Abstract
Background
Primary or acquired chemoresistance is a key link in the high mortality rate of ovarian cancer. There is no reliable method to predict chemoresistance in ovarian cancer. We hypothesized that specific methylation characteristics could distinguish chemoresistant and chemosensitive ovarian cancer patients.
Methods
In this study, we used 450 K Infinium Methylation BeadChip to detect the different methylation CpGs between ovarian cancer patients. The differential methylation genes were analyzed by GO and KEGG Pathway bioinformatics analysis. The candidate CpGs were confirmed by pyrosequencing. The expression of abnormal methylation gene was identified by QRT-PCR and IHC. ROC analysis confirmed the ability to predict chemotherapy outcomes. Prognosis was evaluated using Kaplan–Meier.
Results
In advanced high-grade serous ovarian cancer, 8 CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625) remained hypermethylated in chemoresistant patients. The sensitivity, specificity and AUC of 8 CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625) methylation to predict chemotherapy sensitivity were 63.60–97.00%, 46.40–89.30% and 0.774–0.846. PFS of 6 candidate genes (ITGB6:cg21105318, cg07896068; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934) hypermethylation patients was significantly shorter. The expression of NCALD and LAMA3 in chemoresistant patients was lower than that of chemosensitive patients. Spearman analysis showed that NCALD and LAMA3 methylations were negatively correlated with their expression.
Conclusions
As a new biomarker of chemotherapy sensitivity, hypermethylation of NCALD and LAMA3 is associated with poor PFS in advanced high-grade serous ovarian cancer. In the future, further research on NCALD and LAMA3 will be needed to provide guidance for clinical stratification of demethylation therapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13148-021-01133-2.
Keywords: Ovarian cancer, Chemotherapy resistance, DNA methylation, 450 K Infinium Methylation BeadChip, Prognosis
Background
Ovarian cancer is the most lethal cancer of the female reproductive system. In 2019, 22,530 new cases and 13,980 death occurred in the USA [1]. For the first diagnosis of ovarian cancer patients, the standard treatment is the optimal surgical cytoreduction combined with platinum-based chemotherapy [2]. In the past decade, the survival rate of ovarian cancer has changed only a little, and the 5-year survival rate has remained below 30% [3, 4]. Primary or acquired chemoresistance is a key link in the high mortality rate of ovarian cancer [5, 6]. If the patient’s sensitivity to traditional chemotherapy can be assessed before treatment, doctors can guide chemoresistant patients to undergo clinical trials to seek survival opportunities. Therefore, it is necessary to use robust and sensitive biomarkers to predict chemotherapy outcome in ovarian cancer patients.
At present, there is no reliable method to predict chemoresistance in ovarian cancer. DNA methylation as a biomarker has the following advantages: chemical stability, quantitative detection, chemoresistance-related methylation changes usually occur before the start of chemoresistance, noninvasive detection (can be detected in the patient's body fluids) [7]. With the update of DNA methylation detection platform and technology, more and more genes involved in ovarian cancer chemoresistance have been reported. Except BRCA1, DNA damage repair pathway-related genes, PTEN, RASSF1, MDR1 and FANCF gene hypermethylation were positively correlated with chemotherapy sensitivity. In recent years, new DNA methylation studies related to chemotherapy resistance of ovarian cancer include at least MLH1 [8], SERPINE1 [9], TRIB2 [10], KLF4 [11], FZD10 [12], ZNF671 [13], ABCB1 [14], hMSH2 [15] and other genes. This shows that the regulatory mechanism of DNA methylation in ovarian cancer chemotherapy resistance is complex and diverse. A variety of methylated genes interact with each other, which together leads to chemotherapy resistance in ovarian cancer.
Relative to genetic mutations, DNA methylation can be reversed. Demethylation drugs can reverse abnormal methylation, improve the sensitivity of ovarian cancer patients to chemotherapy drugs, improve efficacy and prolong survival [16–18]. Currently, there are few results on genome-wide methylation in chemoresistant ovarian cancer patients. Here, we used 450 K Infinium Methylation BeadChip to study the genome-wide methylation characteristics of chemotherapy resistance in ovarian cancer.
Materials and methods
Patients
We collected the initial surgical samples of ovarian cancer patients (only carcinomas and not borderline tumors) from the Guangxi Medical University Cancer Hospital. A total of 108 frozen samples (epithelial ovarian cancer) were used for 450 K Infinium Methylation BeadChip, pyrosequencing and QRT-PCR. 132 paraffin samples (advanced high-grade serous ovarian carcinoma) were used for immunohistochemistry. All patients had complete chemotherapy outcome records and postoperative pathological diagnosis. All patients provided written informed consent and were approved by the institutional review committee of Guangxi Medical University Cancer Hospital.
Follow-up
OS (overall survival) was defined as the time from the diagnosis to the death from ovarian cancer, and the survival data of the last follow-up survivors were recorded as censored data. PFS (progression-free survival) was defined as the time from initial treatment to tumor progression. Response to treatment was evaluated using the Response Evaluation Criteria In Solid Tumors (RECIST 1.1 criteria) [19]. Platinum-resistant and refractory ovarian cancer was defined as those whose disease had progressed during first-line platinum-based chemotherapy or relapsed within 6 months after the last platinum treatment [20]. Platinum-sensitive ovarian cancer was defined as relapse more than 6 months after platinum-based chemotherapy [21].
Study design
In this study, we included two stages to identify and validate chemoresistance-related CpGs in ovarian cancer. In the discovery stage, 450 K Infinium Methylation BeadChip was used for screening, and enrichment analysis was used to select biologically meaningful CpGs. The relationship between abnormal methylation and chemotherapy resistance and prognosis was analyzed. In the verification stage, candidate CpGs were verified by pyrosequencing. To clarify whether differential CpGs play a biological function, we used QRT-PCR and immunohistochemistry to detect gene expression.
450K Infinium Methylation BeadChip
Qualified DNA was extracted from 108 samples. DNA was modified by Epitect bisulfite kit (Qiagen, Cat. No. 59110, Germany) and analyzed by 450 K Infinium Methylation BeadChip (Illumina, San Diego, CA, USA) in Shanghai Jingneng company. The methylation level was scored with standardized beta score values ranging from 0 (unmethylated) to 1 (fully methylated). Limma package (R) calculates differential methylation sites between chemotherapy-resistant and chemotherapy-sensitive patients (P ≤ 0.01, |Diff Beta Score| ≥ 0.1). Go (http://geneontology.org/) and KEGG (http://www.genome.jp/kegg/) databases were used to enrich the differential methylation genes. The candidate CpGs were selected according to the Diff Beta Score value and biological function.
Literature search strategy
Gene search strategies were based on Yan's article [22]. Used ‘ovarian cancer’ or ‘ovarian carcinoma,’ ‘DNA methylation’ or ‘methylation,’ ‘resistant’ or ‘resistance’ or ‘chemoresistance’ as keywords, we screened methylated genes associated with the regulation of drug resistance in ovarian cancer from an advanced search in the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/). The search date was updated to May 4, 2021.
Pyrosequencing
We used pyrosequencing to quantitatively determine the methylation level of candidate CpGs. Pyrosequencing primers were designed using PyroMark Assay Design 2.0 software. The primer sequences are shown in Additional file 1: Table S1. Bisulfite modified DNA amplified by PyroMark PCR Kit (Qiagen, Cat. No. 978703, Germany). The reaction steps were as follows: polymerase activation (95°, 3 min), 40 cycles of denaturation (94°, 30 s), annealing (52°, 30 s), extension (72°, 1 min) and final extension 72°, 7 min. The PCR products were qualitatively and quantitatively analyzed by 1% agarose gel electrophoresis.
QRT-PCR
RNA extraction kit (Thermo Scientific, Cat. No. K0731, USA) for RNA extraction. Reverse transcription kit (Thermo Scientific, Cat. No. K1622, USA) for reverse transcription of cDNA. The polymerase chain reaction was performed used a fluorescent quantitative PCR kit (Taraka, Cat. No. DRR820A, Japan). The primer sequences are shown in Additional file 1: Table S2. The 2−ΔCt method calculated the relative expression levels of candidate genes. GAPDH was used as an internal control, and all experiments were repeated three times.
Immunohistochemistry
NCALD, ITGB6 and LAMA3 concentration were 1:400 (Abcam, Cat. No.ab155161, UK), 1:20 (Abcam, Cat. No. ab197672, UK) and 1:50 (Abcam, Cat. No. ab217213, UK). NCALD, ITGB6, LAMA3 were mainly expressed in the cytoplasm, and a small amount was expressed in the cell membrane. Two pathologists read the pathological sections independently. The score criteria were as follows: Positive cell ratio of < 1, 1–25%, 25–50%, 50–75% and 75–100% were assigned 0, 1, 2, 3, 4 points, respectively. Stain intensity of no coloring, light yellow, yellow, brown was assigned 0, 1, 2, 3 points, respectively. The product of positive cell ratio and stain intensity was stain index. Stain index ≤ 6 points were classified as a low expression, while > 6 points were classified as a high expression [23].
Statistical analysis
Except for 450 K Infinium Methylation BeadChip, the rest of the statistical analysis was performed using SPSS 17.0. ROC analysis confirmed the ability to predict chemotherapy outcomes. The cutoff point corresponding to the maximum Youden's index was the cutoff value. According to the cutoff value, patients with ovarian cancer were divided into hypermethylation and hypomethylation. T test (measurement data) and chi-square test (categorical data) were used for comparison between the two groups. The association between CpGs and prognosis was assessed by the Kaplan–Meier plotter. Spearman analyzed the relationship between two variables. P < 0.05 was considered statistically significant.
Results
Study population
The median age of patients was 50.98 ± 10.40 years old. The clinical characteristics are shown in Table 1. Among the frozen samples, 85 patients had FIGO stage III–IV and 23 patients had FIGO stage I–II. There were 91 patients with serous type, 14 patients with mucinous type and 3 patients with endometrioid histology types. There were 74 patients with high-grade serous carcinoma and 17 patients with low-grade serous carcinoma. There were 11 patients with grade 3 and 6 patients with grade 1–2 in mucinous carcinoma and endometrioid carcinoma. According to the postoperative chemotherapy scheme, there were 57 patients with TP (paclitaxel plus cisplatin) and 51 patients with TC (paclitaxel plus carboplatin). CpGs were verified in FIGO stage III–IV high-grade serous ovarian cancer, so the corresponding gene expression detection was carried out in advanced high-grade serous paraffin samples.
Table 1.
Clinical characteristics of patients
| Clinical characteristics | Frozen samples (N = 108) | Paraffin samples (N = 132) | All (N = 240) |
|---|---|---|---|
| Age | 48.12 ± 11.47 | 53.32 ± 8.79 | 50.98 ± 10.40 |
| FIGO stage | |||
| I–II | 23 | – | 23 |
| III–IV | 85 | 132 | 217 |
| Grade (serous) | |||
| 3 | 74 | 132 | 206 |
| 1 | 17 | – | 17 |
| Grade (mucinous and endometrioid) | |||
| 3 | 11 | – | 11 |
| 2 | 1 | – | 1 |
| 1 | 5 | – | 5 |
| Histology types | |||
| Serous | 91 | 132 | 223 |
| Mucinous | 14 | – | 14 |
| Endometrioid | 3 | – | 3 |
| Surgical debulking | |||
| Optimal | 76 | 96 | 172 |
| Suboptimal | 32 | 36 | 68 |
| Chemotherapy outcome | |||
| Chemoresistant | 55 | 56 | 111 |
| Chemosensitive | 53 | 76 | 129 |
| Postoperative chemotherapy scheme | |||
| TP (paclitaxel plus cisplatin) | 57 | 58 | 115 |
| TC (paclitaxel plus carboplatin) | 51 | 74 | 125 |
Genome-wide DNA methylation between chemoresistant patients and chemosensitive patients in epithelial ovarian cancer
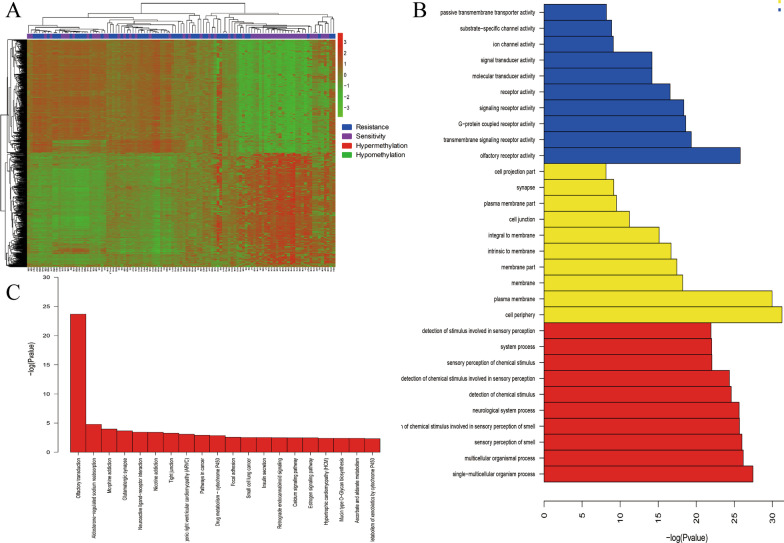
The 450 K Infinium Methylation BeadChip contains 450,000 CpGs, covering 96% of CpG islands. Almost all the methylation genes annotated by NCBI are covered. Since most functionally related DNA methylation occurs on the CpG island of gene promoters, we prefer to select candidate CpG in the promoter region. 7263 CpGs showed significant differences between chemoresistant and chemosensitive epithelial ovarian cancer patients (55 chemoresistant and 53 chemosensitive patients), corresponding to 2654 genes. See Fig. 1a. Compared with chemosensitive patients, there are 6051 hypermethylated CpG loci (corresponding to 2162 genes) and 1212 hypomethylated CpG loci (corresponding to 452 genes) in chemoresistant patients. The difference CpGs in promoter region corresponds to 1058 genes, and the difference CpGs in the 5′UTR region corresponds to 305 genes. The signal pathways of differential methylation genes enriched in KEGG include drug metabolism-cytochrome P450, focal adhesion, calcium signaling pathway, PI3K-Akt signaling pathway and ErbB signaling pathway. Similarly, the biological process of GO enrichment includes the multicellular organismal process, cell adhesion, cell migration and calcium ion binding. See Fig. 1b, c and Additional file 1: Tables S3–S4.
Fig. 1.
Genome-wide DNA methylation in epithelial ovarian cancer: a heat map of differential methylated genes. b The biological process of differential methylation genes (GO). c The signal pathways of differential methylation genes (KEGG)
We systematically searched the literature in the PubMed database and obtained 53 methylated genes related to chemotherapy resistance in ovarian cancer. Among them, 22 methylation genes related to ovarian resistance reported in the literature are enriched in our Methylation BeadChip results. The difference of BRCA1, CD133, ASS1, ABCG2, TGFBI, RGS10, UCHL1, CLDN4, HOXA10, DOK2, AGR2 and OXCT1 gene in our 450 K Infinium Methylation BeadChip is consistent with that reported in the literature. The difference of DNAJC15, RASSF1, HOXA9 and SFRP5 gene in our 450 K Infinium Methylation BeadChip is contrary to that reported in the literature. There is no significant difference in MLH1, FBXO32, PTEN, MAL, TUBB3, FANCF gene in our Methylation BeadChip, see Table 2 [9–12, 15, 24–65]. Because there are few studies on hypomethylation, we prefer to study hypermethylation genes in ovarian cancer chemoresistance. Based on the Diff Beta Score value, CpG region, KEGG and GO analysis, we selected 9 candidate CpGs with fewer reports in the current research literature, corresponding to 4 methylated genes (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; PIK3R3: cg27584146; LAMA3: cg20937934, cg13270625). Diff Beta Score is given in Table 3.
Table 2.
The methylated genes and ovarian cancer multidrug resistance in the literature
| Author | Year | N | Population | Gene | Tissue | Methods | Cell | Methylation level in chemoresistant tissue/cell | Expression | Drugs | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strathdee | 2005 | 41 | UK | MCJ | Advanced EOC | Bisulfite sequencing | – | Hypermethylation | Downregulation | Cisplatin | [24] |
| Lee | 2009 | 61 | UK | MAL | SOC | Bisulfite sequencing | – | Hypomethylation | Upregulation | Platinum | [25] |
| Watanabe/Gifford | 2007/2004 | 36/138 | Japan/UK | hMLH1 | Advanced EOC | MSP | – | Hypermethylation | Downregulation | Platinum | [26, 27] |
| Wang | 2010 | – | – | BRCA1 | – | Bisulfite sequencing | COC1/COC1CisR | Hypomethylation | Upregulation | Cisplatin | [28] |
| Chou | 2010 | 96 | China | FBXO32 | OC | MSP | – | Hypermethylation | Downregulation | Cisplatin | [29] |
| Nicholson | 2009 | 54 | UK | ASS1 | EOC | Bisulfite sequencing | – | Hypermethylation | Downregulation | Cisplatin | [63] |
| GAO | 2019 | – | – | RASSF1A | – | MSP | A2780/A2780CisR | Hypermethylation | Downregulation | Cisplatin | [31] |
| GAO | 2019 | – | – | MDR1 | – | MSP | A2780/A2780CisR | Hypomethylation | Upregulation | Cisplatin | [31] |
| Dai | 2012 | – | – | PTEN | – | Bisulfite sequencing | OVCAR3 | Hypermethylation | Downregulation | Taxol | [32] |
| Li | 2009 | – | – | DR4 | – | MSP | 2008, Hey, NMP-1, OVCAR3, SKOV3, A2780 | Hypermethylation | Downregulation | Platinum | [33] |
| Bram | 2009 | – | – | ABCG2 | – | COBRA | IGROV1 | Hypomethylation | Upregulation | Platinum | [34] |
| Chiang | 2013 | 136 | China | BLU | Advanced HGSOC | MSP | SKOV3, OVCAR3 | Hypermethylation | Downregulation | Platinum | [35] |
| Wang | 2012 | 40 | China | TGFBI | EOC | MSP | SKOV3/SKOV3CisR, SKOV3/TR, A2780/TR | Hypermethylation | Downregulation | Cisplatin and palitaxel | [36] |
| Ali | 2013 | – | – | RGS10 | – | Bisulfite sequencing | A2780/A2780CisR, CAOV3, SKOV3 | Hypermethylation | Downregulation | Cisplatin | [37] |
| Okochi-Takada | 2006 | 17 | Japan | UCHL1 | OC | MSP | 13 ovarian cancer cell lines | Hypermethylation | Downregulation | Cisplatin | [38] |
| Staub | 2007 | – | – | HSulf-1 | – | MSP | SKOV3, OV207 | Hypermethylation | Downregulation | Platinum | [39] |
| Su | 2010 | 126 | China | SFRP5 | EOC | MSP | – | Hypermethylation | Downregulation | Cisplatin | [40] |
| IZUTSU | 2008 | 66 | Japan | TUBB3 | OC | Bisulfite sequencing | OVCAR-3, JHOC-8 | Hypomethylation | Upregulation | Paclitaxel | [41] |
| Pattamadilok | 2008 | 59 | Thailand | LINE-1 | EOC | COBRA | – | Hypomethylation | Unknown | Platinum | [42] |
| Jiang | 2014 | – | – | HOXA10 | – | MSP | SKOV3, HEY | Hypomethylation | Upregulation | Platinum | [43] |
| Matei | 2012 | 17 | America | HOXA9, HOXA11 | OC | Genome-wide methylation study | – | Hypermethylation | Unknown | Platinum | [44] |
| Taniguchi | 2003 | 19 | America | FANCF | OC | MSP | – | Hypomethylation | Upregulation | Cisplatin | [45] |
| YANG | 2018 | – | – | OXCT1 | – | Genome-wide methylation study | 8 ovarian cancer cell lines | Hypermethylation | Downregulation | Cisplatin | [46] |
| Zhao | 2021 | 483 | GEO and TCGA | AGR2, HSPA2, ACAT2 | SOC | Genome-wide methylation study | – | Hypermethylation | Unknown | Platinum | [47] |
| Pan | 2017 | – | – | SERPINE1 | – | MassArray EpiTYPER | A2780CP | Hypomethylation | Upregulation | Carboplatin | [9] |
| Zeller | 2012 | – | – | ARMCX2, COL1A1, MDK, MEST | – | Genome-wide methylation study | A2780CisR | Hypermethylation | Downregulation | Cisplatin | [48] |
| Ha | 2018 | – | – | NAGA | – | Genome-wide methylation study | 11 ovarian cancer cell lines | Hypermethylation | Downregulation | Cisplatin | [49] |
| Visco | 2021 | 16 | America | CLDN1 | Advanced SOC | Genome-wide methylation study | – | Hypomethylation | Upregulation | Cisplatin | [50] |
| Bonito | 2016 | 61 | UK | MSX1 | HGSOC | Genome-wide methylation study | – | Hypomethylation | Upregulation | Platinum | [51] |
| Tomar | 2016 | – | – | CSK | HGSOC PDXs | Genome-wide methylation study | – | Hypermethylation | Downregulation | Platinum | [52] |
| Fang | 2018 | 120 | USA | DOK2 | OC | Genome-wide methylation study | OVCAR3, SKOV3 | Hypermethylation | Downregulation | Platinum | [53] |
| Yu | 2011 | – | – | PTK6, PRKCE, BCL2L1 | – | Genome-wide methylation study | A2780CisR | Hypomethylation | Upregulation | Cisplatin | [54] |
| Teschendorff | 2015 | 134 | UK | HOTAIR | OC | Genome-wide methylation study | – | Hypomethylation | Upregulation | Platinum | [55] |
| Tian | 2019 | 16 | China | hMSH2 | EOC | Bisulfite sequencing | – | Hypermethylation | Downregulation | Platinum | [15] |
| Syed | 2011 | 52 | Germany | Plk2 | EOC | MSP | A2780CisR, SKOV3CisR | Hypermethylation | Downregulation | Cisplatin | [56] |
| Leon | 2016 | – | – | TMEM88 | OC xenografts | Genome-wide methylation study | – | Hypomethylation | Upregulation | Platinum | [57] |
| Mase | 2019 | 78 | Japan/China | ZNF671 | HGSOC | Genome-wide methylation study | JHOS2/4, OVCAR3 | Hypermethylation | Downregulation | Platinum | [58] |
| Baba | 2009 | – | – | CD133/PROM1 | – | MSP | OVCAR8/432, A2780, PEO1 | Hypomethylation | Upregulation | Platinum | [59] |
| Shang/Litkouhi | 2013/2007 | – | – | CLDN4 | – | MSP | 2008 | Hypermethylation | Downregulation | Cisplatin | [60, 61] |
| Witham | 2008 | – | – | DNAJC15 | – | Bisulfite sequencing | OVCAR3/4/5/8, SKOV3 | Hypermethylation | Downregulation | Platinum | [62] |
| Kritsch | 2017 | – | – | TRIB2 | – | Genome-wide methylation study | A2780CisR, SKOV3CisR | Hypermethylation | Downregulation | Cisplatin | [10] |
| Lund | 2017 | – | – | KLF4 | – | Genome-wide methylation study | M019iCisR, OC002CisR | Hypermethylation | Downregulation | Cisplatin | [11] |
| Tomar | 2017 | 45 | Netherlands | FZD10 | HGSOC | Genome-wide methylation study | 10 ovarian cancer cell lines | Hypomethylation | Upregulation | Platinum | [12] |
| Vaclavikova | 2019 | 61 | Czech Republic | ABCB1 | EOC | Bisulfite sequencing | – | Hypomethylation | Upregulation | Platinum | [63] |
| Yao/Duan | 2004/1999 | – | – | TRAG-3/CSAG2 | – | COBRA | SKOV3 | Hypomethylation | Upregulation | Taxol | [30, 65] |
EOC epithelial ovarian cancer, SOC serous ovarian cancer, HGSOC high-grade serous ovarian cancer, MSP methylation-specific polymerase chain reaction, COBRA combined bisulfite restriction analysis, CisR cisplatin resistant, TR palitaxel resistant, CP carboplatin resistant
Table 3.
Hypermethylated genes in chemoresistant ovarian cancer patients (epithelial ovarian cancer)
| Genes | Gene description | Diff beta score | P value |
|---|---|---|---|
| NCALD | Neurocalcin delta | 0.202116802 | 2.16E−08 |
| SLC1A6 | Solute carrier family 1 | 0.169488766 | 3.19E−08 |
| RXRG | Retinoid X receptor, gamma | 0.165189407 | 6.28E−06 |
| ITGB6 | Integrin, beta 6 | 0.162725093 | 1.07E−05 |
| DLG2 | Disks, large homolog 2 (Drosophila) | 0.161362933 | 1.22E−03 |
| CTBP2 | C-terminal binding protein 2 | 0.156564368 | 4.62E−06 |
| LAMA3 | Laminin, alpha 3 | 0.15511936 | 1.84E−07 |
| OR1E2 | Olfactory receptor, family 1, subfamily E, member 2 | 0.151817648 | 8.27E−08 |
| OR5T3 | Olfactory receptor, family 5, subfamily T, member 3 | 0.151025609 | 1.57E−07 |
| OR4B1 | Olfactory receptor, family 4, subfamily B, member 1 | 0.150708678 | 4.06E−08 |
| EPB41L1 | Erythrocyte membrane protein band 4.1-like 1 | 0.149492829 | 3.13E−05 |
| OR8H3 | Olfactory receptor, family 8, subfamily H, member 3 | 0.149428771 | 8.58E−08 |
| GABRA6 | Gamma-aminobutyric acid (GABA) A receptor, alpha 6 | 0.14743259 | 1.24E−07 |
| OR10A5 | Olfactory receptor, family 10, subfamily A, member 5 | 0.146965271 | 2.03E−06 |
| RIMS1 | Regulating synaptic membrane exocytosis 1 | 0.146489176 | 6.40E−07 |
| GCNT3 | Glucosaminyl (N-acetyl) transferase 3, mucin type | 0.144933199 | 7.31E−06 |
| MYH4 | Myosin, heavy chain 4, skeletal muscle | 0.14130487 | 1.29E−06 |
| PIK3R3 | Phosphoinositide-3-kinase, regulatory subunit 3 (gamma) | 0.13020729 | 1.13E−03 |
Genome-wide DNA methylation between chemoresistant patients and chemosensitive patients in advanced high-grade serous ovarian cancer
High-grade serous ovarian cancer (HGSOC) is the most common ovarian cancer subtype and accounts for 80% of the deaths caused by the disease. Advanced (FIGO stage III and IV) HGSOC is one of the hardest human malignancies to treat. We performed genome-wide methylation analysis in advanced high-grade serous ovarian cancer (33 chemoresistant and 28 chemosensitive patients). In advanced high-grade serous ovarian cancer, 3446 CpGs showed significant differences between chemoresistant and chemosensitive patients, corresponding to 855 genes. Compared with chemosensitive patients, there are 2707 hypermethylated CpGs (corresponding to 1611 genes) and 739 hypomethylated CpGs (corresponding to 344 genes) in advanced high-grade serous chemoresistant patients. In advanced high-grade serous ovarian cancer, 8 CpGs remained hypermethylated in chemoresistant patients (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625).The difference between the 6 CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707) in chemoresistant patients and sensitive patients is more than 0.2.
The ability of candidate CpGs to predict chemotherapy sensitivity in advanced high-grade serous ovarian cancer
There is a need to develop and validate biomarkers for chemotherapy response and survival in advanced high-grade serous ovarian cancer (N = 61). The sensitivity, specificity and AUC of 8 candidate CpGs methylation to predict chemotherapy sensitivity were 63.60–97.00%, 46.40–89.30% and 0.774–0.846. In SPSS, 8 CpGs were included in binary logistic regression to produce a predicted value. ROC analysis was performed on the predicted value. The sensitivity, specificity and AUC of 8 candidate CpGs methylation combined to predict chemotherapy sensitivity were 69.70%, 92.90% and 0.867 (95% CI 0.774–0.960, P < 0.001), see Table 4. ROC curve is shown in Fig. 2.
Table 4.
The ability of CpGs to predict chemotherapy sensitivity in advanced HGSOC
| CpGid | AUC | 95% CI | P | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| cg21105318 | 0.774 | 0.658–0.890 | 0.000 | 42.77 | 0.970 | 0.464 |
| cg07896068 | 0.811 | 0.703–0.918 | 0.000 | 78.21 | 0.636 | 0.893 |
| cg18437633 | 0.785 | 0.667–0.902 | 0.000 | 81.02 | 0.667 | 0.786 |
| cg27637873 | 0.846 | 0.749–0.944 | 0.000 | 77.79 | 0.818 | 0.786 |
| cg26782361 | 0.835 | 0.735–0.936 | 0.000 | 74.21 | 0.727 | 0.821 |
| cg16265707 | 0.814 | 0.708–0.919 | 0.000 | 72.26 | 0.636 | 0.857 |
| cg20937934 | 0.787 | 0.672–0.902 | 0.000 | 63.65 | 0.879 | 0.571 |
| cg13270625 | 0.787 | 0.672–0.901 | 0.000 | 60.16 | 0.758 | 0.714 |
| Combined | 0.867 | 0.774–0.960 | 0.000 | 68.63 | 0.697 | 0.929 |
Fig. 2.
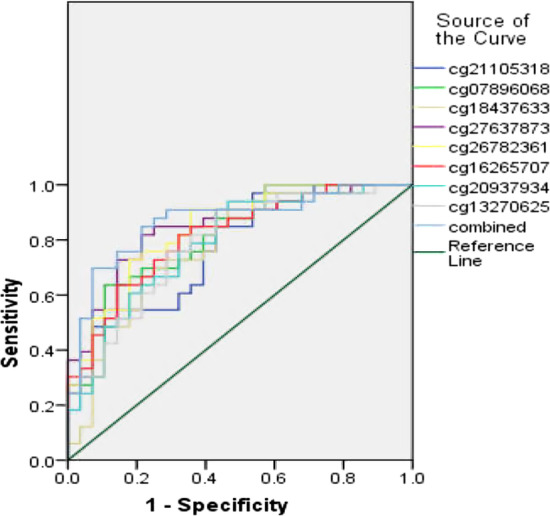
ROC curve of candidate CpGs to predict chemotherapy sensitivity in advanced HGSOC
Hypermethylation of CpGs are associated with poor PFS in advanced high-grade serous ovarian cancer (the total group)
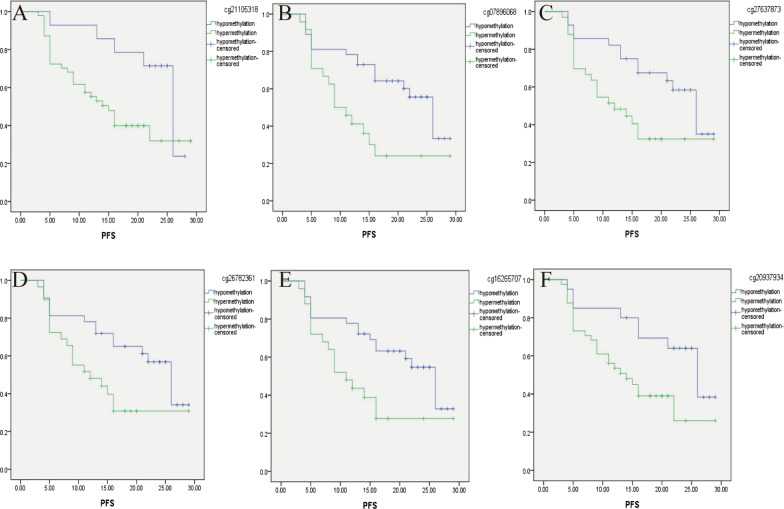
According to the ROC analysis result of the 450 K Infinium Methylation BeadChip, the cutoff values of 8 CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625) methylation were 42.77, 78.21, 81.02, 77.79, 74.21, 72.26, 63.65 and 60.16, respectively, in advanced high-grade serous ovarian cancer (N = 61). According to the cutoff value of 8 CpGs methylation, patients were divided into hypomethylation patients and hypermethylation patients. Kaplan–Meier analysis showed that 6 candidate genes (ITGB6:cg21105318, cg07896068; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934) hypermethylation can divide patients into high-risk patients and low-risk patients to chemotherapy. Compared with hypomethylation patients, PFS of 6 candidate genes (ITGB6:cg21105318, cg07896068; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934) hypermethylation patients was significantly shorter, see Table 5. The survival curve is shown in Fig. 3a–f. There was no significant difference between OS.
Table 5.
The relationship between CpGs methylation and PFS in advanced HGSOC
| CpGid | Advanced HGSOC (the total group) | Advanced HGSOC with complete debulking | Advanced HGSOC with incomplete debulking | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (months) | 95% CI | P | Mean (months) | 95% CI | P | Mean (months) | 95% CI | P | |
| cg21105318 | |||||||||
| Hypomethylation | 22.98 | 19.43–26.52 | 0.020 | 25.63 | 22.65–28.60 | 0.086 | 20.33 | 12.63–28.04 | 0.08 |
| Hypermethylation | 16.18 | 13.13–19.23 | 18.07 | 14.00–22.14 | 12.39 | 9.00–15.78 | |||
| cg07896068 | |||||||||
| Hypomethylation | 20.55 | 17.44–23.66 | 0.006 | 23.50 | 19.92–27.09 | 0.004 | 16.14 | 11.04–21.25 | 0.343 |
| Hypermethylation | 13.66 | 9.74–17.57 | 14.14 | 8.43–19.86 | 12.30 | 8.04–16.56 | |||
| cg27637873 | |||||||||
| Hypomethylation | 21.36 | 18.02–24.70 | 0.010 | 23.89 | 20.20–27.58 | 0.015 | 16.67 | 10.43–22.91 | 0.437 |
| Hypermethylation | 15.18 | 11.59–18.78 | 16.27 | 11.00–21.53 | 13.00 | 9.10–16.90 | |||
| cg26782361 | |||||||||
| Hypomethylation | 20.66 | 17.33–23.99 | 0.019 | 22.86 | 18.89–26.83 | 0.068 | 17.17 | 11.55–22.79 | 0.168 |
| Hypermethylation | 15.08 | 11.35–18.81 | 17.18 | 11.84–22.51 | 11.25 | 8.04–14.46 | |||
| cg16265707 | |||||||||
| Hypomethylation | 20.37 | 17.21–23.52 | 0.013 | 22.89 | 19.27–26.52 | 0.022 | 16.23 | 10.76–21.71 | 0.309 |
| Hypermethylation | 14.37 | 10.41–18.33 | 16.14 | 10.22–22.06 | 12.55 | 8.56–16.53 | |||
| cg20937934 | |||||||||
| Hypomethylation | 21.97 | 18.07–25.87 | 0.023 | 24.30 | 20.15–28.45 | 0.073 | 18.00 | 10.04–25.96 | 0.212 |
| Hypermethylation | 15.63 | 12.35–18.91 | 16.80 | 12.19–21.42 | 12.88 | 9.44–16.33 | |||
Fig. 3.
CpGs hypermethylation associated with poor PFS in advanced HGSOC (a ITGB6/cg21105318, b ITGB6/cg07896068, c NCALD/cg27637873, d NCALD/cg26782361, e NCALD/cg16265707 and f LAMA3/cg20937934)
Relationship between candidate CpGs methylation and PFS in advanced HGSOC patients with different surgical outcomes
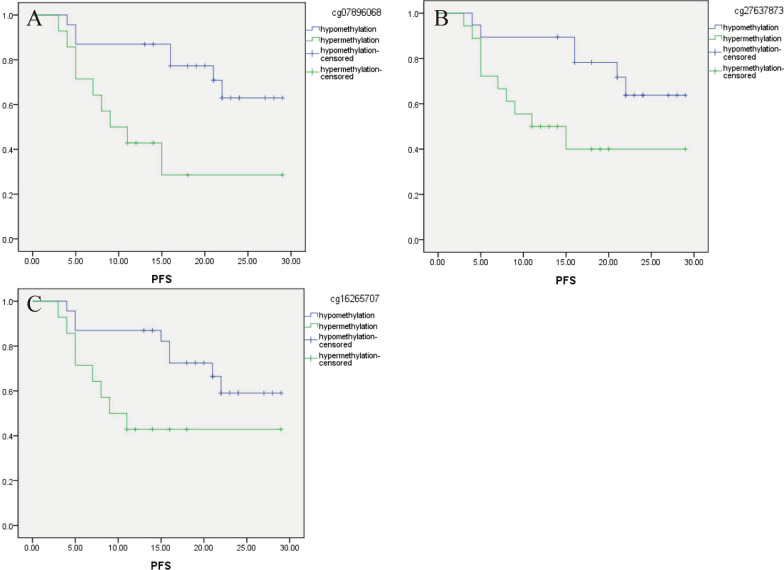
Patients with complete debulking have a better progression-free survival. To exclude the effect of the surgical outcome on prognosis, we analyzed the relationship between 6 CpGs and PFS in patients with different surgical outcomes. In advanced high-grade serous ovarian cancer with complete debulking (N = 37), PFS of 3 candidate CpGs (ITGB6:cg07896068; NCALD: cg27637873, cg16265707) hypermethylation patients was significantly shorter. The survival curve is shown in Fig. 4a–c. There was no significant difference between PFS in advanced high-grade serous ovarian cancer with incomplete debulking (N = 24), see Table 5.
Fig. 4.
CpGs hypermethylation associated with poor PFS in advanced HGSOC with complete debulking (a ITGB6/cg07896068, b NCALD/cg27637873 and c NCALD/ cg16265707)
Identification of candidate CpGs by pyrosequencing in advanced high-grade serous ovarian cancer
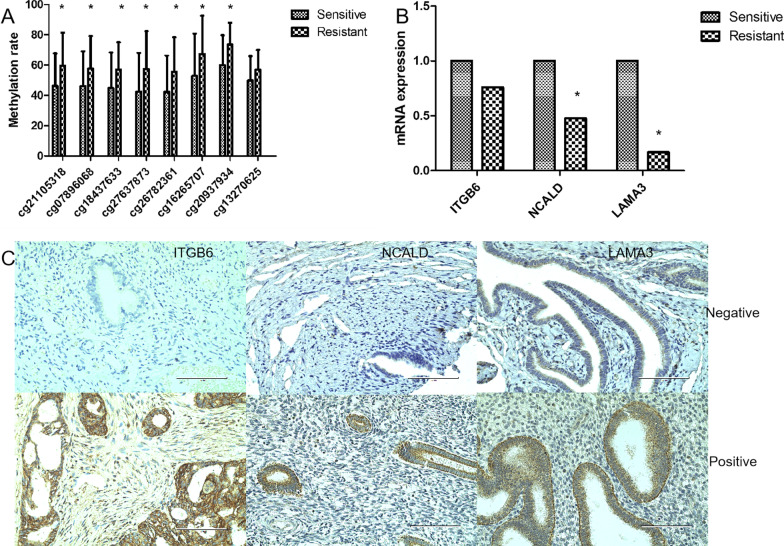
Considering that there are certain false positives on the 450 K Infinium Methylation BeadChip, we further verified the 8 candidate CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625) used pyrosequencing in advanced high-grade serous ovarian cancer. In the pyrosequencing results, the methylation of 7 CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934) in the chemoresistant patients were still higher than that in the chemosensitive patients. There was no statistically significant difference in cg13270625 (LAMA3) methylation level between chemoresistant patients and chemosensitive patients. The methylation rate is shown in Fig. 5a.
Fig. 5.
a The methylation rate of CpGs in advanced HGSOC (pyrosequencing). b Expressions of hypermethylated genes (ITGB6, NCALD and LAMA3) in advanced HGSOC (QRT-PCR). c Immunohistochemical stain (400 ×)
Low expression of NCALD and LAMA3 in advanced high-grade serous ovarian cancer
In advanced high-grade serous ovarian cancer, 8 CpGs hypermethylated in chemoresistant patients (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625).The mRNA and protein expressions of the corresponding 3 hypermethylated genes (ITGB6, NCALD and LAMA3) in advanced high-grade serous ovarian cancer were detected. QRT-PCR results showed that the expression of NCALD and LAMA3 in chemoresistant patients was lower than those in chemosensitive patients (P = 0.039, P = 0.008). The expression of ITGB6 was not statistically different between chemoresistant patients and chemosensitive patients, see Fig. 5b. In the tissue array composed of 132 advanced high-grade serous ovarian cancer patient samples, immunohistochemistry also showed that the expression of NCALD and LAMA3 in chemoresistant patients was lower than that of chemosensitive patients (57.89% VS 39.58%, P = 0.048; 54.67% VS 42.55%, P = 0.041). The expression of ITGB6 was not statistically different between chemoresistant patients and chemosensitive patients (55.56% VS 52.08%, P = 0.71), which was consistent with mRNA expression results. The staining chart is shown in Fig. 5c.
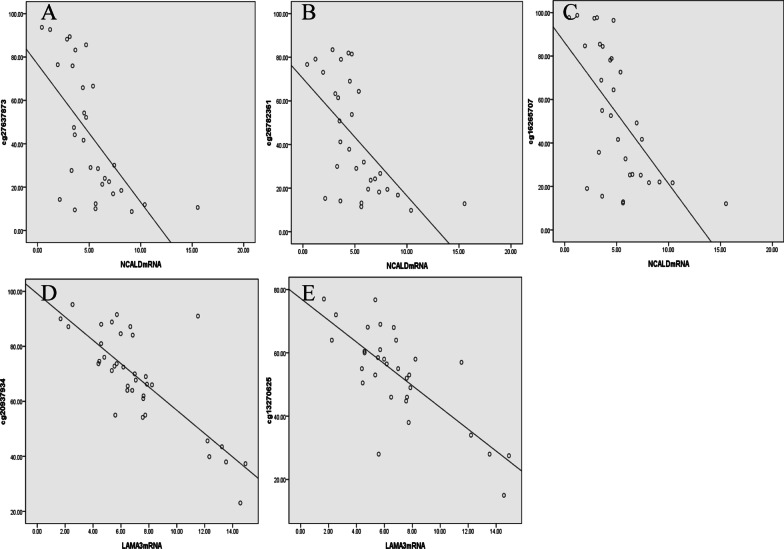
NCALD and LAMA3 expression are negatively correlated with methylation in advanced high-grade serous ovarian cancer
In advanced high-grade serous ovarian cancer, NCALD and LAMA3 have hypermethylation and low expression in chemoresistant patients. Spearman analysis showed that there was a negative correlation between the methylation of NCALD (cg27637873, cg26782361, cg16265707) and LAMA3 (cg20937934, cg13270625) and their mRNA expression. The correlation coefficients between the methylation of NCALD (cg27637873, cg26782361, cg16265707) and mRNA expression were -0.669, -0.636 and -0.657, respectively (P < 0.05). The correlation coefficients between the methylation of LAMA3 (cg20937934, cg13270625) and mRNA expression were − 0.726 and − 0.649, respectively (P < 0.05). See Fig. 6a–e. The hypermethylation of NCALD and LAMA3 in promoters may be the cause of its downregulation in chemoresistance ovarian cancer patients.
Fig. 6.
The relationship between NCALD and LAMA3 methylation and mRNA expression in advanced HGSOC (a cg27637873 methylation and NCALD expression. b cg26782361 methylation and NCALD expression. c cg16265707 methylation and NCALD expression. d cg20937934 methylation and LAMA3 expression. e cg13270625 methylation and LAMA3 expression)
Correlation between candidate genes in advanced high-grade serous ovarian cancer
The regulatory mechanism of DNA methylation in the development of chemotherapy resistance in ovarian cancer is complex and diverse. A variety of methylation genes interact with each other, which together leads to chemotherapy resistance in ovarian cancer. We found that among the 8 candidate CpGs (ITGB6:cg21105318, cg07896068, cg18437633; NCALD: cg27637873, cg26782361, cg16265707; LAMA3: cg20937934, cg13270625) in advanced high-grade serous ovarian cancer, the methylation level was highly positively correlated between any two CpGs, and the CpG correlation coefficient of the same gene was higher. See Additional file 1: Table S5. Further analysis of gene expression correlation revealed that NCALD protein expression was positively correlated with LAMA3 and ITGB6 protein expression. See Additional file 1: Table S6.
Discussion
Clinically, there are limited ways to treat chemotherapy resistance in ovarian cancer. However, demethylation drugs have been shown to resensitize ovarian cancer patients to platinum chemotherapy. It can be seen that abnormal methylation is a key factor in the formation of chemotherapy resistance in ovarian cancer. In this study, we used 450 K Infinium Methylation BeadChip to detect 7263 different CpGs in ovarian cancer chemoresistant and chemosensitive patients. We systematically searched the literature in the PubMed database and obtained 54 methylated genes related to chemotherapy resistance in ovarian cancer. Among them, 22 methylation genes related to ovarian resistance reported in the literature are enriched in our Methylation BeadChip results. The difference of BRCA1, CD133, ASS1, ABCG2, TGFBI, RGS10, UCHL1, CLDN4, HOXA10, DOK2, AGR2 and OXCT1 gene in our 450 K Infinium Methylation BeadChip is consistent with that reported in the literature. BRCA1 is a drug-related gene in ovarian cancer that has received attention. It has been reported to be involved in many cellular processes, including DNA repair and recombination, cell cycle regulation, chromatin remodeling and ubiquitination [66]. Ignatov's study showed that PFS was significantly prolonged in patients with BRCA1 promoter methylation in recurrent ovarian cancer (18.5 months vs 12.8 months, P = 0.008) [67]. Stefansson confirmed that BRCA1 hypermethylation increased platinum sensitivity in ovarian cancer cell lines, xenograft tumors and clinical samples [68]. However, some studies have reported that BRCA1 methylation reduces the sensitivity of the tumors to platinum drugs. For example, Wang’s study showed that with the progress of ovarian cancer, the methylation rate of the BRCA1 promoter increased significantly. Hypermethylation of the BRCA1 gene can lead to the loss of BRCA1 protein and RNA, which makes the disease of these patients develop faster and shorten the survival time than those without BRCA1 methylation [69]. Patch also proposed that, compared with BRCA1/2 gene mutation and expression downregulation, BRCA1 promoter methylation is related to platinum resistance [70]. In our Methylation BeadChip results, BRCA1 is hypomethylated in resistant patients, which is consistent with the results of Stefansson and Ignatov. The relationship between BRCA1 methylation and drug resistance in ovarian cancer still needs further verification. In our Methylation BeadChip results, combined with the region of CpGs, KEGG, GO and prognosis analysis, 9 new candidate CpGs methylation were selected to be associated with drug resistance of epithelial ovarian cancer. Ovarian cancer is not a single disease and can be subdivided into at least five different histological subtypes that have different identifiable risk factors, cells of origin, molecular compositions, clinical features and treatments [71]. High-grade serous ovarian cancer (HGSOC) is the most common ovarian cancer subtype. The vast majority of HGSOC cases are diagnosed at advanced stages (FIGO stage III and IV) with 5-year survival rates of approximately 39% and 17%, respectively [72]. In advanced high-grade serous ovarian cancer, 8 CpGs remained hypermethylated in chemoresistant patients.
Ovarian cancer is a highly heterogeneous disease characterized by multiple histological subtypes. Molecular diversity has been shown to occur within specific histological subtypes of ovarian cancer, between different tumors of an individual patient, as well as within individual tumors. Therefore, there are no clinically validated markers for chemotherapy sensitivity in ovarian cancer. Genome-wide DNA methylation detection helps to understand the complex characteristics of DNA methylation mutations. At present, studies on genome-wide DNA methylation of chemotherapy resistance in ovarian cancer have been largely limited to the level of cell and animal xenografts [52, 73]. Very few studies have examined the genome-wide DNA methylation characteristics of chemoresistant patients. Tomar [12] detected the genome-wide methylation of 8 chemosensitive ovarian cancer patients and 10 chemoresistant ovarian cancer patients. It was found that there were 45 differentially methylated and expressed genes between patients with two chemotherapy outcomes; In the same patient, pyrosequencing confirmed 9 different methylation genes. In the verification set, there are 4 candidate genes (FZD10, FAM83A, MYO18B and MKX) that have at least one CpG site with significant differences between patients with two chemotherapy outcomes. Compared with previous studies on the genome-wide methylation of ovarian cancer chemotherapy resistance, our study has several advantages. First, compared with cell or animal xenografts models, our genome-wide methylation and expression data were obtained from clinical surgical specimens, with long-term follow-up and chemotherapy outcomes. Second, genome-wide methylation was detected in 53 chemosensitive patients and 55 chemoresistant patients. The sample size was significantly increased. Third, unlike previous studies that only focused on screening a large number of markers, we also compared the diagnostic efficacy of abnormal methylation markers to predict chemoresistance. This provides a direction for the early identification of chemoresistant ovarian cancer patients in clinical. We identified 3 new methylation genes (ITGB6, NCALD and LAMA3) with different chemotherapy outcomes in advanced high-grade serous ovarian cancer. Patients with ITGB6, NCALD and LAMA3 hypermethylation have a poor prognosis. The methylation of the NCALD and LAMA3 is negatively correlated with their mRNA expression.
NCALD (neurocalcin delta) is a member of the neuron calcium sensor family, which is involved in the calcium signal pathway and G protein coupled receptor signal pathway. A bioinformatics study in 2020 showed that NCALD expression is regulated by DNA methylation and microRNAs. TCGA data found that the expression of NCALD in platinum-resistant patients was lower than that in platinum-sensitive patients. Patients with low NCALD expression have poor overall survival (OS) and progression-free survival (PFS) [74]. Our findings are consistent with this report. Epigenetic inactivation of NCALD may be one of the key factors leading to chemoresistance in ovarian cancer patients. LAMA3 (laminin, alpha 3) is an important component of the cell basement membrane and plays an important role in the process of cell adhesion, cell migration and embryo differentiation. As an epigenetic inactivation gene, LAMA3 has been reported in various cancer development and chemoresistance studies. However, there is only one study of LAMA3 in ovarian cancer. Tang [75] found that the methylation of LAMA3 in ovarian cancer tissues was higher than that in adjacent tissues and normal tissues. The expression of LAMA3 in ovarian cancer tissues was lower than that in adjacent tissues and normal tissues. The relationship between LAMA3 and ovarian cancer chemoresistance has not been reported. Our study found for the first time that LAMA3 was abnormally hypermethylated and silenced in chemoresistant ovarian cancer patients, which may be a target gene of epigenetic therapy. Although NCALD and LAMA3 are genes that show both methylation and expression changes, the methylation of the ITGB6 gene may also play a role in the chemoresistance of ovarian cancer.
Because the mechanism of chemoresistance in ovarian cancer is complex and diverse, it is very difficult to predict the chemotherapy outcome of ovarian cancer. Abnormal methylation can stratify ovarian cancer patients according to the chemotherapy outcome. In advanced high-grade serous ovarian cancer, the sensitivity, specificity and AUC of 8 CpGs methylation to predict chemotherapy sensitivity were 63.60–97.00%, 46.40–89.30% and 0.774–0.846. PFS of 6 candidate genes hypermethylation patients was significantly shorter. Residual lesions after primary surgery are another important prognostic factor in patients with advanced ovarian cancer. Patients with complete debulking have a better progression-free survival. Incomplete debulking cannot improve the prognosis, and it may even lead to more perioperative morbidity. Therefore, we analyzed the relationship between CpGs methylation and PFS in patients who with complete debulking or incomplete debulking, respectively. In advanced high-grade serous ovarian cancer with complete debulking, PFS of 3 candidate CpGs (ITGB6:cg07896068; NCALD:cg27637873, cg16265707) hypermethylation patients was significantly shorter. In advanced high-grade serous ovarian cancer with incomplete debulking, there was no significant difference between candidate CpGs hypermethylation and PFS. It is suggested that the methylation of 3 CpGs is more valuable in predicting the prognosis of patients with complete debulking.
Like Zhang's research, we also observed ‘batch effect’ in pyrosequencing [76]. Abnormal DNA methylation was found to be associated not only with disease [77, 78], but also with patient age, FIGO stage and histological type. This suggests that we need to stratify potential clinical factors when analyzing methylation related to chemotherapy resistance. Interestingly, we found that among the 8 CpGs in advanced high-grade serous ovarian cancer, the methylation level was highly positively correlated between any two CpGs, and the CpG correlation coefficient of the same gene was higher. Corresponding 3 hypermethylated genes, NCALD protein expression was positively correlated with LAMA3 and ITGB6 protein expression. This shows that NCALD, LAMA3 and ITGB6 may influence each other and participate in the chemotherapy resistance of ovarian cancer together.
There are several shortcomings in our study. First, there are challenges in the clinical collection of matching tumor samples before and after chemotherapy. This study is lateral study. The cancer samples selected are the initial surgical samples. However, our data can still show that the acquisition of these gene methylations is the potential molecular characteristic to obtain chemotherapy resistance and poor prognosis. Second, it is particularly important to predict the response to chemotherapy in ovarian cancer patients with incomplete debulking. Our data reveal that candidate CpGs hypermethylation is associated with worse PFS only in advanced HGSOC patients with complete debulking, but not in advanced HGSOC patients with incomplete debulking. It is suggested that the prognosis prediction of advanced HGSOC patients with incomplete debulking is more complicated. In the future, we will need to combine other biomarkers (such as BRCA1/2 mutational status) or further optimize the model for these specific populations. Third, the biological mechanism of candidate markers such as NCALD and LAMA3 is unclear. We speculate that there is a certain connection between the differentially methylated genes and the combined effect that leads to chemotherapy resistance. This functional mechanism needs to be further studied through confirmatory studies.
Conclusions
In summary, our study shows extensive methylation differences in chemosensitive and chemoresistant ovarian cancer patients. In advanced high-grade serous ovarian cancer, ITGB6, NCALD and LAMA3 hypermethylation indicate chemotherapy resistance and poor prognosis. Important new findings include the identification of two new key genes, NCALD and LAMA3, which may drive the acquired resistance of ovarian cancer. For the first time, the role of DNA methylation in regulating the function of NCALD and LAMA3 genes in advanced high-grade serous ovarian cancer was pointed out. This not only enriches the new gene pool for chemoresistance mechanisms of ovarian cancer, but also provides direction for finding stratified markers of epigenetic therapy.
Supplementary Information
Additional file 1. Table S1. Amplification primers and sequencing primers for Pyrosequencing. Table S2. Primers sequence for QRT-PCR. Table S3. Pathway analysis of different methylation sites between sensitive and resistant patients in KEGG (epithelial ovarian cancer). Table S4. Go analysis of different methylation sites in sensitive and resistant patients (epithelial ovarian cancer). Table S5. Correlation between candidate cpgs methylation (advanced HGSOC). Table S6. Correlation between candidate genes expression (advanced HGSOC).
Acknowledgements
Not applicable.
Abbreviations
- CpGs
Cytosine-phosphate-guanine site
- GO
Geneontology databases
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- QRT-PCR
Quantitative real-time PCR
- IHC
Immunohistochemistry
- ROC
Receiver operating characteristic
- ITGB6
Integrin beta 6
- NCALD
Neurocalcin delta
- LAMA3
Laminin alpha 3
- AUC
Area under curve
- OS
Overall survival
- PFS
Progression-free survival
- PCR
Polymerase chain reaction
- PIK3R3
Phosphoinositide-3-kinase, regulatory subunit 3 (gamma)
- FIGO
The International Federation of Gynecology and Obstetrics
Authors’ contributions
LF contributed to data curation, formal analysis, investigation, software, validation, visualization and writing—original draft. BY and ZH contributed to data curation, investigation, software and visualization. LL contributed to conceptualization, funding acquisition, methodology, project administration, resources, supervision and writing—review and editing. All authors read and approved the final manuscript.
Funding
This work was supported by project supported by the Scientific Research and Technological Development Plan of Guangxi Zhuang Autonomous Region (No. 1140003A-33).
Availability of data and materials
Not applicable.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, De Santis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Wang ZC, Luo L, et al. The clinical value of the combined detection of sEGFR, CA125 and HE4 for epithelial ovarian cancer diagnosis. Eur Rev Med Pharmacol Sci. 2020;24(2):604–610. doi: 10.26355/eurrev_202001_20036. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Wu Y, Zhang H, et al. Clinical value of serum HE4, CA125, CA72-4, and ROMA index for diagnosis of ovarian cancer and prediction of postoperative recurrence. Clin Lab. 2019 doi: 10.7754/Clin.Lab.2018.181030. [DOI] [PubMed] [Google Scholar]
- 5.Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Banerjee S, Pignata S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J Clin Oncol. 2019;37(27):2437–2448. doi: 10.1200/JCO.19.00194. [DOI] [PubMed] [Google Scholar]
- 7.Pan Y, Liu G, Zhou F, et al. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med. 2018;18(1):1–14. doi: 10.1007/s10238-017-0467-0. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan JM, Wilson A, Koo C, et al. Platinum-based chemotherapy induces methylation changes in blood DNA associated with overall survival in patients with ovarian cancer. Clin Cancer Res. 2017;23(9):2213–2222. doi: 10.1158/1078-0432.CCR-16-1754. [DOI] [PubMed] [Google Scholar]
- 9.Pan JX, Qu F, Wang FF, et al. Aberrant SERPINE1 DNA methylation is involved in carboplatin induced epithelial-mesenchymal transition in epithelial ovarian cancer. Arch Gynecol Obstet. 2017;296(6):1145–1152. doi: 10.1007/s00404-017-4547-x. [DOI] [PubMed] [Google Scholar]
- 10.Kritsch D, Hoffmann F, Steinbach D, et al. Tribbles 2 mediates cisplatin sensitivity and DNA damage response in epithelial ovarian cancer. Int J Cancer. 2017;141(8):1600–1614. doi: 10.1002/ijc.30860. [DOI] [PubMed] [Google Scholar]
- 11.Lund RJ, Huhtinen K, Salmi J, et al. DNA methylation and transcriptome changes associated with cisplatin resistance in ovarian cancer. Sci Rep. 2017;7(1):1469. doi: 10.1038/s41598-017-01624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomar T, Alkema NG, Schreuder L, et al. Methylome analysis of extreme chemoresponsive patients identifies novel markers of platinum sensitivity in high-grade serous ovarian cancer. BMC Med. 2017;15(1):116. doi: 10.1186/s12916-017-0870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mase S, Shinjo K, Totani H, et al. ZNF671 DNA methylation as a molecular predictor for the early recurrence of serous ovarian cancer. Cancer Sci. 2019;110:1105–1116. doi: 10.1111/cas.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaclavikova R, Klajic J, Brynychova V, et al. Development of high-resolution melting analysis for ABCB1 promoter methylation: Clinical consequences in breast and ovarian carcinoma. Oncol Rep. 2019;42(2):763–774. doi: 10.3892/or.2019.7186. [DOI] [PubMed] [Google Scholar]
- 15.Tian H, Yan L, Xiao-Fei L, et al. Hypermethylation of mismatch repair gene hMSH2 associates with platinum-resistant disease in epithelial ovarian cancer. Clin Epigenet. 2019;11(1):153. doi: 10.1186/s13148-019-0748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebert M, Renneville A, Bally C, et al. A phase II study of guadecitabine in higher-risk myelodysplastic syndrome and low blast count acute myeloid leukemia after azacitidine failure. Haematologica. 2019;104:1565–1571. doi: 10.3324/haematol.2018.207118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oza AM, Matulonis UA, Alvarez Secord A, et al. A randomized phase 2 trial of epigenetic priming with guadecitabine and carboplatin in platinum-resistant, recurrent ovarian cancer. Clin Cancer Res. 2019;26:1009–1016. doi: 10.1158/1078-0432.CCR-19-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matei D, Ghamande S, Roman L, et al. A phase I clinical trial of guadecitabine and carboplatin in platinum-resistant, recurrent ovarian cancer: clinical, pharmacokinetic, and pharmacodynamic analyses. Clin Cancer Res. 2018;24(10):2285–2293. doi: 10.1158/1078-0432.CCR-17-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogani G, Matteucci L, Tamberi S, et al. RECIST 1.1 criteria predict recurrence-free survival in advanced ovarian cancer submitted to neoadjuvant chemotherapy. Eur J Obstet Gynecol Reprod Biol. 2019;237:93–99. doi: 10.1016/j.ejogrb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Blagden SP, Hamilton AL, Mileshkin L, et al. Phase IB Dose escalation and expansion study of AKT inhibitor afuresertib with carboplatin and paclitaxel in recurrent platinum-resistant ovarian cancer. Clin Cancer Res. 2019;25(5):1472–1478. doi: 10.1158/1078-0432.CCR-18-2277. [DOI] [PubMed] [Google Scholar]
- 21.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 22.Yan B, Yin F, Wang QI, et al. Integration and bioinformatics analysis of DNA-methylated genes associated with drug resistance in ovarian cancer. Oncol Lett. 2016;12(1):157–166. doi: 10.3892/ol.2016.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Q, Duan P, Li L, et al. Expression of placenta growth factor is associated with unfavorable prognosis of advanced-stage serous ovarian cancer. Tohoku J Exp Med. 2018;244(4):291–296. doi: 10.1620/tjem.244.291. [DOI] [PubMed] [Google Scholar]
- 24.Strathdee G, Vass JK, Oien KA, et al. Demethylation of the MCJ gene in stage III/IV epithelial ovarian cancer and response to chemotherapy. Gynecol Oncol. 2005;97(3):898–903. doi: 10.1016/j.ygyno.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Lee PS, Teaberry VS, Bland AE, et al. Elevated MAL expression is accompanied by promoter hypomethylation and platinum resistance in epithelial ovarian cancer. Int J Cancer. 2010;126(6):1378–1389. doi: 10.1002/ijc.24797. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Ueda H, Etoh T, et al. A change in promoter methylation of hMLH1 is a cause of acquired resistance to platinum-based chemotherapy in epithelial ovarian cancer. Anticancer Res. 2007;27(3B):1449–1452. [PubMed] [Google Scholar]
- 27.Gifford G, Paul J, Vasey PA, et al. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin Cancer Res. 2004;10(13):4420–4426. doi: 10.1158/1078-0432.CCR-03-0732. [DOI] [PubMed] [Google Scholar]
- 28.Wang YQ, Zhang JR, Li SD, et al. Aberrant methylation of breast and ovarian cancer susceptibility gene 1 in chemosensitive human ovarian cancer cells does not involve the phosphatidylinositol 3'-kinase-Akt pathway. Cancer Sci. 2010;101(7):1618–1623. doi: 10.1111/j.1349-7006.2010.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou JL, Su HY, Chen LY, et al. Promoter hypermethylation of FBXO32, a novel TGF-beta/SMAD4 target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer. Lab Invest. 2010;90(3):414–425. doi: 10.1038/labinvest.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X, Hu JF, Li T, et al. Epigenetic regulation of the taxol resistance-associated gene TRAG-3 in human tumors. Cancer Genet Cytogenet. 2004;151(1):1–13. doi: 10.1016/j.cancergencyto.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Yang F, Chen W, et al. Multidrug resistance affects the prognosis of primary epithelial ovarian cancer. Oncol Lett. 2019;18(4):4262–4269. doi: 10.3892/ol.2019.10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai F, Zhang Y, Zhu X, et al. Anticancer role of MUC1 aptamer-miR-29b chimera in epithelial ovarian carcinoma cells through regulation of PTEN methylation. Target Oncol. 2012;7(4):217–225. doi: 10.1007/s11523-012-0236-7. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Hu W, Shen DY, et al. Azacitidine enhances sensitivity of platinum-resistant ovarian cancer cells to carboplatin through induction of apoptosis. Am J Obstet Gynecol. 2009;200(2):177e1–179. doi: 10.1016/j.ajog.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Bram EE, Stark M, Raz S, et al. Chemotherapeutic drug-induced ABCG2 promoter demethylation as a novel mechanism of acquired multidrug resistance. Neoplasia. 2009;11(12):1359–1370. doi: 10.1593/neo.91314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang YC, Chang MC, Chen PJ, et al. Epigenetic silencing of BLU through interfering apoptosis results in chemoresistance and poor prognosis of ovarian serous carcinoma patients. Endocr Relat Cancer. 2013;20(2):213–227. doi: 10.1530/ERC-12-0117. [DOI] [PubMed] [Google Scholar]
- 36.Wang N, Zhang H, Yao Q, et al. TGFBI promoter hypermethylation correlating with paclitaxel chemoresistance in ovarian cancer. J Exp Clin Cancer Res. 2012;31(1):6. doi: 10.1186/1756-9966-31-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali MW, Cacan E, Liu Y, et al. Transcriptional suppression, DNA methylation, and histone deacetylation of the regulator of G-protein signaling 10 (RGS10) gene in ovarian cancer cells. PLoS ONE. 2013;8(3):e60185. doi: 10.1371/journal.pone.0060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okochi-Takada E, Nakazawa K, Wakabayashi M, et al. Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int J Cancer. 2006;119(6):1338–1344. doi: 10.1002/ijc.22025. [DOI] [PubMed] [Google Scholar]
- 39.Staub J, J Chien, Y Pan, et al. Epigenetic silencing of HSulf-1 in ovarian cancer: implications in chemoresistance. Oncogene, 2007. 26(34): p. 4969-78. [DOI] [PubMed]
- 40.Su HY, Lai HC, Lin YW, et al. Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through Wnt signaling pathway. Int J Cancer. 2010;127(3):555–567. doi: 10.1002/ijc.25083. [DOI] [PubMed] [Google Scholar]
- 41.Izutsu N, Maesawa C, Shibazaki M, et al. Epigenetic modification is involved in aberrant expression of class III beta-tubulin, TUBB3, in ovarian cancer cells. Int J Oncol. 2008;32(6):1227–1235. doi: 10.3892/ijo_32_6_1227. [DOI] [PubMed] [Google Scholar]
- 42.Pattamadilok J, Huapai N, Rattanatanyong P, et al. LINE-1 hypomethylation level as a potential prognostic factor for epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18(4):711–717. doi: 10.1111/j.1525-1438.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Chu Y, Tang W, et al. Transcription factor WT1 and promoter CpG hypomethylation coactivate HOXA10 expression in ovarian cancer. Curr Pharm Des. 2014;20(11):1647–1654. doi: 10.2174/13816128113199990545. [DOI] [PubMed] [Google Scholar]
- 44.Matei D, Fang F, Shen C, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012;72(9):2197–2205. doi: 10.1158/0008-5472.CAN-11-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi T, Tischkowitz M, Ameziane N, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9(5):568–574. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 46.Yang SD, Ahn SH, Kim JI. 3-Oxoacid CoA transferase 1 as a therapeutic target gene for cisplatin-resistant ovarian cancer. Oncol Lett. 2018;15(2):2611–2618. doi: 10.3892/ol.2017.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Ma S, Wang L, et al. A polygenic methylation prediction model associated with response to chemotherapy in epithelial ovarian cancer. Mol Ther Oncolytics. 2021;20:545–555. doi: 10.1016/j.omto.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeller C, Dai W, Steele NL, et al. Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene. 2012;31(42):4567–4576. doi: 10.1038/onc.2011.611. [DOI] [PubMed] [Google Scholar]
- 49.Ha YN, Sung HY, Yang SD, et al. Epigenetic modification of α-N-acetylgalactosaminidase enhances cisplatin resistance in ovarian cancer. Korean J Physiol Pharmacol. 2018;22(1):43–51. doi: 10.4196/kjpp.2018.22.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visco ZR, Sfakianos G, Grenier C, et al. Epigenetic regulation of Claudin-1 in the development of ovarian cancer recurrence and drug resistance. Front Oncol. 2021;11:620873. doi: 10.3389/fonc.2021.620873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonito NA, Borley J, Wilhelm-Benartzi CS, et al. Epigenetic regulation of the homeobox gene MSX1 associates with platinum-resistant disease in high-grade serous epithelial ovarian cancer. Clin Cancer Res. 2016;22(12):3097–3104. doi: 10.1158/1078-0432.CCR-15-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomar T, de Jong S, Alkema NG, et al. Genome-wide methylation profiling of ovarian cancer patient-derived xenografts treated with the demethylating agent decitabine identifies novel epigenetically regulated genes and pathways. Genome Med. 2016;8(1):107. doi: 10.1186/s13073-016-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang F, Cardenas H, Huang H, et al. Genomic and epigenomic signatures in ovarian cancer associated with resensitization to platinum drugs. Cancer Res. 2018;78(3):631–644. doi: 10.1158/0008-5472.CAN-17-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu W, Jin C, Lou X, et al. Global analysis of DNA methylation by methyl-capture sequencing reveals epigenetic control of cisplatin resistance in ovarian cancer cell. PLoS ONE. 2011;6(12):e29450. doi: 10.1371/journal.pone.0029450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teschendorff AE, Lee SH, Jones A, et al. HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med. 2015;7:108. doi: 10.1186/s13073-015-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syed N, Coley HM, Sehouli J, et al. Polo-like kinase Plk2 is an epigenetic determinant of chemosensitivity and clinical outcomes in ovarian cancer. Cancer Res. 2011;71(9):3317–3327. doi: 10.1158/0008-5472.CAN-10-2048. [DOI] [PubMed] [Google Scholar]
- 57.de Leon M, Cardenas H, Vieth E, et al. Transmembrane protein 88 (TMEM88) promoter hypomethylation is associated with platinum resistance in ovarian cancer. Gynecol Oncol. 2016;142(3):539–547. doi: 10.1016/j.ygyno.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mase S, Shinjo K, Totani H, et al. ZNF671 DNA methylation as a molecular predictor for the early recurrence of serous ovarian cancer. Cancer Sci. 2019;110(3):1105–1116. doi: 10.1111/cas.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28(2):209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 60.Shang X, Lin X, Manorek G, et al. Claudin-3 and claudin-4 regulate sensitivity to cisplatin by controlling expression of the copper and cisplatin influx transporter CTR1. Mol Pharmacol. 2013;83(1):85–94. doi: 10.1124/mol.112.079798. [DOI] [PubMed] [Google Scholar]
- 61.Litkouhi B, Kwong J, Lo CM, et al. Claudin-4 overexpression in epithelial ovarian cancer is associated with hypomethylation and is a potential target for modulation of tight junction barrier function using a C-terminal fragment of Clostridium perfringens enterotoxin. Neoplasia. 2007;9(4):304–314. doi: 10.1593/neo.07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alessandrini F, Pezzè L, Menendez D, et al. ETV7-mediated DNAJC15 repression leads to doxorubicin resistance in breast cancer cells. Neoplasia. 2018;20(8):857–870. doi: 10.1016/j.neo.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaclavikova R, Klajic J, Brynychova V, et al. Development of high-resolution melting analysis for ABCB1 promoter methylation: clinical consequences in breast and ovarian carcinoma. Oncol Rep. 2019;42(2):763–774. doi: 10.3892/or.2019.7186. [DOI] [PubMed] [Google Scholar]
- 64.Nicholson LJ, Smith PR, Hiller L, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009;125(6):1454–1463. doi: 10.1002/ijc.24546. [DOI] [PubMed] [Google Scholar]
- 65.Duan Z, Feller AJ, Toh HC, et al. TRAG-3, a novel gene, isolated from a taxol-resistant ovarian carcinoma cell line. Gene. 1999;229(1–2):75–81. doi: 10.1016/S0378-1119(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 66.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ignatov T, Eggemann H, Costa SD, et al. BRCA1 promoter methylation is a marker of better response to platinum-taxane-based therapy in sporadic epithelial ovarian cancer. J Cancer Res Clin Oncol. 2014;140(9):1457–1463. doi: 10.1007/s00432-014-1704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stefansson OA, Villanueva A, Vidal A, et al. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics. 2012;7(11):1225–1229. doi: 10.4161/epi.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang YQ, Yan Q, Zhang JR, et al. Epigenetic inactivation of BRCA1 through promoter hypermethylation in ovarian cancer progression. J Obstet Gynaecol Res. 2013;39(2):549–554. doi: 10.1111/j.1447-0756.2012.01979.x. [DOI] [PubMed] [Google Scholar]
- 70.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 71.Matulonis UA, Sood AK, Fallowfield L, et al. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goh J, Mohan GR, Ladwa R, et al. Frontline treatment of epithelial ovarian cancer. Asia Pac J Clin Oncol. 2015;11(Suppl 6):1–16. doi: 10.1111/ajco.12449. [DOI] [PubMed] [Google Scholar]
- 73.Granados ML, Hudson LG, Samudio-Ruiz SL. Contributions of the epidermal growth factor receptor to acquisition of platinum resistance in ovarian cancer cells. PLoS ONE. 2015;10(9):e0136893. doi: 10.1371/journal.pone.0136893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong C, Yin F, Zhu D, et al. NCALD affects drug resistance and prognosis by acting as a ceRNA of CX3CL1 in ovarian cancer. J Cell Biochem. 2020;121:4470–4483. doi: 10.1002/jcb.29670. [DOI] [PubMed] [Google Scholar]
- 75.Tang L, Wang P, Wang Q, et al. Correlation of LAMA3 with onset and prognosis of ovarian cancer. Oncol Lett. 2019;18(3):2813–2818. doi: 10.3892/ol.2019.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Petropoulos S, Liu J, et al. The signature of liver cancer in immune cells DNA methylation. Clin Epigenet. 2018;10:8. doi: 10.1186/s13148-017-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puglia MH, Lillard TS, Morris JP, et al. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci U S A. 2015;112(11):3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim D, Kubzansky LD, Baccarelli A, et al. Psychological factors and DNA methylation of genes related to immune/inflammatory system markers: the VA normative aging study. BMJ Open. 2016;6(1):e009790. doi: 10.1136/bmjopen-2015-009790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Amplification primers and sequencing primers for Pyrosequencing. Table S2. Primers sequence for QRT-PCR. Table S3. Pathway analysis of different methylation sites between sensitive and resistant patients in KEGG (epithelial ovarian cancer). Table S4. Go analysis of different methylation sites in sensitive and resistant patients (epithelial ovarian cancer). Table S5. Correlation between candidate cpgs methylation (advanced HGSOC). Table S6. Correlation between candidate genes expression (advanced HGSOC).
Data Availability Statement
Not applicable.