DAA treatment regimens contained a wide spectrum of agents, including asunaprevir, beclabuvir, daclatasvir, ledipasvir, sofosbuvir and telaprevir. Approximately half (51%) of participants had chronic HCV infection for greater than 20 years and the majority had a history of intravenous drug use (59%) based on self-reported risk behaviours. Most subjects had HCV genotype-1 A/B (96%) while only 4% had genotype-2 or 4. The mean BMI at follow-up was 28 ±6 kg/m2 . The monoinfected (HCV) group and coinfected (HIV–HCV) group were similar with respect to age and demographic characteristics, however, the clinical characteristics varied slightly between the HCV and HIV–HCV groups. The HCV group had significantly higher mean BMI (P=0.03) and mean CAP score (P=0.03) compared with the HIV–HCV group. The HIV–HCV group had slightly higher alanine aminotransferase (ALT) levels (P=0.03). 94% of the HIV–HCV subgroup were virally suppressed with an HIV viral level <40 copies/ml at last follow-up and had a mean CD4+ T-cell count of 705 ±324 cell/mm3 .
Table 1.
Clinical characteristics at last follow-up
| Clinical characteristics | Total (n=162) | Monoinfected (HCV; n=107) |
Coinfected (HIV–HCV; n=55) |
|---|---|---|---|
| Duration of follow-up, years | 4.1 ±1.7 | 4.1 ±1.6 | 4.0 ±1.9 |
| Age, years | 60 ±7 | 60 ±7 | 60 ±8 |
| BMI, kg/m2 | 28 ±6 | 29 ±6a | 27 ±5a |
| CAP, dB/m | 247 ±59 | 254 ±58a | 232 ±59a |
| Sex | |||
| Male, n (%) | 105 (65) | 67 (63) | 38 (69) |
| Female, n (%) | 57 (35) | 40 (37) | 17 (31) |
| Race | |||
| White, n (%) | 36 (22) | 22 (21) | 14 (25) |
| Black, n (%) | 124 (77) | 84 (79) | 40 (73) |
| Mixed race/other, n (%) | 2 (1) | 1 (1) | 1 (2) |
| Ethnicity: Hispanic or Latino, n (%) | 11 (7) | 4 (4) | 7 (13) |
| Risk | |||
| Drinks per week | 1 ±2 | 0 ±1 | 1 ±3 |
| HCV infection >20 years, n (%) | 81 (51) | 50 (47) | 31 (58) |
| History of IVDU, n (%) | 95 (59) | 61 (58) | 34 (62) |
| Diabetes, n (%) | 30 (19) | 19 (18) | 11 (20) |
| Smoking pack years | 10 ±10 | 11 ±11 | 9 ±9 |
| Lab data | |||
| AST, U/l | 24 ±12 | 23 ±10 | 26 ±16 |
| ALT, U/l | 21 ±15 | 18 ±9a | 25 ±22a |
| HIV viral load <40, n (%) | 51 (94) | ||
| CD4+ T-cell count, cells/mm3 | 705 ±324 |
Values are mean ±sd unless indicated otherwise.
P<0.05 between HCV and HIV–HCV groups. ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; CAP, controlled attenuation parameter; IVDU, intravenous drug use.
According to liver biopsy data at baseline, 57% of subjects had a fibrosis score of F0–F1, whereas 26% had F3/F4 fibrosis and there was no difference between HCV-monoinfected and HIV-coinfected subjects (Table 2). With respect to steatosis grading, 10% had S2 or greater steatosis at baseline. At last follow-up using transient elastography, there was an overall reduction in those falling into the F3 and F4 category and an increase in those categorized with F0–1 and F2 fibrosis (P<0.003 for pre- versus post-SVR fibrosis). 45% of those with F3 or F4 at baseline had F2 or lower fibrosis at follow-up. The frequency of steatosis as measured by CAP score at last follow-up increased slightly from 10% to 12% for the entire cohort. The monoinfected group had an increase in the prevalence of steatosis from 10% to 15%, whereas the HIV–HCV group decreased in frequency of S2 steatosis at last follow-up from 10% to 4%.
Table 2.
Baseline and follow-up fibrosis and steatosis grading
| Fibrosis scores | Total (n=162) | Monoinfected (HCV; n=107) |
Coinfected (HIV–HCV; n=55) |
|---|---|---|---|
| Baseline liver biopsy | |||
| F0–F1, n (%) | 93 (57) | 61 (57) | 32 (58) |
| F2, n (%) | 27 (17) | 17 (16) | 10 (18) |
| F3, n (%) | 21 (13) | 13 (12) | 8 (15) |
| F4, n (%) | 21 (13) | 16 (15) | 5 (9) |
| Steatosis ≥ Grade 2, n (%) | 14 (10) | 9 (10) | 5 (10) |
| Follow-up Fibroscan | |||
| F0–F1, n (%) | 104 (64) | 69 (64) | 35 (64) |
| F2, n (%) | 29 (18) | 16 (15) | 13 (24) |
| F3, n (%) | 16 (10) | 10 (9) | 6 (11) |
| F4, n (%) | 13 (8) | 12 (11) | 1 (2) |
| Steatosis (CAP ≥311 dB/m), n (%) | 17 (12) | 15 (15)a | 2 (4)a |
P<0.05 for HCV versus HIV–HCV comparison. Steatosis grade available n=146 at baseline and n=145 at follow-up. CAP, controlled attenuation parameter.
Next, we categorized subjects as stable, regressor or progressor with respect to fibrosis. 89% of the cohort had stable fibrosis, while 7% had regression and 4% had progression of fibrosis. There was no statistically significant difference in the distribution of those who were stable, regressors or progressors between the HCV and HIV–HCV subgroups. HIV-coinfected subjects represented 34% of those with stable fibrosis, 33% of regressors and 33% of progressors. There was no difference in regression/progression groups with respect to age, estimated weekly alcohol intake, diabetes, aspartate transaminase, ALT, CD4+ T-cell count or change in BMI during follow-up (data not shown). However, BMI at last follow-up, CAP score and smoking pack years differed significantly (Figure 1). Mean BMI of those with stable fibrosis or regression of fibrosis was significantly lower than the BMI of the progressor group (P=0.004, P=0.01, respectively). Similarly, mean CAP score among those with stable fibrosis was also significantly lower compared with the progressor group (P=0.005). In a sub-analysis excluding those without confirmed fasting prior to transient elastography measurements (n=14 excluded: 1 progressor, 3 regressors and 10 with stable fibrosis), difference in CAP score remained statistically significant. The progressor subgroup had a disproportionately higher frequency of steatosis at last follow-up (50%) compared with the stable (9%) and regressor (27%) subgroups (P=0.01). With regards to smoking, those with progression of fibrosis had significantly more pack years compared with those without progression (P<0.0001).
Figure 1.
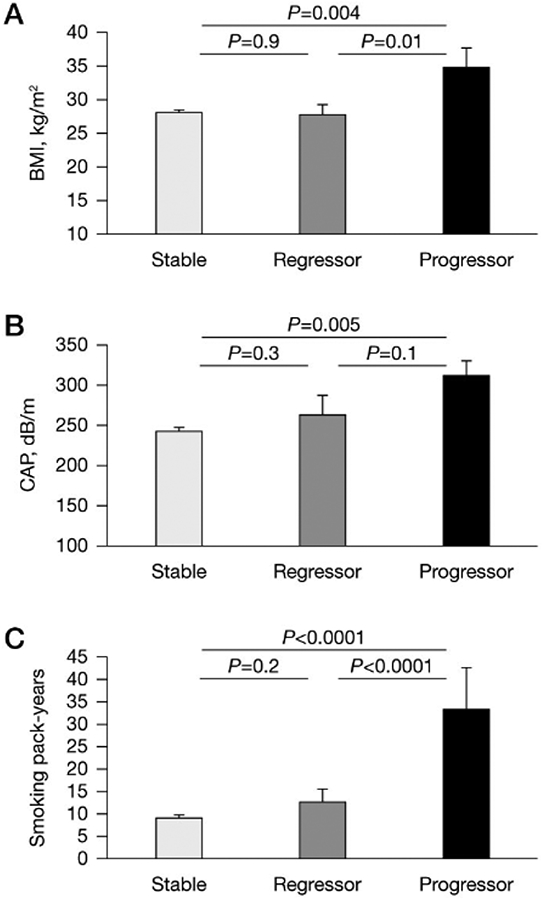
Clinical characteristics associated with changes in hepatic fibrosis after HCV clearance
Participants categorized by change in fibrosis at last follow-up as stable (n=144), regressor (n=12) and progressor (n=6), comparing mean (A) body mass index (BMI), (B) controlled attenuation parameter (CAP) and (C) smoking pack-years between each group.
In order to identify the key factors associated with fibrosis stage at last follow-up we performed a regression analysis which included age, HIV coinfection, BMI, CAP score, smoking pack years, weekly alcohol intake, baseline fibrosis score, and duration of follow-up. We found that baseline fibrosis score (P<0.0001), HIV coinfection (P=0.009), lower CAP score (P<0.05) and lower smoking pack-years (P=0.0007) were associated with lower fibrosis score at last follow-up (Additional file 1). When excluding subjects that were not confirmed to have fasted for transient elastography, HIV (P=0.008) and smoking pack-years (P=0.003) and CAP (P=0.04) remained significant.
In a sensitivity analysis using fibrosis stage cutoffs for NAFLD instead of hepatitis to categorize subjects as progressors, regressors, or stable, BMI, CAP, and smoking pack years remained significant factors associated with fibrosis progression in univariate analyses (Additional file 2). When using NAFLD cutoffs for fibrosis stage in the multivariable regression, baseline fibrosis stage (P<0.0001) and smoking pack years (P=0.007) were the only significant factors associated with fibrosis.
Discussion
Although chronic HCV is now a widely treatable disease, questions remain regarding the long-term outcomes for the many patients who achieve SVR following decades of infection. The majority of patients will be able to achieve and maintain SVR which has been shown to produce reductions in hepatic inflammation and subsequent regression of liver fibrosis in groups with and without HIV coinfection [1-3]. A few studies limited to individuals with advanced fibrosis (F3/F4 pre-treatment) using transient elastography demonstrated acute reductions in liver stiffness at the end of treatment or within 1 year post-SVR [12,13]. Further, Lledó et al. [14], showed that while there was no significant effect of HIV coinfection on change in elastography at SVR, 40% of patients experienced a reduction greater than 30% in liver stiffness (kPa) 12 weeks after treatment. However, few studies have characterized the factors associated with regression or progression of hepatic fibrosis with up to 4 years of follow-up after HCV clearance with contemporary therapies. The present study prospectively examined factors associated with regression and progression of hepatic fibrosis after successful treatment of HCV in patients with and without HIV. Increased BMI, hepatic steatosis and smoking were identified as significant co-factors in the subset of patients who had progression of fibrosis despite HCV clearance. Somewhat unexpectedly, HIV coinfection was not associated with worsening of fibrosis. These observations may be useful in determining how to monitor and counsel patients following HCV clearance.
Smoking has been suggested as a cofactor for progression of fibrosis in various organs including lungs, kidneys and the liver in a variety of diseases including NAFLD, primary biliary cholangitis, and HCV, possibly through increases in oxidative stress and inflammation. For example, Pessione et al. [15] found a strong independent relationship between hepatic fibrosis score and tobacco consumption. In this cross-sectional study of patients with chronic HCV, higher consumption of tobacco measured by smoking pack-years was related to greater severity of fibrosis [15]. Further, Xiong et al. [16] found that a cigarette smoking history of 10 pack years or greater was associated with advanced fibrosis in chronic hepatitis B (CHB) infection and impaired fibrosis regression with CHB treatment. The present study found that the relationship between smoking and hepatic fibrosis was also significant after HCV clearance. Studies suggest that chemicals in cigarette smoke may have fibrogenic effects, specifically nicotine, which may affect hepatic cells via nicotinic acetylcholine receptors. In non-alcoholic steatohepatitis, nicotine has been shown to induce fibrogenic pathways and upregulate collagen1-α2 and the pro-fibrogenic cytokine transforming growth factor beta 1 (TGF-β1) [17]. Most studies have not analysed other methods of nicotine and tobacco use, however, research trends are shifting to analysing the effects of e-cigarette use on the liver in animal models as this method of nicotine delivery grows in popularity. This novel observation linking smoking to fibrosis progression after HCV clearance deserves further investigation and provides a potential modifiable target to optimize liver health in the growing number of patients who have been successfully treated.
In the present study, subjects categorized as progressors had increased BMI compared with those who did not progress in univariate analysis. Previous studies have identified obesity as a factor associated with rapid progression of liver disease in patients with active HCV infection [18]. Ortiz et al. [18] describes a BMI ≥25 kg/m2 as the cutoff for risk of rapid hepatic fibrosis progression in a study of 114 patients with chronic HCV without SVR. Interestingly, BMI did not remain a significant factor in multivariate analysis once hepatic steatosis was also included, indicating the potential mechanism by which increased adiposity promotes hepatic fibrosis.
We observed that the presence of hepatic steatosis following viral clearance was an important indicator of progression of fibrosis in multivariate analysis. One study by Noureddin et al. [19] evaluated a cohort of 101 subjects without HCV genotype-3 approximately 1 year after SVR and identified a similar finding. The study found that after achieving SVR, patients with steatosis (≥248 dB/m) continued to have clinically significant liver fibrosis (defined as ≥7 kPa) while those without steatosis did not have fibrosis [19]. While steatosis has classically been associated with genotype-3, both Noureddin et al. [19] and the present study did not include patients with genotype-3. We observed a positive relationship between BMI and steatosis measured by CAP scores. Interestingly, the present study did not find a relationship between CAP score and self-reported alcohol use, although it is known that alcohol increases steatosis and also causes oxidative stress, inflammation and damage to the liver [20]. Furthermore, there was no significant relationship between alcohol intake and progression or regression of fibrosis score at last follow-up, however, significant alcohol intake was not highly prevalent in this cohort.
Unexpectedly, HIV was associated with having a lower fibrosis score at last follow-up in multivariate analysis which accounted for fibrosis grade at baseline. This may be due to the fact that the coinfected group as a whole was less enriched with co-factors associated with fibrosis progression (that is, BMI and CAP scores). In a study of 214 patients who achieved SVR following DAA therapy for HCV (60% coinfected with HIV), Malin et al. [21] showed no difference in short-term changes in transient elastography between HCV-monoinfected and HIV-coinfected participants at a median follow-up of 12 weeks. Although HIV has been identified as a cofactor associated with rapid progression of fibrosis in active HCV infection [22], observations by Malin et al. [21], Lledó et al. [14], and the current study suggest that HIV may have less of an impact on fibrosis in the context of both acute and long-term viral clearance. Further studies with a larger sample of HIV-infected patients are needed to understand the impact of HIV on the long-term regression or progression of hepatic fibrosis following HCV viral therapy.
The present study had several limitations. It is important to recognize that while histopathological determination of severity of fibrosis was obtained prior to HCV therapy, less invasive transient elastography was employed for longitudinal follow-up staging of fibrosis. Transient elastography, however, has been well-established and validated to METAVIR staging as a non-invasive method for evaluating liver fibrosis in chronic HCV and in HIV [23,24] and has been used in other cohort studies to track changes in fibrosis which was staged initially by liver biopsy pre-therapy [4]. Furthermore, we used conservative cutoffs for both fibrosis and steatosis as well as stringent definition of a 2-stage change in fibrosis for progression and regression to mitigate the potential variability in the continuous measurement scale of transient elastography. In the current study only one quarter of the cohort had advanced fibrosis (F3–F4) pre-treatment and 29% of those showed regression of fibrosis; this may have limited the ability to identify factors associated with fibrosis regression. While research suggest that biopsy length and number of portal tracts are important to optimize accuracy of histopathology determinations [25], limited data on these characteristics prevented application of such criteria in the current study. Another limitation in the study was the relatively small number of subjects in the progressor group. Nonetheless, the risk factors identified were statistically robust in multivariate analyses and are logical in the context of existing reports and the current understanding of liver disease. In addition, since participants, by definition, no longer have HCV at follow-up, we repeated analyses using transient elastography fibrosis cutoffs established for NAFLD and all the key findings remained significant. This study should be replicated in larger cohorts to further validate these observations.
In conclusion, we show a significant relationship between BMI, hepatic steatosis, and smoking pack years and progression of hepatic fibrosis post-SVR in hepatitis C patients both with and without HIV. The influence of BMI was largely attributable to the effect of adiposity on the increased risk of hepatic steatosis. The relationship between HIV coinfection and fibrosis change post-SVR should be further investigated. Our observations suggest that counselling and attention to modifiable risk factors such as maintaining a healthy body weight, avoidance of possible hepatoxic effects of tobacco and nicotine may benefit patients treated for chronic HCV and reduce the risk of liver disease progression.
Supplementary Material
Additional file 2: A figure showing participants categorized by change in fibrosis based on NAFLD established cutoffs for fibrosis stage at last follow-up can be found at https://www.intmedpress.com/uploads/documents/4531_Balmaceda_Addfile2.pdf
Additional file 1: A table showing nominal logistic regression of fibrosis stage at last follow-up can be found at https://www.intmedpress.com/uploads/documents/4531_Balmaceda_Addfile1.pdf
Acknowledgements
This work was support by the Intramural Research program of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute.
Footnotes
Disclosure statement
C Gross holds stock in Merck, Pfizer and Johnson & Johnson. S Kottilil has received research grants paid to the university from Merck Inc. and Gilead Sciences Inc. and participated in advisory boards for Merck Inc. and Gilead Sciences Inc. The remaining authors declare no competing interests.
Trial Registration Number: NCT01350648.
References
- 1.ANRS CO13 HEPAVIH Cohort. Regression of liver stiffness after sustained hepatitis C virus (HCV) virological responses among HIV/HCV-coinfected patients. AIDS 2015; 29: 1821–1830. doi: 10.1097/QAD.0000000000000787 [DOI] [PubMed] [Google Scholar]
- 2.Facciorusso A, Del Prete V, Turco A, Buccino RV, Nacchiero MC, Muscatiello N. Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: results from a 5-year cohort study. J Gastroenterol Hepatol 2018; 33: 942–949. doi: 10.1111/jgh.14008 [DOI] [PubMed] [Google Scholar]
- 3.Pietsch V, Deterding K, Attia D, et al. Long-term changes in liver elasticity in hepatitis C virus-infected patients with sustained virologic response after treatment with direct-acting antivirals. United European Gastroenterol J 2018; 6: 1188–1198. doi: 10.1177/2050640618786067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casado JL, Esteban MA, Banon S, et al. Fibrosis regression explains differences in outcome in HIV-/HCV-coinfected patients with cirrhosis after sustained virological response. Dig Dis Sci 2015; 60: 3473–3481. doi: 10.1007/s10620-015-3773-y [DOI] [PubMed] [Google Scholar]
- 5.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010; 52: 833–844. doi: 10.1002/hep.23744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aleman S, Rahbin N, Weiland O, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis 2013; 57: 230–236. doi: 10.1093/cid/cit234 [DOI] [PubMed] [Google Scholar]
- 7.Labarga P, Fernandez-Montero JV, de Mendoza C, Barreiro P, Pinilla J, Soriano V. Liver fibrosis progression despite HCV cure with antiviral therapy in HIV-HCV-coinfected patients. Antivir Ther 2015; 20: 329–334. doi: 10.3851/IMP2909 [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996; 24: 289–293. doi: 10.1002/hep.510240201 [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Conde M, Montes-Ramirez ML, Miralles P, et al. Comparison of transient elastography and liver biopsy for the assessment of liver fibrosis in HIV/hepatitis C virus-coinfected patients and correlation with noninvasive serum markers. J Viral Hepat 2010; 17: 280–286. doi: 10.1111/j.1365-2893.2009.01180.x [DOI] [PubMed] [Google Scholar]
- 10.de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012; 32: 911–918. doi: 10.1111/j.1478-3231.2012.02820.x [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51: 454–462. doi: 10.1002/hep.23312 [DOI] [PubMed] [Google Scholar]
- 12.Persico M, Rosato V, Aglitti A, et al. Sustained virological response by direct antiviral agents in HCV leads to an early and significant improvement of liver fibrosis. Antivir Ther 2018; 23: 129–138. doi: 10.3851/IMP3186 [DOI] [PubMed] [Google Scholar]
- 13.Giannini EG, Crespi M, Demarzo M, et al. Improvement in hepatitis C virus patients with advanced, compensated liver disease after sustained virological response to direct acting antivirals. Eur J Clin Invest 2019; 49: e13056 doi: 10.1111/eci.13056 [DOI] [PubMed] [Google Scholar]
- 14.Lledó GM, Carrasco I, Benitez-Gutierrez LM, et al. Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection. AIDS 2018; 32: 2347–2352. [DOI] [PubMed] [Google Scholar]
- 15.Pessione F, Ramond MJ, Njapoum C, et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology 2001; 34: 121–125. doi: 10.1053/jhep.2001.25385 [DOI] [PubMed] [Google Scholar]
- 16.Xiong M, Li J, Yang S, et al. Impacts of cigarette smoking on liver fibrosis and its regression under therapy in male patients with chronic hepatitis B. Liver Int 2019; 39: 1428–1436. [DOI] [PubMed] [Google Scholar]
- 17.Soeda J, Morgan M, McKee C, et al. Nicotine induces fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on hepatic stellate cells. Biochem Biophys Res Commun 2012; 417: 17–22. doi: 10.1016/j.bbrc.2011.10.151 [DOI] [PubMed] [Google Scholar]
- 18.Ortiz V, Berenguer M, Rayon JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol 2002; 97: 2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x [DOI] [PubMed] [Google Scholar]
- 19.Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J Gastroenterol 2018; 24: 1269–1277. doi: 10.3748/wjg.v24.i11.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zakhari S Bermuda Triangle for the liver: alcohol, obesity, and viral hepatitis. J Gastroenterol Hepatol 2013; 28 Suppl 1: 18–25. doi: 10.1111/jgh.12207 [DOI] [PubMed] [Google Scholar]
- 21.Malin JJ, Boesecke C, Schwarze-Zander C, et al. Liver stiffness regression after successful hepatitis C treatment is independent of HIV coinfection. HIV Med 2019; 20: 230–236. doi: 10.1111/hiv.12705 [DOI] [PubMed] [Google Scholar]
- 22.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30: 1054–1058. doi: 10.1002/hep.510300409 [DOI] [PubMed] [Google Scholar]
- 23.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48: 835–847. doi: 10.1016/j.jhep.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Morse CG, McLaughlin M, Proschan M, et al. Transient elastography for the detection of hepatic fibrosis in HIV-monoinfected adults with elevated aminotransferases on antiretroviral therapy. AIDS 2015; 29: 2297–2302. doi: 10.1097/QAD.0000000000000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003; 39: 239–244. doi: 10.1016/S0168-8278(03)00191-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: A figure showing participants categorized by change in fibrosis based on NAFLD established cutoffs for fibrosis stage at last follow-up can be found at https://www.intmedpress.com/uploads/documents/4531_Balmaceda_Addfile2.pdf
Additional file 1: A table showing nominal logistic regression of fibrosis stage at last follow-up can be found at https://www.intmedpress.com/uploads/documents/4531_Balmaceda_Addfile1.pdf


