Abstract
The controlled presentation of proteins from and within materials remains of significant interest for many bioengineering applications. Though “smart” platforms offer control over protein release in response to a single external cue, no strategy has been developed to trigger delivery in response to user-specified combinations of environmental inputs, nor to independently control the release of multiple species from a homogenous material. In this work, we introduce a modular semisynthetic scheme to govern the release of site-specifically modified proteins from hydrogels following Boolean logic. A sortase-mediated transpeptidation reaction was used to generate recombinant proteins C-terminally tethered to gels through environmentally sensitive degradable linkers. By varying the connectivity of multiple stimuli-labile moieties within these customizable linkers, we exhaustively demonstrate YES/OR/AND control of protein release in response to one and two-input combinations involving enzyme, reductant, and light. Tethering of multiple proteins each through a different stimuli-sensitive linker permits their independent and sequential release from a common material. We expect these methodologies to enable new opportunities in tissue engineering and therapeutic delivery.
Keywords: protein delivery, hydrogels, boolean logic, degradation, sortase
Table of Contents Entry:
The programmed release of site-specifically modified proteins from hydrogel biomaterials in response to precise combinations of environmental inputs is specified using Boolean YES/OR/AND logic. Sequential and independently controlled release of proteins from a common material is afforded through well-defined installation of degradable moieties tethering the protein to the gel.
Graphical Abstract
Hydrogels are attractive vehicles for the controlled delivery of proteins due to their readily tunable physical and chemical properties including stiffness, geometry, chemical functionality, degradability, and mesh size.[1–3] Despite extensive research on gel-based platforms that swell or degrade in response to biologically relevant signals (e.g., temperature,[4–7] pH,[6,8–12] enzyme,[12–19] light,[18,20–23] redox,[24,25] other biomolecules[26,27]), which enable the simultaneous release of several proteins, strategies that permit independent triggered release of many species from a single material remain elusive. Moreover, the ability to regulate release without sacrificing protein stability or activity represents an open challenge within the biomaterials community. Towards both ends, we present here the first robust synthetic strategy that affords user-programmable release of site-specifically modified proteins from gels. By tethering proteins of interest to hydrogel networks through degradable linkers of defined molecular architecture, we gain Boolean YES/OR/AND logic-based control over protein release in response to complex sets of inputs. This approach yields biomacromolecular delivery only when user-specified combinations of external cues are present, permitting the sequential and independent triggered release of multiple proteins from gel biomaterials.
On-demand protein release experiments were performed using poly(ethylene glycol) (PEG)-based hydrogels. PEG is an inert hydrophilic polymer that exhibits low biofouling and is useful in preventing non-specific adsorption to gels that could inhibit protein delivery.[2] A strain-promoted azide-alkyne cycloaddition (SPAAC) reaction between a four-arm PEG tetrabicyclononyne (PEG-tetraBCN) and a linear PEG diazide (N3-PEG-N3) was used to generate near-ideal step-growth polymer networks. SPAAC is the most common biocompatible click reaction;[28] we have previously exploited SPAAC’s excellent reaction selectivity to form hydrogels in the presence of living cells and serum-containing culture medium.[18,29,30] Azide-functionalized proteins that are included within the gel formulation at physiologically relevant concentrations (<100 μM) are homogenously tethered throughout the network with minimal impact on material mechanical properties.
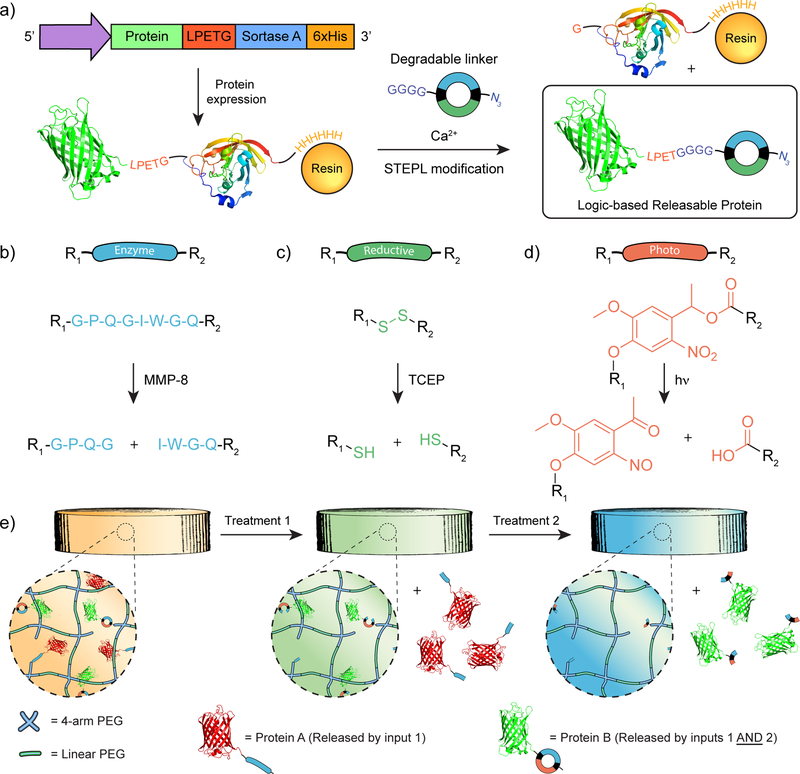
To introduce azido functionality onto proteins of interest in a site-specific manner, thereby yielding a monodisperse population with uniform activity, we exploit a sortase-mediated reaction.[31,32] Staphylococcus aureus sortase A is a calcium-assisted transpeptidase that recognizes the C-terminal sorting signal “LPXTG”, forming an acyl-enzyme intermediate with the protein while simultaneously displacing the C-terminal portion of the sorting signal’s threonine residue. The thioester of the acyl-enzyme intermediate can be nucleophilically displaced with a polyglycine probe, covalently modifying the C-terminus while regenerating the sortase A enzyme. We implemented “sortagging” through the Sortase-Tag Enhanced Protein Ligation (STEPL) technique (Figure 1A).[33] In STEPL, E. coli is used to recombinantly express a single fusion protein construct containing the protein of interest, the sorting sequence LPETG, a (GGS)5 flexible linker, sortase A, and a 6xHis-Tag. Upon addition of calcium and a customizable probe containing an N-terminal polyglycine moiety, an intramolecular sortagging event ligates the probe to the protein of interest and cleaves the 6xHis-functionalized sortase. When this reaction is performed during immobilized metal affinity chromatography of the protein, sortase A remains bound to the Ni-NTA column, enabling site-specific protein labeling and purification in a single step. Our group has previously implemented the STEPL system to create protein-polymer hydrogel biomaterials,[34] and has demonstrated that many proteins retain native bioactivity after sortagging.[35]
Figure 1.
Synthesis and logic-based release of site-specifically modified proteins from hydrogels. (a) A one-step purification/functionalization sortase-mediated transpeptidation reaction[33] enables degradable polyglycine probes to be affixed to the C-termini of a protein of interest. Degradable linkers are based on orthogonal cleavage reactions between: (b) MMP and the proteolytically sensitive peptide sequence GPQG↓IWGQ, (c) a reductant and disulfide bond, and (d) near-UV light and the oNB moiety. (e) Multiple proteins tethered to a hydrogel through different logical linkers are independently released when exposed to varying chemical environments. Use of a linker with a cyclic architecture can function as a logical AND gate, requiring two unique stimuli to trigger protein release.
Taking advantage of its unique ability to install non-natural functionalities onto the C-terminus of recombinant proteins, we used STEPL to introduce both an azide necessary for hydrogel tethering and a modular degradable sequence to regulate triggered protein release. Recently, we developed programmable hydrogel biomaterials that degrade in response to precise combinations of external stimuli specified through the controlled arrangement of degradable groups within material crosslinkers.[36] We sought to extend this biocomputational scheme to control the release of site-specifically modified proteins via linkers that cleave in response to user-defined input combinations governed by Boolean YES/OR/AND logic. We hypothesized that the linker between the protein and azide can function as a YES-gate for controlling protein release when it contains a single degradable moiety, an OR-gate (denoted with logic symbol ∨) when two unique cleavable moieties are included in series, and as an AND-gate (denoted by logic symbol ∧) when two unique degradable moieties are present in parallel. To formulate linkers containing an N-terminal polyglycine moiety for sortagging and a C-terminal azide for gel conjugation, precisely connected through multiple labile bonds with defined topology, we opted to create linkers using peptide chemistry.
To assess this approach for modular logic-based protein release, we selected three distinct stimuli-labile moieties (Figure 1B–1D): (1) the proteolytically sensitive peptide sequence, GPQG↓IWGQ, which is cleaved by matrix metalloproteinase (MMP) enzymes;[14,37,38] (2) disulfide bonds, which are reduced by tris(2-carboxyethyl)phosphine (TCEP) and other reducing agents; and (3) an ortho-nitrobenzyl ether (oNB) group, which undergoes irreversible photoscission upon exposure to near-UV light (λ = 365 nm)[18,39,40]. Since each degradable moiety is susceptible to a different class of external stimulus (i.e., enzyme, chemical environment, light), orthogonal control over chemical cleavage was expected (Figure 1E).
We used the enzyme- (E), reductive- (R), and light- (P) degradable moieties to construct nine distinct linkers for C-terminal protein modification by STEPL that collectively spanned every permutation of YES-, OR-, and AND-gated responses involving the E, R, and P inputs (Methods S1–S9). Each linker contained a stimuli-degradable region flanked by an N-terminal polyglycine moiety (GGGG) for sortagging and a C-terminal azide (N3) for gel tethering. All peptide linkers were synthesized using solid-phase peptide synthesis, purified, and characterized by mass spectrometry.
Logic-based degradable linkers were installed onto the C-terminus of several recombinant proteins of interest using STEPL (Methods S10–S23). While this method can be used to modify virtually any monomeric protein species, initial efforts focused on Enhanced Green Fluorescent Protein (EGFP), whose fluorescence serves as a surrogate for its activity and provides a convenient way to visualize and quantify protein release from hydrogels. Each of the nine degradable polyglycine probes were sortagged onto EGFP (Methods S11–S19). All proteins were isolated with quantitative terminal functionalization in excellent purity as confirmed by mass spectrometry.
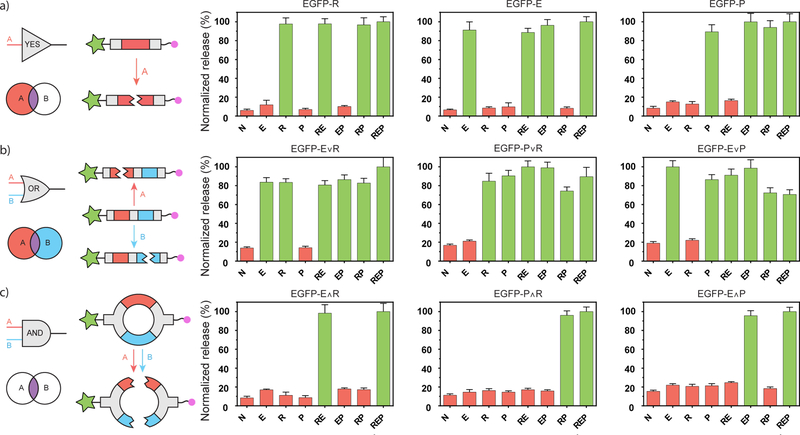
To characterize protein release in response to different combinations of environmental stimuli, each of the nine EGFP variants were individually tethered into PEG-based gels. Release of each EGFP variant was quantified for all eight possible input combinations of enzyme, reductant, and light using the supernatant fluorescence corresponding to EGFP (Fig 2., Method S24). Since overall material response time depends on hydrogel geometry, input identity and concentration, species diffusivity, as well as cleavage kinetics of the degradable moiety in response to proper stimulus, time-course experiments were performed to identify treatment conditions appropriate for assessing Boolean responsiveness (Fig. S1, Method S25). Under the identified conditions, the YES logic-based systems (EGFP-E-N3, EGFP-R-N3, EGFP-P-N3) behaved as engineered, releasing protein in conditions containing the programmed cue with a ~10-fold greater selectivity than conditions that did not. This high selectivity demonstrates chemical orthogonality amongst the three input/substrate pairs. The OR logic-based systems (EGFP-E∨R-N3, EGFP-P∨R-N3, EGFP-E∨P-N3) also behaved as expected, releasing protein in the presence of either programmed cue. The AND logic-based systems (EGFP-E∧R-N3, EGFP-P∧R-N3, and EGFP-E∧P-N3) all offered protein release only when both requisite environmental cues were present. To our knowledge, these AND-based materials represent the first systems that require more than one input to release a protein payload from a hydrogel. We also note the modularity of the approach, where the same protein can be readily sortagged with different species to yield a wide variety of user-defined responsiveness to combinations of environmental factors.
Figure 2.
Logic-based EGFP variants exhibit programmable protein release in response to environmentally presented inputs combinations. Logical schematic and response profiles of the (a) YES-, (b) OR-, and (c) AND-gated proteins. Each region of the Venn diagram corresponds to a unique combination of inputs and indicates whether the material is expected to degrade (colored) or remain intact (white). Protein (green star) is C-terminally linked to the gel (pink circle) through linkers containing multiple stimuli-labile bonds (red and blue). Plot titles correspond to the linker identity, with x-axis labels indicating treatment conditions (N is no treatment, E is MMP enzyme, R is a chemical reductant, P is UV light). Green bars signify conditions expected to result in protein release; red bars indicate conditions expected not to yield protein release. Error bars correspond to ±1 standard deviation about the mean for n = 3 experimental replicates.
We next sought to leverage the precise control this approach affords over the environmentally triggered release by demonstrating the independent and differential release of multiple proteins from the same material. Hydrogels were formulated with both EGFP and a red fluorescent protein, mCherry (also synthesized and modified by STEPL, Methods S20–22), uniformly tethered via different logically degradable linkages. These materials were sequentially treated with enzyme, masked UV light (400 μm line patterns), and reductant; after each step, fluorescent microscopy was used to visualize the spatial presentation of each gel-bound protein (Figure 3). When a hydrogel containing YES logical proteins EGFP-P-N3 and mCherry-E-N3 was treated with enzyme, mCherry was fully released while EGFP remained attached to the gel. Subsequent exposure to masked light induced patterned EGFP release. EGFP remained in the photopatterned configuration upon reductive treatment. Next, a second hydrogel containing the OR logic-based proteins EGFP-P∨R-N3 and mCherry-E∨R-N3 was exposed to enzyme, inducing the full release of mCherry while not affecting EGFP presentation. Exposure to masked UV light resulted in selective EGFP release in a photopatterned line configuration; subsequent exposure to a reductant fully released EGFP. Finally, a third hydrogel containing AND logically tethered proteins EGFP-E∧P-N3 and mCherry-P∧R-N3 was exposed to enzyme and displayed no protein release. Subsequent treatment with masked UV light induced EGFP release in exposed regions but did not elicit mCherry release. Upon reductive treatment, EGFP presentation was unchanged while mCherry was released from the previously light-exposed regions, inducing the development of the interspaced line pattern. In every case, the observed environmentally triggered protein release matched the engineered response, highlighting this platform’s unique ability to permit programmable, independently triggered delivery of multiple proteins from a single material.
Figure 3.
Sequential and spatiotemporally varied release of EGFP and mCherry from a single gel in response to environmental cues. (a-d) YES-based logical release of EGFP-P-N3 and mCherry-E-N3; (e-h) OR-based logical release of EGFP-P∨R-N3 and mCherry-E∨R-N3; (i-l) AND-based logical release of EGFP-E∧P-N3 and mCherry-P∧R-N3 prior to treatment (N) and upon sequential enzyme (E), masked light exposure (P), and reductive (R) treatments. Gels were imaged using fluorescent microscopy. Left and right images for each condition respectively correspond to the fluorescence of EGFP (green) and mCherry (red) within the same gel. Insets depict full hydrogel. Scale bars = 400 μm.
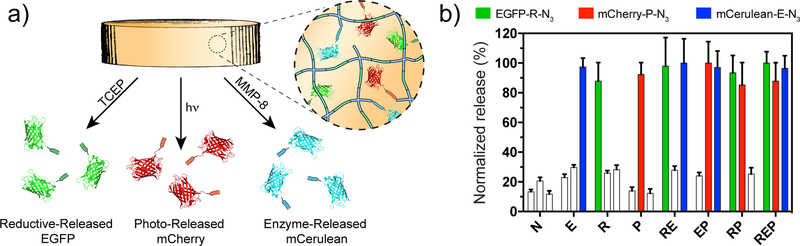
To demonstrate independent control of three biomacromolecules from the same material, we formulated gels with three distinct fluorescent proteins tethered through different YES-logic degradable linkers: EGFP-R-N3, mCherry-P-N3, and a blue fluorescent protein construct, mCerulean-E-N3 (also synthesized and modified by STEPL, Method S23). Spectrally separated excitation and emissions for these fluorophores enabled independent quantification of each species following gel treatments. As expected, proteins were released only in the presence of the input corresponding to their linker (Figure 4). To our knowledge, this is the first approach that enables independent programmable control over the environmentally triggered delivery of multiple proteins from a single material.
Figure 4.
Independently triggered release of three distinct proteins from a single gel. (a) Experimental schematic of a hydrogel with homogenously tethered EGFP-R-N3, mCherry-P-N3, and mCerulean-E-N3. (b) Release for each protein under every relevant environment (EGFP, mCherry, mCerulean from left to right for each treatment condition). Colored bars (green = EGFP, red = mCherry, blue = mCerulean) indicate conditions expected to yield release while unshaded bars indicate conditions not expected to yield release. Error bars correspond to ±1 standard deviation about the mean for n = 3 experimental replicates.
In this work, we have established a robust strategy to program release of site-specifically modified proteins from hydrogels in response to user-defined environmental signals. We exploited a versatile sortase-mediated transpeptidation reaction to quantitatively functionalize the C-termini of a variety of proteins with degradable linkers that tether species to gels. Nine unique linkers exhaustively spanning each Boolean YES-, OR-, and AND-gated response to three distinct input classes (i.e., enzyme, light, reductive environments) enabled triggered protein release in accordance with the engineered logical function. We demonstrated the first biomaterial systems that require more than one external cue to release a protein payload and the only approach that enables independently triggered release of multiple proteins from a single homogenous material. Given the synthetic modularity of the presented approach, we expect that this platform will yield many new opportunities in controlled therapeutic delivery for disease treatment and tissue regeneration. We are actively employing these strategies to autonomously guide stem cell differentiation within materials, sequentially delivering stimulatory proteins in response to phenotype-specific inputs.
Experimental Section
Synthesis of logical peptide linkers for sortagging:
Peptides were synthesized via standard Fmoc solid-phase peptide synthesis and further modified with solution-phase reactions. Intramolecular peptide stapling was introduced via either oxidative disulfide formation or by copper(I)-catalyzed azide-alkyne cycloaddition. Peptides were purified using reversed-phase high-pressure liquid chromatography (RP-HPLC) on a silica C18 column. Purity was confirmed by matrix-assisted laser desorption/ionization time of flight mass spectrometry. Complete experimental details are given in Methods S1–S9.
STEPL-based sortagging for C-terminal protein labeling:
Each fusion protein containing: the protein of interest (i.e., EGFP, mCherry, mCerulean), sorting sequence LPETG, (GGS)5 flexible linker, sortase A, and a 6xHis-Tag, was expressed in BL21(DE3) E. coli. Cells were lysed via sonication, and the 6xHis-tagged fusion protein was immobilized on Ni-NTA. To promote the STEPL reaction, sortaggable peptide (10x) and calcium chloride (0.1 mM) were added to the Ni-NTA resin and reacted at 37 °C for 4 hours with mild agitation. The flow-through containing the sortagged protein was collected and purified via centifugal membrane filtration. Complete experimental details are given in Methods S10–23.
Logic-based protein release in response to environmental stimuli:
Hydrogels (10 μL) were formulated from PEG-tetraBCN[18] (Mn ~ 20,000 Da, 2 mM), N3-PEG-N3[41] (Mn ~ 3,500 Da, 4 mM), and sortagged azide-functionalized logical proteins (0.1 mM total, equal concentration of all proteins) in MMP buffer (50 mM Tris, 200 mM NaCl, 5 mM CaCl2, 1 μM ZnCl2, pH = 7.5) in the bottom of microcentrifuge tubes (0.6 mL). The protein and PEG-tetraBCN were pre-reacted for 4 hours prior to mixing with N3-PEG-N3. After one hour, formed hydrogels were washed for 24 hours in MMP buffer to remove unconjugated protein.
All treatments were performed at 4 °C in MMP buffer (100 μL). Samples not receiving a given input were maintained in MMP buffer in parallel to treated gels. Samples receiving the reductive input were treated with TCEP.HCl (2 μL, 100 mM in MMP buffer) and incubated overnight. Excess TCEP was quenched with hydroxyethyl disulfide (5 μL, 100 mM in MMP buffer). Subsequently, samples receiving the enzyme input were treated with MMP-8 (2.5 μL, 0.4 mg mL−1 in MMP buffer) overnight. Finally, samples receiving the light input were exposed to UV light (λ = 365 nm, 20 mW cm−2, 10 minutes) and all samples were incubated in MMP buffer. Three days later, protein release was quantified by measuring the fluorescence corresponding to EGFP (λex = 475 nm, λem = 510 nm), mCherry (λex = 575 nm, λem = 610 nm), and mCerulean (λex = 433 nm, λem = 475 nm) in the supernatant. All data was collected in experimental triplicate. Complete experimental details are given in Method S25.
Supplementary Material
Acknowledgements
The authors thank Drs. R. Warden-Rothman and A. Tsourkis (University of Pennsylvania) for providing the pSTEPL plasmid, Dr. B. Badeau for assistance in synthesizing N3-oNB-OSu, and S. Adelmund for providing BCN-OSu. We gratefully acknowledge support from S. Edgar at the UW Mass Spectrometry Center as well as that from the National Institutes of Health and N. Peters at the UW W. M. Keck Microscopy Center (S10 OD016240). This work was supported by a University of Washington Faculty Startup Grant (C.A.D.), as well as a CAREER Award (DMR 1652141, C.A.D.) and a grant (DMR 1807398, C.A.D.) from the National Science Foundation. Authors J. A. Shadish and B. A. Badeau contributed equally to this work.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Prathamesh Milind Gawade, Department of Chemical Engineering, University of Washington, 3781 Okanogan Lane NE, Seattle, WA 98195, USA.
Jared A. Shadish, Department of Chemical Engineering, University of Washington, 3781 Okanogan Lane NE, Seattle, WA 98195, USA
Barry A. Badeau, Department of Chemical Engineering, University of Washington, 3781 Okanogan Lane NE, Seattle, WA 98195, USA
Cole A. DeForest, Department of Chemical Engineering, University of Washington, 3781 Okanogan Lane NE, Seattle, WA 98195, USA Department of Bioengineering, University of Washington, 3720 15th Ave NE, Seattle, WA 98105, USA; Institute for Stem Cell & Regenerative Medicine, University of Washington, 850 Republican Street, Seattle, WA 98109, USA.
References
- [1].Vermonden T, Censi R, Hennink WE, Chem. Rev 2012, 112, 2853. [DOI] [PubMed] [Google Scholar]
- [2].Lin CC, Anseth KS, Pharm. Res 2009, 26, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Badeau BA, DeForest CA, Annu. Rev. Biomed. Eng 2019, 21, annurev. [DOI] [PubMed] [Google Scholar]
- [4].Censi R, Vermonden T, van Steenbergen MJ, Deschout H, Braeckmans K, De Smedt SC, van Nostrum CF, di Martino P, Hennink WE, J. Control. Release 2009, 140, 230. [DOI] [PubMed] [Google Scholar]
- [5].Vermonden T, Jena SS, Barriet D, Censi R, Van Der J, Hennink WE, Siegel RA, 2011, 43, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huynh CT, Nguyen MK, Lee DS, Chem. Commun. (Camb) 2012, 48, 10951. [DOI] [PubMed] [Google Scholar]
- [7].Sim HJ, Thambi T, Lee DS, J. Mater. Chem. B 2015, 3, 8892. [DOI] [PubMed] [Google Scholar]
- [8].Chiu Y-L, Chen M-C, Chen C-Y, Lee P-W, Mi F-L, Jeng U-S, Chen H-L, Sung H-W, Soft Matter 2009, 5, 962. [Google Scholar]
- [9].Nguyen MK, Park DK, Lee DS, Biomacromolecules 2009, 10, 728. [DOI] [PubMed] [Google Scholar]
- [10].Nguyen MK, Huynh CT, Gao GH, Kim JH, Huynh DP, Chae SY, Lee KC, Lee DS, Soft Matter 2011, 7, 2994. [Google Scholar]
- [11].Xu W, He X, Zhong M, Hu X, Xiao Y, RSC Adv. 2015, 5, 3157. [Google Scholar]
- [12].Koetting MC, Guido JF, Gupta M, Zhang A, Peppas NA, J. Control. Release 2016, 221, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aimetti AA, Machen AJ, Anseth KS, Biomaterials 2009, 30, 6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Patterson J, Hubbell JA, Biomaterials 2010, 31, 7836. [DOI] [PubMed] [Google Scholar]
- [15].Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, a Shuman J, Freels PD, Gorman III JH, Gorman RC, Spinale FG, Burdick JA, Nat. Mater 2014, 13, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen W-H, Luo G-F, Lei Q, Jia H-Z, Hong S, Wang Q-R, Zhuo R-X, Zhang X-Z, Chem. Commun. (Camb) 2015, 51, 465. [DOI] [PubMed] [Google Scholar]
- [17].Holloway JL, Ma H, Rai R, Hankenson KD, Burdick JA, Macromol. Biosci 2015, 15, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].DeForest CA, Tirrell DA, Nat. Mater 2015, DOI 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- [19].Zhu S, Nih L, Carmichael ST, Lu Y, Segura T, Adv. Mater 2015, 27, 3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tibbitt MW, Han BW, Kloxin AM, Anseth KS, J. Biomed. Mater. Res. - Part A 2012, 100 A, 1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kharkar PM, Kiick KL, Kloxin AM, Polym. Chem 2015, 6, 5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Azagarsamy MA, Anseth KS, Angew. Chemie Int. Ed 2013, 52, 13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruskowitz ER, DeForest CA, Nat. Rev. Mater 2018, 3, 17087. [Google Scholar]
- [24].Chen W, Zheng M, Meng F, Cheng R, Deng C, Feijen J, Zhong Z, Biomacromolecules 2013, 14, 1214. [DOI] [PubMed] [Google Scholar]
- [25].Lv Y, Yang B, Li YM, Wu Y, He F, Zhuo RX, Colloids Surfaces B Biointerfaces 2014, 122, 223. [DOI] [PubMed] [Google Scholar]
- [26].Miyata T, Asami N, Uragami T, Nature 1999, 399, 766. [DOI] [PubMed] [Google Scholar]
- [27].Yang T, Ji R, Deng X-X, Du F-S, Li Z-C, Soft Matter 2014, 10, 2671. [DOI] [PubMed] [Google Scholar]
- [28].Sletten EM, Bertozzi CR, Angew. Chemie Int. Ed 2009, 48, 6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].DeForest CA, Polizzotti BD, Anseth KS, Nat. Mater 2009, 8, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DeForest CA, Anseth KS, Nat. Chem 2011, 3, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mao H, Hart SA, Schink A, Pollok BA, J. Am. Chem. Soc 2004, 126, 2670. [DOI] [PubMed] [Google Scholar]
- [32].Guimaraes CP, Witte MD, Theile CS, Bozkurt G, Kundrat L, Blom AEM, Ploegh HL, Nat. Protoc 2013, 8, 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Warden-Rothman R, Caturegli I, Popik V, Tsourkas A, Anal. Chem 2013, 85, 11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu L, Shadish JA, Arakawa CK, Shi K, Davis J, DeForest CA, Adv. Biosyst 2018, 2, 1800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shadish JA, Benuska GM, DeForest CA, Nat. Mater 2019, DOI 10.1038/s41563-019-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA, Nat. Chem 2018, 10, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA, Proc. Natl. Acad. Sci. U. S. A 2003, 100, 5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].DeForest CA, Anseth KS, Annu. Rev. Chem. Biomol. Eng 2012, 3, 421. [DOI] [PubMed] [Google Scholar]
- [39].Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Science 2009, 324, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kloxin AM, Tibbitt MW, Anseth KS, Nat. Protoc 2010, 5, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].DeForest CA, Sims EA, Anseth KS, Chem. Mater 2010, 22, 4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.