Abstract
The discovery of new inhibitors that can be used in the treatment of viral diseases, including Covid-19, is an area open to research, and there is a need for innovative compounds with increased efficiency that provide inhibition by suppressing enzyme, and receptor mechanisms. The iron(III) and nickel(II) complexes were synthesized by template condensation of 4-methoxy-salicylaldehyde with S-methylthiosemicarbazone derivatives of 1,1,1-trifluoroacetylacetone (for Fe1) and methylacetoacetate (for Ni1). The complex structures having N2O2-chelating thiosemicarbazidato ligand were identified by analytical, spectroscopic, and X-ray crystallography results. Coordination environment of iron(III) center in complex Fe1 has a distorted square pyramidal geometry consisting of the N2O2 donor set and a chlorine atom, while that of Ni1 is square plane with the set. Inhibitory effect of Fe1 compound against SARS-CoV-2 virus specific 3C-like protease enzyme was investigated experimentally. It was determined that the highest inhibition concentration of Fe1 was 100 μM. Percent inhibition activity at this concentration was on average 30.62 ± 3.809%. Binding of both compounds to the 3C-like protease enzyme specific to the SARS-CoV-2 virus was analyzed using docking calculations. As a result of the docking calculation of Fe1, it has been observed that the compound has a binding energy of -7.4 kcal / mol to 3CL-like protease. It has been observed that the protein amino acids GLY143, THR26, and ASN142 contribute to the high binding affinity of the Fe1. The experimental and theoretical results obtained for the two complexes support each other.
Keywords: Iron, Nickel, Thiosemicarbazone, SARS-CoV-2
1. Introduction
Thiosemicarbazone chemistry is an expanding topic by participation of their transition metal complexes having drug potential [[1], [2], [3], [4], [5], [6]]. For pharmacological purposes, even though platinum, palladium and copper ions are mostly preferred in complex formations of thiosemicarbazones, iron(III) and nickel(II) have also been included in studies in recent years [[7], [8], [9], [10]].
Metal complexes of S-alkylthiosemicarbazones with salen-like donor atom set (N2O2) have been exhibited remarkable potentials as anti-cancer drug active ingredients. In an in vitro study, a series of palladium(II) complexes obtained from acetylacetone-S-alkyl thiosemicarbazones were reported to have much lower IC50 values than cisplatin on HepG2 and Hep3B hepatocellular carcinomas, HCT116 colorectal carcinoma cells [6]. This performance has been associated with their xanthine oxidase inhibitions between 0.42 -12.01 μg/ml of IC50 values. Remarkable cytotoxicity data were obtained also with the iron(III) and nickel(II) complexes containing this type of tetradentate thiosemicarbazones [7,9,10]. An iron(III) complex with a tetradentate S-methylthiosemicarbazidato ligand showing a meaningful cytotoxic effect against HT-29 and HeLa cell lines was found to be linked to CT-DNA with intercalation mode and calculated its intrinsic DNA-binding constant (Kb) as 1 × 105 M−1 from data of the experiments performed with calf thymus DNA [11]. Moreover, enzyme inhibition capabilities [6], antidiabetic [12] and antioxidant properties [[13], [14], [15]] of metal complexes with N2O2-thiosemicarbazone ligands have been reported.
The 2019 coronavirus disease outbreak (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in more than 3 million deaths in less than a year. SARS-CoV-2 infection has a wide range of clinical manifestations, including both asymptomatic cases and rapid deaths [16]. Activation of the immune system and production of inflammatory cytokines are essential for natural anti-viral immune responses [17]. However, hyperactivation of the immune system causes an acute increase in circulating pro-inflammatory cytokine levels, leading to a "cytokine burst" [18]. Cytokine burst is clinically characterized by systemic inflammation, hyperferritinemia, hemodynamic instability, and multi-organ failure [19].
The transition metal complexes of thiosemicarbazones show various biological activities and have been the subject of many studies [[20], [21], [22], [23], [24], [25], [26]]. In addition to these studies, which mostly focus on the search for cancer-preventing active substances, there are also studies examining antiviral effects. Frequently, thiosemicarbazones have been studied as organic molecules in antiviral property studies, and metal complexes are few in number. In a study in which platinum(II) and palladium(II) complexes of thiophene-2-carboxaldehyde thiosemicarbazone derivatives were examined, activity against DNA and RNA viruses was investigated [27]. Complexes of the same metals with pyridine-2-carbaldehyde thiosemicarbazone exhibited considerable efficacy against herpes simplex virus 1 (HSV-1) [28]. In another study; The efficiency of thiosemicarbazones copper(II) and nickel(II) complexes against HIV viruses has been determined [29].
The biological activity potential of metal complexes of such N2O2-chelating thiosemicarbazone ligands have been known since 2007 [30]. An iron and a nickel(II) centered complexes bearing such thiosemicarbazones were synthesized and structurally defined. In the study, the inhibitory effects of the two compounds against SARS-CoV2 3CL-like protease were investigated considering the enzyme inhibition abilities and the antiviral effect potentials of thiosemicarbazone compounds. The virus contains four non-structural proteins: papain-like (PLpro) and 3-chymotrypsin-like (3CLpro) proteases, RNA polymerase and helicase. Both proteases (PLpro and 3CLpro) are involved in transcription and replication of the virus. Among the four proteins, 3CLpro is mainly involved in the replication of the virus [31]. One of the main target proteins in inhibiting virus replication is 3CL main protease enzyme. In the study, first, the binding affinity of the substances against the 3CL main protease enzyme by autodocking was examined, and then the inhibitory effect of the Fe1 substance with high binding affinity was experimentally investigated.
2. Materials and methods
2.1. Apparatus and methods
The elemental analyses were determined on a Thermo Finnigan Flash EA 1112 Series Elemental Analyzer. IR spectra were obtained using ATR unit on Agilent Carry 630 spectrophotometer. 1H NMR spectra were measured on Varian UNITY INOVA 500 MHz NMR spectrometer. UV-Visible spectra were recorded on Ocean Optics QE65000 diode array spectrophotometer. Magnetic moment measurements were carried out by Gouy technique with an MK I model device of Sherwood Scientific at room temperature.
Suitable crystals of Fe1 and Ni1 were selected for data collection which was performed on a Bruker D8-QUEST diffractometer equipped with a graphite-monochromatic Mo-Kα radiation at 296 K. The H atoms were located from different maps and then treated as riding atoms with C-H distances of 0.93-0.96 Å. We used these procedures for our analysis: solved by direct methods; SHELXS-2013 [32]; refined by full-matrix least-squares methods; SHELXL-2013 [33]; data collection: Bruker APEX2 [34]; molecular graphics: MERCURY [35]; solution: WinGX [36]. Powder XRD patterns were recorded by XPERT-PRO diffractometer system using Cu-Kα1 radiation with λ = 1.5406 Å.
2.2. Synthesis
The starting materials, S-methylthiosemicarbazone derivatives of 1,1,1-Trifluoroacetylacetone (1) and methylacetoacetate (2) were obtained in the form of hydroiodide salt by using literature metods [[37], [38], [39]]. The cream colored reaction products were recrystallized from ethanol-dichloromethane (1:1) and dried in vacuo.
(1) Yield: 2.58 g, 70.0%; m.p. (°C): 147; Calc. for C7H11N3OSF3I (Mr=369.14), %: C, 22.78; H, 3.00; N, 11.38; S, 13.04. Found, %: C, 22.49; H, 2.83; N, 11.54; S, 12.24. IR (cm−1): νas(NH2) 3354, νs(NH2) 3249, ν(OH) 3042, δ(NH2), ν(C=N) 1642-1541. UV-Vis [in 10−5 M CHCl3, λmax (nm), log ε (dm3 cm−1 mol−1)]: 242 (5.03), 362 (3.15). 1H NMR (ppm): 9.84 (s, 2H, NH2), 9.46 (s, 1H, OH), 3.70 (s, 2H, -CH2-), 2.63 (s, 3H, S-CH3), 2.12 (s, 3H, C-CH3).
(2) Yield: 1.92 g, 58.0%; m.p. (°C): 117; Calc. for C7H14N3O2SI (Mr=331.17), %: C, 25.39; H, 4.26; N, 12.69; S, 9.68. Found, %: C, 25.51; H, 4.03; N, 12.47; S, 9.23. IR (cm−1): νas(NH2) 3260, νs(NH2) 3165, ν(OH) 3081, δ(NH2), ν(C=N) 1722-1574. UV-Vis [in 10−5 M CHCl3, λmax (nm), log ε (dm3 cm−1 mol−1)]: 241 (5.03), 364 (3.26). 1H NMR (ppm): 9.38 (s, 2H, NH2), 7.18 (s, 1H, OH), 3.65 (s, 3H, O-CH3), 3.18 (s, 2H, -CH2-), 2.68 (s, 3H, S-CH3), 2.22 (s, 3H, C-CH3).
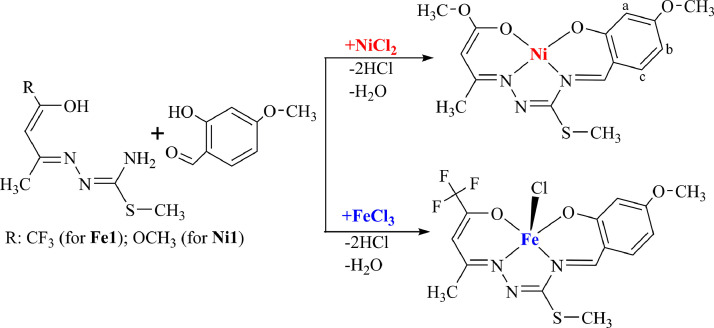
Monochloro-N1-1,1,1-Trifluoroacetylacetone-N4-4-methoxysalicylidene-S-methyl-thiosemicarbazidato iron(III) (Fe1)
To a solution of compound 1 (0.37 g, 1.0 mmol) and 4-methoxysalicylaldehyde (0.15 g, 1.0 mmol) in ethanol (10 ml) was added dropwise a solution of FeCl3.6H2O (0.27 g, 1.0 mmol) in ethanol (5 ml) and the mixture was stirred at 70°C for 10 min. Then it was cooled to room temperature and Et3N (0.1 mL) was added to the mixture. After 8 hours a black-looking crystalline product was separated by filtration, washed with 5 mL of ethanol-ether (1:1) and dried in vacuo (Fig. 1 ).
Fig. 1.
Synthesis scheme of the complexes.
Yield: 0.14 g, 30.0%; m.p. (°C): 310. μeff (BM): 5.86. Calc. for C15H14FeClF3N3O3S (Mr= 464.64), %: C, 38.77; H, 3.04; N, 9.04; S, 6.90. Found, %: C, 38.56; H, 3.33; N, 8.69; S, 6.37. IR (cm−1): ν(C=N1) 1615; ν(N2=C) 1606; ν(N4=C) 1581; ν(C-O) 1160, 1124. UV-Vis [in 10−5 M CHCl3, λmax (nm), log ε (dm3 cm−1 mol−1)]: 239 (4.79), 309 (4.98), 422 (4.65)
N1-methylacetoacetate-N4-4-methoxysalicylidene-S-methyl-thiosemicarbazidato nickel(II) (Ni1)
To a solution of compound 2 (0.33 g, 1.0 mmol) and 4-methoxysalicylaldehyde (0.15 g, 1.0 mmol) in ethanol (10 ml) was added dropwise a solution of NiCl2.6H2O (0.24 g, 1.0 mmol) in ethanol (5 ml). The reaction mixture was stirred at 70°C for 10 min. After cooling, 0.1 mL of Et3N was added to the mixture and allowed to stand at room temperature for 24 h. Red crystalline product was separated by filtration, washed with 5 mL of ethanol ether (1:1) and dried in vacuo (Fig. 1).
Yield: 0.14 g, 35.0%; m.p. (°C): 199, μeff is equal approx. 0 BM. Calc. for C15H17NiN3O4S (Mr=394.07), %: C, 45.72; H, 4.35; N, 10.66; S, 8.14. Found, %: C, 45.43; H, 4.64; N, 10.28; S, 7.65. IR (cm−1): ν(C=N1) 1617; ν(N2=C) 1591; ν(N4=C) 1574; ν(C-O) 1149, 1111. UV-Vis [in 10−5 M CHCl3, λmax (nm), log ε (dm3 cm−1 mol−1)]: 240 (4.99), 262 (4.94), 305 (4.91), 332 (4.84), 341 (4.83), 432 (4.78), 485 (4.59). 1H NMR (ppm): 7.68 (s, 1H, N4=CH), 7.25 (d, 1H, c), 6.55 (s, 1H, a), 6.38 (d, 1H, b), 4.62 (s, 1H, =CH), 3.83 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 2.60 (s, 3H, S-CH3), 2.28 (s, 3H, C-CH3).
2.3. Docking calculations
In the study, binding pose and affinity of both compounds to the 3C-like protease enzyme specific to the SARS-CoV-2 virus were analyzed via molecular docking study using AutoDock VINA package [40]. In the docking calculation, 3C-like protease structure was taken from the Protein Data Bank (PDB code 6LU7). The protonation of residues was calculated at pH 7 using PROPKA [41]. The grid box was centered to the previously defined binding pocket, and the box size was set to 24 Å for each axis [42]. First, rigid docking procedure was followed. Next, flexible docking was carried out with the same grid box and size parameters. In the flexible docking, the side chain of residues THR25, HIS41, MET49, GLN142, and GLN189 were specified as flexible.
2.4. SARS-CoV2 3CL protease activity assay
The inhibitory activity of complex Fe1 compound, which shows high binding affinity against the SARS-CoV-2 virus-specific 3C-like protease enzyme according to the auto-docking results, was also examined with the commercially available BPS Bioscience 3CL Protease assay kit (Catalog # 79955). Experiments were made according to manufacturer's protocol. To understand inhibition percent of Fe1, its 0.1, 0.5, 25, 100 μM concentrations were used. Fe1 was dissolved in DMSO and a 10 mM stock solution was prepared. Its 0.1, 0.5, 25, 100 μM concentrations were prepared 3CL protease assay buffer included BPS Bioscience 3CL Protease assay kit. Concentrations were created so that there would be no more than 1% DMSO. 3CL protease assay buffer with the same amount of DMSO was analyzed as a control. Absorbance values of the controls subtracted from other wells. GC376 included in the kit was used as inhibitor control. The inhibition level of the substances was analyzed by comparing with GC376.
3. Results and Discussion
3.1. Structural characterization
The template condensation of the starting materials (1 and 2) with 4-methoxysalicylaldehyde in the presence of nickel(II) or iron(III) in the 1:1:1 molar ratio yielded the solid complexes. The formation and purity of the complexes were confirmed by elemental analysis, IR, UV-Vis and 1H NMR spectra (for Ni1) as well as single crystal X-ray diffraction analysis (Figure S1-S4). The ν(NH2), ν(OH), and also δ(NH2) bands disappeared in the infrared spectra of the complexes due to reactions of hydroxy and thioamide groups on 1 and 2. Similarly, no signals of the OH and NH2 groups were observed in the 1H NMR spectra of complex Ni1 (Figure S3). The resulting N4=CH and C=CH signals which are singlets and equivalent to the integral value of one proton, confirmed the chelate formation around nickel(II). The spectra showed the expected chemical shift values of the protons belonging to O-CH3, S-CH3 and C-CH3 groups in addition to the aromatic protons in the range of 7.25-6.38 ppm.
The electronic spectrum of the complexes showed π→π* and n→π* bands, which are transitions due to imine, thioamide groups and the phenyl ring, in the range of 239-341 nm. The bands recorded at 432 and 485(shoulder) nm for Ni1 and 422 nm for Fe1 can be assigned to charge transfer transitions. Because of quite low intensities, d–d transitions belonging to both complexes could be not observed in the spectra.
The μeff value of Fe1 (5.86 BM) indicates the five unpaired d5 structure. Magnetic measurement result of Ni1 shows diamagnetic form that is attributed to square-planar structure.
3.2. Crystallography
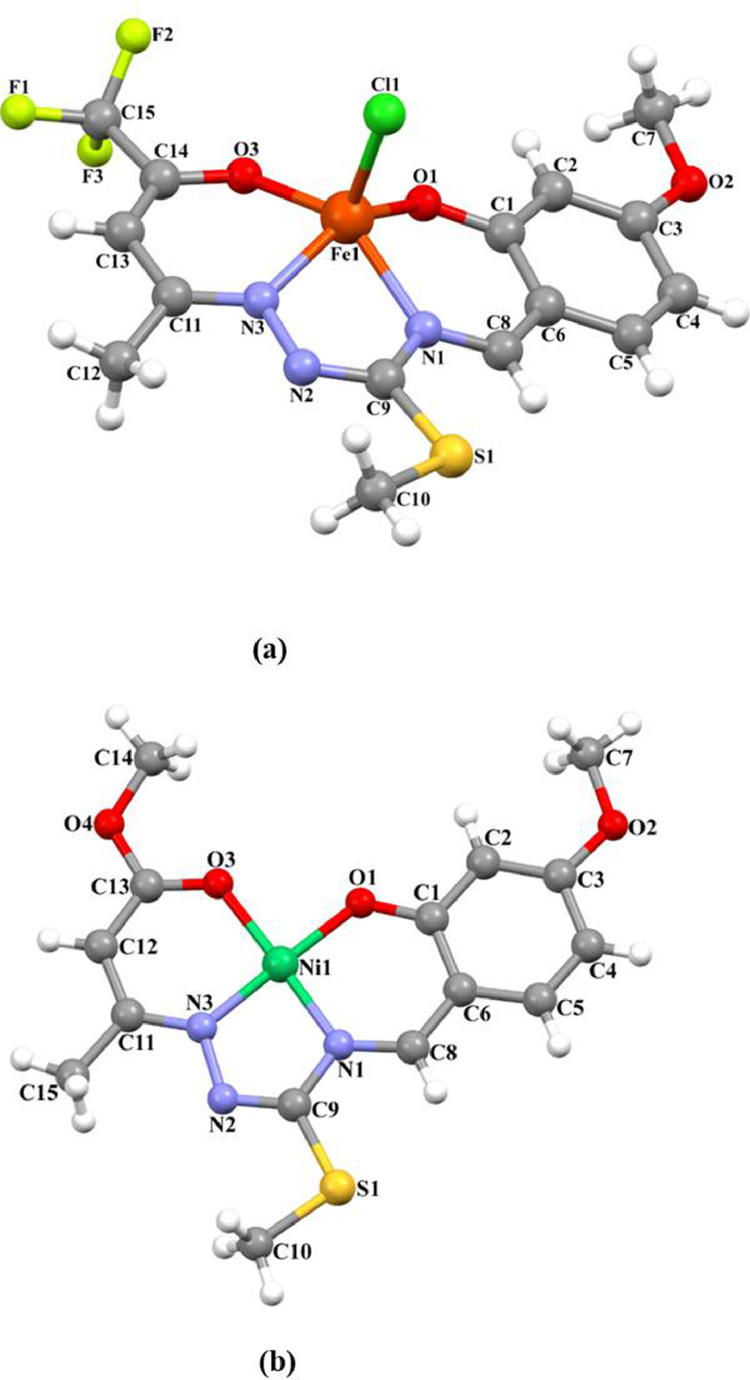
The molecular structures of Fe1 and Ni1, with the atom numbering schemes, are shown in Fig. 2 . In Fe1, the iron(III) ion is coordinated by two oxygen atoms [Fe1-O1=1.897(2) Å and Fe1-O3=1.907(2) Å] and two nitrogen atoms [Fe1-N1=2.067(3) Å and Fe1-N3=2.076(3) Å] from thiosemicarbazone ligand and chlorine atom [Fe1-Cl1=2.2146(11) Å]. The geometry of the Fe1 atom can be evaluated by the Addison distortion index tau (τ = (β-α)/60, where α and β are the two largest coordinated angles in the complex). In a five-coordinate geometry, τ = 0 for a square pyramidal geometry and τ=1 for a trigonal bipyramidal geometry. The τ value is calculated as 0.05 and this value shows that Fe1 atom has a distorted square pyramidal geometry (Table 1 ). In Fe1 crystal, atom C4 in the molecule at (x, y, z) acts as hydrogen-bond donor to the one of flour atoms in molecule at (x, y, z+1), so forming a C(11) chain which is running parallel to the [001] direction (Figure S1a, S2).
Fig. 2.
The molecular structures of Fe1 (a) and Ni1 (b) showing the atom numbering schemes.
Table 1.
Crystal data and structure refinement parameters for Fe1 and Ni1.
| Fe1 | Ni1 | |
|---|---|---|
| Empirical formula | C15H14ClF3FeN3O3S | C15H17N3NiO4S |
| Formula weight | 464.65 | 394.08 |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a (Å) | 8.1510 (6) | 4.8494 (6) |
| b (Å) | 9.2058 (7) | 11.8570 (16) |
| c (Å) | 13.3198 (10) | 15.331 (2) |
| α (°) | 85.271 (3) | 106.763 (6) |
| β (°) | 74.269 (4) | 96.289 (5) |
| γ (°) | 80.017 (3) | 99.336 (6) |
| V (Å3) | 946.81 (12) | 821.42 (19) |
| Z | 2 | 2 |
| Diffractometer | BRUKER D8-QUEST | |
| Temperature (K) | 296 | |
| Dc (g cm−3) | 1.630 | 1.593 |
| μ (mm−1) | 1.10 | 1.33 |
| θ range (°) | 3.2-26.4 | 3.5-26.5 |
| Measured refls. | 17755 | 17602 |
| Independent refls. | 3844 | 3147 |
| Rint | 0.040 | 0.068 |
| S | 1.13 | 1.12 |
| R1/wR2 | 0.050/0.103 | 0.081/0.173 |
| Δρmax/Δρmin (eÅ−3) | 0.35/-0.33 | 0.58/-0.55 |
Table 2.
Selected bond distances (Å, °).
| Fe1 | |||
|---|---|---|---|
| Fe1-O1 | 1.897(2) | Fe1-O3 | 1.907(2) |
| Fe1-N1 | 2.067(3) | Fe1-N3 | 2.076(3) |
| Fe1-Cl1 | 2.2146(11) | ||
| O1-Fe1-O3 | 92.51(10) | O1-Fe1-N1 | 87.07(10) |
| O3-Fe1-N1 | 144.71(11) | O1-Fe1-N3 | 147.74(11) |
| O3-Fe1-N3 | 87.47(11) | N1-Fe1-N3 | 74.99(11) |
| O1-Fe1-Cl1 | 108.50(8) | O3-Fe1-Cl1 | 108.66(9) |
| N1-Fe1-Cl1 | 104.86(8) | N3-Fe1-Cl1 | 102.00(9) |
| Ni1 | |||
|---|---|---|---|
| N1-Ni1 | 1.842(6) | N3-Ni1 | 1.839(6) |
| Ni1-O1 | 1.842(5) | Ni1-O3 | 1.873(5) |
| N3- Ni1-O1 | 178.7(3) | N3-Ni1-N1 | 83.6(3) |
| O1-Ni1-N1 | 95.3(2) | N3-Ni1-O3 | 94.5(2) |
| O1-Ni1-O3 | 86.6(2) | N1-Ni1-O3 | 178.0(2) |
Table 3.
Hydrogen bond parameters (Å, °).
| D-H•••A | D-H | H•••A | D•••A | D-H•••A |
|---|---|---|---|---|
| Fe1 | ||||
| C4—H4•••F1i | 0.93 | 2.45 | 3.249 (12) | 146 |
| Ni1 | ||||
| C4—H4•••O4i | 0.93 | 2.53 | 3.408 (9) | 159 |
Symmetry code: (i) x, y, z+1 for Fe1; (i) x+1, y+1, z for Ni1.
In the nickel(II) complex, the metal center is coordinated by two oxygen atoms [Ni1-O1=1.842(5) Å and Ni1-O3=1.873(5) Å] and two nitrogen atoms [Ni1-N1=1.842(6) Å and Ni1-N3=1.839(6) Å] of the ligand backbone, thus showing a distorted square planar coordination geometry. The C4 atom in the Ni1 molecule at (x, y, z) acts as hydrogen-bond donor to the O4 atom in the molecule at (x+1, y+1, z), so forming a C(10) chain which is running parallel to the [110] direction (Figure S1b, S2).
3.3. X-ray powder diffraction
The powder XRD diffraction is carried out for complexes Fe1 and Ni1 which shows prominent sharp peaks, with the evaluating the diffraction patterns of complexes given in Figures S6 and S7 which reveals crystalline nature of the complexes.
The Miller indices (hkl) along with observed and calculated d angles, 2θ values, and relative intensities are given in Table S3. The average crystalline sizes of the complexes were calculated using Debye Scherrer equation (D = K λ / β Cos θ) Where D = Particle size, K = Dimensionless shape factor, λ = X-ray wavelength (1.5406 Å) β = full width at half maximum of the diffraction peak, θ = Diffraction angle. The complexes of Fe1-Ni1 have a crystalline size of 32.09 for Fe1 and 47.63 nm for Ni1 respectively suggesting that the complexes are in a nanocrystalline phase.
3.4. Antiviral activity
Serious acute respiratory syndrome coronavirus (SARS-CoV), SARS-CoV2 and the Middle East respiratory syndrome coronavirus (MERS-CoV) have caused fatal outbreaks of pneumonia. Other members of the Coronaviridae family of zoonotic origin are endemic in the human population and account for up to 30% of mild respiratory infections [[43], [44], [45], [46]]. The discovery of new inhibitors that can be used in the treatment of viral diseases, including Covid-19, is an area open to research, and there is a need for innovative compounds with increased efficiency that provide inhibition by suppressing enzyme, and receptor mechanisms.
Although there are studies showing that different thiosemicarbazone compounds have antiviral effects, there are limited studies on their antiviral effects against the SARS-CoV2 virus [[47], [48]]. In these studies, the binding affinities against SARS-CoV2 were examined bioinformatically. However, the direct effects of thiosemicarbazone compounds against the SARS-CoV2 virus, natural immunity and cell death caused by inflammation induced by cytokine stimulation are unknown.
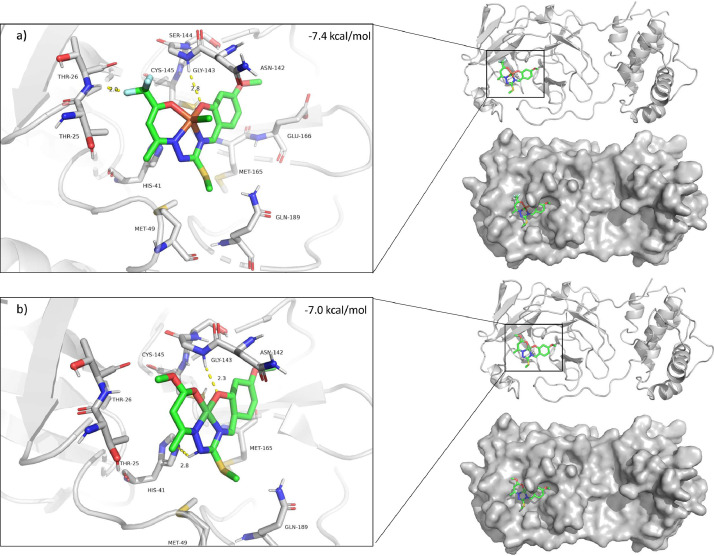
To predict the binding affinity and pose of both compounds to the 3C-like protease enzyme specific to the SARS-CoV-2 virus, docking calculations were performed. As a result of the rigid docking calculation the best docking pose docking score estimated to be -7.4 kcal/mol for Fe1, and -7.0 kcal/mol for Ni1. The residues GLY143 and THR26 backbone have hydrogen bond interaction with Fe1. For Ni1, similar hydrogen bond interaction was observed with GLY143, and in addition HIS41 is in the range of making hydrogen bond interaction (Fig. 3 ). Next, in order to assess possible conformations of the residues side chains that may affect binding were selected through manually inspection for flexible docking. As a result of the flexible docking procedure, the highest docking score is -7.8 for both Fe1 and Ni1. It has been observed in Fe1 binding pose that ASN142 side chain amine group located in between Fe1-O3 and Fe1-Cl atoms, similar position is observed for Ni1 as well (Figure S5).
Fig. 3.
The best binding pose of molecular rigid docking results of Fe1 (a) and Ni1 (b).
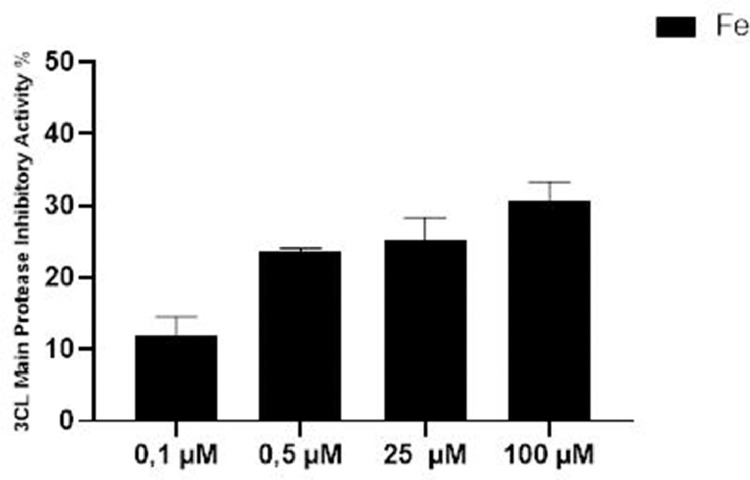
In accordance with the results obtained, inhibitory effect of Fe1 compound showing higher binding affinity against SARS-CoV-2 virus specific 3C-like protease enzyme was investigated experimentally. It was determined that the highest inhibition concentration of Fe1 compound was 100 μM. Percent inhibition activity at this concentration was on average 30.62 ± 3.809% (Fig. 4 ).
Fig. 4.
3CL main protease inhibitory activity of Fe1.
Tang and colleagues, in a randomized controlled study of 150 patients, found that hydroxychloroquine had no significant effect on accelerating viral clearance. Moreover, side effects were observed in patients treated with 800-1200mg of hydroxychloroquine per day compared to patients treated without hydroxychloroquine [49].
In another study, placebo-controlled, randomized remdesivir trials were conducted in patients with Covid-19. It was found that there was no significant difference between the time to clinical recovery in the remdesivir-treated group and the time to recovery in the placebo-treated group. Although the number of patients using remdesivir within 10 days of symptoms onset was higher than in the placebo group, the 28-day mortality was similar for both groups. No significant difference was observed between the two groups in terms of oxygen support duration, length of hospital stay, and clinical recovery rates on the 14th and 28th days [50].
In our previous studies in the context of antiproliferation, it was found that iron(III) complexes of such N2O2-chelating thiosemicarbazones are more active than those with other metal ions, nickel(II), manganese(III) etc. [7,8,11]. Experiments with 3 CL-like protease enzyme also confirmed the activity superiority of iron(III) complex
4. Conclusion
The new iron(III) and nickel(II) complexes with N2O2-thiosemicarbazones were synthesized and carried out the purity and structural confirmation. The inhibitory effects of the two compounds against SARS-CoV2 3CL-like protease were investigated by experimental and theoretical methods. Complex Fe1 was more effective than Ni1 and proved that it can be a potent inhibitor for such enzymes.
The experimental and theoretical results showed once again that the metal specie in such thiosemicarbazone complexes cause significant differences in biological activity. Within the scope of the investigation of antiviral effects iron(III)-thiosemicarbazone complexes, it is obvious that complex Fe1 may be pioneer to achieve the better ones. This study includes the first knowledges related to inhibitory activity of such thiosemicarbazone complexes on 3CL-like protease enzyme.
Supplementary Material: Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 1997452 for Fe1 and 1997453 for Ni1. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
CRediT authorship contribution statement
Belkis Atasever Arslan: Conceptualization, Methodology, Supervision, Investigation, Visualization, Writing – review & editing. Büşra Kaya: Visualization, Investigation, Validation. Onur Şahin: Visualization, Investigation, Validation. Sefer Baday: Investigation, Formal analysis, Validation. Cemil Can Saylan: Investigation, Formal analysis, Validation. Bahri Ülküseven: Writing – review & editing, Supervision, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge to Scientific and Technological Research Application and Research Center, Sinop University, Turkey, for the use of the Bruker D8-QUEST diffractometer. The authors declare patent application (Turk Patent and Trademark Office, Ref. no. PT2020-22067). There are no further patents, products in development or marketed products to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2021.131166.
Appendix. Supplementary materials
References
- 1.Singh D., Singh R.V. Synthetic and biochemical aspects of titanocene- and zirconocene-dichloride chelates of thiosemicarbazones derived from heterocyclic ketones. J. Inorg. Biochem. 1993;15:227–234. [Google Scholar]
- 2.Baldini M., Belicchi-Ferrari M., Bisceglie F., Dall’Aglio P.P., Pelosi G., Pinelli S., Tarasconi P. Copper(II) Complexes with Substituted Thiosemicarbazones of α-Ketoglutaric Acid: synthesis, X-ray Structures. DNA Binding Stud., Nuclease Biol. Activity. 2004;43:7170–7179. doi: 10.1021/ic049883b. Inorganic Chemistry. [DOI] [PubMed] [Google Scholar]
- 3.Trudu F., Amato F., Vaňhara P., Pivetta T., Peña-Méndez E.M., Havel J. Coordination compounds in cancer: past, present and perspectives. J. Appl. Biomed. 2015;13:79–103. [Google Scholar]
- 4.Kovala-Demertzi D., Demertzis M.A., Miller J.R., Papadopoulou C., Dodorou C., Filousis G. Platinum(II) complexes with 2-acetyl pyridine thiosemicarbazone: synthesis, crystal structure, spectral properties, antimicrobial and antitumour activity. J. Inorg. Biochem. 2001;86:555–563. doi: 10.1016/s0162-0134(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 5.Garoufis A., Hadjikakou S.K., Hadjiliadis N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 2009;253:1384–1397. [Google Scholar]
- 6.Özerkan D., Ertik O., Kaya B., Erdem Kuruca S., Yanardag R., Ülküseven B. Novel palladium (II) complexes with tetradentate thiosemicarbazones. Synthesis, characterization, in vitro cytotoxicity and xanthine oxidase inhibition. Invest. New Drugs. 2019;37:1187–1197. doi: 10.1007/s10637-019-00751-1. [DOI] [PubMed] [Google Scholar]
- 7.Atasever B., Ülküseven B., Bal-Demirci T., Erdem-Kuruca S., Solakoğlu Z. Cytotoxic activities of new iron(III) and nickel(II) chelates of some S-methyl-thiosemicarbazones on K562 and ECV304 cells. Invest. New Drugs. 2010;28:421–432. doi: 10.1007/s10637-009-9272-2. [DOI] [PubMed] [Google Scholar]
- 8.Bal-Demirci T., Congur G., Erdem A., Erdem-Kuruca S., Özdemir N., Akgün-Dar K., Varol B., Ülküseven B. Iron(III) and nickel(II) complexes as potential anticancer agents: synthesis, physicochemical and structural properties, cytotoxic activity and DNA interactions. New J. Chem. 2015;39:5643. [Google Scholar]
- 9.Kaya B., Atasever-Arslan B., Kalkan Z., Gür H., Ülküseven B. Apoptotic mechanisms of nickel (II) complex with N1-acetylacetone- N4-4-methoxy-salicylidene-S-allyl-thiosemicarbazone on HL60 leukemia cells. Gen. Physiol. Biophys. 2016;35:451–458. doi: 10.4149/gpb_2016006. [DOI] [PubMed] [Google Scholar]
- 10.Süleymanoğlu M., Kaya B., Erdem-Kuruca S., Ülküseven B. Iron(III) and nickel(II) complexes of tetradentate thiosemicarbazones: Synthesis, atructure, cytotoxicity, and lipophilicity. J. Biochem. Mol. Toxicol. 2019:22383. doi: 10.1002/jbt.22383. [DOI] [PubMed] [Google Scholar]
- 11.Kaya B., Yılmaz Z.K., Şahin O., Aslim B., Tükenmez Ü., Ülküseven B. Structural analysis and biological functionalities of iron(III)-and manganese(III)-thiosemicarbazone complexes: in vitro anti‑proliferative activity on human cancer cells, DNA binding and cleavage studies. J. Biol. Inorg. Chem. 2019;24:365–376. doi: 10.1007/s00775-019-01653-6. [DOI] [PubMed] [Google Scholar]
- 12.Yanardag R., Bal Demirci T., Ulkuseven B., Bolkent S., Tunalı S., Bolkent S. Synthesis, characterization and antidiabetic properties of N(1)-2,4-dihydroxybenzylidene-N(4)-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium(IV) Eur. J. Med. Chem. 2009;44:818–826. doi: 10.1016/j.ejmech.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Ceylan B.İ., Yilmaz A., Bölükbaşı O., Türker Acar E., Özyürek M., Kurt Y., Ülküseven B. A square-pyramidal iron(III) complex obtained from 2-hydroxy-benzophenone-S-allylthiosemicarbazone: Synthesis, characterization, electrochemistry, quantum chemical studies and antioxidant capability. J. Coordination Chem., 73 NO. 2020;1:120–136. [Google Scholar]
- 14.Bal-Demirci T., Şahin M., Özyürek M., Kondakçı E., Ülküseven B. Synthesis, antioxidant activities of the nickel (II), iron (III) and oxovanadium (IV) complexes with N2O2 chelating thiosemicarbazones. Spectrochim. Acta, Part A. 2014;126:317. doi: 10.1016/j.saa.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Kaya B., Şahin O., Bener M., Ülküseven B. Iron (III) and nickel (II) complexes with S-alkyl (n-C1-6)-thiosemicarbazidato ligands: Synthesis, structural characterization, and antioxidant features. J. Mol. Struct. 2018;1167:16. [Google Scholar]
- 16.Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S., Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowe JE. Fetal and Neonata Physiology. Fifth Edition. 2017. Host Defense Mechanisms Against Viruses; pp. 1175–1197. In: Polin R., Abman S., Rowitch D., Benitz W., editors. [Google Scholar]
- 18.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze MG. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., Schreiner P., Neale G., Vogel P., Webby R., Jonsson C.B., Kanneganti TD. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–168. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palanimuthu D., Shinde S.V., Somasundaram K., Samuelson AG. In vitro and in vivo anticancer activity of copper bis(thiosemicarbazone) complexes. J. Med. Chem. 2013;56:722–734. doi: 10.1021/jm300938r. [DOI] [PubMed] [Google Scholar]
- 21.Khan A.S., Yusuf M. Synthesis, spectral studies and in vitro antibacterial activity of steroidal thiosemicarbazone and their palladium(II) complexes. Eur. J. Med. Chem. 2009;44:2270–2274. doi: 10.1016/j.ejmech.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Karatepe M., Karatas F. Antioxidant, pro-oxidant effect of the thiosemicarbazone derivative Schiff base (4-(1-phenylmethylcyclobutane-3-yl)- 2-(2-hydroxy-benzylidenehydrazino) thiazole) and its metal complexes on rats. Cell Biochem. Funct. 2006;24:547–554. doi: 10.1002/cbf.1266. [DOI] [PubMed] [Google Scholar]
- 23.Padhye S., Afrasiabi Z., Sinn E., Fok J., Mehta K., Rath N. Antitumor metallothiosemicarbazonates: structure and antitumor activity of palladium complex of phenanthrenequinone thiosemicarbazone. Inorg. Chem. 2005;44:1154–1156. doi: 10.1021/ic048214v. [DOI] [PubMed] [Google Scholar]
- 24.Rebolledo A.P., Vieites M., Gambino D., Piro O.E., Castellano E.E., Zani C.L., Souza-Fagundes E.M., Teixeira L.R., Batista A.A., Beraldo H. Palladium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: spectral characterization, structural studies and cytotoxic activity. J. Inorg. Biochem. 2005;99:698–706. doi: 10.1016/j.jinorgbio.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Hall I.H., Lackey C.B., Kistler T.D., RWJr Durh.am, Jouad E.M., Khan M., Thanh X.D., Djebbar-Sid S., Benali-Baitich O., Bouet GM. Cytotoxicity of copper and cobalt complexes of furfural semicarbazone and thiosemicarbazone derivatives in murine and human tumor cell lines. Pharmazie. 2000;55:937–941. [PubMed] [Google Scholar]
- 26.Stacy A.E., Palanimuthu D., Bernhardt P.V., Kalinowski D.S., Jansson P.J., Richardson DR. Zinc(II)-Thiosemicarbazone Complexes Are Localized to the Lysosomal Compartment Where They Transmetallate with Copper Ions to Induce Cytotoxicity. J. Med. Chem. 2016;59:4965–4984. doi: 10.1021/acs.jmedchem.6b00238. [DOI] [PubMed] [Google Scholar]
- 27.Karaküçük-İyidoğan A., Taşdemir D., Oruç-Emre E.E., Balzarini J. Novel platinum (II) and palladium (II) complexes of thiosemicarbazones derived from 5-substitutedthiophene-2-carboxaldehydes and their antiviral and cytotoxic activities. Eur. J. Med. Chem. 2011;46:5616–5624. doi: 10.1016/j.ejmech.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varadinova T., Kovala-Demertzi D., Rupelieva M., Demertzis M., Genova P. Antiviral activity of platinum(II) and palladium(II) complexes of pyridine-2-carbaldehyde thiosemicarbazone. Acta Virol. 2001;45:87–94. [PubMed] [Google Scholar]
- 29.Pelosi G., Bisceglie F., Bignami F., Ronzi P., Schiavone P., Re M.C., Casoli C., Pilotti E.J. Antiretroviral Activity of Thiosemicarbazone Metal Complexes. Med. Chem. 2010;53(24):8765–8769. doi: 10.1021/jm1007616. [DOI] [PubMed] [Google Scholar]
- 30.Bal T., Atasever B., Solakoğlu Z., Erdem-Kuruca S., Ülküseven B. Synthesis, characterisation and cytotoxic properties of the N1, N4-diarylidene-S-methyl- thiosemicarbazone chelates with Fe (III) and Ni (II) Eur. J. Med. Chem. 2007;42(2):161–167. doi: 10.1016/j.ejmech.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Abdusalam A.A.A., Murugaiyah V. Identification of Potential Inhibitors of 3CL Protease of SARS-CoV-2 From ZINC Database by Molecular Docking-Based Virtual Screening. Front. Mol. Biosci. 2020;17(7) doi: 10.3389/fmolb.2020.603037. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheldrick G.M. A short history of SHELX. Acta Cryst. 2008;A64:112. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 33.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015;C71:3. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.APEX2, Bruker AXS Inc. Madison Wisconsin USA (2013).
- 35.Macrae C.F., Sovago I., Cottrell S.J., Galek P.T.A., McCabe P., Pidcock E., Platings M., Shields G.P., Stevens J.S., Towler M., Wood P.A. J. Appl. Cryst. 2020;53:226–235. doi: 10.1107/S1600576719014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrugia L.J. J. Appl. Cryst. 2012;45:849–854. [Google Scholar]
- 37.Yamazaki C. J. Chem. 1975;53:610–615. [Google Scholar]
- 38.Gradinaru J., Forni A., Simonov Y., Popovici M., Zecchin S., Gdaniec M., Fenton D.E. Inorg. Chim. Acta. 2004;357:2728–2736. [Google Scholar]
- 39.Kaya B., Ülküseven B., Şahin O., Şahin Z.S. J. Mol. Struct. 2019;1191:337–344. [Google Scholar]
- 40.Trott O., Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolinsky T.J., Czodrowski P., Li H., Nielsen J.E., Jensen J.H., Klebe G., Baker NA. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007;35(suppl_2):W522–W525. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo S., Park J.W., An D., Yoon J., Paik H., & Hwang S. (2020). Supercomputer-aided Drug Repositioning at Scale: Virtual Screening for SARS-CoV-2 Protease Inhibitor. Under review in DTMBIO.
- 43.Arslan B.A., Timucin AC. Immunotherapy approaches on innate immunity for SARS-Cov-2. Acta Virol. 2020;64(4):389–395. doi: 10.4149/av_2020_401. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. .e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D., Boons G.J., Bosch B.J., Rey F.A., de Groot R.J., Veesler D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haribabu J., Srividya S., Mahendiran D., Gayathri D., Venkatramu V., Bhuvanesh N., Karvembu R. Synthesis of Palladium(II) Complexes via Michael Addition: Antiproliferative Effects through ROS-Mediated Mitochondrial Apoptosis and Docking with SARS-CoV-2. Inorg. Chem. 2020;59(23):17109–17122. doi: 10.1021/acs.inorgchem.0c02373. [DOI] [PubMed] [Google Scholar]
- 48.Haroon M., Akhtar T., Khalid M., Ali S., Zahra S., Ul Haq I., Alhujaily M. Synthesis, antioxidant, antimicrobial and antiviral docking studies of ethyl 2-(2-(arylidene)hydrazinyl)thiazole-4-carboxylates. J Biosci. 2021 doi: 10.1515/znc-2021-0042. Apr 26. [DOI] [PubMed] [Google Scholar]
- 49.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., Wu Y., Xiao W., Liu S., Chen E., Chen W., Wang X., Yang J., Lin J., Zhao Q., Yan Y., Xie Z., Li D., Yang Y., Liu L., Qu J., Ning G., Shi G., Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.