Abstract

Codeposition of Pt and Au on Ni wire was performed using a simple treatment of immersing Ni wire in aqueous solutions containing both K2PtCl4 and HAuCl4. For evaluating the electrochemical properties of the thus-prepared electrodes, cyclic voltammograms (CVs) of 1.0 M ethanol in 1.0 M NaOH aqueous solutions were recorded. Compared with Pt- or Au-deposited Ni wire electrodes prepared by treating Ni wire in aqueous solutions of a single component, e.g., 1.0 mM K2PtCl4 or 1.0 mM HAuCl4, a noteworthy increase in the electrocatalytic current was observed for the oxidation of ethanol with a PtAu-codeposited Ni (PtAu/Ni) wire electrode even when it was prepared in an aqueous solution containing both 0.10 mM K2PtCl4 and 0.10 mM HAuCl4. In addition, the shape and the peak potentials of CVs recorded using PtAu/Ni wire electrodes were found to be different from those recorded with the Pt- or Au-deposited Ni wire electrodes. Because the CV responses typical of the PtAu/Ni wire electrodes were observed even when a PtAu/Ni wire electrode was prepared in an aqueous solution containing both 0.010 mM K2PtCl4 and 1.0 mM HAuCl4, it is considered that a small amount of Pt was effectively modified or incorporated and affected the electrochemical properties significantly. The CV results for ethanol oxidation were compared with those for the electrocatalytic oxidations of methanol, 1-propanol, and 2-propanol. Besides, the CV results recorded with the present PtAu/Ni wire electrodes are discussed in comparison with some previous results obtained using other PtAu nanoelectrocatalysts.
1. Introduction
Direct alcohol fuel cells have been attracting much attention in recent years.1−4 In particular, Pd-based1 or Pt-free2 electrocatalysts have been developed for the oxidation of alcohols. However, on the other hand, Pt-based electrocatalysts are still used for the oxidation of alcohols in both acidic and alkaline media.3,4 Also, noble-metal-based binary or ternary nanoelectrocatalysts have been attracting significant attention.3,4
We are studying the modification or deposition of noble metals, such as Au and Pd, utilizing galvanic replacement reactions, and reported several results in recent years.5−10 The deposition of Au could be performed on Ni5 and Ti6 wire electrodes, which was reported together with their possibility in electroanalysis. The deposition of Pd could be performed on Ni wire7 and Ni microparticles,8 so that the thus-prepared Pd-deposited Ni materials were found to be useful for the electrocatalytic oxidation of ethanol in alkaline solutions.7,8 Furthermore, the codeposition of Pd and Au on Ni wire was studied, and it was found that the deposition of PdAu was significantly promoted by treating Ni wire in aqueous solutions containing both K2PdCl4 and HAuCl4.9 Exhibiting the electrocatalytic responses similar to those observed with Pd electrocatalysts, the codeposition of PdAu on Ni materials was found to be promising in preparing electrocatalysts for the oxidation of ethanol.9
Following our progress mentioned above, in this work, we explored the codeposition of Pt and Au on Ni wire by simply immersing it in aqueous solutions containing both K2PtCl4 and HAuCl4. Herein, we report the electrocatalytic properties of PtAu-codeposited Ni (PtAu/Ni) wire electrodes for the oxidation of alcohols and compare the electrochemical responses with those reported previously using PtAu nanoelectrocatalysts.
In our paper about codeposition of PdAu on Ni wire, we collected and cited previous works on PdAu bimetallic electrocatalysts.9 Concerning PtAu bimetallic electrocatalysts, there are also several previous works11−19 when we collected only the works for the electrocatalytic oxidation of ethanol in alkaline media. Various kinds of PtAu-nanostructured electrocatalysts have been reported with different names, such as Au/Pt bimetallic nanodendrites,11 hollow nanoporous Au/Pt core–shell catalysts with nanochannels,12 AuPt-alloyed nanochains,13 porous PtAu-alloyed nanoflowers,14 AuPt nanodendrites,15 AuPt alloy nanowires,16 Au multipod nanoparticle core–Pt shell nanoparticles,17 porous flowerlike PtAu nanocrystals,18 and Au@Pt star-like nanocrystals.19 These PtAu nanoelectrocatalysts were prepared in suspensions, modified on glassy carbon electrodes, and utilized in the electrochemical measurements. Also, the PtAu nanoelectrocatalysts have been reported with suitable supporting materials, e.g., carbon supports (Vulcan carbon),20−24 graphene-related materials,25−27 and Bi2O3.28 Compared with the PdAu nanoelectrocatalysts cited in our previous work,9 one of the features of the PtAu nanoelectrocatalysts would be the diversity of the reported nanomaterials. In addition, their electrocatalytic responses for ethanol oxidation were different, while cyclic voltammograms (CVs) reported for the oxidation of ethanol with PdAu nanoelectrocatalysts until now were similar to those recorded with Pd nanoelectrocatalysts including our previous work.9
Before we explored the codeposition of Pt and Au on Ni wire, we had reported that the deposition of single component of Pt on Ni wire was not easy in comparison with the cases of Pd or Au on Ni wire.7 The difficulty in the same approach, i.e., immersing Ni wire in aqueous solutions of K2PtCl4 or K2PtCl6, had prevented us to report the results, although afterward it was found that the predeposition of Ag on Ni wire was effective in modifying Pt.10
Because the present approach shows that the PtAu/Ni wire electrodes significantly increase the electrocatalytic oxidation currents for ethanol and other alcohols, the electrochemical properties of the present PtAu/Ni wire electrodes are discussed by carefully comparing the CV responses with those reported until now. Some specific features of the present PtAu/Ni wire electrodes are presented including a disadvantage that the current decreases in repeated scans, although the behaviors in repeated scans are unclear in some previous works.
In addition to the use of NiO-based materials as cathodes for fuel cells, NiO nanostructures have been studied for ethanol oxidation in alkaline media, although no typical electrocatalytic responses of ethanol were shown.29 In this work, it is characteristic that pure Ni metal wire was used as the conducting support for PtAu nanoelectrocatalysts. Because the successful Pd deposition on Ni microparticles has been shown via the galvanic replacement reaction previously,8 the possibility of PtAu deposition on Ni microparticles would be expected from this work.
2. Results and Discussion
2.1. Codeposition of Pt and Au on Ni Wire via Galvanic Replacement Reactions
Previously, we reported that the codeposition of Pd and Au on Ni wire via the galvanic replacement reactions was remarkably promoted when PdCl42– and AuCl4– coexisted in aqueous solutions.9 Thus, at first, we similarly carried out the immersion of Ni wire in an aqueous solution containing both 0.10 mM PtCl42– and 0.10 mM AuCl4– for 10 min, and then, the CVs of ethanol oxidation were recorded with the thus-prepared PtAu codeposited Ni (PtAu/Ni) wire electrode.
For comparison, the CVs for the oxidation of 1.0 M ethanol in 1.0 M NaOH aqueous solution were recorded with Au or Pt electrodes. The CVs in Figure 1A were recorded using an Au-deposited Ni (Au/Ni) wire electrode prepared by immersing Ni wire in an aqueous solution of 1.0 mM HAuCl4 for 10 min, and the CVs in Figure 1B were recorded using an Au disk electrode. The CVs in Figure 2A were recorded using a Pt-deposited Ni (Pt/Ni) wire electrode prepared by immersing Ni wire in an aqueous solution of 1.0 mM K2PtCl4 for 10 min, and the CVs in Figure 2B were recorded using a Pt disk electrode. From the CVs in Figures 1 and 2, the differences in the electrocatalytic responses for ethanol between Au and Pt would be easily recognized: the differences in the peak potentials and the ratio of the oxidation peak currents in the forward and reversed scans. Additionally, for the Au electrodes, the CV response was almost constant after the second scan in the repeated scans with a constant peak current value of ca. 6 mA cm–2. In contrast, for the Pt electrodes, the current of the electrocatalytic oxidation of ethanol increased in the repeated scans, and the peak current value was less than 1 mA cm–2 even at the 10th scan.
Figure 1.

CVs of 1.0 M ethanol in 1.0 M NaOH aqueous solutions recorded with (A) an Au-deposited Ni wire electrode prepared by immersing a piece of Ni wire in an aqueous solution of 1.0 mM HAuCl4 for 10 min at 30 °C and (B) an Au disk electrode. The CVs are composed of 2nd, 5th, and 10th scans in the consecutive scans. Scan rate: 50 mV/s.
Figure 2.

CVs of 1.0 M ethanol in 1.0 M NaOH aqueous solutions recorded with (A) a Pt-deposited Ni wire electrode prepared by immersing a piece of Ni wire in an aqueous solution of 1.0 mM K2PtCl4 for 10 min at 30 °C and (B) a Pt disk electrode. The CVs are composed of 2nd, 5th, and 10th scans in the consecutive scans. Scan rate: 50 mV/s.
However, Figure 3A,B show CVs for the oxidation of 1.0 M ethanol in 1.0 M NaOH aqueous solution recorded with a PtAu/Ni wire electrode prepared by immersing Ni wire into an aqueous solution containing both 0.10 mM K2PtCl4 and 0.10 mM HAuCl4 for 10 min. Because the current magnitude varied in the repeated scans, the CV responses of the 1st to the 3rd scan are shown in Figure 3A, and those of the 3rd to the 10th scan are shown in Figure 3B. Figure 3C shows the transformation of the peak currents with the scanned cycle number.
Figure 3.
(A and B) CVs of 1.0 M ethanol in 1.0 M NaOH aqueous solution recorded with a PtAu/Ni wire electrode prepared by immersing a piece of Ni wire in an aqueous solution containing both 0.10 mM K2PtCl4 and 0.10 mM HAuCl4 for 10 min at 30 °C. (A) The 1st to the 3rd and (B) the 3rd to the 10th scans in the consecutive scans. Scan rate: 50 mV/s. (C) Transformation in the peak current with the scan cycle number.
From the results in Figure 3, we can extract some features of the CV responses recorded with PtAu/Ni as follows:
-
(1)
The peak current of the positive-going scans was generally so large that the maximum value approached 80 mA cm–2 but gradually diminished in the repeated scans after reaching the maximum value.
-
(2)
The peak potential of the positive-going scans was apparently negative to that on Au (Figure 1) and slightly positive to that on Pt (Figure 2).
-
(3)
The peak potentials of the positive- and negative-going scans were almost the same, and the peak current of the negative-going scans was much smaller (<20 mA cm–2) than that of the positive-going scans, as shown in Figure 3C.
For the ethanol oxidation in alkaline solutions with Au, Pt, or Pd electrocatalysts, it is known that the oxidation currents in the negative-going scans start to increase at the potentials where metal oxides were reduced to metals. Hence, the CV responses in Figure 3, in particular, having feature (3) mentioned above, imply that the electrocatalytic oxidation scheme of ethanol on PtAu/Ni is essentially different from that on the single component of Au or Pt. This is in contrast to our previous result that the electrocatalytic oxidation of ethanol with PdAu/Ni was regarded as similar to that with Pd.9
For comparison, Figure 4 summarizes the CVs recorded with the PtAu/Ni (Figure 4A), Au/Ni, and Pt/Ni (Figure 4B) wire electrodes. Remarkably increased oxidation current with the PtAu/Ni wire electrode would be recognized in Figure 4 despite the lower concentration of K2PtCl4 and HAuCl4 (both 0.10 mM) used while preparing the PtAu/Ni electrode.
Figure 4.

CVs of 1.0 M ethanol in 1.0 M NaOH aqueous solution recorded with (A) a PtAu/Ni wire electrode (the 3rd scan in Figure 3A) and (B) with Pt/Ni and Au/Ni wire electrodes (the 10th scans in Figures 1A and 2A) summarized for comparison.
2.2. Effects of [PtCl42–]/[AuCl4–] in Preparing the PtAu/Ni Wire Electrodes on the Electrocatalytic Oxidation of Ethanol
Because the electrocatalytic oxidation of ethanol with the PtAu/Ni wire electrodes exhibited CVs different from those with Pt/Ni and Au/Ni wire electrodes, we explored how the electrochemical properties change depending on the ratio of [PtCl42–]/[AuCl4–] in aqueous solutions in which Ni wire was immersed. After several trials, we could find an interesting tendency for the CV responses to change when [AuCl4–] > [PtCl42–]. Figure 5 shows the CVs depending on [PtCl42–]/[AuCl4–] when [AuCl4–] was fixed to 1.0 mM (Figure 5A) and 0.10 mM (Figure 5B).
Figure 5.
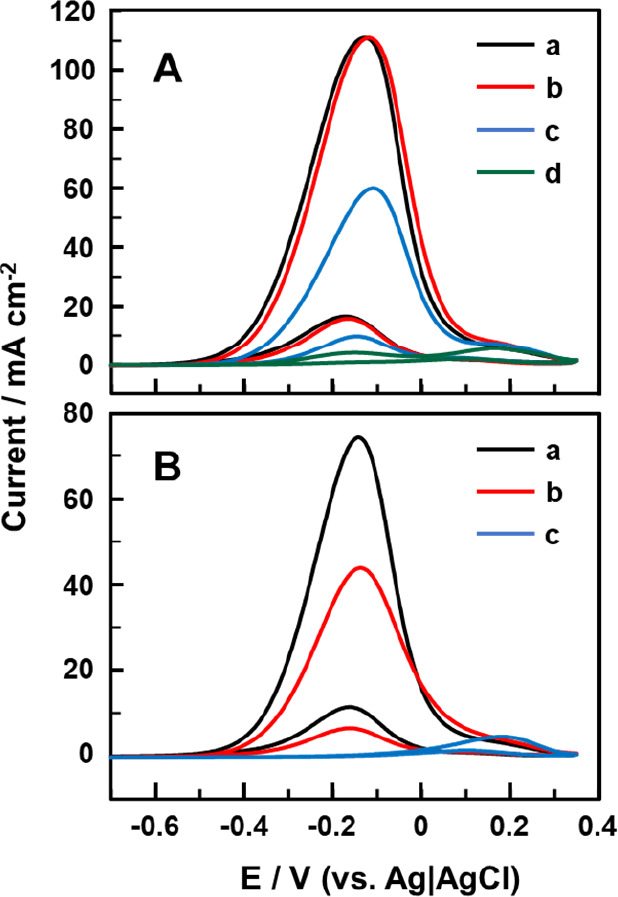
CVs of 1.0 M ethanol in 1.0 M NaOH aqueous solutions recorded with PtAu/Ni wire electrodes. When preparing the PtAu/Ni wire electrodes, we used aqueous solutions containing (A) both 1.0 mM AuCl4– and (a) 0.10, (b) 0.010, (c) 0.0010, or (d) 0.00010 mM PtCl42– and (B) both 0.10 mM AuCl4– and (a) 0.10, (b) 0.010, or (c) 0.0010 mM PtCl42–. For each CV, the maximum one in the repeated scans was shown. Scan rate: 50 mV/s.
With regard to the results in Figure 5, it was characteristic that, even when the amount of Pt was lower, we could observe the CV responses typical of the ethanol oxidation with PtAu/Ni wire electrodes. The CV responses in Figure 5A(a),(b) were almost the same, indicating that 0.010 mM PtCl42– was enough to govern the CV responses with 1.0 mMAuCl4–. Although a further decrease in [PtCl42–]/[AuCl4–] changed the response to Au eventually (Figure 5A(d)), it was impressive that the oxidation peak typical of the ethanol oxidation with PtAu/Ni emerged in the positive-going scan even when [PtCl42–]/[AuCl4–] was 1/1000, as shown in Figure 5A(c). With 0.10 mMAuCl4–, more PtCl42– was necessary to show the CV responses typical of PtAu/Ni in terms of [PtCl42–]/[AuCl4–], but the pure Au-like response was observed when [PtCl42–] was decreased to 0.0010 mM (Figure 5B(c)). Under the conditions that the oxidation peak current decreased in the positive-going scans (i.e., Figure 5A(c),B(b)), slight positive shifts of the peak potential were observed. This might be related to the increased contribution of Au.
2.3. Surface Images of PtAu/Ni Wire Electrodes
Since the codeposition of Pt and Au brought about huge differences in the electrocatalytic currents, as summarized in Figure 4, the surface images of the PtAu/Ni wire electrodes were observed using a field-emission scanning electron microscope (FE-SEM) to compare with those of Pt/Ni and Au/Ni wire electrodes. Figure 6A,B shows typical FE-SEM images of the surfaces of Pt/Ni and Au/Ni wire electrodes, respectively. By immersing Ni wire in an aqueous solution containing 1.0 mM PtCl42– or 0.10 mM AuCl4– for 10 min, Pt nanoparticles (PtNPs) or Au nanoparticles (AuNPs) were recognized to deposit on Ni surfaces, as shown in Figure 6A,B via the galvanic replacement reactions. The sparse deposition of PtNPs in Figure 6A would be in accordance with the small oxidation current in Figure 2A. The deposition of AuNPs in Figure 6B is apparently denser than that in Figure 6A. Here, note that [AuCl4–] was 0.10 mM for Figure 6B, while [PtCl42–] was 1.0 mM for Figure 6A. Thus, from Figure 6A,B, we understand that PtNPs do not deposit well on the surface of Ni via the galvanic replacement reaction of eq 1, although the potential difference would be enough (eqs 2 and 3) and larger than that between Ni and PdCl42– (eqs 2 and 4)
| 1 |
| 2 |
| 3 |
| 4 |
Figure 6.

Typical FE-SEM images of the surfaces of (A) Pt/Ni, (B) Au/Ni, and (C) PtAu/Ni wire electrodes prepared by immersing a piece of Ni wire in aqueous solutions containing (A) 1.0 mM K2PtCl4, (B) 0.10 mM HAuCl4, and (C) both 0.10 mM K2PtCl4 and 0.10 mM HAuCl4, for 10 min at 30 °C.
Figure 6C shows a typical FE-SEM image of the surface of a PtAu/Ni wire electrode prepared by immersing Ni wire in an aqueous solution containing both 0.10 mM PtCl42– and 0.10 mM AuCl4– for 10 min. From this image, it is recognized that nanocrystals whose size was ca. 50 nm were deposited very densely and closely on the Ni surface by sticking with each other. Judging from the preparation conditions, it is obvious that 0.10 mM PtCl42– coexisting with 0.10 mM AuCl4– has caused significant changes in the deposition of nanocrystals, as shown in Figure 6B,C.
However, Figure 7 shows the changes in the deposition of nanocrystals affected by 0.010 mM PtCl42– coexisting with 1.0 mM AuCl4–. The deposition of AuNPs after the treatment with 1.0 mM AuCl4– (Figure 7A) was apparently different from that with 0.10 mM AuCl4– (Figure 6B), and Figure 7B shows that the small amount (1/100 in molar ratio) of PtCl42– somewhat promoted the surface deposition of nanocrystals. Here, we stress that the CV response in Figure 5A(b) was recorded with the PtAu/Ni wire electrode prepared with 0.010 mM PtCl42– and 1.0 mM AuCl4– whose surface image corresponded to Figure 7B. Because pure Au/Ni wire electrodes show different CV responses (see Figure 1), significant effects of a smaller amount of PtCl42– on CV responses would be recognized also from minor changes in the surface images.
Figure 7.

Typical FE-SEM images of the surfaces of (A) Au/Ni and (B) PtAu/Ni wire electrodes prepared by immersing a piece of Ni wire in aqueous solutions containing (A) 1.0 mM HAuCl4 or (B) both 0.010 mM K2PtCl4 and 1.0 mM HAuCl4, for 10 min at 30 °C.
To know the surface dispersion of Pt with Au on PtAu/Ni, we tried to observe the EDS element mapping, as we had carried out for PdAu/Ni.9 However, because the energies of Pt and Au are very close, the detection and the mapping of Pt were not easy. For the surfaces of PtAu/Ni prepared with 0.10 mM PtCl42– and 0.10 mM AuCl4–, the amount analysis of the elements showed that the ratio of Au/Pt was ca. 5:1 in the analysis software, but the peaks of Pt were not well-separated from those of Au. So, it is inferred that the deposition of Au would be dominant compared with that of Pt. When the amount of Pt was decreased, unfortunately, we could not detect Pt well in the element mapping.
2.4. Electrocatalytic Oxidation of Alcohols on PtAu/Ni Wire Electrodes
Before discussing the CV responses typical of the ethanol oxidation with PtAu/Ni wire electrodes (Figure 3), we recorded CVs for the oxidation of methanol, 1-propanol, and 2-propanol in alkaline solutions. The results showed that the CV responses for the oxidations of methanol (Figure 8A) and 1-propanol (Figure 8B) resembled those of ethanol (Figure 3B), while the current magnitudes of the peaks around −0.2 V were different. In Figure 8B, a smaller peak was observed around 0.2 V; in Figure 8C, for 2-propanol, the current around 0.2 V was relatively increased.
Figure 8.
CVs of 1.0 M (A) methanol, (B) 1-propanol, or (C) 2-propanol in 1.0 M NaOH aqueous solutions with a PtAu/Ni wire electrode prepared by immersing a piece of Ni wire in an aqueous solution containing both 0.10 mM K2PtCl4 and 0.10 mM HAuCl4 for 10 min at 30 °C. For all CVs, the 3rd to the 10th scans in the consecutive scans are shown for comparison. Scan rate: 50 mV/s.
For the electrocatalytic oxidations of these alcohols, the differences with Au, Pd, and Pt electrodes have been reported previously.30,31 In addition, some special attention has been devoted to the differences between methanol and ethanol with Pt,32 1-propanol and 2-propanol with Pt and Pd,33 and Pt and Au for 2-propanol.34 To discuss the results in Figure 8, we observed CVs of methanol, 1-propanol, and 2-propanol with Au and Pt disk electrodes (the data are not shown) similar to the cases of ethanol (Figures 1B and 2B). Consequently, we could find the following points:
-
(1)
No electrocatalytic responses were observed for the oxidation of methanol with Au and 2-propanol with Pt.
-
(2)
The peak potentials of the oxidation of methanol and 1-propanol with Pt were negative to those of the present peaks around −0.2 V in Figure 8, which is similar to the case of ethanol.
-
(3)
The CVs of the oxidation of 1-propanol and 2-propanol on Au were similar to those of ethanol on Au (Figure 1B), but the electrocatalytic current was the largest for 2-propanol.
Thus, the peaks around −0.2 V in Figure 8A–C, which decreased with the repeated scans, could be assigned to the electrocatalytic oxidations with PtAu/Ni similar to the case of ethanol (Figure 3). Also, the peaks around 0.2 V in Figure 8B,C were inferred to be recognized reflecting the degree of the electrocatalytic oxidation with Au. In general, in comparison with the previous works,30−33 our results with PtAu/Ni for four alcohols would resemble each other except the peaks around 0.2 V, indicating the CV responses different from those on Pt. This is in contrast to our previous results that the CV responses of methanol and ethanol were different with Pd/Ni7 and PdAu/Ni9 wire electrodes.
2.5. Considerations about CV Responses on PtAu/Ni Wire Electrodes
We compared the present CV responses recorded using PtAu/Ni wire electrodes with those of PtAu bimetallic nanoelectrocatalysts reported until now.11−28 However, it was difficult to find exactly identical CV results. For example, in ref (11), the oxidation peak in the positive-going scan with AuPt bimetallic nanodendrites was reported as negative to that with Au and positive to that with Pt accompanying a small oxidation peak in the reversed negative scan at the same potential as the positive-going scan. This response resembles the present one, but another oxidation peak was markedly observed in the reversed negative-going scan at the potential where Pt oxides were reduced to Pt.11 Similar CV responses have been reported for AuPt alloy nanowires,16 Au@Pt star-like nanocrystals,19 and also Pt-Au heteronanocrystals including the response coming from Au parts.35 Therefore, the CV responses in refs (11), (16), (19), and (35) might be one of the typical CV responses for ethanol oxidation with PtAu electrocatalysts. However, in the present CV responses with PtAu/Ni (Figure 3), there is no oxidation current around the reduction of Pt oxides in the negative-going scans.
Judging from the oxidation peaks whose features are mentioned in Figure 3, we think that the responses reported using Pt-deposited Au/C21 would be relatively similar. In ref (21), it was the characteristic that Pt was electrodeposited on Au/C at a constant potential. As a result of this electrodeposition, Pt would be located on the surfaces of Au. Thus, it may be reasonable that the CV responses that were somewhat different from those with other PtAu nanoelectrocatalysts were observed. Furthermore, a previous work also showed similar CV responses with AuPt nanoparticles (AuPtNPs), whose core size was 2 nm encapsulated with organic shells, for methanol oxidation in alkaline media.36 Although the work in ref (36) was for methanol oxidation, the present results of Figures 3 and 8A showed that the CV responses for ethanol and methanol were similar. Hence, the electrocatalytic reactions on the present PtAu/Ni may have some similarities with the previous case of AuPtNPs.36
Full understanding of the CV responses on the present PtAu/Ni (Figure 3) would not be easy as well as the reasons of huge differences from those on Pt/Ni and Au/Ni (Figure 4). However, from the CV responses and the similarities to the previous works,21,36 we considered some plausible views in the following.
2.5.1. Dispersions of Pt and Au on the Surfaces of Nanocrystals Formed on Ni
If there are some island areas of Au or Pt on the nanocrystals formed on Ni and if they work as Au or Pt electrodes, Au-like or Pt-like CV responses should have been observed to some extent even with PtAu/Ni. However, the present results in Figures 3 and 4 showed that the CV responses did not include the oxidation current on each single metal, although their currents on Au/Ni and Pt/Ni were quite small, as shown in Figures 1A and 2A. Actually, Au-rich preparations (e.g., Figure 5A(d),B(c)) showed Au-like CV responses. Therefore, to enhance the electrocatalytic oxidation current, the mixing of Pt and Au at the atomic level on the surfaces may be expected or interfacial areas of Pt/Au may affect the electrochemical responses significantly to govern the CV responses. For the former expectation, synergetic electrocatalytic activity involving adjacent Pt and Au atoms has been proposed for the CV responses in ref (36).36,37 The latter presumption would agree with the result that a large oxidation peak (at 0.19 V vs Ag|AgCl) is observed with PtAu hetero-nanocrystals in addition to the responses on Pt and Au parts.35 However, unfortunately, we could not observe Pt element mapping with EDS analysis as already mentioned.
2.5.2. Accumulation of Pt in the Growth Process of PtAu Nanocrystals on Ni
As we discussed concerning Figures 2A and 6A, the direct deposition of Pt on Ni was unfavorable and very minor compared with that of Au on Ni. In addition, if some Pt atoms deposited on the surfaces of nanocrystals, the galvanic replacement reaction of eq 5 should proceed judging from the potentials of eqs 3 and 6
| 5 |
| 6 |
Hence, in the growing process of PtAu nanocrystals, the accumulated amount of Pt is expected to be smaller than that of Au. If the nanocrystals deposited on Ni contain Au mainly, some condensation of Pt in the vicinity of the surfaces of nanocrystals would be expected. This may be in relation with the fact that the smaller amounts of Pt effectively affected to show the typical CV responses (Figure 5).
2.5.3. Importance of the Coexistence of Pt and Au
Concerning the present CV responses, we pointed out that the CVs reported using Pt-deposited Au/C21 would be similar. Since Pt was modified on the surfaces of Au/C via electrodeposition in this case,21 we tried a stepwise modification of Pt on Au nanocrystals on Ni. That is, by treating Ni wire at first in an aqueous solution of AuCl4– and then in an aqueous solution of PtCl42–, we prepared modified electrodes and the CV responses were observed (the data are not shown). As a result, the shape and peak potentials of CVs were almost the same as those recorded with PtAu/Ni, but the current was apparently smaller. Thus, to show the CV responses in Figure 3, it is expected that the coexistence of Pt with Au in the growth of nanocrystals would be important although the amount of Pt is smaller. Only the surface modification of Pt on Au nanocrystals would be insufficient to build up unique surfaces of PtAu/Ni that caused the enhanced current.
2.6. Current Decrease in Repeated CV Scans on PtAu/Ni
The oxidation peaks of alcohols gradually decreased in the repeated scans with PtAu/Ni as we show in Figures 3 and 8. In our previous studies, the changes in CVs at the 2nd, 5th, and 10th scans were shown for the results recorded with Pd/Ni wire7 and PdAu/Ni wire9 electrodes. Also, while the CVs with Au were almost constant in the repeated scans in Figure 1, those with Pt in Figure 2 showed an increase in the oxidation currents. Because the current decrease usually means the degradation of electrocatalysts, we think that the changes in the current magnitude in repeated scans would be important for considering properties of electrocatalysts.
For the present results, we think that the current decrease in the repeated scans with PtAu/Ni would be remarkable. However, general comparisons with other PtAu nanoelectrocatalysts are not easy because previous papers tend to show one typical CV. Only when the poisoning, durability, or long-term stability is evaluated, some results of repeated scans seem to be shown.
Among the previous works on ethanol oxidation, ref (35) reported that the decrease from the 100th scan to the 300th scan was small (ca. 20%). So, the present current decrease in Figure 3 might be a disadvantageous point of the present PtAu/Ni. Concerning this point, we have the following results:
-
(1)
After observing the current decrease, some recoveries of the used electrodes were tried to exhibit higher current. However, at present, it was difficult. That is, the CV responses having higher current values were observed only in the first use of the electrodes.
-
(2)
We tried the redox cycling treatments in an aqueous solution of NaOH and then recorded CVs for ethanol oxidation. As a result, the oxidation currents for ethanol observed after the redox cycles in NaOH were much smaller. So, some poisoning by OH– would be expected.
-
(3)
A huge oxidation current may cause local pH changes38 and local consumption of ethanol. Actually, a positive-going scan having a high current oxidation, as shown in Figure 4A, was calculated to be equivalent to ca. 0.014 C. To consider these points, practically, we carried out several CV scans by changing the solution every time after finishing a single scan. In this experiment, we found that the current decreased similar to the consecutive repeated scans. Thus, it is inferred that the poisoning actually occurred in the potential scans.
-
(4)
In ref (19), an unusual chronoamperometric response has been reported for ethanol oxidation with Au@Pt star-like nanocrystals. In relation with the current decrease in CVs with PtAu/Ni, we observed the chronoamperometric response. However, the result showed a simple decay, not special ones (the data are not shown).
3. Conclusions
Following our previous work on PdAu-codeposited Ni (PdAu/Ni) wire electrodes for the oxidation of ethanol,9 we prepared PtAu/Ni wire electrodes by simply immersing Ni wire in aqueous solutions containing both K2PtCl4 and HAuCl4. Using the thus-prepared PtAu/Ni wire electrodes, we could observe increased electrocatalytic currents for ethanol oxidation as well as the characteristic CVs, whose shapes and peak potentials were different from those on Pt/Ni or Au/Ni, as summarized in Figure 4. The changes implied that Pt and Au were mixed at the atomic level in the nanodeposits on Ni to show the electrochemical properties different from Pt or Au. Furthermore, the small amount of Pt was found to affect or govern the CV responses.
Compared with our previous works on Pd/Ni7 and PdAu/Ni9 wire electrodes, the present current magnitude for ethanol oxidation approaching 80 mA cm–2 or more (see Figure 5) was the largest. In addition, the surface image of PtAu nanocrystals deposited on Ni (Figure 6C) denoted that the deposition of PtAu was significantly promoted by the coexistence of PtCl42– and AuCl4–. Therefore, the present preparation, i.e., by immersing Ni wire for 10 min at 30 °C with lower concentrations of precursor ions (0.1 mM level), would have potentials as a surface modification method to give higher electrocatalytic properties peculiar to PtAu.
At present, the exact mechanisms for promoting the deposition of nanocrystals and for causing a huge current increase are unclear. Because the deposition reactions proceeded by just immersing Ni wire, we could discuss the progresses of deposition based on the redox potentials. However, the surface state and the electrochemical characteristics of PtAu/Ni were different from those of PdAu/Ni,9 although the potential relationships are relatively close. Since the detailed surface or element analysis was not easy due to the resolutions, further works including the studies on other bimetallic systems would be necessary.
In addition, while some different CV responses have been reported depending on the sort of alcohols on Au, Pt, or Pd previously,30−34 the CV responses of ethanol on PtAu/Ni were clarified to be similar to those for methanol, 1-propanol, and 2-propanol on PtAu/Ni in spite of the variation of the maximum currents (see Figures 3 and 8). So, we may expect electrocatalytic mechanisms, which are common for the monohydric alcohols, but are different from those with pure noble metals. For these details, our studies are in progress including the CV measurements of ethylene glycol and glycerol on PtAu/Ni over monohydric alcohols.
In this paper, we described the decrease in the electrocatalytic current in the repeated scans. Since the current decrease implies the degradation of PtAu/Ni electrocatalysts, the information might be negative for the proposed materials. However, such information would be important for considering the reaction mechanisms and improving the electrocatalytic properties. Judging from the different CV responses summarized in Figure 4, we believe that the present results obtained with PtAu/Ni are worthwhile reporting, specifying the differences of the present CV responses from other PtAu electrocatalysts and mentioning the changes in the electrocatalytic performances in the repeated scans.
4. Experimental Section
4.1. Apparatus and Materials
Scanning electron microscopic (SEM) images were obtained with a Sigma 500 instrument, Carl Zeiss Microscopy. EDS analysis was performed using the instrument (XFlash 6130, Bruker) coupled with FE-SEM (field-emission scanning electron microscopy). CVs were recorded using a potentiostat (PGSTAT 128N, Metrohm Autolab). The platinum wire and Ag|AgCl (3.0 M NaCl) electrode (BAS, Inc.) were employed as the counter and reference electrodes, respectively. Hence, the potential values of CVs are vs Ag|AgCl. Ni wire (diameter of 0.30 mm, 99+% degree, Nilaco Co.) was used for preparing Pt-, Au-, and PtAu-modified Ni electrodes. For the purpose of comparisons, an Au disk (diameter of 1.6 mm, BAS, Inc.) or a Pt disk (diameter of 1.6 mm, BAS, Inc.) electrode was used as a working electrode.
HAuCl4·3H2O, K2PtCl4,and K2PtCl6 were purchased from Sigma-Aldrich. Other reagents were obtained from Sigma-Aldrich or Wako Pure Chemicals. All aqueous solutions were prepared with ultrapure water obtained from a water purification system (Arium pro, Sartorius) with a specific resistance >18 MΩ cm.
4.2. Preparation of PtAu-Modified Ni Wire Electrodes
Before the surface modification, a piece of Ni wire (diameter of 0.30 mm, ca. 7 cm length) was washed in acetone with 10-min sonication and then in 1.0 M HCl aqueous solution with 10-min sonication.7,9 The washed Ni wire was immersed in an aqueous solution of the precursor ions: HAuCl4 for Au, K2PtCl4 for Pt, and both K2PtCl4 and HAuCl4 for PtAu modifications. Although K2PtCl6 was examined in the initial stage of this work, the results were not very different from those obtained with K2PtCl4. So, only the results obtained with K2PtCl4 were shown. All the treatments were carried out using 10-mL glass bottles. The temperature was kept at 30 °C using a thermostat bath.
For electrochemical evaluations, 5.0 mm of the modified Ni wire working electrode was exposed to the electrolyte and the rest was wrapped by water-proof tape, and CVs of alcohols in alkaline solutions were recorded. To evaluate the current magnitude, the current value of CVs was represented after dividing by a geometrical surface area of the 5.0 mm Ni cylinder (0.30 mm diameter).
Acknowledgments
M.O. thanks financial support from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant nos. 18K05170 and 21K05111). G.C. and Y.H. acknowledge the financial support from the National Natural Science Foundation of China (nos. 51672074 and 1774082), the Natural Science Foundation of Hubei Province (Grant no. 2019CFA006), and the Program for Science and Technology Innovation Team in Colleges of Hubei Province (Grant no. T201901).
The authors declare no competing financial interest.
References
- Yin Z.; Lin L.; Ma D. Construction of Pd-based nanocatalysts for fuel cells: Opportunities and challenges. Catal. Sci. Technol. 2014, 4, 4116–4128. 10.1039/C4CY00760C. [DOI] [Google Scholar]
- Ozoemena K. I. Nanostructured platinum-free electrocatalysts in alkaline direct alcohol fuel cells: Catalyst design, principles and applications. RSC Adv. 2016, 6, 89523–89550. 10.1039/C6RA15057H. [DOI] [Google Scholar]
- Bai J.; Liu D.; Yang J.; Chen Y. Nanocatalysts for electrocatalytic oxidation of ethanol. ChemSusChem 2019, 12, 2117–2132. 10.1002/cssc.201803063. [DOI] [PubMed] [Google Scholar]
- Lyu F.; Cao M.; Mahsud A.; Zhang Q. Interfacial engineering of noble metals for electrocatalytic methanol and ethanol oxidation. J. Mater. Chem. A 2020, 8, 15445–15457. 10.1039/D0TA03199B. [DOI] [Google Scholar]
- Umeya Y.; Kobayashi Y.; Kawashimo T.; Ahn S.; Chang G.; Oyama M. Preparation of Gold Modified Nickel Wire Electrodes for Electroanalysis via a Galvanic Replacement Reaction. Electroanalysis 2018, 30, 1370–1377. 10.1002/elan.201800077. [DOI] [Google Scholar]
- Terazawa D.; Kawashimo T.; Cai Z.; Chang G.; He Y.; Oyama M. Citrate-driven modification of gold on titanium wire electrodes by the treatment in aqueous solutions of HAuCl4. J. Electroanal. Chem. 2020, 872, 113991 10.1016/j.jelechem.2020.113991. [DOI] [Google Scholar]
- Kobayashi Y.; Yoshida H.; Ahn S.; Chang G.; Oyama M. Palladium Deposition on Nickel Wire Electrodes by a Galvanic Replacement Reaction. ACS Appl. Energy Mater. 2019, 2, 2337–2343. 10.1021/acsaem.9b00182. [DOI] [Google Scholar]
- Kobayashi Y.; Cai Z.; Chang G.; He Y.; Oyama M. Palladium Deposition on Nickel Microparticles by a Galvanic Replacement Reaction for Electrocatalytic Oxidation of Ethanol. ACS Appl. Energy Mater. 2019, 2, 6023–6030. 10.1021/acsaem.9b01132. [DOI] [Google Scholar]
- Funo S.; Cai Z.; Chang G.; He Y.; Oyama M. Codeposition of Palladium and Gold on Nickel Wire Electrodes via Galvanic Replacement Reactions for Ethanol Oxidation. ACS Appl. Energy Mater. 2020, 3, 7083–7090. 10.1021/acsaem.0c01120. [DOI] [Google Scholar]
- Sato F.; Funo S.; Cai Z.; Chang G.; He Y.; Oyama M. Modification with platinum of silver-deposited nickel wire electrodes for electrocatalytic oxidation of alcohols. Electrochem. Commun. 2021, 124, 106939 10.1016/j.elecom.2021.106939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Wang D.; Liu D.; Huang J.; You T. Synthesis and electrocatalytic activity of Au/Pt bimetallic nanodendrites for ethanol oxidation in alkaline medium. J. Colloid Interface Sci. 2012, 367, 342–347. 10.1016/j.jcis.2011.09.087. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Zhu A.; Huang R.; Zhang Q.; Liu Q. Hollow nanoporous Au/Pt core–shell catalysts with nanochannels and enhanced activities towards electro-oxidation of methanol and ethanol. Int. J. Hydrogen Energy 2014, 39, 8246–8256. 10.1016/j.ijhydene.2014.03.193. [DOI] [Google Scholar]
- Zhang Q.; Ju K.; Huang X.; Wang A.; Wei J.; Feng J. Metformin mediated facile synthesis of AuPt alloyed nanochains with enhanced electrocatalytic properties for alcohol oxidation. Electrochim. Acta 2015, 182, 305–311. 10.1016/j.electacta.2015.09.112. [DOI] [Google Scholar]
- Song P.; Mei L.; Wang A.; Fang K.; Feng J. One-pot surfactant-free synthesis of porous PtAu alloyed nanoflowers with enhanced electrocatalytic activity for ethanol oxidation and oxygen reduction reactions. Int. J. Hydrogen Energy 2016, 41, 1645–1653. 10.1016/j.ijhydene.2015.11.021. [DOI] [Google Scholar]
- Wang A.; Ju K.; Zhang Q.; Song P.; Wei J.; Feng J. Folic acid bio-inspired route for facile synthesis of AuPt nanodendrites as enhanced electrocatalysts for methanol and ethanol oxidation reactions. J. Power Sources 2016, 326, 227–234. 10.1016/j.jpowsour.2016.06.115. [DOI] [Google Scholar]
- Gan Q.; Tao L.; Zhou L.; Zhang X.; Wang S.; Li Y. Directional coalescence growth of ultralong Au93Pt7 alloy nanowires and their superior electrocatalytic performance in ethanol oxidation. Chem. Commun. 2016, 52, 5164–5166. 10.1039/C6CC01391K. [DOI] [PubMed] [Google Scholar]
- Moon Y.; Mai H. D.; Yoo H. Platinum Overgrowth on Gold Multipod Nanoparticles: Investigation of Synergistic Catalytic Effects in a Bimetallic Nanosystem. ChemNanoMat 2017, 3, 196–203. 10.1002/cnma.201600322. [DOI] [Google Scholar]
- Li S.; Xu H.; Xiong Z.; Zhang K.; Wang C.; Yan B.; Guo J.; Du Y. Monodispersed porous flowerlike PtAu nanocrystals as effective electrocatalysts for ethanol oxidation. Appl. Surf. Sci. 2017, 422, 172–178. 10.1016/j.apsusc.2017.05.246. [DOI] [Google Scholar]
- Peng Y.; Li L.; Tao R.; Tan L.; Qiu M.; Guo L. One-pot synthesis of Au@Pt star-like nanocrystals and their enhanced electrocatalytic performance for formic acid and ethanol oxidation. Nano Res. 2018, 11, 3222–3232. 10.1007/s12274-017-1851-5. [DOI] [Google Scholar]
- Da Silva S. G.; Silva J. C. M.; Buzzo G. S.; De Souza R. F. B.; Spinacé E. V.; Neto A. O.; Assumpção M. H. M. T. Electrochemical and fuel cell evaluation of PtAu/C electrocatalysts for ethanol electro-oxidation in alkaline media. Int. J. Hydrogen Energy 2014, 39, 10121–10127. 10.1016/j.ijhydene.2014.04.169. [DOI] [Google Scholar]
- Jin C.; Zhang J.; Huo Q.; Dong R. Significant activity improvement of Au/C by Pt deposition for electrooxidation of ethanol. J. Electroanal. Chem. 2015, 736, 112–116. 10.1016/j.jelechem.2014.11.011. [DOI] [Google Scholar]
- Pearson A.; O’Mullane A. P. A simple approach to improve the electrocatalytic properties of commercial Pt/C. Chem. Commun. 2015, 51, 11297–11300. 10.1039/C5CC03834K. [DOI] [PubMed] [Google Scholar]
- Dutta A.; Mondal A.; Datta J. Tuning of platinum nano-particles by Au usage in their binary alloy for direct ethanol fuel cell: Controlled synthesis, electrode kinetics and mechanistic interpretation. J. Power Sources 2015, 283, 104–114. 10.1016/j.jpowsour.2015.01.113. [DOI] [Google Scholar]
- Wang H.; Jiang K.; Chen Q.; Xie Z.; Cai W. Carbon monoxide mediated chemical deposition of Pt or Pd quasi-monolayer on Au surfaces with superior electrocatalysis for ethanol oxidation in alkaline media. Chem. Commun. 2016, 52, 374–377. 10.1039/C5CC06551H. [DOI] [PubMed] [Google Scholar]
- Gnanaprakasam P.; Jeena S. E.; Selvaraju T. Hierarchical electroless Pt deposition at Au decorated reduced graphene oxide via a galvanic exchanged process: An electrocatalytic nanocomposite with enhanced mass activity for methanol and ethanol oxidation. J. Mater. Chem. A 2015, 3, 18010–18018. 10.1039/C5TA04293C. [DOI] [Google Scholar]
- Dutta A.; Ouyang J. Enhanced electrocatalytic performance on polymer-stabilized graphene decorated with alloy nanoparticles for ethanol oxidation reaction in alkaline media. Monodispersed porous flowerlike PtAu nanocrystals as effective electrocatalysts for ethanol oxidation. Appl. Catal., B 2014, 158–159, 119–128. 10.1016/j.apcatb.2014.04.018. [DOI] [Google Scholar]
- Hu X.; Lin C.; Wei L.; Hong C.; Zhang Y.; Zhuang N. High electrocatalytic performance of graphene nanoribbon supported PtAu nanoalloy for direct ethanol fuel cell and theoretical analysis of anti-CO poisoning. Electrochim. Acta 2016, 187, 560–566. 10.1016/j.electacta.2015.11.100. [DOI] [Google Scholar]
- Sarkar S.; Jana R.; Vadlamani H.; Ramani S.; Mumbaraddi D.; Peter S. C. Facile Aqueous-Phase Synthesis of the PtAu/Bi2O3 Hybrid Catalyst for Efficient Electro-Oxidation of Ethanol. ACS Appl. Mater. Interfaces 2017, 9, 15373–15382. 10.1021/acsami.7b00083. [DOI] [PubMed] [Google Scholar]
- Amin S.; Tahira A.; Solangi A. R.; Mazzaro R.; Ibupoto Z. H.; Fatima A.; Vomiero A. Functional Nickel Oxide Nanostructures for Ethanol Oxidation in Alkaline Media. Electroanalysis 2020, 32, 1052–1059. 10.1002/elan.201900662. [DOI] [Google Scholar]
- Ren J.; Yang Y.; Zhang B.; Tiana N.; Cai W.; Zhou Z.; Sun S. H–D kinetic isotope effects of alcohol electrooxidation on Au, Pd and Pt electrodes in alkaline solutions. Electrochem. Commun. 2013, 37, 49–52. 10.1016/j.elecom.2013.10.009. [DOI] [Google Scholar]
- Wang B.; Tao L.; Cheng Y.; Yang F.; Jin Y.; Zhou C.; Yu H.; Yang Y. Electrocatalytic Oxidation of Small Molecule Alcohols over Pt, Pd, and Au Catalysts: The Effect of Alcohol’s Hydrogen Bond Donation Ability and Molecular Structure Properties. Catalysts 2019, 9, 387 10.3390/catal9040387. [DOI] [Google Scholar]
- Ruiz-Camacho B.; Medina-Ramirez A.; Villicana Aguilera M.; Minchaca-Mojica J. I. Pt supported on mesoporous material for methanol and ethanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2019, 44, 12365–12373. 10.1016/j.ijhydene.2019.01.180. [DOI] [Google Scholar]
- Liu J.; Ye J.; Xu C.; Jiang S. P.; Tong Y. Electro-oxidation of methanol, 1-propanol and 2-propanol on Pt and Pd in alkaline medium. J. Power Sources 2008, 177, 67–70. 10.1016/j.jpowsour.2007.11.015. [DOI] [Google Scholar]
- Liu Y.; Zeng Y.; Liu R.; Wu H.; Wang G.; Cao D. Poisoning of acetone to Pt and Au electrodes for electrooxidation of 2-propanol in alkaline medium. Electrochim. Acta 2012, 76, 174–178. 10.1016/j.electacta.2012.04.130. [DOI] [Google Scholar]
- Mourdikoudis S.; Chirea M.; Zanaga D.; Altantzis T.; Mitrakas M.; Bals S.; Liz-Marzán L. M.; Pérez-Juste J.; Pastoriza-Santos I. Governing the morphology of Pt–Au heteronanocrystals with improved electrocatalytic performance. Nanoscale 2015, 7, 8739–8747. 10.1039/C4NR07481E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Njoki P. N.; Lin Y.; Mott D.; Wang W.; Zhong C. Characterization of Carbon-Supported AuPt Nanoparticles for Electrocatalytic Methanol Oxidation Reaction. Langmuir 2006, 22, 2892–2898. 10.1021/la0529557. [DOI] [PubMed] [Google Scholar]
- Mott D.; Luo J.; Njoki P. N.; Lin Y.; Wang L.; Zhong C. Synergistic activity of gold-platinum alloy nanoparticle catalysts. Catal. Today 2007, 122, 378–385. 10.1016/j.cattod.2007.01.007. [DOI] [Google Scholar]
- Figueiredo M. C.; Aran-Ais R. M.; Climent V.; Kallio T.; Feliu J. M. Evidence of Local pH Changes during Ethanol Oxidation at Pt Electrodes in Alkaline Media. ChemElectroChem 2015, 2, 1254–1258. 10.1002/celc.201500151. [DOI] [Google Scholar]




