Abstract

A basic understanding of the high-temperature pyrolysis process of jet fuels is not only valuable for the development of combustion kinetic models but also critical to the design of advanced aeroengines. The development and utilization of alternative jet fuels are of crucial importance in both military and civil aviation. A direct coal liquefaction (DCL) derived liquid fuel is an important alternative jet fuel, yet fundamental pyrolysis studies on this category of jet fuels are lacking. In the present work, high-temperature pyrolysis studies on a DCL-derived jet fuel and its blend with the traditional RP-3 jet fuel are carried out by using a single-pulse shock tube (SPST) facility. The SPST experiments are performed at averaged pressures of 5.0 and 10.0 bar in the temperature range around 900–1800 K for 0.05% fuel diluted by argon. Major intermediates are obtained and quantified using gas chromatography analysis. A flame-ionization detector and a thermal conductivity detector are used for species identification and quantification. Ethylene is the most abundant product for the two fuels in the pyrolysis process. Other important intermediates such as methane, ethane, propyne, acetylene, and 1,3-butadiene are also identified and quantified. The pyrolysis product distributions of the pure RP-3 jet fuel are also performed. Kinetic modeling is performed by using a modern detailed mechanism for the DCL-derived jet fuel and its blends with the RP-3 jet fuel. Rate-of-production analysis and sensitivity analysis are conducted to compare the differences of the chemical kinetics of the pyrolysis process of the two jet fuels. The present work is not only valuable for the validation and development of detailed combustion mechanisms for alternative jet fuels but also improves our understanding of the pyrolysis characteristics of alternative jet fuels.
1. Introduction
Alternative jet fuels are of significant importance to meet the needs from both military and civil aviation energy goals. A series of alternative jet fuels including the Fischer–Tropsch (FT) synthetic paraffinic kerosene,1 hydroprocessed renewable jet fuels derived from camelina and tallow,2 alcohol-to-jet fuels,3 hydrorefined algal oil,4 and gas-to-liquid FT synthetic kerosene5 are being considered. One of the essential properties for alternative jet fuels is the requirement of high energy density to ensure the range and payload of volume-limited air crafts.6 For this purpose, highly strained multi-cyclic hydrocarbons with high energy density have been developed and studied, such as the RP-2 and JP-10 jet fuels employed in rocket and military aeroengines.7 To achieve higher energy density alternative kerosene, many researchers have made great efforts to synthesize energy-dense jet fuels containing polycyclic hydrocarbons using different methods from different feedstocks.6,8 For example, high energy density fuels such as 1,1,3-trimethylcyclohexane, dimers of isophorone, substituted cyclohexanes, and polycyclic alkanes were produced from isophorone, which was a promising feedstock.8−11 The synthesized polycyclic alkanes usually exhibit higher energy density compared with the traditional jet fuels and are being considered for usage in aeroengines.
Besides the traditional synthesis method for the development of alternative high energy density jet fuels consisting of multi-cyclic hydrocarbons, the direct coal liquefaction (DCL) process for the production of liquid fuels reveals natural advantages due to the large number of aromatic hydrocarbons in coal molecules, which can be directly converted to multi-cyclic hydrocarbons from the high-pressure hydrogenation in the DCL process.12 However, unlike the FT synthetic process that has been commercialized for a long time and employed in a wide number of countries to produce liquid jet fuels,13 the large-scale commercial production of jet fuels from the DCL process via high-pressure hydrogenation is very resource-consuming and technologically difficult.12 Even though, the million-ton DCL production plant for liquid jet fuels has been constructed with the progress in high-efficiency catalysts and process optimization in recent years for both strategic and environmental reasons.12,14,15
The usage of the DCL-derived jet fuel for aviation applications requires a basic understanding of their corresponding physical properties and combustion properties including ignition, extinction, pyrolysis, heat release, and pollution formation.16 As demonstrated previously, the major components in DCL fuels are cycloalkanes with two or three rings due to the special molecular structure characteristics of coal.12 There have been extensive experimental and kinetic modeling studies on pyrolysis and combustion properties of monocyclic alkanes such as cyclohexane,17−19 methylcyclohexane,20,21 ethyl cyclohexane,22 n-propyl cyclohexane,23 and n-butyl cyclohexane24 due to their large amount of existence in traditional gasoline and jet fuels. However, very few studies have been reported on the real DCL-derived jet fuels besides some studies on representative polycyclic alkanes including decalin,25−27 tetralin,28,29 and JP-10.30−32 In addition, the current aviation fuel testing, approval, and airworthiness certification processes limit the usage of the DCL-derived jet fuel as the single fuel in current aeroengines, and the DCL-derived jet fuel should also be used by blending with traditional jet fuels.33,34 Hence, experimental and kinetic modeling studies on the DCL-derived jet fuel and its blends with the traditional jet fuel are one of the key procedures toward practical application of the DCL-derived jet fuel. Previously, Yang et al. studied the high-temperature ignition properties of the DCL-derived jet fuel and its blend with the traditional RP-3 jet fuel.35 The experimental and modeling study results indicate that the species profiles of small hydrocarbon compounds during the oxidation of the DCL-derived jet fuel and RP-3 show very different phenomena, even though the high-temperature ignition properties between the two different fuels are similar.35 Thus, pyrolysis studies of DCL-derived jet fuel are necessary to obtain a better understanding of its combustion properties and to develop more predictable combustion kinetic mechanisms, that is, within the HyChem framework.31,36,37
Besides the use of the DCL-derived jet fuel for civil aviation, pyrolysis of alternative jet fuels with high energy density is of crucial importance in the development of advanced hypersonic aircrafts because the jet fuel can be used to relieve the great heat load via the endothermic pyrolysis process.38−41 Thus, extensive experimental and modeling studies have been performed to investigate the pyrolysis of traditional jet fuels including RP-3, JP-10, and Jet-A.31,42−44 A jet-stirred reactor and a flow reactor are widely used experimental facilities for the study of fuel pyrolysis process under low-temperature conditions.20,45,46 However, most of the interested pyrolysis kinetics and product distributions are under high-temperature conditions, which requires the design of novel experimental facilities. One of the efficient approaches is the use of shock tube apparatus in combination with advanced laser absorption to measure the pyrolysis speciation.31,47,48 However, such analytical instruments are usually species dependent and expensive. In contrast, the single-pulse shock tube (SPST) has been implemented by several groups in recent years for fuel pyrolysis studies due to the simplicity in post-shock sampling and analysis.3,42,49,50 A series of fuels from small hydrocarbons to complex real fuels were investigated via the SPST experimental method.42,44,49 However, there is still a significant lack of understanding of pyrolysis characteristics of polycyclic alkanes and DCL-derived jet fuels.
Based on the above considerations, fundamental pyrolysis studies of DCL-derived jet fuels together with their blend with traditional jet fuels are required to develop predictable detailed combustion kinetic models and to promote the fuel approval and airworthiness certification processes. Toward this end, the major aim of this work is to provide such fundamental data, evaluate modern detailed combustion kinetics mechanisms, and identify reaction pathways to improve our understanding of jet fuel combustion chemistry. To do so, the pyrolysis characteristics of the DCL-derived jet fuel and its 50/50 blend with the RP-3 jet fuel in volume are studied by using the SPST experimental facility and kinetic modeling. The paper is organized as follows. First, the experimental conditions investigated and the experimental setups used are presented. The experimental results are then described in detail and compared with modern detailed kinetic mechanisms from the literature. The pyrolysis characteristics of the DCL-derived jet fuel are also compared with pure traditional jet fuels. Finally, kinetic model analysis including reaction pathway analysis and sensitivity analysis is performed using detailed combustion kinetic mechanisms, and implications for future studies are recommended.
2. Experimental Methods
2.1. NUC-SPST Facility
Pyrolysis experiments are performed using the SPST at the North University of China (NUC), which is composed of a 1.5 m driver section and a 3.05 m driven section with an inner diameter of 44 mm. The details of the experimental facility and procedures have been described previously.44 Briefly, the driver and driven sections are separated via a polycarbonate diaphragm. A pressure vessel named a dump tank in SPST is used to consume the reflected shock waves and to ensure the reaction mixture solely under heated condition. The incident shock velocity is measured using four PCB 113B21 piezoelectric pressure transducers mounted on the sidewall of the driven section. The pressure–time profiles are measured by a Kistler 603CBA piezoelectric pressure transducer located at the end of the driven section. All pressure traces are recorded via two digital TiePie Handyscope HS4 oscilloscopes. The reflected shock wave pressure (P5) and temperature (T5) are determined using the one-dimensional normal shock relations employed by the program Gaseq51 from the initial temperature/pressure, the measured incident shock velocity, and the thermodynamic properties of the reaction mixtures. The pyrolysis time is defined as the time interval between the arrival of the reflected shock wave and the 80% of the pressure signal recorded by the Kistler pressure sensor. The shock-heated products are sampled from the end wall using a solenoid valve protruding 10 mm into the SPST and are analyzed using an Agilent 7820A gas chromatograph. A flame-ionization detector and a thermal conductivity detector are used for reaction products.
The reaction mixture is prepared in stainless steel mixture tanks according to Dalton’s law of partial pressure and is maintained for at least 12 h before experiments to ensure complete vaporization and homogeneity. A heating system with seven thermocouples placed along the mixing tank, a shock tube, and a sampling tube is used to maintain the experimental system with a temperature of 398 K to avoid adsorption of the liquid jet fuel. The jet fuel is provided by the Aviation Fuel and Chemical Airworthiness Certification Centre of CAAC, and the purity is larger than 99.9%. The purities of Ar and Kr are 99.99%. Helium is used as a driver gas in the SPST, and the purity is also 99.99%. Kr is used as an internal standard gas, and the system is calibrated using a 16 gas GC standard obtained from Beijing Haipubeifen gas Ltd China. The calibrated standard is used to calculate the concentration of the pyrolytic products, while the effective carbon number method is used to estimate the concentrations of species with no calibration standard. Table 1 lists the detailed experimental conditions, and the pyrolysis of 0.05% fuel diluted by Ar at about 5 and 10 bar is investigated. The high-diluted experimental condition is adopted to equalize the temperature because the jet fuel pyrolysis process is endothermic.
Table 1. Pyrolysis Experimental Conditions in This Work.
| fuel | XFuel (mol %) | XAr (mol %) | XKr (mol %) | Avg. P5 (bar) | T5 range (K) | Avg. reaction time (ms) |
|---|---|---|---|---|---|---|
| DCL-derived jet fuel | 0.05 | 99.45 | 0.5 | 4.8 | 1000–1700 | 1.65 |
| 0.05 | 99.45 | 0.5 | 10.2 | 1060–1600 | 1.73 | |
| 50/50 blend of DCL-derived and RP-3 jet fuels | 0.05 | 99.45 | 0.5 | 5.1 | 1200–1750 | 1.73 |
| 0.05 | 99.45 | 0.5 | 10.0 | 1080–1600 | 1.72 |
2.2. Experimental Uncertainty
The uncertainty in reflected temperatures is mainly induced by the uncertainty in the shock attenuation and non-ideal shock reflection from the interactions between the shock wave and the boundary layer. Using a standard error analysis procedure, the experimental temperature uncertainty is approximately ±2% based on the analysis by Petersen et al.52 For the uncertainty of reaction time, several test experiments under different temperature conditions during the SPST debugging period are performed, and each experiment is repeated three times to measure the averaged reaction time as described previously.44 The results show that the uncertainty is less than 5% for all the experiments. Thus, the uncertainty in the reaction time is ±5%. For the measured species concentrations, the uncertainty in the calibrated species concentration using repetitive sampling of the standard gas is approximately ±10%, and the uncertainty in the estimated species concentration using the effective carbon number method is approximately ±20%.53,54 It has been previously confirmed that the carbon balance mainly relies on the absorption in the mixture tank, shock tube, sampling tube, and the GC analysis method.44,49 Previous studies using the current facilities reveal that the experimental system is accurate to describe the carbon balance within 15% unceratinty.44,49 Therefore, the uncertainty of the measured properties in this work are generally consistent with the other related facilities and fuel pyrolysis studies.49,53
3. Kinetic Modeling
Kinetic modeling is performed by using Cantera software55 assuming a closed homogeneous batch reactor at a constant volume. The residence/reaction time approach is adopted to simulate the SPST results because it is simple, and the modeling results show no significant differences compared with the method based on the recorded pressure profiles.56 The three-component surrogate model composed of 9% n-dodecane, 35% decalin, and 56% n-pentylcyclohexane in mole fraction is used to mimic the DCL-derived jet fuel, while the four-component surrogate model composed of 25.6% n-dodecane, 21.4% decalin, 49% n-pentylcyclohexane, and 4% n-propylbenzene is used for the 50/50 blend jet fuel.35 The used surrogate models are based on previous detailed analysis on the measured physical properties, that is, density, H/C ratio, lower heating values, and molecular compositions of the real jet fuels.35
4. Results and Discussion
4.1. Experimental and Modeling Results of the DCL Jet Fuel
Figure 1 shows the major species profiles as a function of temperature for 0.05% DCL-derived jet fuel pyrolysis experiment at pressures of 5 and 10 bar together with the kinetic modeling results. It can be seen that the kinetic modeling results using the detailed combustion mechanism can well capture most of the product distributions along the temperature profiles except that large deviations exist for ethylene. A similar reactivity trend was also found for the RP-3 jet fuel.44 One of the major reasons may be due to the overemphasized importance of ethylene in the high pyrolysis process of large hydrocarbons from the successive β-scission reactions.37 In addition, the current detailed mechanism taking no account of the formation of large polycyclic aromatic hydrocarbons probably may further aggravate the production yield of ethylene since the formation of ethylene is the most important reaction path. The pressure change does not affect the variation tendencies of all the detected products; however, the absolute yields of the products are influenced. From Figure 1, the pressure mainly affects the yields for methane (CH4) and ethane (C2H6), while the effect on propene (C3H6) and 1,3-butadiene are small because the formation of these alkene products is mainly through β-scission reactions, which is more affected by temperature. The product yields of CH4 and C2H6 exhibit an opposite trend with pressure probably induced by the competition relationship between the two pressure-dependent reactions, that is, CH3 + H(+M) = CH4(+M) and CH3 + CH3(+M) = C2H6(+M) that controls the transformation between CH4 and C2H6. Under high-temperature conditions, both the experimental and kinetic modeling results indicate that the pressure can also affect the product yields of ethylene, acetylene. One of the major reasons for this phenomenon can be attributed to the mutual transformation among ethane, ethylene, acetylene, and the other small molecules through high-temperature pressure-dependent pyrolysis reactions as revealed by Zeng et al. via rate-of-production (ROP) analysis.44 To further check the pressure effect on the pyrolysis process, we perform additional kinetic modeling studies for the DCL-derived jet fuel at the same conditions with a pressure of 20 bar, and the results compared with 5 and 10 bar are provided as the Supporting Information. Similar reactivity can be observed as shown in Figure 1.
Figure 1.

Species profiles as a function of temperature for 0.05% DCL jet fuel pyrolysis experiment at 5 and 10 bar together with kinetic modeling results. The black square symbols and the red triangle symbols represent the experimental results, while the solid and dashed lines represent the kinetic modeling results at 5 and 10 bar, respectively.
From the formation of major products, the pyrolysis process of the DCL-derived jet fuel under the studied conditions starts above 1100 K. C2H4 is the most abundant product for the studied two experimental conditions, which is the same as previously studied other jet fuels.31,42,44,46,48 It is worth noting that the quantity of acetylene (C2H2) increases significantly as the temperature increases mainly due to the high-temperature cracking process of C2H4, 1,3-butadiene (C4H6), and so on.44,57,58 CH4 is another major product, and its yield gradually increases as the temperature increases. The yield of CH4 reaches a maximum value around 1600 K for both the studied experimental conditions. However, the consumption of CH4 is not detected under the studied temperatures due to the stable structure of CH4. The variation tendencies as a function of temperature for C3H6, C2H6, allene (a-C3H4), propyne (p-C3H4), and C4H6 are similar, and the yields of these products first increase to maximum values as the temperature increases approximately from 1100 K to values around 1300–1400 K and then decrease significantly as the temperature continues to increase, indicating that these products are unstable at higher temperatures.
4.2. Experimental and Modeling Results of the 50/50 Blend Jet Fuel
The experimental and kinetic modeling results for pyrolysis of the 50/50 blend of DCL-derived and RP-3 jet fuels with a concentration of 0.05% diluted by Ar at 5 and 10 bar are explicitly shown in Figure 2. It can be seen that the variation tendencies of the pyrolysis products as a function of temperature are very similar compared with that of the DCL-derived jet fuel. The pressure effect on the pyrolysis product distributions is also similar. For the 50/50 blend jet fuel, the yield of C2H4 is slightly lower than that of the DCL-derived jet fuel, which could correspond with a lower yield of C2H2. The pyrolysis product yields of C3H6 and C2H6 are close to that of the DCL-derived jet fuel, and small differences for a-C3H4 and (p-C3H4) are observed between the DCL-derived and blend jet fuels. It should be noted that the yield of C4H6 from the DCL-derived jet fuel is larger than that from the 50/50 blend fuel. The experimental observations are in accordance with previous ROP analysis during the ignition studies on RP-3 and DCL-derived jet fuels.35
Figure 2.
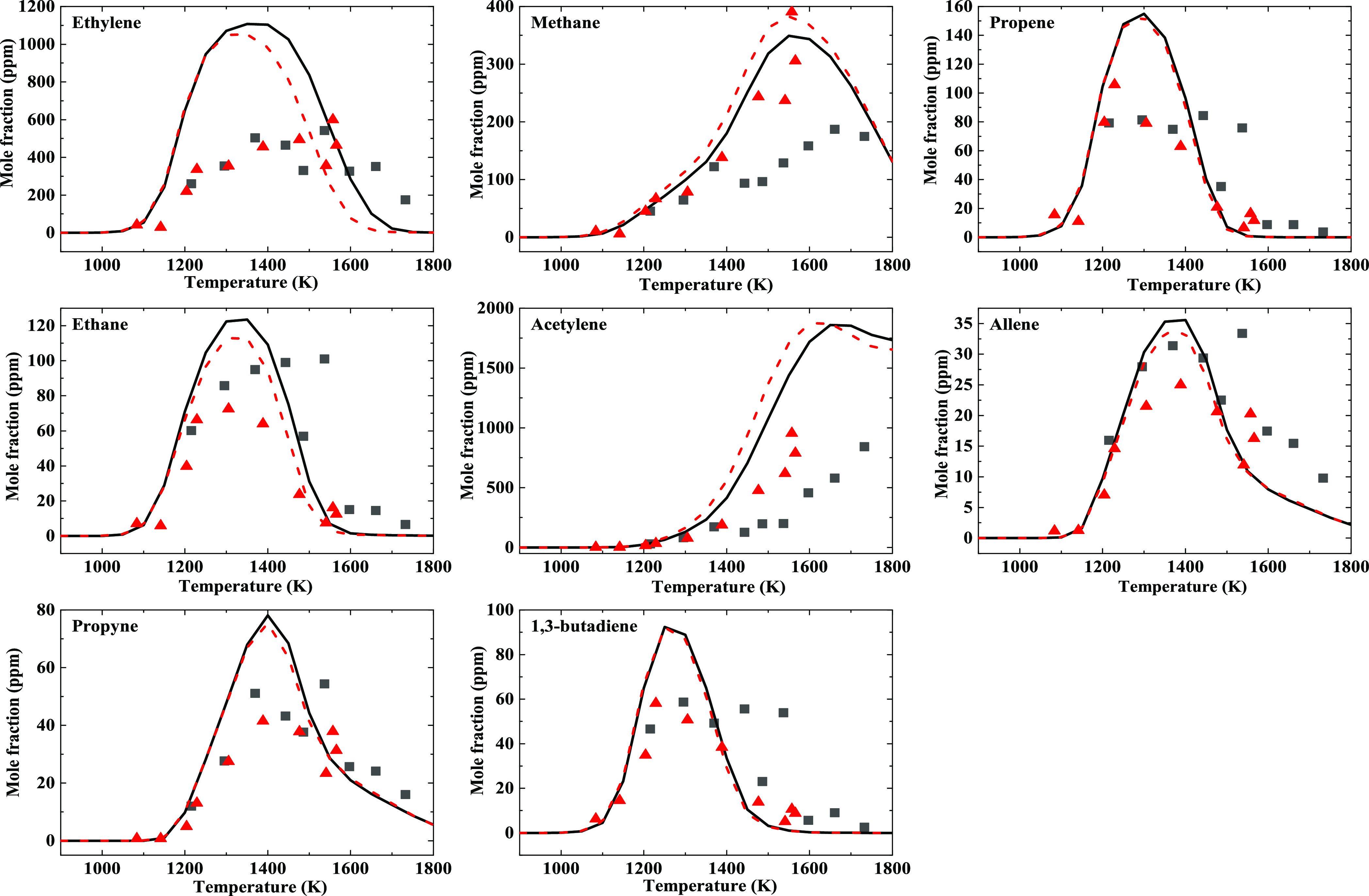
Species profiles as a function of temperature for 0.05% 50/50 blend jet fuel pyrolysis experiment at 5 and 10 bar together with kinetic modeling results. The black square symbols and the red triangle symbols represent the experimental results, while the solid and dashed lines represent the kinetic modeling results at 5 and 10 bar, respectively.
4.3. Comparison with RP-3 Jet Fuel Pyrolysis
Figure 3 shows comparison of the major species profiles as a function of temperature for RP-3 fuel, DCL-derived jet fuel, and their 50/50 blend fuel with a concentration of 0.05% fuel diluted by Ar at 5 bar. However, it is worth noting that the absolute production yield is also affected by the real molecular formula, which is still unknown accurately. Hence, the major purpose of Figure 3 is to demonstrate the product distribution tendencies and the major product differences among the three jet fuels. It can be seen that the product yields of ethylene, methane, ethane, and propene from RP-3 pyrolysis are generally larger than that from the DCL-derived jet fuel and the 50/50 blend fuel. The product distributions of the blend fuel are much prone to exhibit similar tendencies as the DCL-derived jet fuel. Another notable difference is that the acetylene yield from the blend fuel is lower than the other two jet fuels, indicating that the blend jet fuel may have a low soot tendency because acetylene is the key precursor toward soot formation. Figure 3 also explicitly shows that the formation of 1,3-butadiene is larger than the other two fuels, probably due to the large existence of two- and three-ring cycloalkane compounds since the decompositions of these compound tend to form larger alkene molecules.28,29,59
Figure 3.

Comparisons of major species profiles for RP-3, DCL-derived jet fuel, and their 50/50 blend fuel with a concentration of 0.05% fuel diluted by Ar at 5 bar.
4.4. ROP and Sensitivity Analysis
ROP analysis has been conducted for 0.05% fuel concentration diluted by Ar at T = 1400 K and 10 bar with the reaction time at 1.70 ms for the three jet fuels, respectively. At the defined time, the jet fuels are in fact completely consumed, and the ROP analysis reveals the major reactions toward the formation of these pyrolysis products. The ROP analysis results for the three fuels reveal that the dominant reactions controlling the consumption and formation of the measured ethylene, methane, ethane, acetylene, and C3 species including allene, propyne, and propene are directly related to the mutual transformation reactions involving C0–C3 species, which is the same as discussed in previous studies.44 The major difference lies in the formation and consumption reactions of 1,3-butadiene, which is explicitly shown in Figure 4. The most important consumption reaction of 1,3-butadiene formed from the three jet fuels is the same, that is, C4H6 + H = C2H4 + C2H3. However, the dominant reactions related to the formation of 1,3-butadiene are different between RP-3 and DCL-derived jet fuels. The decomposition reaction of cyclohexene (cC6H10) that can be formed easily due to the large number of cycloalkanes in the DCL-derived jet fuel is the most important reaction for the formation of 1,3-butadiene. For RP-3, the consumption and formation reactions of 1,3-butadiene are mostly directly related to the C2–C4 species.
Figure 4.

Relative contributions to the formation and consumption of 1,3-butadiene of the three jet fuels. The species cC6H10, SAXC6H11, and SAXC4H7 denotes cyclohexene, 1-hexen-3-yl, and 1-butene-3-yl, respectively. Other small molecules can be found in the detailed mechanism.
To further identify the differences of the chemical kinetics between RP-3 and DCL-derived jet fuels, sensitivity analysis is performed to identify important reactions that affect the yields of major products including ethylene, methane, acetylene, and 1,3-butadiene. The results are shown in Figure 5. It is shown that the most sensitive reactions for methane are identical for the two fuels. However, slight differences are found for ethylene and acetylene. Specifically, the reaction C4H6 + H = C2H4 + C2H3 exhibits large sensitivity factors of acetylene and ethylene for the DCL-derived jet fuel due to the large production yield of C4H6. From Figure 5, it is obvious that the cycloalkane compounds existing in the DCL-derived jet fuel show significant effect on the formation of 1,3-butadiene. The abstraction from decalin and the decomposition reaction of the C2H3cC6H11 radical show large positive effect on the formation of 1,3-butadiene. The sensitivity analysis results are in good accordance with that from ROP analysis, and the results indicate that future work on the development of accurate surrogate models of DCL-derived jet fuel and the optimization of combustion mechanism of 1,3-butadiene60,61 are critical in the study of combustion properties of the DCL-derived jet fuel.
Figure 5.
Important sensitive reactions for the major products including ethylene, methane, acetylene, and 1,3-butadiene from pyrolysis of DCL-derived jet fuel (a) and RP-3 jet fuels (b). Sensitivity analysis is performed for 0.05% fuel concentration at T = 1400 K and 10 bar with a reaction time of 1.70 ms. RDECALIN-3 and PXC6H13 denote 3-decalyl and 1-hexyl radicals, respectively.
5. Conclusions
The practical use and airworthiness certification process of the alternative jet fuel requires a basic understanding of its combustion properties. The pyrolysis of the alternative jet fuel is not only crucial in the development of their combustion kinetic models but also plays an important role in the development of advanced hypersonic aircrafts. For this purpose, this work reports the first experimental and kinetic modeling study on the pyrolysis of an alternative jet fuel from the DCL process and its 50/50 blend with the traditional RP-3 jet fuel. The SPST facility is employed to perform the pyrolysis experiments for 0.05% fuel concentration diluted in Ar at averaged pressures of 5.0 and 10.0 bar in the temperature range around 900–1800 K with the reaction time around 1.70 ms. The major products including ethylene, methane, acetylene, propene, allene, propyne, and 1,3-butadiene are detected and quantified. Ethylene is the most abundant product, and acetylene significantly increases as the temperature increases. The pyrolysis characteristics of the DCL-derived jet fuel are systematically compared with that of the traditional RP-3 jet fuel. ROP analysis and sensitivity analysis are conducted to identify the important reactions related to the pyrolysis process of the three jet fuels. It is shown that the formation of 1,3-butadiene is the major difference between RP-3 and the DCL-derived jet fuel. Future work on the development of an accurate surrogate model to mimic the real molecular compositions of the DCL-derived jet fuel and optimization of the immature 1,3-butadiene combustion mechanism should be valuable for the study of DCL-derived jet fuels.
Acknowledgments
J.L. acknowledges foundations from the International Scientific Cooperation Projects of Key R&D Programs in Shanxi Province (no. 201803D421101), Research Project Supported by Shanxi Scholarship Council of China (no. 2020-115), and The Young Academic Leaders Support Program of North University of China (no. QX201810); Q.-D. Wang acknowledges the financial support by the Fundamental Research Funds for the Central Universities of China (no. 2020ZDPYMS05).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02530.
Experimental results of jet fuel pyrolysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Naik C. V.; Puduppakkam K. V.; Modak A.; Meeks E.; Wang Y. L.; Feng Q.; Tsotsis T. T. Detailed chemical kinetic mechanism for surrogates of alternative jet fuels. Combust. Flame 2011, 158, 434–445. 10.1016/j.combustflame.2010.09.016. [DOI] [Google Scholar]
- Valco D.; Gentz G.; Allen C.; Colket M.; Edwards T.; Gowdagiri S.; Oehlschlaeger M. A.; Toulson E.; Lee T. Autoignition behavior of synthetic alternative jet fuels: An examination of chemical composition effects on ignition delays at low to intermediate temperatures. Proc. Combust. Inst. 2015, 35, 2983–2991. 10.1016/j.proci.2014.05.145. [DOI] [Google Scholar]
- Guzman J.; Kukkadapu G.; Brezinsky K.; Westbrook C. Experimental and modeling study of the pyrolysis and oxidation of an iso-paraffinic alcohol-to-jet fuel. Combust. Flame 2019, 201, 57–64. 10.1016/j.combustflame.2018.12.013. [DOI] [Google Scholar]
- Zhu Y.; Li S.; Davidson D. F.; Hanson R. K. Ignition delay times of conventional and alternative fuels behind reflected shock waves. Proc. Combust. Inst. 2015, 35, 241–248. 10.1016/j.proci.2014.05.034. [DOI] [Google Scholar]
- Dagaut P.; Karsenty F.; Dayma G.; Diévart P.; Hadj-Ali K.; Mzé-Ahmed A.; Braun-Unkhoff M.; Herzler J.; Kathrotia T.; Kick T.; Naumann C.; Riedel U.; Thomas L. Experimental and detailed kinetic model for the oxidation of a Gas to Liquid (GtL) jet fuel. Combust. Flame 2014, 161, 835–847. 10.1016/j.combustflame.2013.08.015. [DOI] [Google Scholar]
- Zhang X.; Pan L.; Wang L.; Zou J.-J. Review on synthesis and properties of high-energy-density liquid fuels: Hydrocarbons, nanofluids and energetic ionic liquids. Chem. Eng. Sci. 2018, 180, 95–125. 10.1016/j.ces.2017.11.044. [DOI] [Google Scholar]
- Colket M.; Heyne J.; Rumizen M.; Gupta M.; Edwards T.; Roquemore W. M.; Andac G.; Boehm R.; Lovett J.; Williams R.; Condevaux J.; Turner D.; Rizk N.; Tishkoff J.; Li C.; Moder J.; Friend D.; Sankaran V. Overview of the national jet fuels combustion program. AIAA J. 2017, 55, 1087–1104. 10.2514/1.j055361. [DOI] [Google Scholar]
- Chung H. S.; Chen C. S. H.; Kremer R. A.; Boulton J. R.; Burdette G. W. Recent developments in high-energy density liquid hydrocarbon fuels. Energy Fuels 1999, 13, 641–649. 10.1021/ef980195k. [DOI] [Google Scholar]
- Wang W.; Liu Y.; Li N.; Li G.; Wang W.; Wang A.; Wang X.; Zhang T. Synthesis of renewable high-density fuel with isophorone. Sci. Rep. 2017, 7, 6111. 10.1038/s41598-017-06556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.; Zhang L.; Zhang X.; Han P.; Xie J.; Pan L.; Zou D.-R.; Liu S.-H.; Zou J.-J. Synthesis of high-density and low-freezing-point jet fuel using lignocellulose-derived isophorone and furanic aldehydes. Sustainable Energy Fuels 2018, 2, 1863–1869. 10.1039/c8se00197a. [DOI] [Google Scholar]
- Ryan C. F.; Moore C. M.; Leal J. H.; Semelsberger T. A.; Banh J. K.; Zhu J.; McEnally C. S.; Pfefferle L. D.; Sutton A. D. Synthesis of aviation fuel from bio-derived isophorone. Sustainable Energy Fuels 2020, 4, 1088–1092. 10.1039/c9se01014a. [DOI] [Google Scholar]
- Mochida I.; Okuma O.; Yoon S.-H. Chemicals from Direct Coal Liquefaction. Chem. Rev. 2014, 114, 1637–1672. 10.1021/cr4002885. [DOI] [PubMed] [Google Scholar]
- Khodakov A. Y.; Chu W.; Fongarland P. Advances in the Development of Novel Cobalt Fischer–Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. 10.1021/cr050972v. [DOI] [PubMed] [Google Scholar]
- Liu W.; Wang J.; Bhattacharyya D.; Jiang Y.; DeVallance D. Economic and environmental analyses of coal and biomass to liquid fuels. Energy 2017, 141, 76–86. 10.1016/j.energy.2017.09.047. [DOI] [Google Scholar]
- Huang Y.; Rolfe A.; Rezvani S.; Herrador J. M. H.; Franco F.; Pinto F.; Snape C.; Hewitt N. Converting brown coal to synthetic liquid fuels through direct coal liquefaction technology: Techno-economic evaluation. Int. J. Energy Res. 2020, 44, 11827–11839. 10.1002/er.5823. [DOI] [Google Scholar]
- Curran H. J. Developing detailed chemical kinetic mechanisms for fuel combustion. Proc. Combust. Inst. 2019, 37, 57–81. 10.1016/j.proci.2018.06.054. [DOI] [Google Scholar]
- Buda F.; Heyberger B.; Fournet R.; Glaude P.-A.; Warth V.; Battin-Leclerc F. Modeling of the gas-phase oxidation of cyclohexane. Energy Fuels 2006, 20, 1450–1459. 10.1021/ef060090e. [DOI] [Google Scholar]
- Serinyel Z.; Herbinet O.; Frottier O.; Dirrenberger P.; Warth V.; Glaude P. A.; Battin-Leclerc F. An experimental and modeling study of the low- and high-temperature oxidation of cyclohexane. Combust. Flame 2013, 160, 2319–2332. 10.1016/j.combustflame.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirjean B.; Buda F.; Hakka H.; Glaude P. A.; Fournet R.; Warth V.; Battin-Leclerc F.; Ruiz-Lopez M. The autoignition of cyclopentane and cyclohexane in a shock tube. Proc. Combust. Inst. 2007, 31, 277–284. 10.1016/j.proci.2006.07.247. [DOI] [Google Scholar]
- Bissoonauth T.; Wang Z.; Mohamed S. Y.; Wang J.-y.; Chen B.; Rodriguez A.; Frottier O.; Zhang X.; Zhang Y.; Cao C.; Yang J.; Herbinet O.; Battin-Leclerc F.; Sarathy S. M. Methylcyclohexane pyrolysis and oxidation in a jet-stirred reactor. Proc. Combust. Inst. 2019, 37, 409–417. 10.1016/j.proci.2018.05.086. [DOI] [Google Scholar]
- Narayanaswamy K.; Pitsch H.; Pepiot P. A chemical mechanism for low to high temperature oxidation of methylcyclohexane as a component of transportation fuel surrogates. Combust. Flame 2015, 162, 1193–1213. 10.1016/j.combustflame.2014.10.013. [DOI] [Google Scholar]
- Husson B.; Herbinet O.; Glaude P. A.; Ahmed S. S.; Battin-Leclerc F. Detailed Product Analysis during Low- and Intermediate-Temperature Oxidation of Ethylcyclohexane. J. Phys. Chem. A 2012, 116, 5100–5111. 10.1021/jp301043r. [DOI] [PubMed] [Google Scholar]
- Pousse E.; Porter R.; Warth V.; Glaude P. A.; Fournet R.; Battin-Leclerc F. Lean methane premixed laminar flames doped by components of diesel fuel II: n-Propylcyclohexane. Combust. Flame 2010, 157, 75–90. 10.1016/j.combustflame.2009.06.022. [DOI] [Google Scholar]
- Mao Y.; Wang S.; Wu Z.; Qiu Y.; Yu L.; Ruan C.; Chen F.; Zhu L.; Lu X. An experimental and kinetic modeling study of n-butylcyclohexane over low-to-high temperature ranges. Combust. Flame 2019, 206, 83–97. 10.1016/j.combustflame.2019.04.043. [DOI] [Google Scholar]
- Comandini A.; Dubois T.; Abid S.; Chaumeix N. Comparative Study on Cyclohexane and Decalin Oxidation. Energy Fuels 2014, 28, 714–724. 10.1021/ef402046n. [DOI] [Google Scholar]
- Wang M.; Zhang K.; Kukkadapu G.; Wagnon S. W.; Mehl M.; Pitz W. J.; Sung C.-J. Autoignition of trans-decalin, a diesel surrogate compound: Rapid compression machine experiments and chemical kinetic modeling. Combust. Flame 2018, 194, 152–163. 10.1016/j.combustflame.2018.04.019. [DOI] [Google Scholar]
- Yu L.; Wu Z.; Qiu Y.; Qian Y.; Mao Y.; Lu X. Ignition delay times of decalin over low-to-intermediate temperature ranges: Rapid compression machine measurement and modeling study. Combust. Flame 2018, 196, 160–173. 10.1016/j.combustflame.2018.06.014. [DOI] [Google Scholar]
- Li Y.; Zou J.; Yuan W.; Cao C.; Zhang Y.; Qi F.; Yang J. Unraveling chemical structure of laminar premixed tetralin flames at low pressure with photoionization mass spectrometry and kinetic modeling. Int. J. Chem. Kinet. 2021, 53, 154–163. 10.1002/kin.21431. [DOI] [Google Scholar]
- Raza M.; Qian Y.; Wang S.; Mao Y.; Zhu J.; Lu X. The experimental study of autoignition of tetralin at intermediate-to-high temperatures. Fuel 2020, 266, 117081. 10.1016/j.fuel.2020.117081. [DOI] [Google Scholar]
- Gao C. W.; Vandeputte A. G.; Yee N. W.; Green W. H.; Bonomi R. E.; Magoon G. R.; Wong H.-W.; Oluwole O. O.; Lewis D. K.; Vandewiele N. M.; Van Geem K. M. JP-10 combustion studied with shock tube experiments and modeled with automatic reaction mechanism generation. Combust. Flame 2015, 162, 3115–3129. 10.1016/j.combustflame.2015.02.010. [DOI] [Google Scholar]
- Tao Y.; Xu R.; Wang K.; Shao J.; Johnson S. E.; Movaghar A.; Han X.; Park J.-W.; Lu T.; Brezinsky K.; Egolfopoulos F. N.; Davidson D. F.; Hanson R. K.; Bowman C. T.; Wang H. A Physics-based approach to modeling real-fuel combustion chemistry - III. Reaction kinetic model of JP10. Combust. Flame 2018, 198, 466–476. 10.1016/j.combustflame.2018.08.022. [DOI] [Google Scholar]
- Vandewiele N. M.; Magoon G. R.; Van Geem K. M.; Reyniers M.-F.; Green W. H.; Marin G. B. Kinetic Modeling of Jet Propellant-10 Pyrolysis. Energy Fuels 2015, 29, 413–427. 10.1021/ef502274r. [DOI] [Google Scholar]
- ASTM . Standard Specification for Aviation Turbine Fuels. In ASTM D1655-20d; ASTM International: West Conshohocken, PA, 2020; Vol. ASTM D1655-20d.
- ASTM . Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons. In ASTM D7566-20c; ASTM International: West Conshohocken, PA, 2020; Vol. ASTM D7566-20c.
- Yang Z.-Y.; Zeng P.; Wang B.-Y.; Jia W.; Xia Z.-X.; Liang J.; Wang Q.-D. Ignition characteristics of an alternative kerosene from direct coal liquefaction and its blends with conventional RP-3 jet fuel. Fuel 2021, 291, 120258. 10.1016/j.fuel.2021.120258. [DOI] [Google Scholar]
- Xu R.; Wang K.; Banerjee S.; Shao J.; Parise T.; Zhu Y.; Wang S.; Movaghar A.; Lee D. J.; Zhao R.; Han X.; Gao Y.; Lu T.; Brezinsky K.; Egolfopoulos F. N.; Davidson D. F.; Hanson R. K.; Bowman C. T.; Wang H. A physics-based approach to modeling real-fuel combustion chemistry - II. Reaction kinetic models of jet and rocket fuels. Combust. Flame 2018, 193, 520–537. 10.1016/j.combustflame.2018.03.021. [DOI] [Google Scholar]
- Wang H.; Xu R.; Wang K.; Bowman C. T.; Hanson R. K.; Davidson D. F.; Brezinsky K.; Egolfopoulos F. N. A physics-based approach to modeling real-fuel combustion chemistry - I. Evidence from experiments, and thermodynamic, chemical kinetic and statistical considerations. Combust. Flame 2018, 193, 502–519. 10.1016/j.combustflame.2018.03.019. [DOI] [Google Scholar]
- Huang H.; Spadaccini L. J.; Sobel D. R. Fuel-cooled thermal management for advanced aeroengines. J. Eng. Gas Turbines Power 2004, 126, 284–293. 10.1115/1.1689361. [DOI] [Google Scholar]
- Xing Y.; Fang W. J.; Xie W. J.; Guo Y. S.; Lin R. S. Thermal Cracking and Heat Sink Measurement of Model Compounds of Endothermic Hydrocarbon Fuels under Supercritical Conditions. Acta Chim. Sin. 2008, 66, 2243–2247. [Google Scholar]
- Gascoin N.; Abraham G.; Gillard P. Synthetic and jet fuels pyrolysis for cooling and combustion applications. J. Anal. Appl. Pyrolysis 2010, 89, 294–306. 10.1016/j.jaap.2010.09.008. [DOI] [Google Scholar]
- Zhong B.-J.; Peng H.-S. Experimental Study on the Combustion of Thermally Cracked Endothermic Hydrocarbon Fuel. Combust. Sci. Technol. 2020, 192, 213–228. 10.1080/00102202.2018.1559838. [DOI] [Google Scholar]
- Han X.; Liszka M.; Xu R.; Brezinsky K.; Wang H. A high pressure shock tube study of pyrolysis of real jet fuel Jet A. Proc. Combust. Inst. 2019, 37, 189–196. 10.1016/j.proci.2018.05.136. [DOI] [Google Scholar]
- Zhao P.; Han S.; Li X.; Zhu T.; Tao X.; Guo L. Comparison of RP-3 Pyrolysis Reactions between Surrogates and 45-Component Model by ReaxFF Molecular Dynamics Simulations. Energy Fuels 2019, 33, 7176–7187. 10.1021/acs.energyfuels.9b01321. [DOI] [Google Scholar]
- Zeng P.; Wang B.-Y.; He R.; Liang J.; Yang Z.-Y.; Xia Z.-X.; Wang Q.-D. Single-Pulse Shock Tube Pyrolysis Study of RP-3 Jet Fuel and Kinetic Modeling. ACS Omega 2021, 6, 11039–11047. 10.1021/acsomega.1c00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire F. H.; Carstensen H.-H.; Herbinet O.; Battin-Leclerc F.; Marin G. B.; Van Geem K. M. Experimental and modeling study of the pyrolysis and combustion of dimethoxymethane. Combust. Flame 2018, 190, 270–283. 10.1016/j.combustflame.2017.12.001. [DOI] [Google Scholar]
- Jin Z.-H.; Chen J.-T.; Song S.-B.; Tian D.-X.; Yang J.-Z.; Tian Z.-Y. Pyrolysis study of a three-component surrogate jet fuel. Combust. Flame 2021, 226, 190–199. 10.1016/j.combustflame.2020.12.016. [DOI] [Google Scholar]
- Hanson R. K. Applications of quantitative laser sensors to kinetics, propulsion and practical energy systems. Proc. Combust. Inst. 2011, 33, 1–40. 10.1016/j.proci.2010.09.007. [DOI] [Google Scholar]
- Wang K.; Xu R.; Parise T.; Shao J.; Movaghar A.; Lee D. J.; Park J.-W.; Gao Y.; Lu T.; Egolfopoulos F. N.; Davidson D. F.; Hanson R. K.; Bowman C. T.; Wang H. A physics-based approach to modeling real-fuel combustion chemistry - IV. HyChem modeling of combustion kinetics of a bio-derived jet fuel and its blends with a conventional Jet A. Combust. Flame 2018, 198, 477–489. 10.1016/j.combustflame.2018.07.012. [DOI] [Google Scholar]
- Nagaraja S. S.; Liang J.; Dong S.; Panigrahy S.; Sahu A.; Kukkadapu G.; Wagnon S. W.; Pitz W. J.; Curran H. J. A hierarchical single-pulse shock tube pyrolysis study of C2-C6 1-alkenes. Combust. Flame 2020, 219, 456–466. 10.1016/j.combustflame.2020.06.021. [DOI] [Google Scholar]
- Sela P.; Shu B.; Aghsaee M.; Herzler J.; Welz O.; Fikri M.; Schulz C. A single-pulse shock tube coupled with high-repetition-rate time-of-flight mass spectrometry and gas chromatography for high-temperature gas-phase kinetics studies. Rev. Sci. Instrum. 2016, 87, 105103. 10.1063/1.4963844. [DOI] [PubMed] [Google Scholar]
- Morley C.Gaseq: a Chemical Equilibrium Program for Windows, 0.79, 2005.
- Petersen E. L.; Rickard M. J. A.; Crofton M. W.; Abbey E. D.; Traum M. J.; Kalitan D. M. A facility for gas- and condensed-phase measurements behind shock waves. Meas. Sci. Technol. 2005, 16, 1716–1729. 10.1088/0957-0233/16/9/003. [DOI] [Google Scholar]
- Nagaraja S. S.; Kukkadapu G.; Panigrahy S.; Liang J.; Lu H.; Pitz W. J.; Curran H. J. A pyrolysis study of allylic hydrocarbon fuels. Int. J. Chem. Kinet. 2020, 52, 964–978. 10.1002/kin.21414. [DOI] [Google Scholar]
- Scanlon J. T.; Willis D. E. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985, 23, 333–340. 10.1093/chromsci/23.8.333. [DOI] [Google Scholar]
- Goodwin D. G.; Speth R. L.; Moffat H. K.; Weber B. W.. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes, version 2.4.0, 2018.
- Han X.; Mehta J. M.; Brezinsky K. Temperature approximations in chemical kinetics studies using single pulse shock tubes. Combust. Flame 2019, 209, 1–12. 10.1016/j.combustflame.2019.07.022. [DOI] [Google Scholar]
- Lockhart J. P. A.; Goldsmith C. F.; Randazzo J. B.; Ruscic B.; Tranter R. S. An Experimental and Theoretical Study of the Thermal Decomposition of C4H6 Isomers. J. Phys. Chem. A 2017, 121, 3827–3850. 10.1021/acs.jpca.7b01186. [DOI] [PubMed] [Google Scholar]
- Pilla G. L.; Davidson D. F.; Hanson R. K. Shock tube/laser absorption measurements of ethylene time-histories during ethylene and n-heptane pyrolysis. Proc. Combust. Inst. 2011, 33, 333–340. 10.1016/j.proci.2010.06.146. [DOI] [Google Scholar]
- Zeng M.; Li Y.; Yuan W.; Li T.; Wang Y.; Zhou Z.; Zhang L.; Qi F. Experimental and kinetic modeling study of laminar premixed decalin flames. Proc. Combust. Inst. 2017, 36, 1193–1202. 10.1016/j.proci.2016.05.037. [DOI] [Google Scholar]
- Wang Q.-D.; Panigrahy S.; Yang S.; Martinez S.; Liang J.; Curran H. J. Development of Multipurpose Skeletal Core Combustion Chemical Kinetic Mechanisms. Energy Fuels 2021, 35, 6921–6927. 10.1021/acs.energyfuels.1c00158. [DOI] [Google Scholar]
- Bai J.; Cavallotti C.; Zhou C.-W. Theoretical kinetics analysis for ȮH radical addition to 1,3-butadiene and application to model prediction. Combust. Flame 2020, 221, 228–240. 10.1016/j.combustflame.2020.07.036. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



