Abstract
Background
Older adults (≥50 years) represent the fastest-growing population of people who use cannabis, potentially due to the increasing promotion of cannabis as medicine by dispensaries and cannabis websites. Given healthy aging and cannabis use are both associated with cognitive decline, it is important to establish the effects of cannabis on cognition in healthy aging.
Objective
This systematic scoping review used preferred reporting items for systematic reviews and meta-analyses guidelines to critically examine the extent of literature on this topic and highlight areas for future research.
Method
A search of six databases (PubMed, EMBASE, PsycINFO, Web of Science, Family and Society Studies Worldwide, and CINAHL) for articles published by September 2019, yielded 1,014 unique results.
Results
Six articles reported findings for older populations (three human and three rodent studies), highlighting the paucity of research in this area. Human studies revealed largely null results, likely due to several methodological limitations. Better-controlled rodent studies indicate that the relationship between ∆9-tetrahydrocannabinol (THC) and cognitive function in healthy aging depends on age and level of THC exposure. Extremely low doses of THC improved cognition in very old rodents. Somewhat higher chronic doses improved cognition in moderately aged rodents. No studies examined the effects of cannabidiol (CBD) or high-CBD cannabis on cognition.
Conclusions
This systematic scoping review provides crucial, timely direction for future research on this emerging issue. Future research that combines neuroimaging and cognitive assessment would serve to advance understanding of the effects of age and quantity of THC and CBD on cognition in healthy aging.
Keywords: Older adult, Cannabis, ∆9-Tetrahydrocannabinol, Cannabidiol, Cognitive function
Introduction
Older adults (≥50 years) comprise the fastest-growing population of people who use cannabis in Western society (Azofeifa et al., 2016; Fahmy, Hatch, Hotopf, & Stewart, 2012; Han et al., 2017; Kostadinov & Roche, 2017). More than half of older people who use cannabis report using cannabis medicinally (Choi, DiNitto, & Marti, 2017; Sexton, Cuttler, & Mischley, 2019), potentially due to the perception of cannabis as having medicinal effects (Bobitt et al., 2019), a notion perpetuated by dispensaries and popular cannabis websites (Boatwright & Sperry, 2018; Cavazos-Rehg et al., 2019; Luc, Tsang, Thrul, Kennedy, & Moran, 2020). This proclivity to use cannabis medicinally likely accounts for why older adults tend to select cannabis high in cannabidiol (CBD; the major non-psychoactive cannabis compound) compared with young people who tend to select cannabis high in ∆9-tetrahydrocannabinol (THC; the main psychoactive cannabis compound; Choi et al., 2017; Sexton et al., 2019). The proliferation of cannabis use among older adults may have significant public health consequences, since increases in life expectancy mean that older adults are projected to comprise around 40% of the Western population by 2060 (Australian Bureau of Statistics, 2017; Office for National Statistics, 2017; U.S. Census Bureau, 2018). Of concern are the effects of cannabis use on cognition, given this group’s underlying susceptibility to cognitive decline.
Although crystalized cognitive functions (e.g., vocabulary) tend to remain stable or strengthen with age (Harada, Natelson Love, & Triebel, 2013), some older adults experience noticeable concurrent declines in fluid cognitive functions including memory, learning, inhibition, attention, decision-making, cognitive flexibility, and processing speed (Eppinger, Hämmerer, & Li, 2011; Fraundorf, Hourihan, Peters, & Benjamin, 2019; Kray & Lindenberger, 2000; Marschner et al., 2005; Mell et al., 2005; Samson & Barnes, 2013; Tucker-Drob, Brandmaier, & Lindenberger, 2019; Weiler, Bellebaum, & Daum, 2008). Significant heterogeneity exists among individuals regarding the degree and extent of age-related cognitive decline (de Frias, Lövdén, Lindenberger, & Nilsson, 2007). Nonetheless, cognitive decline has significant, negative effects for mental health and wellbeing (Burholt, Windle, & Morgan, 2016; Hill et al., 2016; Parikh, Troyer, Maione, & Murphy, 2015; Wilson et al., 2013), underscoring the need to examine factors that may exacerbate age-related cognitive decline. One such factor may be cannabis use, given the known detrimental effects of cannabis on cognition in younger populations.
Cannabis impairs cognition in younger people, likely due to the detrimental effects of THC on the developing brain (Broyd, van Hell, Beale, Yücel, & Solowij, 2016; Crane, Schuster, Fusar-Poli, & Gonzalez, 2013; Gorey, Kuhns, Smaragdi, Kroon, & Cousijn, 2019; National Academies of Sciences, Engineering, and Medicine 2017; Scott et al., 2018). Acute cannabis intoxication (≤6 hr post use) is associated with greater impulsivity and poorer working memory in young people who chronically use cannabis (Crean, Crane, & Mason, 2011). Conversely, cannabis has limited non-acute effects (7 hours to 3 weeks after use) on cognition, with a meta-analysis finding a remediation in cannabis-related cognitive deficits following 72 hours of abstinence (Scott et al., 2018). Nonetheless, non-acute cannabis effects may persist among people who use cannabis chronically (Crean et al., 2011; Gonzalez, Carey, & Grant, 2002; Grant, Gonzalez, Carey, Natarajan, & Wolfson, 2003; Schreiner & Dunn, 2012; Scott et al., 2018). Together, these studies highlight the importance of considering duration since last use and chronicity of cannabis use, when examining the effects of cannabis on cognition.
Cannabis use is also associated with altered brain morphology and function among younger populations, with significant effects on the cerebellum, and medial temporal and frontal cortices (Batalla et al., 2013; Lorenzetti et al., 2016; Yücel et al., 2016). Cannabis use has repeatedly been associated with reductions in hippocampal volume (Batalla et al., 2013), likely perpetuated by the dense concentration of cannabinoid receptors in this region (Glass, Faull, & Dragunow, 1997). Despite significant morphological changes and altered brain function resulting from cannabis use, a review by Lorenzetti et al. (2016) found adolescents who used cannabis did not markedly differ from controls, on cognitive task performance. This may point to the compensatory recruitment of other brain regions to mitigate cannabis-induced cognitive impairment. Ultimately, these studies indicate that research should employ both cognitive assessments and neuroimaging in order to obtain a comprehensive picture of the effects of cannabis on cognition.
The effects of cannabis use on cognition in older adults may be complicated, however, by a number of age-related factors, including (i) an increased selection of high-CBD cannabis, which has anti-inflammatory properties (Burstein, 2015; Mori et al., 2017) and may attenuate the cognitive impacts of low-grade inflammation seen in aging (Fard & Stough, 2019; Patterson, 2015); (ii) a greater use of oral routes of cannabis administration (Sexton et al., 2019) and a slowing of the metabolism, resulting in extended periods of intoxication (Sagar & Gruber, 2018); (iii) age-related changes in the dopamine system (Karrer, Josef, Mata, Morris, & Samanez-Larkin, 2017), which is instrumental in several cognitive domains affected by age including reward-based decision-making (Berry, Jagust, & Hsu, 2019), and is affected by cannabis use (Yoo, DiMuzio, & Filbey, 2019); and (iv) age-related changes in brain morphology and function, including to regions such as the hippocampus, which undergoes significant changes during aging (Lister & Barnes, 2009), has a dense concentration of cannabinoid receptors (Glass et al., 1997), and has consistently been found to be affected by cannabis use in younger populations (Batalla et al., 2013; Yücel et al., 2016). In addition, the differential cognitive effects of alcohol between younger and older adults (Boissoneault, Sklar, Prather, & Nixon, 2014; Salmon & Forester, 2012; Sklar, Gilbertson, Boissoneault, Prather, & Nixon, 2012) further underscores the need to examine the effect of cannabis on cognition among older adults.
Ultimately, the proliferation of cannabis use among older adults who may already be susceptible to cognitive decline, the known detrimental effects of cannabis on cognitive function in young people, and the differential cognitive effects of alcohol between younger and older adults, highlights the need to examine the effects of cannabis use on cognitive function among older adults. Although reviews have examined how cannabis affects cognitive function in older adults with underlying pathology (e.g., dementia; National Academies of Sciences, Engineering, and Medicine 2017; Scott, Brennan, & Benitez, 2019), comparatively little is known about the extent of research examining the effects of cannabis use in healthy aging.
Recent reviews of animal studies found the effect of THC on cognitive function to be age-dependent. Although even low doses were detrimental in younger rodents, THC exerted pro-cognitive effects on memory and learning in older populations (Calabrese & Rubio-Casillas, 2018; Yosef Sarne, 2019). Another recent review provided an important overview of some studies examining the effects of cannabis use on aging, with a focus on molecular systems and some consideration of cognition (Yoo et al., 2019). Nevertheless, a systematic review of existing literature including both human and animal models, more detailed synthesis, and critical appraisal of cannabis effects on healthy aging and cognition across species would ensure that all relevant studies are captured and provide crucial direction for future research on this emerging issue. As a result, the aim of this systematic scoping review is to determine the current extent of the literature, summarize available findings, and identify gaps in knowledge regarding the effects of cannabis use on cognitive function in healthy aging.
Materials and Methods
This systematic scoping review was conducted in line with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009; Tricco et al., 2018).
Eligibility Criteria
Papers published by September 2019 were included if they examined the effects of whole plant or phytocannabinoids (THC or CBD) on cognitive function in healthy, older adult humans (≥50 years) or animals (e.g., mice ≥ 12 months, the approximate equivalent to older adulthood in humans; Flurkey, M, & Harrison, 2007). Age ≥ 50 was chosen in order to capture the fastest-growing population of healthy-aging people who use cannabis (Azofeifa et al., 2016; Fahmy et al., 2012; Han et al., 2017; Kostadinov & Roche, 2017). No restrictions were placed on publication date and studies could examine either acute or non-acute effects of cannabis on cognition. Studies had to be in English and include a baseline or comparison group not exposed to cannabis. Studies that focused exclusively on populations with underlying pathology or substance use disorders (other than cannabis use disorder), or that conflated cannabis with other substances and examined effects on cognition via a single, polysubstance use variable, were excluded. Missing or unclear data were clarified by emailing corresponding authors.
Information Sources
Six large databases (PubMed, EMBASE, PsycINFO, Web of Science, Family and Society Studies Worldwide, and CINAHL) were searched using a specialized search strategy, developed with a librarian experienced in systematic reviews (see Supplementary material for the full PubMed electronic search strategy). To ensure saturation of the literature, additional publications were identified via: (i) Grey literature (i.e., research not published as a peer-reviewed article), including conference abstracts and dissertations (searched via ProQuest) and (ii) reference lists of included articles and relevant reviews.
Results were exported to Rayyan QCRI, an online service for systematic reviews. Title and abstracts and eligible full text pdfs were independently reviewed, and data for each included study were independently extracted by two reviewers (NP and TJW), blind to each other’s decisions. Disagreements between reviewers were resolved through consensus following each review stage. Extracted data included participants (sample size, age range, mean age, and sex), exposure (cannabis use or administration definition), comparison group (definition of control/reference group), outcome information (measures), and main findings.
Results
The search resulted in 1,014 unique articles for title and abstract screening, yielding 134 articles for full-text review, leading to six articles included in the review (see Fig. 1 for the PRISMA flow chart).
Fig. 1.
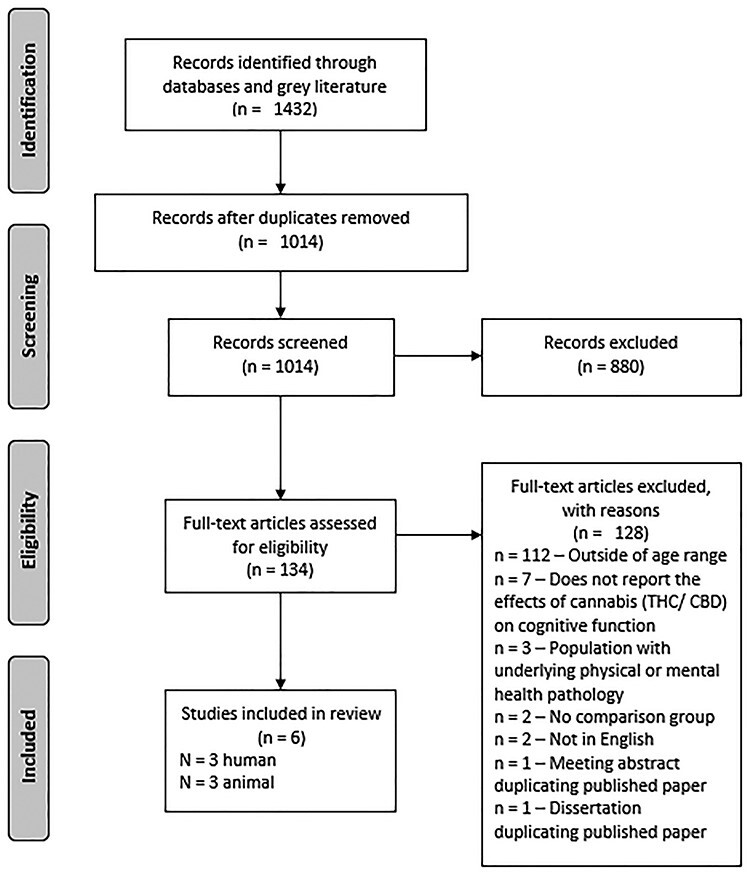
Preferred reporting items for systematic reviews and meta-analyses flow chart. Note: THC = tetrahydrocannabinol, CBD = cannabidiol.
Human Studies
Table 1 outlines the details of the human studies. Most studies (n = 110; 87%) were excluded at full-text review because they did not report results for older adults, resulting in three studies and a collective sample of 3,462 people who use cannabis and 7,917 controls (never used cannabis). None of the studies assessed time since last cannabis use (e.g., via a urine toxicology screen and self-report questions). Thus, it is unclear whether these studies examined the non-acute effects of cannabis use on cognition. All studies used assessments of cognitive functions including working, episodic, and delayed memory, vocabulary knowledge, oral reading skills, cognitive flexibility, processing speed, reaction time, and learning. Burggren et al. (2018) also assessed clinician-reported cognitive function via the mini-mental state examination (MMSE).
Table 1.
Human studies examining the relationship between cannabis use and cognitive function in middle-to-older-aged adults (>50 years)
| Author | Sample characteristics | Cannabis use | Cognitive task/s | Main findings |
|---|---|---|---|---|
| Thayer et al. (2019) |
N = 56a People who currently use cannabis: n = 28; Mage = 66.79, SD = 5.28; range = 60–80; females = 10 (36%) Controls: n = 28; Mage = 69.79, SD = 5.71, range = 61–83; females = 17 (61%) |
≥weekly for ≥past year | NIH Toolbox Cognition Battery—Flanker inhibitory control and attention test; picture sequence memory test; list sorting working memory test; picture vocabulary test; oral reading recognition test; dimensional change card sorting test; pattern comparison processing speed test | No significant difference in cognitive performance between people who use cannabis and controls on any of the cognitive outcomes |
| Burggren et al. (2018) |
N = 50 People with a history of heavy cannabis use: n = 24; Mage = 65.4, range = 58.2–72.6; females = 8 (33%) Controls: n = 26; Mage = 67.7, range = 60.6–74.8; females = 12 (46%) |
≥20 days per month, initiated during adolescence (<20 years), continuing for ≥ 1 year, with ≤ 2 uses per month after age 35 | Buschke–Fuld selective reminding test—consistent long-term retrieval and delayed recall; Wechsler Memory Scale-II—Logical Memory I and II and Verbal Paired Associates I and II; Rey–Osterrieth complex figure—delayed recall; trail-making test—Parts A and B; Stroop test—word reading speed and interference; Wechsler adult intelligence Scale-III—digit symbol; Verbal Fluency FAS and animal naming Tests; MMSE | No significant difference in cognitive performance
between people with a history of heavy cannabis use and controls
on any of the cognitive outcomes No group differences were found for the MMSE |
| Dregan and Gulliford (2012) |
N = 11,419;
females = 5,734 (50%); all
participants were aged 42 at baseline. People who currently use cannabis: n = 736 People who used cannabis in their lifetime: n = 2,674 Controls: n = 7,863 |
Current cannabis use: Used in the past
12 months; Lifetime cannabis use: Lifetime use, but not in the past 12 months |
Immediate and delayed word recall; animal-naming test; letter-cancelation test | No significant effect of current cannabis use at
age 42 and cognitive function at age 50. People who had used cannabis in their lifetime at age 42 had significantly better scores on memory (immediate and delayed word recall) and executive function (comprised of the animal-naming test and the letter-cancelation test) at age 50, compared with controls. |
Note: Mage = Mean age; MMSE = mini-mental state examination
aOnly 28 people who use cannabis and 10 controls completed cognitive outcomes.
The definition of cannabis use differed among studies (i.e., lifetime, past 12-month, or former heavy use). Dregan and Gulliford (2012) did not report information regarding the duration, quantity, and frequency of cannabis use among their older adult population. Participants in Thayer, YorkWilliams, Hutchison, and Bryan (2019) reported an average age of onset of cannabis use at 20.04 years (SD = 8.11; range = 14–58 years), with an average duration of use of 23.55 years (SD = 8.11 years; range = 14–58 years) and used cannabis an average of 63.46 days (SD = 24.87; range = 12–90 days), in the past 90 days. Burggren et al. (2018) reported an average duration of use of 11.3 years (SD = 13.0 years), or 4,181.2 (SD = 4,784.6) lifetime uses. None of the studies reported quantity of cannabis use and only Thayer et al. (2019) reported route of administration (i.e., 57% smoked, 32% used edibles, and 11% used both) and cannabis strain preference (although a majority of participants—71%—were unsure of strain characteristics).
Dregan and Gulliford (2012) found a significant relationship between cannabis use and cognitive function. Although current cannabis use at age 50 was not associated with cognition, older adults who used cannabis at least once in their lifetime by age 42 had better cognitive function 8 years later at age 50 compared with controls. Conversely, Thayer et al. (2019) found no significant difference between older adults who use cannabis and older adult controls on attention, episodic memory, working memory, vocabulary, reading, executive function, and processing speed. Similarly, Burggren et al. (2018) found no significant difference between older adults with a history of heavy cannabis use and older adult controls, on neuropsychological domains of encoding and delayed memory, processing speed, and executive function. Further, older adult cannabis users did not differ from controls on MMSE scores.
Thayer et al. (2019) and Burggren et al. (2018) also examined the effects of cannabis use on brain morphology, via magnetic resonance imaging (MRI). Thayer et al. (2019) found no significant difference between older adults who use cannabis and controls on cerebrospinal fluid (CSF) volume, gray or white matter, or hippocampus volume. Further, after adjusting for false-discovery rates, there were no significant differences between older adults who used cannabis compared to age-matched controls on the right putamen or left pallidum. Results only showed a reliable difference in the left putamen, lingual cortex, and the rostral middle frontal cortex. Cortical volume was not associated with cognitive performance. Burggren et al. (2018) found older adults with a history of heavy cannabis use had cortical thinning of the hippocampus compared with older adult controls. However, there was no significant difference between older adults with a history of heavy cannabis use and older adult controls on parietal lobe morphology (a region with comparatively less cannabinoid receptors than the hippocampus), thus highlighting the significant effect of previous heavy cannabis use on hippocampus morphology. Finally, Burggren et al. (2018) found no significant association between cortical thickness in any brain region and performance on any of the neuropsychological domains.
Animal Studies
A summary of the three rodent studies is provided in Table 2. The three studies administered different doses of THC, intraperitoneally (either by injection or osmotic minipump). Bilkei-Gorzo et al. (2017) administered 3 mg/kg THC per day for 28 days, whereas Aso, Andrés-Benito, and Ferrer (2016) administered a daily injection of THC and CBD in a 1:1 ratio (0.75 mg/kg) for 5 weeks. Conversely, Sarne, Toledano, Rachmany, Sasson, and Doron (2018) exmained the effects of a single, 0.002 mg/kg injection of THC on cognition. All studies employed a washout period ranging from 5 days to 4 weeks, before cognitive testing, thus examining the non-acute effects of cannabis on cognition.
Table 2.
Animal studies examining the relationship between cannabis use and cognitive function in middle-to-older-aged mice (>12 months)
| Author | Sample | Cannabinoid administration | Cognitive task/s | Main findings |
|---|---|---|---|---|
| Bilkei-Gorzo et al. (2017) | Mice (C57BL/6 J); young = 2 months, mature = 12 months, old = 18 months; male | THC (3 mg/kg per day), for 28 days. Followed by a 5-day washout period | Novel object location recognition test; Partner recognition test; Morris Water Maze | THC improved spatial learning, learning flexibility, and long-term spatial memory in mature and old mice, compared with age-matched controls |
| THC-treated young mice had worse memory performance, compared with age-matched controls. | ||||
| Sarne et al. (2018) | Mice; young = 2 months, older = 24 months; female | Single injection of THC (0.002 mg/kg). Followed by a 3-week washout period (before object recognition task) or 4-week washout period (before place recognition task) | Object and place recognition; Y-Maze; Morris Water Maze; active and passive avoidance | THC-treated older mice preferred the novel object over the familiar object and had better place recognition. Vehicle-treated older mice failed to distinguish between the novel and the familiar objects and had poorer place recognition. |
| THC-treated older mice spent a significantly greater time in the novel arm of the Y-maze compared with vehicle-treated elderly mice. | ||||
| Young mice spent a significantly greater time in the novel arm of the Y-maze compared with vehicle-treated older mice. This difference disappeared when young mice were compared with THC-treated older mice. | ||||
| Significant improvement in performance on the Morris Water Maze (spatial learning) among THC-treated, but not vehicle-treated older mice. | ||||
| Aso et al. (2016) | Wild-type mice, non-aged controls = 3 months; aged = 12 months; male | THC 0.75 mg/kg + CBD 0.75 mg/kg, daily injection for 5 weeks. Followed by a 10 day washout period | Two object recognition test | Vehicle-treated aged mice had significantly poorer memory performance than 3-month-old wild-types. |
| THC + CBD did not significantly affect memory performance of 3-month-old wild-types | ||||
| No significant effect of THC + CBD on memory performance in aged mice, compared to age-matched controls. |
Note: THC = tetrahydrocannabinol, CBD = cannabidiol.
Sarne et al. (2018) used female, whereas the others used male mice. All studies assessed memory, whereas Bilkei-Gorzo et al. (2017) and Sarne et al. (2018) also assessed learning and flexibility. Bilkei-Gorzo et al. (2017) and Sarne et al. (2018) found THC-treated older mice performed better than vehicle-treated older mice on learning and spatial memory. Conversely, Aso et al. (2016) found no significant difference in memory performance between older mice treated with combined THC and CBD compared to vehicle-treated older mice.
Sarne et al. (2018) also examined the effects of THC versus vehicle on brain morphology, via MRI. Results revealed greater tissue density in 11 brain regions including the entorhinal cortex, amygdala, external capsule-corpus callosum, visual cortex, cingulate cortex, cingulum, caudate-putamen, and mamillary bodies, among THC-treated rodents. No decreases in tissue density were detected in any brain region. Further, although THC treatment had no significant effect on total brain volume, THC treatment was associated with smaller amygdala volume, and larger entorhinal cortex, prefrontal cortex, posterior hippocampus volume.
Discussion
This systematic scoping review examined current research on the relationship between cannabis use and cognitive function in healthy aging and provides a starting point for future research. A systematic search of six large databases found only six articles satisfied the eligibility criteria for this review, thus confirming the paucity of research on the effects of cannabis use on cognition in healthy aging. Most human studies were excluded since they did not report effects for older adults. This became apparent at full-text review for most articles, potentially because the keyword “middle age” covers 45–64 years. Further, although some studies included a few participants aged ≥ 50 years (and thus were tagged with the keywords “middle age” or “older adult”), they did not actually report results for this group specifically and therefore were ineligible for this review.
The scant research in this area indicates that existing findings reported herein should be interpreted with caution, since replication and further research are required. Nonetheless, preliminary hypotheses for future research can be gleaned from the reviewed articles. Only one cannabis use variable was associated with cognitive function in humans. Dregan and Gulliford (2012) found lifetime use (≥1 occasions)—but not past 12-month cannabis use—at age 42 predicted better cognitive function 8 years later. Although this suggests pro-cognitive effects of cannabis, the relationship may in part be confounded by cognitive reserve, which was not adequately controlled in analyses. Cognitive reserve is a multifaceted construct that buffers against the effects of brain insults, leading to better cognitive function in the presence of brain pathology and healthy aging (Satz, Cole, Hardy, & Rassovsky, 2011; Stern, 2009). Future studies should control for cognitive reserve by using a comprehensive assessment of the construct (i.e., by measuring verbal IQ, educational attainment, and occupation history) and/or a longitudinal approach.
Remaining results indicated that former and current cannabis use were not significantly associated with changes in cognitive function in older adults. Burggren et al. (2018) found no difference between people with a history of heavy cannabis use and controls, on memory, attention, processing speed, and executive function in older adulthood. Despite this, older adults with a history of cannabis use had cortical thinning of the hippocampus compared to older adult controls, which in turn were not associated with neuropsychological performance. This result may be indicative of the compensatory recruitment of other brain regions to mitigate the detrimental effects of chronic cannabis exposure, as postulated by reviews of studies conducted with younger populations (Batalla et al., 2013; Lorenzetti et al., 2016). Nonetheless, the rebound in cognition observed in Burggren et al. (2018) extends the results of Scott et al. (2018), which found a remediation of cognitive deficits among young people following 72 hours of abstinence from cannabis.
Thayer et al. (2019) found weekly or greater cannabis use was not associated with memory, response inhibition, or processing speed. Further, older adults who used cannabis and aged-matched controls did not differ significantly on brain morphology, with similar CSF, gray and white matter, and hippocampal volumes, which were unassociated with cognitive performance. The lack of significant difference in hippocampal volume between older adults who use cannabis and aged-matched controls is at odds with the results seen in Burggren et al. (2018) and in other studies of younger people who use cannabis (Batalla et al., 2013 and Lorenzetti et al., 2016). This discrepancy in findings may indicate that chronic, but not infrequent cannabis use is associated with significant changes in hippocampal morphology.
The predominantly null findings among the human studies may also be attributable to the neuropsychological measures used in these studies. There is a dearth of research examining the effects of cannabis on neuropsychological function using the NIH Toolbox Cognition Battery used in Thayer et al. (2019). Panee, Gerschenson, and Chang (2018) used five out of the seven tests from the NIH Toolbox Cognition Battery. Ultimately, Panee et al. (2018) found no significant difference in performance between young adults with a positive urine toxicology screen for THC and controls, on any of the neuropsychological assessments. Conversely, Petker et al. (2019) found younger adults with a positive urine toxicology screen for THC had worse performance on the picture sequence memory test and pattern comparison processing speed tests of the NIH Toolbox Cognition Battery, after controlling for demographic factors, and alcohol and tobacco use. However, there were no significant differences between younger adults who used cannabis and controls on the dimensional change card sort test, Flanker inhibitory control and attention test, and the list sorting working memory test performance. Ultimately, these studies suggest that some assessments from the NIH Toolbox Cognition Battery may be more sensitive to the effects of cannabis than others; however, further research is required in this area.
Some studies have used the measures employed by Burggren et al. (2018) to examine the effects of cannabis on cognition. Studies of adults who less frequently (e.g., > monthly use; Fatjó-Vilas et al., 2020) or previously (but not currently) used cannabis (Lyons et al., 2004) found no difference in Wechsler Memory Scale (WMS) performance compared to age-matched controls. Conversely, Medina et al. (2007) found adolescents who used cannabis (>60 instances of lifetime cannabis use) performed marginally worse than age-matched controls on the WMS, following one month of abstinence. Research using the Rey–Osterrieth complex figure test found no significant effect of former cannabis use on performance in a twin study (Lyons et al., 2004). Other studies have found no significant difference in performance on the Rey–Osterrieth complex figure test, between adolescents who use cannabis heavily, and age-matched controls (Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Winward, Hanson, Tapert, & Brown, 2014).
In separate samples of young people, both Gruber, Dahlgren, Sagar, Gönenc, and Killgore (2012a) and Thames, Arbid, and Sayegh (2014) found young people who used cannabis had significantly worse performance on the Stroop and trail-making test Part B compared with controls. Conversely, Lyons et al. (2004) found no significant effect of history of cannabis use on Stroop performance. Finally, a longitudinal study of people who used medicinal cannabis for three months (predominantly for anxiety, depression, chronic pain, and sleep) had a significant improvement on Part A of the trail-making test and on the Stroop test (Gruber et al., 2016). Together, existing studies indicate that the Rey–Osterrieth complex figure test may not be sensitive to the effects of cannabis use. Further, although the WMS, Stroop, and trail-making test may capture cognitive impairment associated with recent, heavy cannabis use, these tests may have limited utility for examining the effects of former heavy cannabis use on subsequent cognitive function following a period of prolonged abstinence, as examined by Burggren et al. (2018).
Finally, the predominantly null findings among the human studies may also be attributable to the breadth in age and cannabis use characteristics among study participants. For instance, the cannabis samples in both Burggren et al. (2018) and Thayer et al. (2019) comprised of older adults with both early and late onset, long-term and short-term, and frequent and infrequent cannabis use. Further, Dregan and Gulliford (2012) examined cannabis effects at age 50, whereas Thayer et al. (2019) examined cannabis effects among older adults aged 60–80 years. Given early onset (<16 years) and heavy/persistent cannabis use have been found to have a detrimental impact on cognition in younger populations (e.g., Meier et al., 2012; Sagar et al., 2015) and given aging has been associated with decline in some cognitive functions (e.g., Samson & Barnes, 2013), the studies included in this review potentially comprise heterogenous groups of older adults with varying levels of cognitive function. Examining the effects of cannabis on cognition within more homogenous groups (e.g., similar age groups, early onset vs. later onset groups) may provide a better understanding of the relationship between cannabis use and cognition in healthy aging. This notion of heterogenity impacting interpretable outcomes is supported by the animal studies in this review, which include controlled studies suggesting that the effects of cannabis on cognition may vary as a function of age and level of cannabis exposure.
In contrast to the human studies, a chronic low dose of THC (3 mg/kg/day for 28 days) improved memory, spatial learning, and flexibility in mature and old mice (12 and 18 months; Bilkei-Gorzo et al., 2017), whereas a single, extremely low dose (0.002 mg/kg) improved memory performance and spatial learning and was associated with a volumetric increase in entorhinal cortex, prefrontal cortex, and posterior hippocampus, in very old mice (24 months; Sarne et al., 2018). The increase in the volume of the posterior hippocampus seen in Sarne et al. (2018) is at odds with the hippocampal thinning observed in Burggren et al. (2018) and null effects seen in Thayer et al. (2019). These mixed results may be due to the difference in THC exposure among the studies. Namely, Burggren et al. (2018) participants had a history of heavy cannabis use and Thayer et al. (2019) participants reported using cannabis at least weekly for the past year, while the rodents in Sarne et al. (2018) were treated with a single, ultra-low dose of THC (0.002 mg/kg). Thus, these results may highlight the differential effects of THC on brain morphology depending on the level of exposure as suggested by Calabrese and Rubio-Casillas (2018); however, research is required to examine this further. Rodent studies are well-positioned to test this hypothesis.
Chronic administration of a 1:1 ratio of THC and CBD, containing 0.75 mg/kg THC, a dose four times less than that administered in Bilkei-Gorzo et al. (2017) did not affect memory performance in old mice (12 months; Aso et al., 2016). Although a single, extremely low dose of THC (0.002 mg/kg) elicited pro-cognitive effects for very old mice (24 months), a larger (but still low) chronic dose (3 mg/kg/day, for 28 days) was required to exert pro-cognitive effects in old mice (12 months). Ultimately, THC may exert pro-cognitive effects by stimulating an endogenous compensatory mechanism, protecting the brain from further insults (Sarne et al., 2018). These animal studies indicate that carefully controlled observational and acute administration studies are required to fully investigate the validity of the hypotheses regarding age- and dose-dependencies of THC effects in human populations. No studies examined the effects of CBD alone, or high-CBD cannabis on cognitive function in healthy aging in animals or humans. Future research should aim to elucidate the contribution of this powerful antioxidant to cognitive function in healthy aging.
This review is limited by the small number of articles available on the effects of cannabis on cognitive function in healthy aging, and thus, conclusions should be interpreted with caution. The articles examined in this paper also have several limitations. Dregan and Gulliford (2012) and Thayer et al. (2019) used self-report to assess abstinence from cannabis, alcohol, and other substances before cognitive testing; however, they did not confirm self-reported abstinence using biological measures. Thus, some participants may have been experiencing acute intoxication, whereas others may have been experiencing non-acute, withdrawal, or no effects (in instances of prolonged abstinence). Given the known differential effects of acute versus non-acute effects of cannabis on cognition in younger populations (Crean et al., 2011; Gonzalez et al., 2002; Grant et al., 2003; Schreiner & Dunn, 2012; Scott et al., 2018), this may have contributed to the largely null results in human studies. Future research should collect data regarding time since last cannabis use and biologically verify recent cannabis use/abstinence before cognitive testing, in order to ensure the homogeneity of the cannabis sample. Similarly, studies should also biologically verify abstinence from other substances, given acute intoxication has been linked to cognitive impairments (e.g., effects of acute alcohol intoxication on response inhibition; Campbell, Chambers, Allen, Hedge, & Sumner, 2017).
The human studies included in this review also did not control for the route of administration of cannabis, which affects THC blood concentrations (Newmeyer et al., 2016). Future research should account for the route of administration given the high rates of edible use among older adults (Sexton et al., 2019; Thayer et al., 2019). The cognitive tasks used in the rodent studies have limited translational validity (Young, Powell, Risbrough, Marston, & Geyer, 2009) and many conflate cognitive function with motor function and novelty preference, which also decrease with age (Bingham, Martin, Macrae, & Carswell, 2012; Lindner, 1997; Stansfield & Kirstein, 2006). Further research is also required to examine the effects of cannabis use on dopamine function in older adults, given the known effects of cannabis on dopamine function in younger populations (Yoo et al., 2019), and the role of dopamine in general cognition including reward-based decision-making (Berry et al., 2019). This potential interaction is also important since age-related changes in the dopaminergic system have been observed (Karrer et al., 2017) and are linked to detrimental mental health and wellbeing outcomes (Volkow et al., 2014; Volkow et al., 2016). Altered dopamine function in older adults may explain why older adults tend to be less risk averse than younger populations (Fernandes et al., 2018; Pachur, Mata, & Hertwig, 2017), which may lead to negative practical outcomes (e.g., risky financial behaviors).
Conclusions
This systematic scoping review examined the extent of the literature on the effects of cannabis use on cognition in healthy aging older adults. Our work provides crucial, timely direction for future research on this emerging issue. Although dispensaries and popular cannabis websites have flooded the market with the promotion of cannabis as a medicine, this review underscores a dearth of research examining the effects of cannabis use on cognitive function in healthy-aging older adults. Existing human studies have several methodological limitations, potentially accounting for the predominantly null effects. These limitations are exemplified by better-controlled rodent studies, which underscore that the effects of cannabis on cognition in healthy aging may vary as a function of age and level of THC exposure. Ultimately, given the recent increase in cannabis use among older adults, future human research should examine the relationship between both early and later-life cannabis use on cognitive function within more homogenous, older adult samples of people who use cannabis. This research should employ both cognitive assessments and neuroimaging to develop a comprehensive understanding of the effects of cannabis on cognition. Future research should also account for confounding factors including acute versus non-acute effects, cognitive reserve, route of administration, and cannabis strain preference. Further research using animal models are also required to determine the mechanistic effects of THC and CBD (together and in insolation) on cognitive function in healthy aging, using measures that account for motor function and novelty preference.
Supplementary Material
Acknowledgements
The authors alone are responsible for the content and writing of this paper. We would like to thank Karen Heskett for her assistance in developing the search strategy for this review.
Contributor Information
Nina Pocuca, Department of Psychiatry, University of California San Diego, San Diego, CA, USA.
T Jordan Walter, Department of Psychiatry, University of California San Diego, San Diego, CA, USA.
Arpi Minassian, Department of Psychiatry, University of California San Diego, San Diego, CA, USA; Center for Stress and Mental Health, Veteran’s Administration San Diego Hospital, San Diego, CA, USA.
Jared W Young, Department of Psychiatry, University of California San Diego, San Diego, CA, USA; Research Service, VA San Diego Healthcare System, San Diego, CA, USA.
Mark A Geyer, Department of Psychiatry, University of California San Diego, San Diego, CA, USA; Research Service, VA San Diego Healthcare System, San Diego, CA, USA.
William Perry, Department of Psychiatry, University of California San Diego, San Diego, CA, USA.
Funding
Nina Pocuca is supported by an Interdisciplinary Research Fellowship in NeuroAIDS (R25MH081482). Jordan Walter is supported by a T32 fellowship from the National Institute of Mental Health (grant number T32MH018399). This work was supported by the National Institute on Drug Abuse and the Translational Methamphetamine Aids Research Center (grant numbers DA043535, P50 DA26306).
Conflict of Interest
None declared.
References
- Aso, E., Andrés-Benito, P., & Ferrer, I. (2016). Delineating the efficacy of a cannabis-based medicine at advanced stages of dementia in a murine model. Journal of Alzheimer's Disease, 54, 903–912. doi: 10.3233/JAD-160533. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics . (2017). Dataset: Population Projections, Australia, 2017–2066. Retrieved from: https://www.abs.gov.au/ausstats/abs@.nsf/latestProducts/3222.0Media%20Release12017%20(base)%20-%202066.
- Azofeifa, A., Mattson, M. E., Schauer, G., McAfee, T., Grant, A., & Lyerla, R. (2016). National Estimates of Marijuana Use and Related Indicators -- National Survey on Drug Use and Health, United States, 2002-2014. In MMWR Surveillance Summaries (, Vol. 65, pp. 1–25). Atlanta, Georgia: Centers for Disease Control & Prevention (CDC). [DOI] [PubMed] [Google Scholar]
- Batalla, A., Bhattacharyya, S., Yuecel, M., Fusar-Poli, P., Crippa, J. A., Nogue, S. et al. (2013). Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PloS One, 8(2), e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, A. S., Jagust, W. J., & Hsu, M. (2019). Age-related variability in decision-making: Insights from neurochemistry. Cognitive, Affective, & Behavioral Neuroscience, 19(3), 415–434. doi: 10.3758/s13415-018-00678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo, A., Albayram, O., Draffehn, A., Michel, K., Piyanova, A., Oppenheimer, H. et al. (2017). A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nature Medicine, 23(6), 782. doi: 10.1038/nm.4311. [DOI] [PubMed] [Google Scholar]
- Bingham, D., Martin, S. J., Macrae, I. M., & Carswell, H. V. O. (2012). Watermaze performance after middle cerebral artery occlusion in the rat: The role of sensorimotor versus memory impairments. Journal of Cerebral Blood Flow & Metabolism, 32(6), 989–999. doi: 10.1038/jcbfm.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatwright, K. D., & Sperry, M. L. (2018). Accuracy of medical marijuana claims made by popular websites. Journal of Pharmacy Practice. doi: 10.1177/0897190018818907. [DOI] [PubMed] [Google Scholar]
- Bobitt, J., Qualls, S. H., Schuchman, M., Wickersham, R., Lum, H. D., Arora, K. et al. (2019). Qualitative analysis of cannabis use among older adults in Colorado. Drugs and Aging. doi: 10.1007/s40266-019-00665-w. [DOI] [PubMed] [Google Scholar]
- Boissoneault, J., Sklar, A., Prather, R., & Nixon, S. J. (2014). Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs, 75(5), 870–879. doi: 10.15288/jsad.2014.75.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd, S. J., van Hell, H. H., Beale, C., Yücel, M., & Solowij, N. (2016). Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biological Psychiatry, 79(7), 557–567. doi: 10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Burggren, A. C., Siddarth, P., Mahmood, Z., London, E. D., Harrison, T. M., Merrill, D. A. et al. (2018). Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis and Cannabinoid Research, 3(1), 242–251. doi: 10.1089/can.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burholt, V., Windle, G., & Morgan, D. J. (2016). A social model of loneliness: The roles of disability, social resources, and cognitive impairment. The Gerontologist, 57(6), 1020–1030. doi: 10.1093/geront/gnw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein, S. (2015). Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic & Medicinal Chemistry, 23(7), 1377–1385. doi: 10.1016/j.bmc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- Calabrese, E. J., & Rubio-Casillas, A. (2018). Biphasic effects of THC in memory and cognition. European Journal of Clinical Investigation, 48(5), e12920. doi: 10.1111/eci.12920. [DOI] [PubMed] [Google Scholar]
- Campbell, A. E., Chambers, C. D., Allen, C. P. G., Hedge, C., & Sumner, P. (2017). Impairment of manual but not saccadic response inhibition following acute alcohol intoxication. Drug and Alcohol Dependence, 181, 242–254. doi: 10.1016/j.drugalcdep.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Cavazos-Rehg, P. A., Krauss, M. J., Cahn, E., Lee, K. E., Ferguson, E., Rajbhandari, B. et al. (2019). Marijuana promotion online: An investigation of dispensary practices. Prevention Science, 20(2), 280–290. doi: 10.1007/s11121-018-0889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, N. G., DiNitto, D. M., & Marti, C. N. (2017). Nonmedical versus medical marijuana use among three age groups of adults: Associations with mental and physical health status. The American Journal on Addictions, 26(7), 697–706. doi: 10.1111/ajad.12598. [DOI] [PubMed] [Google Scholar]
- Crane, N. A., Schuster, R. M., Fusar-Poli, P., & Gonzalez, R. (2013). Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review, 23(2), 117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean, R. D., Crane, N. A., & Mason, B. J. (2011). An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine, 5(1), 1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias, C. M., Lövdén, M., Lindenberger, U., & Nilsson, L.-G. (2007). Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence, 35(4), 381–392. doi: 10.1016/j.intell.2006.07.011. [DOI] [Google Scholar]
- Dregan, A., & Gulliford, M. C. (2012). Is illicit drug use harmful to cognitive functioning in the Midadult years? A cohort-based investigation. American Journal of Epidemiology, 175(3), 218–227. doi: 10.1093/aje/kwr315. [DOI] [PubMed] [Google Scholar]
- Eppinger, B., Hämmerer, D., & Li, S.-C. (2011). Neuromodulation of reward-based learning and decision making in human aging. Annals of the New York Academy of Sciences, 1235, 1. doi: 10.1111/j.1749-6632.2011.06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy, V., Hatch, S. L., Hotopf, M., & Stewart, R. (2012). Prevalences of illicit drug use in people aged 50 years and over from two surveys. Age and Ageing, 41(4), 553–556. doi: 10.1093/ageing/afs020. [DOI] [PubMed] [Google Scholar]
- Fard, M. T., & Stough, C. (2019). A review and hypothesized model of the mechanisms that underpin the relationship between inflammation and cognition in the elderly. Frontiers in Aging Neuroscience, 11. doi: 10.3389/fnagi.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatjó-Vilas, M., Soler, J., Ibáñez, M. I., Moya-Higueras, J., Ortet, G., Guardiola-Ripoll, M. et al. (2020). The effect of the AKT1 gene and cannabis use on cognitive performance in healthy subjects. Journal of Psychopharmacology.. doi: 10.1177/0269881120928179. [DOI] [PubMed] [Google Scholar]
- Fernandes, C., Pasion, R., Gonçalves, A. R., Ferreira-Santos, F., Barbosa, F., Martins, I. P. et al. (2018). Age differences in neural correlates of feedback processing after economic decisions under risk. Neurobiology of Aging, 65, 51–59. doi: 10.1016/j.neurobiolaging.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Flurkey, K. C., M, J., & Harrison, D. E. (2007). Mouse Models in Aging Research. In Fox, J. G. et al. (Eds.), The mouse in biomedical research (2nd ed., pp. 637–672). Burlington, MA: Elsevier. [Google Scholar]
- Fraundorf, S. H., Hourihan, K. L., Peters, R. A., & Benjamin, A. S. (2019). Aging and recognition memory: A meta-analysis. Psychological Bulletin, 145(4), 339. doi: 10.1037/bul0000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, M., Faull, R. L. M., & Dragunow, M. (1997). Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience, 77(2), 299–318. doi: 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez, R., Carey, C., & Grant, I. (2002). Nonacute (residual) neuropsychological effects of cannabis use: A qualitative analysis and systematic review. The Journal of Clinical Pharmacology, 42(S1), 48S–57S. doi: 10.1177/0091270002238794. [DOI] [PubMed] [Google Scholar]
- Gorey, C., Kuhns, L., Smaragdi, E., Kroon, E., & Cousijn, J. (2019). Age-related differences in the impact of cannabis use on the brain and cognition: A systematic review. European Archives of Psychiatry and Clinical Neuroscience, 269(1), 37–58. doi: 10.1007/s00406-019-00981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, I., Gonzalez, R., Carey, C. L., Natarajan, L., & Wolfson, T. (2003). Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. Journal of the International Neuropsychological Society, 9(5), 679–689. doi: 10.10170S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gruber, S. A., Dahlgren, M. K., Sagar, K. A., Gönenc, A., & Killgore, W. D. S. (2012a). Age of onset of marijuana use impacts inhibitory processing. Neuroscience Letters, 511(2), 89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, S. A., Sagar, K. A., Dahlgren, M. K., Racine, M., & Lukas, S. E. (2012b). Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors, 26(3), 496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, S. A., Sagar, K. A., Dahlgren, M. K., Racine, M. T., Smith, R. T., & Lukas, S. E. (2016). Splendor in the grass? A pilot study assessing the impact of medical marijuana on executive function. Frontiers in Pharmacology, 7(355), 1–12. doi: 10.3389/fphar.2016.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, B. H., Sherman, S., Mauro, P. M., Martins, S. S., Rotenberg, J., & Palamar, J. J. (2017). Demographic trends among older cannabis users in the United States, 2006–13. Addiction, 112(3), 516–525. doi: 10.1111/add.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, C. N., Natelson Love, M. C., & Triebel, K. L. (2013). Normal cognitive aging. Clinics in Geriatric Medicine, 29(4), 737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, N. L., Mogle, J., Wion, R., Munoz, E., DePasquale, N., Yevchak, A. M. et al. (2016). Subjective cognitive impairment and affective symptoms: A systematic review. The Gerontologist, 56(6), e109–e127. doi: 10.1093/geront/gnw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer, T. M., Josef, A. K., Mata, R., Morris, E. D., & Samanez-Larkin, G. R. (2017). Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: A meta-analysis. Neurobiology of Aging, 57, 36–46. doi: 10.1016/j.neurobiolaging.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinov, V., & Roche, A. (2017). Bongs and baby boomers: Trends in cannabis use among older Australians. Australasian Journal on Ageing, 36(1), 56–59. doi: 10.1111/ajag.12357. [DOI] [PubMed] [Google Scholar]
- Kray, J., & Lindenberger, U. (2000). Adult age differences in task switching. Psychology and Aging, 15(1), 126. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Lindner, M. D. (1997). Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiology of Learning and Memory, 68(3), 203–220. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- Lister, J. P., & Barnes, C. A. (2009). Neurobiological changes in the hippocampus during normative aging. Archives of Neurology, 66(7), 829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- Lorenzetti, V., Alonso-Lana, S. J., Youssef, G., Verdejo-Garcia, A., Suo, C., Cousijn, J. et al. (2016). Adolescent cannabis use: What is the evidence for functional brain alteration? Current Pharmaceutical Design, 22(42), 6353–6365. doi: 10.2174/1381612822666160805155922. [DOI] [PubMed] [Google Scholar]
- Luc, M. H., Tsang, S. W., Thrul, J., Kennedy, R. D., & Moran, M. B. (2020). Content analysis of online product descriptions from cannabis retailers in six US states. International Journal of Drug Policy, 75, 102593. doi: 10.1016/j.drugpo.2019.10.017. [DOI] [PubMed] [Google Scholar]
- Lyons, M. J., Bar, J. L., Panizzon, M. S., Toomey, R., Eisen, S., Xian, H. et al. (2004). Neuropsychological consequences of regular marijuana use: A twin study. Psychological Medicine, 34(7), 1239–1250. doi: 10.1017/S0033291704002260. [DOI] [PubMed] [Google Scholar]
- Marschner, A., Mell, T., Wartenburger, I., Villringer, A., Reischies, F. M., & Heekeren, H. R. (2005). Reward-based decision-making and aging. Brain Research Bulletin, 67(5), 382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Medina, K. L., Hanson, K. L., Schweinsburg, A. D., Cohen-Zion, M., Nagel, B. J., & Tapert, S. F. (2007). Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society, 13(5), 807. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, M. H., Caspi, A., Ambler, A., Harrington, H., Houts, R., Keefe, R. S. et al. (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences, 109(40), E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell, T., Heekeren, H. R., Marschner, A., Wartenburger, I., Villringer, A., & Reischies, F. M. (2005). Effect of aging on stimulus-reward association learning. Neuropsychologia, 43(4), 554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. doi: 10.1371/journal.pmed.1000097. [DOI] [PubMed] [Google Scholar]
- Mori, M. A., Meyer, E., Soares, L. M., Milani, H., Guimarães, F. S., & de Oliveira, R. M. W. (2017). Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 75, 94–105. doi: 10.1016/j.pnpbp.2016.11.005. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2017). The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. 10.17226/24625. [DOI] [PubMed] [Google Scholar]
- Newmeyer, M. N., Swortwood, M. J., Barnes, A. J., Abulseoud, O. A., Scheidweiler, K. B., & Huestis, M. A. (2016). Free and glucuronide whole blood Cannabinoids' pharmacokinetics after controlled smoked, vaporized, and oral cannabis Administration in Frequent and Occasional Cannabis Users: Identification of recent cannabis intake. Clinical Chemistry, 62(12), 1579. doi: 10.1373/clinchem.2016.263475. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics . (2017). Table A2–1, Principal projection - UK population in age groups. Retrieved from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/tablea21principalprojectionukpopulationinagegroups.
- Pachur, T., Mata, R., & Hertwig, R. (2017). Who dares, who errs? Disentangling cognitive and motivational roots of age differences in decisions under risk. Psychological Science, 28(4), 504–518. doi: 10.1177/095679761668772. [DOI] [PubMed] [Google Scholar]
- Panee, J., Gerschenson, M., & Chang, L. (2018). Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. Journal of Neuroimmune Pharmacology, 13(1), 113–122. doi: 10.1007/s11481-017-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh, P. K., Troyer, A. K., Maione, A. M., & Murphy, K. J. (2015). The impact of memory change on daily life in normal aging and mild cognitive impairment. The Gerontologist, 56(5), 877–885. doi: 10.1093/geront/gnv030. [DOI] [PubMed] [Google Scholar]
- Patterson, S. L. (2015). Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology, 96, 11–18. doi: 10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petker, T., Owens, M. M., Amlung, M. T., Oshri, A., Sweet, L. H., & MacKillop, J. (2019). Cannabis involvement and neuropsychological performance: Findings from the human connectome project. Journal of Psychiatry & Neuroscience, 44(6), 414. doi: 10.1503/jpn.180115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar, K. A., Dahlgren, M. K., Gönenç, A., Racine, M. T., Dreman, M. W., & Gruber, S. A. (2015). The impact of initiation: Early onset marijuana smokers demonstrate altered stroop performance and brain activation. Developmental Cognitive Neuroscience, 16, 84–92. doi: 10.1016/j.dcn.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar, K. A., & Gruber, S. A. (2018). Marijuana matters: Reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. International Review of Psychiatry, 30(3), 251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, J. M., & Forester, B. (2012). Substance abuse and co-occurring psychiatric disorders in older adults: A clinical case and review of the relevant literature. Journal of Dual Diagnosis, 8(1), 74–84. doi: 10.1080/15504263.2012.648439. [DOI] [Google Scholar]
- Samson, R. D., & Barnes, C. A. (2013). Impact of aging brain circuits on cognition. European Journal of Neuroscience, 37(12), 1903–1915. doi: 10.1111/ejn.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarne, Y. (2019). Beneficial and deleterious effects of cannabinoids in the brain: The case of ultra-low dose THC. The American Journal of Drug and Alcohol Abuse, 45(6), 551–562. doi: 10.1080/00952990.2019.1578366. [DOI] [PubMed] [Google Scholar]
- Sarne, Y., Toledano, R., Rachmany, L., Sasson, E., & Doron, R. (2018). Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiology of Aging, 61, 177–186. doi: 10.1016/j.neurobiolaging.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Satz, P., Cole, M. A., Hardy, D. J., & Rassovsky, Y. (2011). Brain and cognitive reserve: Mediator (s) and construct validity, a critique. Journal of Clinical and Experimental Neuropsychology, 33(1), 121–130. doi: 10.1080/13803395.2010.493151. [DOI] [PubMed] [Google Scholar]
- Schreiner, A. M., & Dunn, M. E. (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Experimental and Clinical Psychopharmacology, 20(5), 420–429. doi: 10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- Scott, E. P., Brennan, E., & Benitez, A. (2019). A systematic review of the neurocognitive effects of cannabis use in older adults. Current Addiction Reports, 6(4), 443–455. doi: 10.1007/s40429-019-00285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. C., Slomiak, S. T., Jones, J. D., Rosen, A. F. G., Moore, T. M., & Gur, R. C. (2018). Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA psychiatry, 75(6), 585–595. doi: 10.1001/jamapsychiatry.2018.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton, M., Cuttler, C., & Mischley, L. K. (2019). A survey of cannabis acute effects and withdrawal symptoms: Differential responses across user types and age. Journal of Alternative and Complementary Medicine, 25, 326–335. doi: 10.1089/acm.2018.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar, A. L., Gilbertson, R., Boissoneault, J., Prather, R., & Nixon, S. J. (2012). Differential effects of moderate alcohol consumption on performance among older and younger adults. Alcoholism: Clinical and Experimental Research, 36(12), 2150–2156. doi: 10.1111/j.1530-0277.2012.01833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield, K. H., & Kirstein, C. L. (2006). Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology, 48(1), 10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames, A. D., Arbid, N., & Sayegh, P. (2014). Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addictive behaviors, 39(5), 994–999. doi: 10.1016/j.addbeh.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer, R. E., YorkWilliams, S. L., Hutchison, K. E., & Bryan, A. D. (2019). Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Research-Neuroimaging, 285, 58–63. doi: 10.1016/j.pscychresns.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D. et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob, E. M., Brandmaier, A. M., & Lindenberger, U. (2019). Coupled cognitive changes in adulthood: A meta-analysis. Psychological Bulletin, 145(3), 273–301. doi: 10.1037/bul0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau . (2018). Projected 5-Year Age Groups and Sex Composition: Main Projections Series for the United States, 2017–2060. Retrieved from https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html
- Volkow, N. D., Swanson, J. M., Evins, A. E., DeLisi, L. E., Meier, M. H., Gonzalez, R. et al. (2016). Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry, 73(3), 292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D., Wang, G.-J., Telang, F., Fowler, J. S., Alexoff, D., Logan, J. et al. (2014). Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proceedings of the National Academy of Sciences, 111(30), E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler, J. A., Bellebaum, C., & Daum, I. (2008). Aging affects acquisition and reversal of reward-based associative learning. Learning & memory, 15(4), 190–197. doi: 10.1101/lm.890408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. S., Boyle, P. A., Segawa, E., Yu, L., Begeny, C. T., Anagnos, S. E. et al. (2013). The influence of cognitive decline on well-being in old age. Psychology and Aging, 28(2), 304–313. doi: 10.1037/a0031196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward, J. L., Hanson, K. L., Tapert, S. F., & Brown, S. A. (2014). Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. Journal of the International Neuropsychological Society, 20(8), 784–795. doi: 10.1017/S1355617714000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, H. B., DiMuzio, J., & Filbey, F. M. (2019). Interaction of cannabis use and aging: From molecule to mind. Journal of Dual Diagnosis.. doi: 10.1080/15504263.2019.1665218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. W., Powell, S. B., Risbrough, V., Marston, H. M., & Geyer, M. A. (2009). Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacology & Therapeutics, 122(2), 150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel, M., Lorenzetti, V., Suo, C., Zalesky, A., Fornito, A., Takagi, M. J. et al. (2016). Hippocampal harms, protection and recovery following regular cannabis use. Translational Psychiatry, 6(1), e710. doi: 10.1038/tp.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


