Abstract
Oral hypofunction, resulting from a combined decrease in multiple oral functions, may affect systemic-condition deterioration; however, few studies have examined the association between oral hypofunction and general health among older adults. In this cross-sectional study, we examined the relationship between oral hypofunction and sarcopenia in community-dwelling older adults. We included 878 adults (268 men and 610 women, mean age 76.5 ± 8.3 years). Tongue coating index, oral moisture, occlusal force, oral diadochokinesis (/pa/,/ta/,/ka/), tongue pressure, mas-ticatory function, and swallowing function were evaluated as indicators of oral hypofunction. Grip strength, gait speed, and skeletal muscle mass index were measured as diagnostic sarcopenia parameters. The association between oral hypofunction and sarcopenia was examined via logistic regression using sarcopenia as the dependent variable. Oral hypofunction prevalence was 50.5% overall, 40.3% in men, and 54.9% in women. The prevalence of sarcopenia was 18.6% overall, 9.7% in men, and 22.5% in women. A logistic regression showed oral hypofunction, age, body mass index, higher-level functional capacity, and serum albumin level were significantly associated with sarcopenia. Sarcopenia occurred at an increased frequency in patients diagnosed with oral hypofunction (odds ratio: 1.59, 95% confidence interval: 1.02–2.47); accordingly, oral hypofunction appears to be significantly associated with sarcopenia.
Keywords: oral hypofunction, sarcopenia, oral function, physical health, older adults, Japan
1. Introduction
Japan has a rapidly aging population. As the population ages, the number of people in need of nursing care, and the cost of social security benefits increase correspondingly [1]. Therefore, extending the healthy life expectancy of older adults has become an urgent issue.
In 2016, the Japanese Society of Gerodontology (JSG) recognized oral hypofunction as a new disease in the oral field [2], and it was introduced into the public insurance system in Japan in 2018 [3]. As of 2021, the public insurance system in Japan defines oral hypofunction as “a disease in which oral function is complexly reduced, not only by aging but also by various factors pertaining to diseases and disorders”. Oral hypofunction is diagnosed by assessing the presence or absence of decreased oral function in seven dimensions, i.e., poor oral hygiene, oral dryness, reduced occlusal force, decreased tongue-lip motor function, decreased tongue pressure, decreased masticatory function, and deterioration of swallowing function. The items examination examined have been determined to be associated with malnutrition [2,4]. Individuals with decreased levels of functionality in three or more of the seven dimensions considered are diagnosed with oral hypofunction.
Decreases in individual levels of oral function have been shown to influence general health deterioration [5,6,7,8,9]. Oral hypofunction is a factor hat influences the deterioration of general health. To date, however, few studies have examined the association between oral hypofunction and general health among older adults [10]. If the relationship between oral hypofunction and systemic diseases is clarified, it may be possible to extend the healthy life expectancy of older adults from the perspective of dentistry.
In this study, we focused on sarcopenia as an outcome of oral hypofunction, as it is a principal geriatric syndrome, and malnutrition is a risk factor for its development [11,12], as is oral hypofunction. The Asian Working Group for Sarcopenia defines sarcopenia as “age-related loss of skeletal muscle mass plus loss of muscle strength and/or reduced physical performance” [12]. Sarcopenia can have a negative impact on daily life and decrease quality of life. Therefore, the prevention of sarcopenia, and timely intervention are needed. If the relationship between oral hypofunction and sarcopenia is clarified, it may be possible to address sarcopenia from the standpoint of dental professionals. The purpose of this study was to clarify the relationship between oral hypofunction and sarcopenia among community-dwelling older adults.
2. Materials and Methods
2.1. Research Design
This cross-sectional study used data collected in 2018 for “The Otassha Study”, a cohort study that evaluated comprehensive health examination data conducted by the Tokyo Metropolitan Institute of Gerontology. Data was collected from people aged 65 years or older living in Itabashi city, Tokyo. Potential participants were informed of the date, time, and venue of the health examination in advance by letter, and the health examination was conducted for those who appeared on the allotted date. The purpose and content of the health examination were explained to all participants in writing and orally, and written consent for participation in the study was obtained from all study participants. Individuals with missing survey data were excluded. All health examination evaluators received training on measurement methods and the use of evaluation instruments in advance, and evaluation criteria were standardized. This study was approved by the Ethics Committee of the Tokyo Metropolitan Institute of Gerontology (2006-17, 2011-48, 2018-Zin1, 16), and was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Statement [13].
2.2. Number of Teeth
Numbers of present and functional teeth were evaluated via an intra-oral examination by dentists and dental hygienists [14]. Present teeth were defined as those in which crowns had erupted, and were excluded if they were not occluded, were stump teeth, or showed significant looseness. Functional teeth included present teeth, pontics for bridges, artificial teeth for removable dentures, and superstructures for implants.
2.3. Oral Hypofunction
To diagnose oral hypofunction, the degree of tongue coating, oral moisture, occlusal force, tongue-lip motor function, tongue pressure, masticatory function, and swallowing function were evaluated by dentists and dental hygienists. The presence or absence of seven items including poor oral hygiene, oral dryness, reduced occlusal force, decreased tongue-lip motor function, decreased tongue pressure, decreased masticatory function, and deterioration of swallowing function were assessed, and participants who experienced decreased functionality of least three items were diagnosed with oral hypofunction based on diagnostic criteria published by the JSG in 2016 [2]. Removable denture users underwent oral function examinations with their removable dentures in place.
2.3.1. Poor Oral Hygiene
Degree of tongue coating was evaluated using the tongue coating index (TCI). To determine TCI values, the tongue was divided into nine blocks, and the degree of tongue coating of each block was visually inspected [15]. Poor oral hygiene was defined as a TCI value ≥ 50% [2].
2.3.2. Oral Dryness
The degree of oral moisture was assessed using an oral moisture checker (Mucus, Life Co., Ltd., Saitama, Japan) [16,17]. The device was pressed against the center of the tongue dorsum at approximately 200 gf to measure mucosal moisture content. Three successive measurements were performed, and the median oral moisture value was used. Oral dryness was defined as an oral moisture level < 27.0 [2].
2.3.3. Reduced Occlusal Force
Occlusal force was measured using a pressure-sensitive sheet (Dental Prescale 50H Type-R, Fuji Film Co., Tokyo, Japan) scanned using an image scanner (Occluzer, FPD-707, Fuji Film Co.) [18,19,20]. Participants bit a 98-μm thick, pressure-sensitive sheet with maximal force at the intercuspal position for 3 s. Occlusal contact points on the pressure-sensitive sheet were read by an image scanner, and occlusal force was calculated. Participants with occlusal force values determined to be <200 N were deemed to have reduced occlusal force [2].
2.3.4. Decreased Tongue–Lip Motor Function
Tongue-lip motor function was measured based on the dexterity of the tongue and lips using an automatic counter (KENKOU-KUN handy, Takei Scientific Instruments Co., Ltd., Niigata, Japan) [21]. Sounds including/pa/,/ta/, and/ka/were repeated as quickly as possible for 5 s each, and the number of syllables pronounced per s was measured to determine the oral diadochokinesis (ODK) value. Decreased tongue-lip motor function was defined as a/pa/,/ta/, or/ka/ODK value of <6.0 times/s [2].
2.3.5. Decreased Tongue Pressure
Tongue pressure was assessed using a tongue pressure measurement device (JMS tongue pressure device TPM-01, JMS Co., Ltd., Hiroshima, Japan) [22,23]. To evaluate tongue pressure, the air-inflated balloon portion of a disposable oral probe was placed at the anterior section of the palate, and each study participant pressed the balloon against their palate using their tongue for 7 s. Participants compressed balloons three times with maximum voluntary effort at 30-s intervals, the mean maximum pressure recorded was used as the tongue pressure value. Participants with a tongue pressure value < 30 kPa were considered to have decreased tongue pressure [2].
2.3.6. Decreased Masticatory Function
Masticatory function was evaluated using gummy jelly (test gummy jelly, UHA Mikakuto Co., Ltd., Osaka, Japan) [24,25]. Participants chewed gummy jelly 30 times, and spat the chewed material onto gauze. Chewed gummy jelly was visually compared with a score chart that defined 10 levels of masticatory function to determine values. Those who scored of ≤2 were considered to have decreased masticatory function [2].
2.3.7. Deterioration of Swallowing Function
Swallowing function was assessed using a 10-item Eating Assessment Tool [26], which is a self-administered questionnaire with 10 questions that assess swallowing function using a 5-point Likert scale ranging from 0 to 4. Participants with a total score of ≥3 were considered to have deteriorated swallowing function [2].
2.4. Sarcopenia
To diagnose sarcopenia, parameters including grip strength, gait speed, and appendicular skeletal muscle mass of participants were measured by researchers with expertise in physical functions assessed. Using the Asian Working Group for Sarcopenia 2019 (AWGS2019) algorithm as a reference, sarcopenia was diagnosed if the participant had either low appendicular skeletal muscle mass and muscle strength levels or low appendicular skeletal muscle and low physical performance. Sarcopenia was considered severe if participants were determined to be positive for both sarcopenia indicators [12].
2.4.1. Low Muscle Strength
To evaluate muscle strength, the grip strength of the dominant hand was measured using a Smedley-type hand dynamometer (Grip-A, Takei Scientific Instruments Co., Ltd., Niigata, Japan). Participants were required to grip of the hand dynamometer in a standing position with their arms lowered at a position that felt natural. Measurements were obtained twice, and the higher value was used to record handgrip strength. A handgrip strength < 28 kg and <18 kg for men and women, respectively, were defined as low muscle strength [12].
2.4.2. Low Physical Performance
Gait speed was measured to evaluate physical performance. Participants were instructed to walk at a normal pace along a straight 11-m walkway with 3-m and 8-m points marked with tape on a flat floor. Time needed to walk the 5-m distance between tape marks was measured to determine gait speed [27]. A gait speed lower than 1 m/s was indicated low physical performance [12].
2.4.3. Low Appendicular Skeletal Muscle Mass
Appendicular skeletal muscle mass was measured using bio-electrical impedance analysis (InBody 770, InBody Inc., Seoul, Korea). Each measured appendicular skeletal muscle mass value was divided by the square of the height (m conversion) of each participant to calculate skeletal muscle mass index (SMI). An SMI value of <7.0 kg/m2 and <5.7 kg/m2 for men and women, respectively, indicated low appendicular skeletal muscle mass [12].
2.5. Other Recorded Variables
Other survey items included the following: age; years of education; body mass index (BMI); drinking and smoking habits; living situation; depression assessed with a questionnaire [9]; higher-level functional capacity assessed using the Japan Science and Technology Agency index of competence (JST-IC) [28], the Japanese version of the Mini Mental State Examination (MMSE-J) for cognitive function [29,30]; medical history recorded through an interview (stroke, heart disease, diabetes, and cancer); and serum albumin and hemoglobin A1c levels.
2.6. Statistical Analysis
Patients with sarcopenia and severe sarcopenia were included in the sarcopenia group when performing statistical analyses. Between-group comparisons of continuous variables were performed using the Mann–Whitney U test, and categorical variables were analyzed with the chi-squared test. The association between oral hypofunction and sarcopenia was examined via logistic regression analysis using the forced entry method, with sarcopenia as the dependent variable. Inclusion of variables in the model was based on existing knowledge of risk factors for sarcopenia. Statistical analyses were performed using IBM SPSS version 27 (IBM Corp., Armonk, NY, USA). As this was a secondary study of the Otassha Study, no prior sample size calculations were performed
3. Results
3.1. Participants
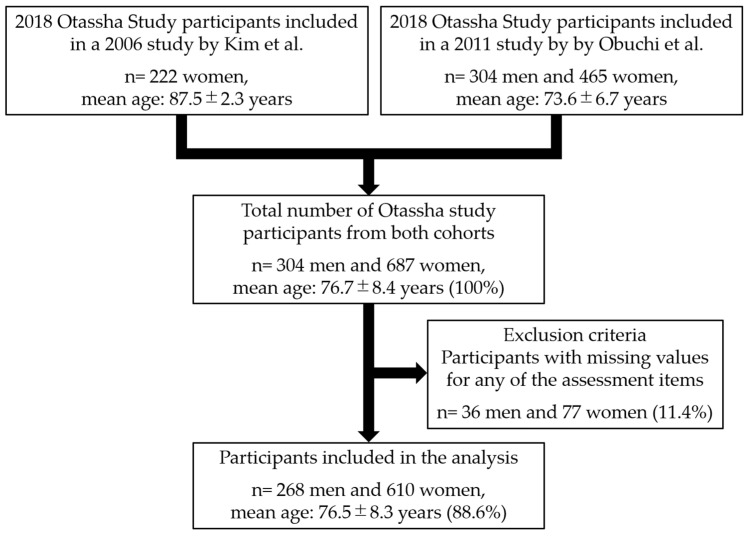
A flowchart detailing the design of this study is shown in Figure 1. There were 991 adults (100%) (304 men and 687 women, mean age: 76.7 ± 8.4 years) who visited the venue and participated in the comprehensive health examination. Among the visitors, 113 (11.4%) with missing survey data were excluded from the analysis. The total number of participants included in the study was 878 adults (88.6%) (268 men and 610 women, mean age: 76.5 ± 8.3 years).
Figure 1.
Flowchart of the study design.
3.2. Characteristics of Study Participants and between Group Comparisons
Characteristics of the study population and survey item differences according to sex, and presence of sarcopenia and oral hypofunction are shown in Table 1 and Table 2. Table 1 and Table 2 contain between-group comparisons of continuous and categorical variables, respectively. The prevalence of oral hypofunction was 50.5% overall, 40.3% in men, and 54.9% in women. The prevalence of oral hypofunction in men and women significantly differed (p < 0.001). The prevalence of sarcopenia and severe sarcopenia was 14.4% and 4.2% overall, 7.2% and 2.2% in men, and 17.4% and 5.1% in women, respectively. The prevalence of sarcopenia (both severe and not severe) in men and women significantly differed (p < 0.001). When sarcopenia and severe sarcopenia were considered collectively, sarcopenia prevalence was 18.6% overall, 9.7% in men, and 22.5% in women. The prevalence of oral hypofunction between the robust not diagnosed with sarcopenia and sarcopenia groups was 45.3% and 73.0%, respectively, and the values were determined to significantly differ (p < 0.001). Among subordinate symptoms of oral hypofunction considered, rates of reduced occlusal force (p < 0.001), decreased tongue-lip motor function (p < 0.001), decreased tongue pressure (p < 0.001), decreased masticatory function (p < 0.001), and deterioration of swallowing function (p = 0.028) were significantly higher in patients of the sarcopenia versus robust group.
Table 1.
Simple comparison of participant characteristics and continuous variables.
| Overall | Women | Men | Robust | Sarcopenia | Robust | Oral Hypofunction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 878) | (n = 610) | (n = 268) | (n = 715) | (n = 163) | (n = 435) | (n = 443) | ||||
| Continuous Variables | Median, (Q1, Q3) |
Median, (Q1, Q3) |
Median, (Q1, Q3) |
p-Value | Median, (Q1, Q3) |
Median, (Q1, Q3) |
p-Value | Median, (Q1, Q3) |
Median, (Q1, Q3) |
p-Value |
| Variables of oral hypofunction | ||||||||||
| Tongue coating index (%) | 22.2, (11.1, 50.0) | 22.2, (11.1, 50.0) | 33.3, (16.7, 61.1) | <0.001 | 22.2, (11.1, 50.0) | 22.2, (11.1, 38.9) | 0.047 | 16.7, (11.1, 38.9) | 33.3, (11.1, 55.6) | <0.001 |
| Oral moisture | 27.1, (24.9, 28.8) | 26.9, (24.8, 28.6) | 27.4, (25.2, 29.3) | 0.008 | 27.2, (25.0, 28.9) | 26.6, (24.4, 28.6) | 0.096 | 27.8, (26.3, 29.3) | 26.1, (24.3, 28.0) | <0.001 |
| Occlusal force (N) | 257.1, (121.0, 417.8) | 213.8, (101.8, 366.4) | 367.7, (221.3, 559.3) | <0.001 | 293.2, (140.4, 435.9) | 163, (72.8, 288.7) | <0.001 | 352.2, (240.0, 517.4) | 155.5, (79.6, 301.2) | <0.001 |
| Oral diadochokinesis/pa/ (time/s) | 6.4, (5.8, 6.8) | 6.2, (5.8, 6.8) | 6.4, (6.0, 6.8) | 0.002 | 6.4, (6.0, 6.8) | 6.0, (5.4, 6.6) | <0.001 | 6.6, (6.2, 7.0) | 6.2, (5.6, 6.6) | <0.001 |
| Oral diadochokinesis/ta/ (time/s) | 6.4, (5.8, 6.8) | 6.2, (5.8, 6.8) | 6.4, (6.0, 6.8) | 0.004 | 6.4, (5.8, 6.8) | 6.0, (5.4, 6.4) | <0.001 | 6.6, (6.2, 7.0) | 6, (5.6, 6.4) | <0.001 |
| Oral diadochokinesis/ka/ (time/s) | 5.8, (5.4, 6.4) | 5.8, (5.4, 6.4) | 6.0, (5.2, 6.4) | 0.917 | 6.0, (5.4, 6.4) | 5.6, (5.0, 6.0) | <0.001 | 6.2, (5.8, 6.6) | 5.6, (5.0, 6.0) | <0.001 |
| Tongue pressure (kPa) | 31.8, (26.3, 36.9) | 31.3, (25.8, 35.7) | 33.4, (27.8, 39) | <0.001 | 32.9, (27.8, 37.8) | 27.2, (20.6, 31.8) | <0.001 | 34.6, (30.8, 39.2) | 28.3, (23.5, 33.2) | <0.001 |
| Gummy jelly score | 5, (3, 6) | 5, (2, 6) | 6, (5, 7) | <0.001 | 5, (4, 6) | 4, (1, 6) | <0.001 | 6, (5, 6) | 4, (1, 6) | <0.001 |
| EAT-10 score | 1, (0, 3) | 1, (0, 3) | 1, (0, 4) | 0.516 | 1, (0, 3) | 1, (0, 5) | 0.004 | 0, (0, 2) | 2, (0, 5) | <0.001 |
| Oral hypofunction score | 3, (2, 4) | 3, (2, 4) | 2, (1, 3) | <0.001 | 2, (1, 3) | 4, (2, 5) | <0.001 | 2, (1, 2) | 4, (3, 4) | <0.001 |
| Variables of sarcopenia | ||||||||||
| Handgrip strength (kg) | 24.0, (18.0, 31.0) | 21.0, (17.0, 25.0) | 35.0, (30.3, 40.0) | <0.001 | 25.0, (21.0, 33.0) | 16, (14, 19) | <0.001 | 26, (21, 35) | 21, (16, 27) | <0.001 |
| Gait speed (m/s) | 1.32, (1.11, 1.52) | 1.32, (1.09, 1.47) | 1.39, (1.22, 1.55) | <0.001 | 1.39, (1.19, 1.52) | 1.09, (0.91, 1.32) | <0.001 | 1.39, (1.22, 1.56) | 1.25, (1.04, 1.43) | <0.001 |
| Skeletal muscle mass index (kg/m2) | 6.1, (5.6, 7.0) | 5.8, (5.3, 6.2) | 7.5, (7.0, 7.9) | <0.001 | 6.3, (5.8, 7.3) | 5.3, (4.9, 5.6) | <0.001 | 6.3, (5.7, 7.4) | 5.9, (5.4, 6.7) | <0.001 |
| Other recorded variables | ||||||||||
| Age (years) | 76, (68, 85) | 78, (70, 86) | 71, (68, 78.8) | <0.001 | 74, (68, 81) | 86, (80, 89) | <0.001 | 72, (67, 79) | 81, (72, 86) | <0.001 |
| Number of present teeth | 23, (14, 27) | 22, (12, 26) | 25, (20, 28) | <0.001 | 24, (17, 27) | 20, (7, 25) | <0.001 | 26, (21, 28) | 19, (8, 25) | <0.001 |
| Number of functional teeth | 28, (27, 28) | 28, (27, 28) | 28, (27, 28) | 0.081 | 28, (27, 28) | 28, (27, 28) | 0.046 | 28, (27, 28) | 28, (27, 28) | 0.028 |
| Body mass index (kg/m2) | 22.5, (20.6, 24.7) | 22.0, (20.2, 24.3) | 23.4, (21.7, 25.2) | <0.001 | 22.8, (20.8, 24.9) | 21.1, (18.8, 22.9) | <0.001 | 22.8, (20.8, 24.8) | 22.3, (20.3, 24.5) | 0.056 |
| Education (years) | 12, (12, 16) | 12, (11, 14) | 16, (12, 16) | <0.001 | 12, (12, 16) | 12, (10, 12) | <0.001 | 12, (12, 16) | 12, (10, 14) | <0.001 |
| JST-IC score | 12, (9, 14) | 12, (9, 14) | 12, (10, 14) | 0.009 | 12, (10, 14) | 9, (7, 12) | <0.001 | 13, (11, 14) | 11, (8, 13) | <0.001 |
| MMSE-J score | 29, (28, 30) | 29, (28, 30) | 29, (28, 30) | 0.138 | 29, (28, 30) | 29, (27, 29) | <0.001 | 29, (28, 30) | 29, (27, 30) | <0.001 |
| Serum albumin (g/dL) | 4.2, (4.1, 4.4) | 4.3, (4.1, 4.4) | 4.2, (4.1, 4.4) | 0.073 | 4.3, (4.1, 4.4) | 4.2, (4.0, 4.4) | 0.001 | 4.3, (4.1, 4.4) | 4.2, (4.1, 4.4) | 0.008 |
| Hemoglobin A1c (%) | 5.7, (5.5, 6.0) | 5.7, (5.5, 6.0) | 5.6, (5.4, 6.0) | 0.282 | 5.7, (5.5, 6.0) | 5.6, (5.4, 6.0) | 0.493 | 5.7, (5.5, 6.0) | 5.7, (5.4, 6.0) | 0.635 |
Abbreviations: EAT-10: 10-item Eating Assessment Tool; JST-IC: Japan Science and Technology Agency Index of Competence; MMSE-J: Japanese version of the mini mental state examination; Q1: first quartile; Q3: third quartile. Statistical analysis was performed using the Mann–Whitney U test.
Table 2.
Simple comparison of participant characteristics and categorical variables.
| Overall | Women | Men | Robust | Sarcopenia | Robust | Oral Hypofunction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 878) | (n = 610) | (n = 268) | (n = 715) | (n = 163) | (n = 435) | (n = 443) | |||||
| Category Variables | N, (%) | N, (%) | N, (%) | p-Value | N, (%) | N, (%) | p-Value | N, (%) | N, (%) | p-Value | |
| Variables of oral hypofunction | |||||||||||
| Poor oral hygiene | 254, (28.9) | 154, (25.2) | 100, (37.3) | <0.001 | 217, (30.3) | 37, (22.7) | 0.052 | 87, (20.0) | 167, (37.7) | <0.001 | |
| Oral dryness | 414, (47.2) | 307, (50.3) | 107, (39.9) | 0.004 | 327, (45.7) | 87, (53.4) | 0.078 | 130, (29.9) | 284, (64.1) | <0.001 | |
| Reduced occlusal force | 352, (40.1) | 291, (47.7) | 61, (22.8) | <0.001 | 256, (35.8) | 96, (58.9) | <0.001 | 72, (16.6) | 280, (63.2) | <0.001 | |
| Decreased tongue–lip motor function | 485, (55.2) | 343, (56.2) | 142, (53.0) | 0.373 | 363, (50.8) | 122, (74.8) | <0.001 | 147, (33.8) | 338, (76.3) | <0.001 | |
| Oral diadochokinesis/pa/ | <6 times/s | 236, (26.9) | 179, (29.3) | 57, (21.3) | 0.013 | 167, (23.4) | 69, (42.3) | <0.001 | 65, (14.9) | 171, (38.6) | <0.001 |
| Oral diadochokinesis/ta/ | <6 times/s | 250, (28.5) | 186, (30.5) | 64, (23.9) | 0.046 | 180, (25.2) | 70, (42.9) | <0.001 | 68, (15.6) | 182, (41.1) | <0.001 |
| Oral diadochokinesis/ka/ | <6 times/s | 440, (50.1) | 309, (50.7) | 131, (48.9) | 0.628 | 327, (45.7) | 113, (69.3) | <0.001 | 134, (30.8) | 306, (69.1) | <0.001 |
| Decreased tongue pressure | 285, (32.5) | 216, (35.4) | 69, (25.7) | 0.005 | 193, (27.0) | 92, (56.4) | <0.001 | 62, (14.3) | 223, (50.3) | <0.001 | |
| Decreased masticatory function | 191, (21.8) | 164, (26.9) | 27, (10.1) | <0.001 | 123, (17.2) | 68, (41.7) | <0.001 | 16, (3.7) | 175, (39.5) | <0.001 | |
| Deterioration of swallowing function | 256, (29.2) | 174, (28.5) | 82, (30.6) | 0.534 | 197, (27.6) | 59, (36.2) | 0.028 | 65, (14.9) | 191, (43.1) | <0.001 | |
| Oral hypofunction | 443, (50.5) | 335, (54.9) | 108, (40.3) | <0.001 | 324, (45.3) | 119, (73.0) | <0.001 | - | - | ||
| Variables of sarcopenia | |||||||||||
| Low muscle strength | 215, (24.5) | 174, (28.5) | 41, (15.3) | <0.001 | 77, (10.8) | 138, (84.7) | <0.001 | 64, (14.7) | 151, (34.1) | <0.001 | |
| Low physical performance | 114, (13.0) | 97, (15.9) | 17, (6.3) | <0.001 | 52, (7.3) | 62, (38.0) | <0.001 | 24, (5.5) | 90, (20.3) | <0.001 | |
| Low appendicular skeletal muscle mass | 348, (39.6) | 279, (45.7) | 69, (25.7) | <0.001 | 185, (25.9) | 163, (100.0) | <0.001 | 141, (32.4) | 207, (46.7) | <0.001 | |
| Sarcopenia | Robust | 715, (81.4) | 473, (77.5) | 242, (90.3) | <0.001 | 715, (100.0) | 0, (0) | <0.001 | 391, (89.9) | 324, (73.1) | <0.001 |
| Sarcopenia | 126, (14.4) | 106, (17.4) | 20, (7.5) | 0, (0) | 126, (77.3) | 42, (9.7) | 84, (19.0) | ||||
| Severe sarcopenia | 37, (4.2) | 31, (5.1) | 6, (2.2) | 0, (0) | 37, (22.7) | 2, (0.5) | 35, (7.9) | ||||
| Other recorded variables | |||||||||||
| Sex | Women | 610, (69.5) | - | - | 473, (66.2) | 137, (84.0) | <0.001 | 275, (63.2) | 335, (75.6) | <0.001 | |
| Body mass index | <18.5 | 85, (9.7) | 72, (11.8) | 13, (4.9) | 0.001 | 51, (7.1) | 34, (20.9) | <0.001 | 37, (8.5) | 48, (10.8) | 0.243 |
| Daily drinking habits | 139, (15.8) | 47, (7.7) | 92, (34.3) | <0.001 | 122, (17.1) | 17, (10.4) | 0.036 | 81, (18.6) | 58, (13.1) | 0.025 | |
| Smoking habit | Never smoked | 617, (70.3) | 528, (86.6) | 89, (33.2) | <0.001 | 483, (67.6) | 134, (82.2) | 0.001 | 282, (64.8) | 335, (75.6) | 0.002 |
| Used to smoke | 209, (23.8) | 60, (9.8) | 149, (55.6) | 186, (26.0) | 23, (14.1) | 121, (27.8) | 88, (19.9) | ||||
| Smoking | 52, (5.9) | 22, (3.6) | 30, (11.2) | 46, (6.4) | 6, (3.7) | 32, (7.4) | 20, (4.5) | ||||
| Living situation | Living alone | 266, (30.3) | 224, (36.7) | 42, (15.7) | <0.001 | 202, (28.3) | 64, (39.3) | 0.006 | 119, (27.4) | 147, (33.2) | 0.060 |
| Depression | 159, (18.1) | 124, (20.3) | 35, (13.1) | 0.010 | 111, (15.5) | 48, (29.4) | <0.001 | 49, (11.3) | 110, (24.8) | <0.001 | |
| Stroke | 46, (5.2) | 24, (3.9) | 22, (8.2) | 0.009 | 42, (5.9) | 4, (2.5) | 0.077 | 17, (3.9) | 29, (6.5) | 0.079 | |
| Heart disease | 143, (16.3) | 97, (15.9) | 46, (17.2) | 0.641 | 110, (15.4) | 33, (20.2) | 0.129 | 67, (15.4) | 76, (17.2) | 0.482 | |
| Diabetes | 98, (11.2) | 62, (10.2) | 36, (13.4) | 0.157 | 80, (11.2) | 18, (11.0) | 0.957 | 49, (11.3) | 49, (11.1) | 0.924 | |
| Cancer | 128, (14.6) | 91, (14.9) | 37, (13.8) | 0.667 | 102, (14.3) | 26, (16.0) | 0.582 | 67, (15.4) | 61, (13.8) | 0.493 | |
| Serum albumin | <4.0 g/dL | 94, (10.7) | 66, (10.8) | 28, (10.4) | 0.870 | 60, (8.4) | 34, (20.9) | <0.001 | 38, (8.7) | 56, (12.6) | 0.061 |
| Hemoglobin A1c | <6.0% | 644, (73.3) | 446, (73.1) | 198, (73.9) | 0.069 | 524, (73.3) | 120, (73.6) | 0.958 | 319, (73.3) | 325, (73.4) | 0.959 |
| 6.0–6.4% | 137, (15.6) | 104, (17.0) | 33, (12.3) | 111, (15.5) | 26, (16.0) | 69, (15.9) | 68, (15.3) | ||||
| 6.5%≤ | 97, (11.0) | 60, (9.8) | 37, (13.8) | 80, (11.2) | 17, (10.4) | 47, (10.8) | 50, (11.3) |
Statistical analysis was performed by chi-square test.
3.3. Variables Associated with Sarcopenia
Results of the multivariable logistic regression analysis in which sarcopenia was the dependent variable are shown in Table 3. Findings showed a significant association between oral hypofunction and sarcopenia. Sarcopenia frequency in the oral hypofunction group was significantly elevated (odds ratio: 1.585, 95% confidence interval: 1.019–2.465). In addition, age, BMI, JST-IC score, and serum albumin level were significantly associated with sarcopenia.
Table 3.
Multivariable logistic regression analysis with sarcopenia as the dependent variable.
| 95% Confidence Intervals | |||||
|---|---|---|---|---|---|
| Independent Variables | p-Value | Odds Ratio | Lower Limit | Upper Limit | |
| Oral hypofunction | 0:No, 1:Yes | 0.041 | 1.585 | 1.019 | 2.465 |
| Age | 1-year increments | <0.001 | 1.111 | 1.075 | 1.148 |
| Sex | 0:Women, 1:Men | 0.967 | 0.987 | 0.519 | 1.876 |
| Body mass index | 0:18.5≤, 1:<18.5 | <0.001 | 4.036 | 2.248 | 7.246 |
| Daily drinking habits | 0:No, 1:Yes | 0.746 | 1.114 | 0.579 | 2.146 |
| Smoking habit | 0:Never smoked | 0.732 | reference | ||
| 1:Used to smoke | 0.478 | 0.793 | 0.418 | 1.506 | |
| 2:Smoking | 0.639 | 0.774 | 0.265 | 2.258 | |
| Living situation | 0:Living with someone1:Living alone | 0.733 | 1.078 | 0.701 | 1.658 |
| Depression | 0:No, 1:Yes | 0.653 | 0.894 | 0.550 | 1.455 |
| Education | 1-year increments | 0.083 | 0.933 | 0.863 | 1.009 |
| JST-IC score | 1-score increments | 0.004 | 0.900 | 0.838 | 0.967 |
| MMSE-J score | 1-score increments | 0.567 | 0.975 | 0.893 | 1.064 |
| Stroke | 0:No, 1:Yes | 0.126 | 0.409 | 0.130 | 1.285 |
| Heart disease | 0:No, 1:Yes | 0.493 | 1.196 | 0.716 | 1.998 |
| Diabetes | 0:No, 1:Yes | 0.868 | 1.067 | 0.493 | 2.310 |
| Cancer | 0:No, 1:Yes | 0.873 | 1.045 | 0.607 | 1.801 |
| Serum albumin | 0:4.0 g/dL≤, 1:<4.0 g/dL | 0.013 | 1.995 | 1.157 | 3.439 |
| Hemoglobin A1c | 0:<6.0% | 0.333 | reference | ||
| 1:6.0–6.4% | 0.138 | 0.650 | 0.368 | 1.148 | |
| 2:6.5%≤ | 0.656 | 0.834 | 0.375 | 1.855 | |
| Constant | <0.001 | 0.001 | |||
Abbreviations: JST-IC: Japan Science and Technology Agency Index of Competence; MMSE-J: Japanese version of the mini mental state examination; Q1: first quartile; Q3: third quartile; B: Partial regression coefficient
4. Discussion
In this study, we focused on sarcopenia [11,12] as an outcome of oral hypofunction because malnutrition is a risk factor for its development. As oral hypofunction is a new disease, associated data is limited. If general health deterioration risk due to the accumulation of decreases in oral function in old age is clarified, the knowledge will likely facilitate the implementation of planning measures aiming to extend healthy life expectancy from the perspective of dentistry.
Oral function evaluation may help confirm the effectiveness of treatment, and predict future general-health deterioration [8]. It is also known that decreased oral function is a prodromal symptom of severe diseases including neuromuscular conditions [31,32,33,34,35,36,37,38]. It is clearly important to conduct oral function examinations on a broad range of patients, since they have the potential to incidentally detect severe diseases at an early stage.
A strength of this study is that we analyzed a comprehensive set of oral-health and function variables. To the best of our knowledge, this was the first study to demonstrate that oral hypofunction, diagnosed in strict accordance with criteria published by the JSG, was associated with sarcopenia in community-dwelling older adults. One previous study reported an association between oral hypofunction and sarcopenia [39]. Although it was an important investigation, it should be noted that prior authors did not apply the diagnostic criteria officially proposed by the JSG. Specifically, masticatory ability was based on self-reports, which could have introduced bias.
In our study, the prevalence of oral hypofunction based on diagnostic criteria published by the JSG was 50.5% overall, 40.3% among men, and 54.9% among women. Previous reports have indicated that the prevalence of oral hypofunction among older adults living in different regions of Japan is 43.6–61.6% overall, 39.0–62.6% among men, and 46.9–63.1% among women [10,39,40,41]. The prevalence of oral hypofunction in our study was within the range previously reported among community-dwelling older adults.
The prevalence of sarcopenia in our study population was 18.6% overall, 9.7% among men, and 22.5% among women. The prevalence of sarcopenia in older Asians using AWGS2019 criteria has ranged from 16.4 to 22.8% overall, 11.5 to 21.8% among men, and 16.7 to 23.1% among women [42,43,44]. The prevalence of men in the present study was lower than that of previous reports. This difference suggests that the present population likely included fewer male participants who tend to have reduced muscle mass, strength, and physical performance.
The results of the logistic regression analysis showed that age, BMI, JST-IC representing higher-level functional capacity, and serum albumin level reflecting nutritional and inflammatory status were significantly associated with sarcopenia. Age and BMI reportedly have a clear association with sarcopenia [12]. Low serum albumin levels have also been reported to be low in those with sarcopenia [45,46]. It has also been shown that higher-level functional capacity is associated with sarcopenia [47,48]. Results of the present study are in accordance with prior findings.
This study revealed a significant association between oral hypofunction and sarcopenia, even after adjusting for effects of multiple variables. Numerous studies have shown that decreased oral function leads to poor nutritional status [9,20,49]. It has also been suggested that a decrease in oral function affects the choice of food intake [50]. These reports potentially support a pathway in which the presence of various decreases in parameters of oral function promote the development of sarcopenia by worsening nutritional status by promoting an unbalanced intake of foods and nutrients. It has been reported that both prosthetic treatment and simplified dietary advice during dental treatment improve oral function and nutritional status in older adults [51,52,53]. When an older adult is found to have overlapping decreases in oral functions, addressing each affected oral function parameter and providing simplified dietary advice may help reduce sarcopenia risk. Hironaka et al. reported that a decrease in oral function may not only directly lead to physical function decline, but may also affect the decline in physical functions indirectly via a pathway mediated by declining social functions [9]. In addition to dental treatment and dietary advice, dental professionals may reduce risk of sarcopenia by providing a comprehensive response that includes addressing social function decline.
As this was a cross-sectional study, it was not possible to determine the direction of the association between oral hypofunction and sarcopenia. To date, few longitudinal studies have examined the impact of various oral function parameter decreases on sarcopenia [8]. Longitudinal studies are needed to clarify the temporal relationship between oral hypofunction and sarcopenia.
5. Conclusions
In this study, we found a significant association between oral hypofunction and sarcopenia among community-dwelling older adults. The prevalence of oral hypofunction based on diagnostic criteria published by the JSG in 2016 was 50.5% overall, 40.3% in men, and 54.9% in women. The prevalence of sarcopenia based on diagnostic criteria of AWGS2019 was 18.6% overall, 9.7% in men and 22.5% in women. Multidisciplinary action is needed to establish comprehensive measures for managing the oral health of patients with oral hypofunction, especially as a means to avoid future general-health deterioration.
Acknowledgments
We would like to express our sincere appreciation to all staff members and participants of the study.
Author Contributions
Conceptualization, H.H. and T.U.; methodology, M.I.; validation, Y.W. and S.O.; formal analysis, Y.K.; investigation, K.M., A.E., H.K. (Hisashi Kawai), Y.F., K.I., and H.K. (Hunkyung Kim); data curation, M.S.; writing—original draft preparation, Y.K.; writing—review and editing, Y.K. and Y.O.; supervision, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant numbers: JP15K01469, JP16K01853 and JP20K10297) and Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology, Japan (grant numbers: 28–30 and 29–42).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Tokyo Metropolitan Institute of Gerontology (2006-17, 2011-48, 2018-Zin1, 16).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available because of ethico-legal restrictions imposed by the Ethics Committee of the Tokyo Metropolitan Institute of Gerontology.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cabinet Office, Governmento of Japan . Annual Report on the Aging Society: 2020. Cabinet Office; Tokyo, Japan: 2020. [Google Scholar]
- 2.Minakuchi S., Tsuga K., Ikebe K., Ueda T., Tamura F., Nagao K., Furuya J., Matsuo K., Yamamoto K., Kanazawa M., et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology. 2018;35:317–324. doi: 10.1111/ger.12347. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y., Kitagawa N., Shichita T., Hatanaka Y., Uchida Y. State of Implementation of Examination and Management of Oral Hypofunction Newly Covered by National Health Insurance. Ronen Shika Igaku. 2020;35:230–232. [Google Scholar]
- 4.Matsuo K., Taniguchi H., Nakagawa K., Kanazawa M., Furuya J., Tsuga K., Ikebe K., Ueda T., Tamura F., Nagao K., et al. Relationships between Deterioration of Oral Functions and Nutritional Status in Elderly Patients in an Acute Hospital. Ronen Shika Igaku. 2016;31:123–133. [Google Scholar]
- 5.Suzuki M., Koyama S., Kimura Y., Ishiyama D., Otobe Y., Nishio N., Ichikawa T., Kunieda Y., Ohji S., Ito D., et al. Relationship between characteristics of skeletal muscle and oral function in community-dwelling older women. Arch. Gerontol. Geriatr. 2018;79:171–175. doi: 10.1016/j.archger.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Hatta K., Ikebe K. Association between oral health and sarcopenia: A literature review. J. Prosthodont. Res. 2020 doi: 10.2186/jpr.JPOR_2019_567. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe Y., Okada K., Kondo M., Matsushita T., Nakazawa S., Yamazaki Y. Oral health for achieving longevity. Geriatr. Gerontol. Int. 2020;20:526–538. doi: 10.1111/ggi.13921. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T., Takahashi K., Hirano H., Kikutani T., Watanabe Y., Ohara Y., Furuya H., Tetsuo T., Akishita M., Iijima K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. Ser. A. 2018;73:1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- 9.Hironaka S., Kugimiya Y., Watanabe Y., Motokawa K., Hirano H., Kawai H., Kera T., Kojima M., Fujiwara Y., Ihara K., et al. Association between oral, social, and physical frailty in community-dwelling older adults. Arch. Gerontol. Geriatr. 2020;89:104105. doi: 10.1016/j.archger.2020.104105. [DOI] [PubMed] [Google Scholar]
- 10.Shimazaki Y., Nonoyama T., Tsushita K., Arai H., Matsushita K., Uchibori N. Oral hypofunction and its association with frailty in community-dwelling older people. Geriatr. Gerontol. Int. 2020;20:917–926. doi: 10.1111/ggi.14015. [DOI] [PubMed] [Google Scholar]
- 11.Ganapathy A., Nieves J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients. 2020;12:1755. doi: 10.3390/nu12061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med Dir. Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Kugimiya Y., Watanabe Y., Shirobe M., Motohashi Y., Motokawa K., Edahiro A., Ohara Y., Ryu M., Igarashi K., Hoshino D., et al. A comparison of colorimetric and visual methods for the assessment of masticatory performance with color-changeable chewing gum in older persons. J. Dent. Sci. 2021;16:380–388. doi: 10.1016/j.jds.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu T., Ueda T., Sakurai K. New method for evaluation of tongue-coating status. J. Oral Rehabil. 2007;34:442–447. doi: 10.1111/j.1365-2842.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima Y., Yoda T., Araki R., Sakai T., Toya S., Ito K., Funayama S., Enoki Y., Sato T. Evaluation of oral wetness using an improved moisture-checking device for the diagnosis of dry mouth. Oral Sci. Int. 2017;14:33–36. doi: 10.1016/S1348-8643(17)30017-4. [DOI] [Google Scholar]
- 17.Takano T., Kugimiya Y., Morita K., Tazawa S., Ueda T., Sakurai K. Intra- and inter-investigator reliabilities of oral moisture measured using an oral moisture-checking device. J. Oral Rehabil. 2020;47:480–484. doi: 10.1111/joor.12919. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T., Kumagai H., Watanabe T., Uchida T., Nagao M. Evaluation of complete denture occlusal contacts using pressure-sensitive sheets. Int. J. Prosthodont. 1997;10:386–391. [PubMed] [Google Scholar]
- 19.Hidaka O., Iwasaki M., Saito M., Morimoto T. Influence of clenching intensity on bite force balance, occlusal contact area, and average bite pressure. J. Dent. Res. 1999;78:1336–1344. doi: 10.1177/00220345990780070801. [DOI] [PubMed] [Google Scholar]
- 20.Inomata C., Ikebe K., Kagawa R., Okubo H., Sasaki S., Okada T., Takeshita H., Tada S., Matsuda K., Kurushima Y., et al. Significance of occlusal force for dietary fibre and vitamin intakes in independently living 70-year-old Japanese: From SONIC Study. J. Dent. 2014;42:556–564. doi: 10.1016/j.jdent.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Ito K., Yoshihara A., Takano N., Ishigami K., Seida Y., Inoue M., Kitahara M., Miyazaki H. A Comparison of Methods for the Measurement of Oral Diadochokinesis. Ronen Shika Igaku. 2009;24:48–54. [Google Scholar]
- 22.Tsuga K., Maruyama M., Yoshikawa M., Yoshida M., Akagawa Y. Manometric evaluation of oral function with a hand-held balloon probe. J. Oral Rehabil. 2011;38:680–685. doi: 10.1111/j.1365-2842.2011.02202.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa M., Fukuoka T., Mori T., Hiraoka A., Higa C., Kuroki A., Takeda C., Maruyama M., Yoshida M., Tsuga K. Comparison of the Iowa Oral Performance Instrument and JMS tongue pressure measurement device. J. Dent. Sci. 2021;16:214–219. doi: 10.1016/j.jds.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nokubi T., Yoshimuta Y., Nokubi F., Yasui S., Kusunoki C., Ono T., Maeda Y., Yokota K. Validity and reliability of a visual scoring method for masticatory ability using test gummy jelly. Gerodontology. 2013;30:76–82. doi: 10.1111/j.1741-2358.2012.00647.x. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi K., Watanabe Y., Kugimiya Y., Shirobe M., Edahiro A., Kaneda K., Hasegawa Y., Ito M., Hirano H., Sakurai K., et al. Validity of a visual scoring method using gummy jelly for evaluating chewing efficiency in a large-scale epidemiological survey. J. Oral Rehabil. 2019;46:409–416. doi: 10.1111/joor.12761. [DOI] [PubMed] [Google Scholar]
- 26.Belafsky P.C., Mouadeb D.A., Rees C.J., Pryor J.C., Postma G.N., Allen J., Leonard R.J. Validity and reliability of the Eating Assessment Tool (EAT-10) Ann. Otol. Rhinol. Laryngol. 2008;117:919–924. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi Y., Kitamura A., Seino S., Murayama H., Amano H., Nofuji Y., Nishi M., Yokoyama Y., Shinozaki T., Yokota I., et al. Gait Performance Trajectories and Incident Disabling Dementia Among Community-Dwelling Older Japanese. J. Am. Med. Dir. Assoc. 2017;18:e13–e192. doi: 10.1016/j.jamda.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Iwasa H., Masui Y., Inagaki H., Yoshida Y., Shimada H., Otsuka R., Kikuchi K., Nonaka K., Yoshida H., Yoshida H., et al. Assessing competence at a higher level among older adults: Development of the Japan Science and Technology Agency Index of Competence (JST-IC) Aging Clin. Exp. Res. 2018;30:383–393. doi: 10.1007/s40520-017-0786-8. [DOI] [PubMed] [Google Scholar]
- 29.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma N., Ura C., Miyamae F., Inagaki H., Ito K., Niikawa H., Ijuin M., Okamura T., Sugiyama M., Awata S. Distribution of Mini-Mental State Examination scores among urban community-dwelling older adults in Japan. Int. J. Geriatr. Psychiatry. 2017;32:718–725. doi: 10.1002/gps.4513. [DOI] [PubMed] [Google Scholar]
- 31.Tamburrini A., Tacconi F., Barlattani A., Mineo T.C. An update on myasthenia gravis, challenging disease for the dental profession. J. Oral Sci. 2015;57:161–168. doi: 10.2334/josnusd.57.161. [DOI] [PubMed] [Google Scholar]
- 32.Palmer P.M., Neel A.T., Sprouls G., Morrison L. Swallow characteristics in patients with oculopharyngeal muscular dystrophy. J. Speech Lang. Hear. Res. 2010;53:1567–1578. doi: 10.1044/1092-4388(2010/09-0068). [DOI] [PubMed] [Google Scholar]
- 33.Borgnakke W.S., Anderson P.F., Shannon C., Jivanescu A. Is there a relationship between oral health and diabetic neuropathy? Curr. Diab. Rep. 2015;15:93. doi: 10.1007/s11892-015-0673-7. [DOI] [PubMed] [Google Scholar]
- 34.Bergendal B., McAllister A. Orofacial function and monitoring of oral care in amyotrophic lateral sclerosis. Acta Odontol. Scand. 2017;75:179–185. doi: 10.1080/00016357.2016.1276212. [DOI] [PubMed] [Google Scholar]
- 35.Kouwenberg C.V., Voermans N.C., Quinlivan R., van den Engel-Hoek L. Mastication and Oral Motor Function in McArdle Disease: Patient Reported Complaints. J. Neuromuscul. Dis. 2018;5:353–357. doi: 10.3233/JND-180320. [DOI] [PubMed] [Google Scholar]
- 36.Jeon Y. Fibromyalgia: Practical considerations for oral health care providers. J. Dent. Anesth. Pain Med. 2020;20:263–269. doi: 10.17245/jdapm.2020.20.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi Y.A., Suh D.I., Chae J.H., Shin H.I. Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int. J. Pediatr. Otorhinolaryngol. 2020;130:109818. doi: 10.1016/j.ijporl.2019.109818. [DOI] [PubMed] [Google Scholar]
- 38.Printza A., Boziki M., Triaridis S., Kiousi V., Arnaoutoglou M., Constantinidis J., Grigoriadis N. Tongue strength, dysphagia questionnaire, pharyngeal secretions and FEES findings in dysphagia management in amyotrophic lateral sclerosis. Auris Nasus Larynx. 2020;48:672–682. doi: 10.1016/j.anl.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M., Hamada T., Tanaka A., Nishi K., Kume K., Goto Y., Beppu M., Hijioka H., Higashi Y., Tabata H., et al. Association of Oral Hypofunction with Frailty, Sarcopenia, and Mild Cognitive Impairment: A Cross-Sectional Study of Community-Dwelling Japanese Older Adults. J. Clin. Med. 2021;10:1626. doi: 10.3390/jcm10081626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kugimiya Y., Watanabe Y., Ueda T., Motokawa K., Shirobe M., Igarashi K., Hoshino D., Takano T., Sakurai K., Taniguchi Y., et al. Rate of oral frailty and oral hypofunction in rural community-dwelling older Japanese individuals. Gerodontology. 2020;37:342–352. doi: 10.1111/ger.12468. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo K., Kito N., Ogawa K., Izumi A., Kishima M., Itoda M., Masuda Y. Improvement of oral hypofunction by a comprehensive oral and physical exercise programme including textured lunch gatherings. J. Oral Rehabil. 2020;48:411–421. doi: 10.1111/joor.13122. [DOI] [PubMed] [Google Scholar]
- 42.Liu X., Hou L., Zhao W., Xia X., Hu F., Zhang G., Hao Q., Zhou L., Liu Y., Ge M., et al. The Comparison of Sarcopenia Diagnostic Criteria using AWGS 2019 with the Other Five Criteria in West China. Gerontology. 2021;67:290–300. doi: 10.1159/000513247. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., He F., Gu X., Chen S., Tong Z., Zhong S. Estimation of sarcopenia prevalence in individuals at different ages from Zheijang province in China. Aging. 2021;13:6066. doi: 10.18632/aging.202567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitamura A., Seino S., Abe T., Nofuji Y., Yokoyama Y., Amano H., Nishi M., Taniguchi Y., Narita M., Fujiwara Y., et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle. 2021;12:30–38. doi: 10.1002/jcsm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Can B., Kara O., Kizilarslanoglu M.C., Arik G., Aycicek G.S., Sumer F., Civelek R., Demirtas C., Ulger Z. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin. Exp. Res. 2017;29:745–752. doi: 10.1007/s40520-016-0626-2. [DOI] [PubMed] [Google Scholar]
- 46.Fukuoka Y., Narita T., Fujita H., Morii T., Sato T., Sassa M.H., Yamada Y. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J. Diabetes Investig. 2019;10:322–330. doi: 10.1111/jdi.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanimoto Y., Watanabe M., Sun W., Sugiura Y., Tsuda Y., Kimura M., Hayashida I., Kusabiraki T., Kono K. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch. Gerontol. Geriatr. 2012;55:e9–e13. doi: 10.1016/j.archger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Wang D.X.M., Yao J., Zirek Y., Reijnierse E.M., Maier A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle. 2020;11:3–25. doi: 10.1002/jcsm.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwasaki M., Motokawa K., Watanabe Y., Shirobe M., Inagaki H., Edahiro A., Ohara Y., Hirano H., Shinkai S., Awata S. A Two-Year Longitudinal Study of the Association between Oral Frailty and Deteriorating Nutritional Status among Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health. 2020;18:213. doi: 10.3390/ijerph18010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoshino D., Hirano H., Edahiro A., Motokawa K., Shirobe M., Watanabe Y., Motohashi Y., Ohara Y., Iwasaki M., Maruoka Y., et al. Association between Oral Frailty and Dietary Variety among Community-Dwelling Older Persons: A Cross-Sectional Study. J. Nutr. Health Aging. 2021;25:361–368. doi: 10.1007/s12603-020-1538-6. [DOI] [PubMed] [Google Scholar]
- 51.Amagai N., Komagamine Y., Kanazawa M., Iwaki M., Jo A., Suzuki H., Minakuchi S. The effect of prosthetic rehabilitation and simple dietary counseling on food intake and oral health related quality of life among the edentulous individuals: A randomized controlled trial. J. Dent. 2017;65:89–94. doi: 10.1016/j.jdent.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki H., Kanazawa M., Komagamine Y., Iwaki M., Jo A., Amagai N., Minakuchi S. The effect of new complete denture fabrication and simplified dietary advice on nutrient intake and masticatory function of edentulous elderly: A randomized-controlled trial. Clin. Nutr. 2018;37:1441–1447. doi: 10.1016/j.clnu.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Kanazawa M., Suzuki H., Komagamine Y., Iwaki M., Amagai N., Minakuchi S. Combined effects of new complete denture fabrication and simplified dietary advice on nutrient intake in edentulous elderly patients for 6 months. Clin. Oral Investig. 2019;23:2245–2252. doi: 10.1007/s00784-018-2669-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available because of ethico-legal restrictions imposed by the Ethics Committee of the Tokyo Metropolitan Institute of Gerontology.