Abstract
Back and Objectives:
To examine, among pediatricians (Peds) and family physicians (FPs): 1) HPV vaccine delivery practices; 2) delivery experiences; and 3) attitudes regarding new 2-dose HPV vaccination schedules.
Methods:
We surveyed nationally representative networks of Peds and FPs by internet/mail from 7/2018–9/2018. Multivariable regression (MV) assessed factors associated with refusal/deferral rates of ≥50% among 11–12 y.o. patients.
Results:
Response rate was 65% (302 Peds; 228 FPs included). Peds strongly recommending HPV vaccine ranged from 99% for ≥15 y.o.(among females) to 83% (males) for 11–12 y.o patients; FPs ranged from 90% (females) for ≥15 y.o. to 66% (males) for 11–12 y.o. (p<.0001 between specialties). 65% of Peds and 42% of FPs always/almost always used presumptive style when discussing HPV vaccine (p<.0001). Overall, 40% used standing orders and 42% had electronic alerts. Proportion reporting ≥50% refusal/deferral among Peds was 19% for female and 23% for male 11–12 y.o.; FPs reported 27% and 36%, respectively. In MV (both genders), refusal/deferral was associated with physicians not “strongly recommending” to 11–12 y.o., not using a presumptive style, perceiving less resistance introducing at 13 than 11–12 y.o. and anticipating an uncomfortable conversation when recommending to 11–12 y.o. 89% of Peds and 79% of FPs reported more adolescents <15 y.o. are completing the HPV series now that only 2 doses are recommended.
Conclusions:
Although most physicians strongly recommend HPV vaccine to 11–12 y.o., our data identifies areas for improvement in recommendation and delivery methods. Most physicians perceive the 2-dose schedule is resulting in higher HPV completion rates.
Table of Contents Summary:
This article reports current HPV delivery practices by primary care physicians, demonstrating areas for improvement in style and strength of recommendations and practice-based delivery methods.
INTRODUCTION
Disease caused by human papillomavirus (HPV) remains a major public health problem globally.1–5 Nearly all cervical and anal cancers, 63–75% of vulvar, vaginal, and penile cancers, and about 70%6 of oropharyngeal cancers are attributable to HPV.2,7–10 Annually in the United States, 33,700 new cancers are related to HPV and 4,175 women die of cervical cancer.11
Effective HPV vaccines have been routinely recommended at age 11–12 years by the Advisory Committee on Immunization Practices (ACIP) since 2006 for females and since 2011 for males.12,13 The recommendations targeting 11–12 year olds were based in part on facilitating implementation since other vaccines are recommended at this age. In addition, HPV vaccines are most effective when given prior to any HPV exposure and vaccination at this age reaches most persons before initiation of sexual activity.
HPV vaccines have demonstrated high efficacy in preventing cervical precancers, other genital cancers, oropharyngeal cancers and genital warts.14 Healthy People 2020 goals include coverage of 80% for all vaccines routinely recommended for U.S. adolescents.15 Although these goals have been met for other adolescent vaccines, in the 2017 NIS-Teen the HPV initiation among 13–17 year-olds was 69% for girls and 63% for boys, and series completion was only 53% for girls and 44% for boys.16 Low HPV vaccination is related to a variety of barriers originating from health care providers and from parents or patients.17–26 Because of the crucial role of provider recommendation in parental decisions to vaccinate27–29, a great deal of research21,30–36 and intervention efforts37–40 have focused on improving provider communication regarding HPV vaccination.
Until 2016, completion of the HPV series was defined as three doses over 6 months. In late 2016, ACIP recommended a 2-dose schedule for adolescents who initiate the HPV vaccination series at ages 9 through 14 years.23 In the 2-dose schedule, the first and second doses of HPV vaccine should be administered at least 6–12 months apart.23 Three doses remain recommended for persons who initiate the series at ages 15 through 26 years, and for immunocompromised persons. In the context of continued suboptimal vaccination rates and recent changes to recommendations, our objectives were to examine the following among nationally representative panels of pediatricians (Peds) and family physicians (FPs): 1) current delivery and communication practices regarding HPV vaccine; 2) attitudes and experiences with HPV vaccine delivery; 3) rates of refusal or deferral of HPV vaccine;; 4) perceived barriers to delivery of HPV vaccine; and 5) knowledge, practices and attitudes regarding the 2-dose HPV vaccination schedule.
METHODS
From July–September, 2018, we administered surveys to national networks of physicians who had agreed to participate in surveys about vaccine policy issues. The Colorado Multi-institutional Review Board approved this study as exempt research.
Study Setting and Population
As part of the Vaccine Policy Collaborative Initiative, a rapid survey mechanism to assess physician attitudes about vaccine issues, we surveyed members of networks of pediatricians (Peds) and family physicians (FPs) recruited from the memberships of the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP). Physicians were eligible if they spent ≥50% of their time providing primary care. We performed quota sampling24 to ensure networks were similar to the AAP and AAFP memberships with respect to region, urban versus rural location, and practice setting. In previous work, we demonstrated that survey responses from network physicians were similar to those of physicians randomly sampled from American Medical Association physician databases with respect to reported demographic characteristics, practice attributes, and attitudes about vaccination issues.24
Survey Design
We used 4-point Likert scales to assess physicians’ strength of recommendation for HPV vaccine for patients in different age groups, frequency of using different vaccine discussion styles, perceived barriers to vaccination (barriers assessed listed in Figure 4) and extent of agreement with statements about the change from a 3-dose to a 2-dose schedule in younger teens and experiences with HPV vaccine delivery. Physicians were classified as using a “presumptive” 41 (or “announcement”)30 style if they reported almost always/always introducing HPV vaccine by saying, “We’ve got three vaccines today: Tdap, HPV and Meningococcal vaccines.” Physicians were classified as using a “conversational”30,41 (or “participatory)41 style if they reported almost always/always saying, “Are you interested in getting HPV vaccine for your child today?” National advisory panels of 6 AAP members and 6 AAFP members representing different states pre-tested the survey and it was then pilot-tested among 13 Peds and 10 FPs.
Figure 4: Physicians’ Perceived Barriers to HPV Vaccination (Peds n=302, FM n=228).
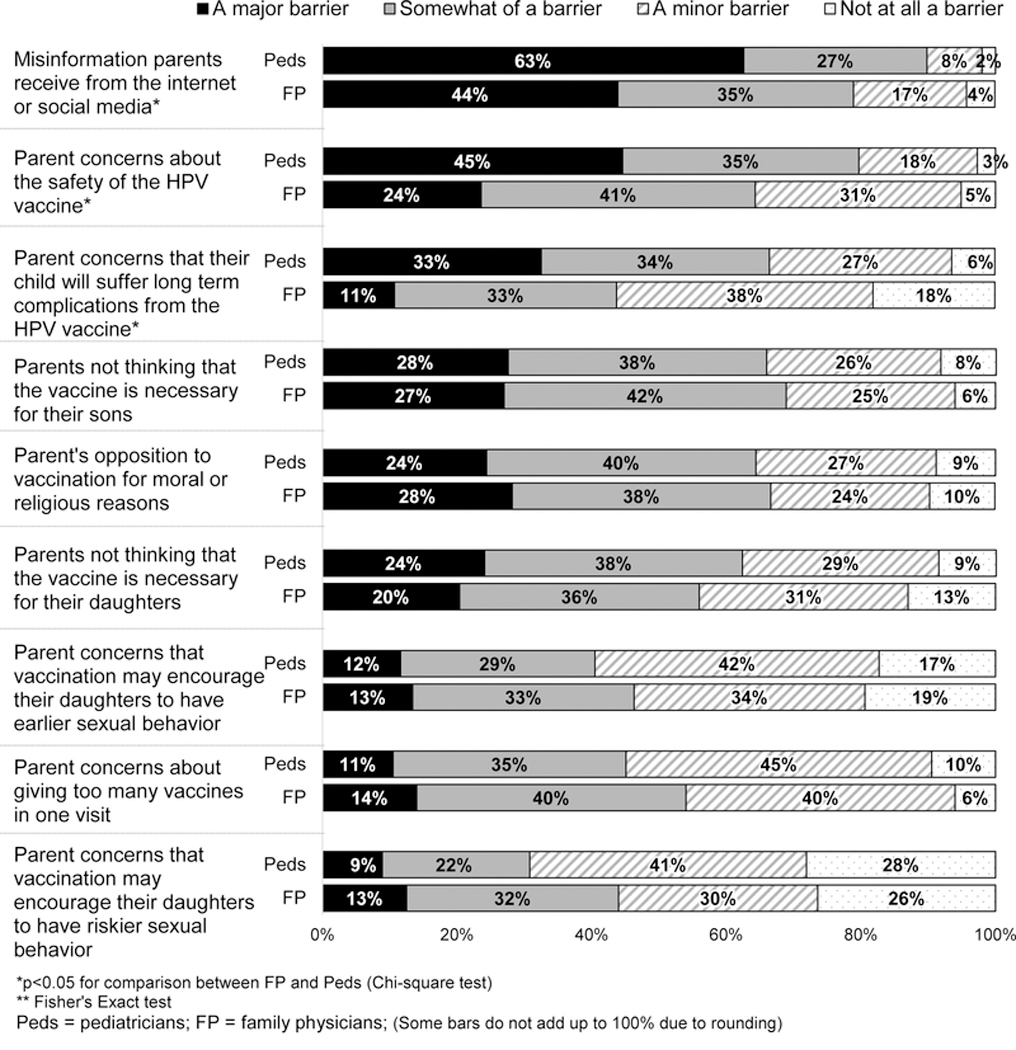
Barriers reported as “Major” by <12% by both specialties: Parent concerns that vaccination may encourage their sons to have earlier sexual behavior, vaccination may encourage their sons to have riskier sexual behavior, vaccine could cause infertility in their daughters/sons, their child will suffer immediate short-term effects from the HPV vaccine, about the efficacy of the HPV vaccine, about waning immunity if the HPV vaccine is given too early; physician concern about giving too many vaccines in one visit, it will result in an uncomfortable conversation with the parent, about the safety of the HPV vaccine for females, about the efficacy of the HPV vaccine for males/females; physician belief that HPV infection is not common enough in males/females to justify a vaccination, pap smears are an adequate way to prevent cerivical cancer, HPV-associated diseases are not severe enough in males/females to justify a vaccination; parents wanting to wait to begin the HPV series until after menarche for girls; failure of some insurance companies to cover HPV vaccination; the ‘up-front’ costs for my practice to purchase the vaccine; Lack of adequate reimbursement for vaccination, the time it will take me to discuss HPV vaccination with my patients and their parents.
Survey Administration
Depending on physician preference, the survey was administered July – September 2018, through the Internet or U.S. mail. We sent the Internet group an initial e-mail with up to 8 e-mail reminders, and the mail group an initial mailing and up to 2 additional mail reminders. Non-respondents in the Internet group were also sent up to 2 mail surveys in case of problems with e-mail. We patterned the mail protocol on Dillman’s Tailored Design Method.42
Statistical analysis
We pooled Internet and mail surveys for analyses because studies have found that physician attitudes are similar when obtained by different methods.42–44 Physicians who did not deliver HPV vaccine in their practice were excluded from analysis. We compared respondents with non-respondents using t-tests for continuous and Pearson’s chi-squared tests for categorical variables. A multivariable analysis was conducted with the outcome of reporting ≥50% refusal/deferral of HPV vaccination among 11–12 year-old patients within each gender. We used a log-Poisson model with robust error estimation to calculate relative risks. Independent variables included physician and practice characteristics (specialty, number of providers in practice, proportion of adolescent patients, proportion of privately insured patients), and physician HPV recommendation style and delivery experiences. We used a cutoff of p<0.25 for inclusion of variables into the model. We used a stepwise backward elimination procedure in which the least significant predictor in the model was eliminated sequentially. At each step, estimates were checked to make sure other variables were not affected by dropping the least significant variable. This resulted in the retention of only those factors that were significant at p<0.05 in the final model, with the exception of specialty which we forced into the final model. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
The overall response rate was 65% (588/908); 70% (317/456) among Peds and 60% (271/452) among FPs. Of respondents, 280 (48%) responded via internet. Fifteen Peds and 43 FPs did not administer HPV vaccine and were excluded. Reasons for not administering HPV vaccine were not explored in this survey. In Table 1, we compare respondents and non-respondents and show additional characteristics available only for respondents. Among FPs, respondents were significantly younger than non-respondents, and had more providers in their practices.
Table 1:
Respondent and Non-Respondent Characteristics by Physician Specialty
| Characteristic | Peds | FP | ||
|---|---|---|---|---|
| Respondents (n=317) |
Non-Respondents (n=139) |
Respondents (n=271) |
Non-Respondents (n=181) |
|
| Mean (SD+) / Median age in years | 52 (10) / 52 | 51 (11) / 50 | 56 (8) / 55* | 58 (8) / 58* |
| Mean (SD) / Median number of providers | 10 (16) / 6 | 16 (55) / 6 | 12 (35) / 6** | 7 (10) / 5** |
| Male, % | 36 | 34 | 59 | 60 |
| Region, % | ||||
| Midwest | 21 | 22 | 31 | 27 |
| Northeast | 23 | 15 | 16 | 16 |
| South | 35 | 44 | 30 | 36 |
| West | 21 | 19 | 24 | 20 |
| Location of Practice, % | ||||
| Rural | 1 | 1 | 7 | 7 |
| Urban-Non-Inner | 46 | 44 | 58 | 55 |
| Urban-Inner | 53 | 56 | 35 | 38 |
| Setting, % | ||||
| Private practice | 80 | 78 | 70 | 76 |
| Univ/Hosp/Public/Other | 16 | 17 | 21 | 19 |
| HMO | 3 | 4 | 8 | 6 |
| Decision-making | ||||
| Independent | 72 | 65 | 51 | 60 |
| Larger system level | 28 | 35 | 49 | 40 |
| Proportion of adolescents 11–18 years old, % | ||||
| 0–9% | 2 | N/A | 74 | N/A |
| 10–19% | 11 | N/A | 21 | N/A |
| 20–29% | 41 | N/A | 4 | N/A |
| ≥30% | 46 | N/A | 2 | N/A |
| Proportion of Hispanic or Latino patients, % | ||||
| 0–9% | 40 | N/A | 65 | N/A |
| 10–24% | 35 | N/A | 20 | N/A |
| 25–49% | 15 | N/A | 9 | N/A |
| ≥50 | 11 | N/A | 6 | N/A |
| Proportion of black or African American patients, % | ||||
| 0–9% | 44 | N/A | 72 | N/A |
| 10–24% | 33 | N/A | 19 | N/A |
| 25–49% | 18 | N/A | 6 | N/A |
| ≥50 | 5 | N/A | 3 | N/A |
| Proportion of patients with private insurance, % | ||||
| 0–24% | 24 | N/A | 23 | N/A |
| 25–49% | 22 | N/A | 21 | N/A |
| 50–74% | 28 | N/A | 29 | N/A |
| 75–100% | 25 | N/A | 26 | N/A |
| Proportion of patients with Medicaid or CHIP, % | ||||
| 0–9% | 24 | N/A | 41 | N/A |
| 10–24% | 22 | N/A | 24 | N/A |
| 25–49% | 28 | N/A | 19 | N/A |
| ≥50% | 25 | N/A | 16 | N/A |
Standard Deviation
p<0.05 for t-test for difference within specialty for respondents vs non-respondents
p<0.05 for Wilcoxon Rank Sum for difference within specialty for respondents vs non-respondents
Peds = pediatricians; FP = family physicians
Delivery and Communication Practices for HPV Vaccine
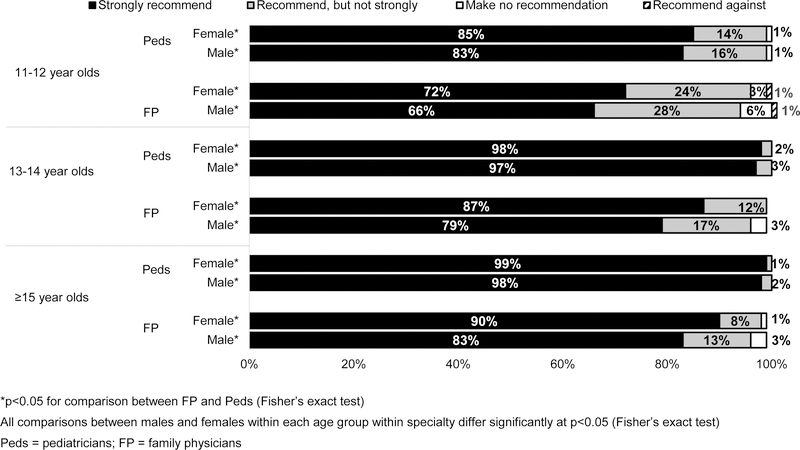
Within each specialty, a larger percentage of physicians made a strong recommendation for HPV vaccination for older adolescents compared with 11–12 year olds (Figure 1). For each age group, a larger percentage of Peds than FPs made a strong recommendation. Sixty-five percent of Peds and 42% of FPs reported almost always/always using a presumptive style, while 16% of Peds and 24% of FPs almost always/always used a conversational style of introduction. Overall, 40% of physicians used standing orders for HPV, 66% had a computer-based system that could report adolescents needing HPV vaccine, and 42% had an electronic alert in the medical record if a patient needed an HPV vaccine (no significant differences by specialty).
Figure 1: Strength of Physician Recommendation for HPV Vaccine by Patient Age and Gender (Peds n=302; FP n=228).
Attitudes and Experiences with HPV Vaccine Delivery
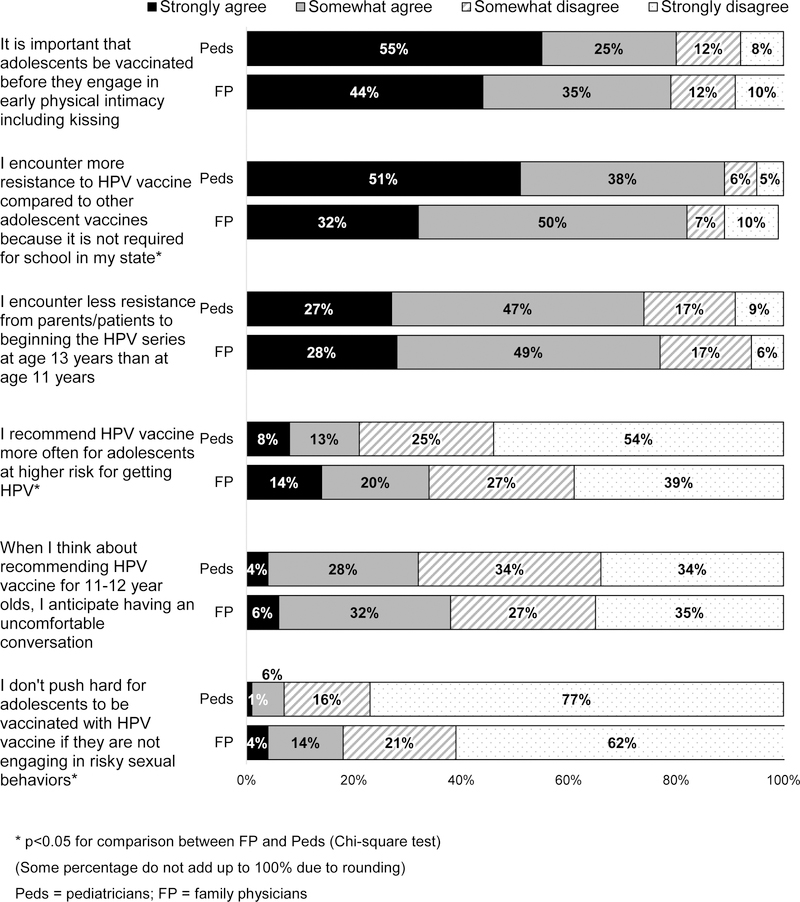
A majority of both specialties strongly or somewhat agreed that it is important that adolescents be vaccinated before they engage in physical intimacy including kissing; that they encounter more resistance to HPV vaccination compared to other adolescent vaccines because it is not required by schools in their state; and that they encounter less resistance from patients/parents to beginning the HPV series at age 13 years than at age 11 years (Figure 3). Around one-third of both specialties reported anticipating an uncomfortable conversation if they recommended vaccination for patients at 11–12 years.
Figure 3: Physician Experiences with HPV Vaccine Discussions (Peds n=302, FM n=228).
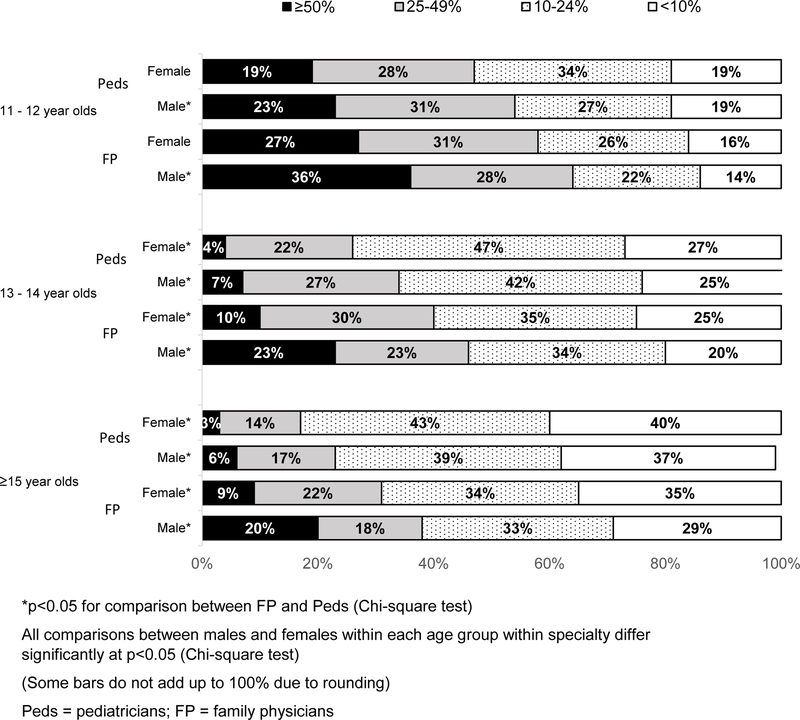
Rates of Refusal or Deferral of HPV Vaccine Physicians reported high rates of refusal/deferral among 11–12 year-old patients, with lower levels among older adolescents (Figure 2); FPs reported higher rates than Peds at all patient ages, and physicians in both specialties were significantly more likely to report refusal/deferral for their male patients than for their female patients.
Figure 2: Proportion of Physicians Reporting Patients/Parents Refusal/Deferral of HPV Vaccine, by Patient Age and Gender (Peds n=302; FP n=228).
Factors significantly associated with physicians reporting a ≥50% refusal/deferral rate among 11–12 year-old patients included: not strongly recommending to 11–12 year-old patients, not using a presumptive recommendation style almost always/always, strongly agreeing that they encounter less resistance to HPV vaccination from patients at age 13 than patients at age 11 years, and anticipating an uncomfortable discussion when recommending to 11–12 year olds (Table 2). The multivariable results for both genders were similar.
Table 2:
Factors Associated with Physicians Reporting a 50% or Higher Refusal/Deferral Rate for HPV Vaccination among their 11–12 year-old Patients by Gender
| Female patients (n=482) | Male patients (n=497) | |||||||
|---|---|---|---|---|---|---|---|---|
| <50% n=376 (78%) Colum n % |
≥50% n=106 (22%) Colum n % |
Biv. p value |
MV RR (95% CI) |
<50% n=356 (72%) Colum n % |
≥50% n=141 (28%) Colum n % |
Biv. p value |
MV RR (95% CI) |
|
| Anticipate having an uncomfortable conversation when recommending HPV vaccine for 11–12 year-olds | 30 70 |
51 49 |
<.0001 | 1.62 (1.17–2.24) Ref. | 30 70 |
46 54 |
0.001 | 1.32 (1.02–1.72) Ref. |
| Strongly/Somewhat agree | ||||||||
| Strongly/Somewhat disagree | ||||||||
| Strongly recommend to 11–12 year-olds | ||||||||
| Yes | 16 | 52 | Ref. 2.14 (1.50–3.04) | 86 | 52 | Ref. 1.90 (1.43–2.53) | ||
| No | 84 | 48 | <.0001 | 14 | 48 | <.0001 | ||
| Practice Specialty | ||||||||
| FP | 39 | 50 | 1.11 (0.81–1.52) Ref. | 38 | 54 | 1.30 (0.99–1.69) Ref. | ||
| Peds | 61 | 50 | 0.04 | 62 | 46 | 0.001 | ||
| Use of a presumptive recommendation style | ||||||||
| Freq/Occ/Rarely/Never | 36 | 66 | 1.61 (1.11–2.32) Ref. | 34 | 67 | 1.74 (1.27–2.38) Ref. | ||
| Almost Always/ Always | 64 | 34 | <.0001 | 66 | 33 | <.0001 | ||
| Perceive less resistance from parents / patients to beginning the HPV series at age 13 than at age 11 years | ||||||||
| Strongly agree | 20 | 54 | 2.33 (1.69–3.22) Ref. | 20 | 47 | 1.84 (1.42–2.38) Ref. | ||
| Others | 80 | 46 | <.0001 | 80 | 53 | <.0001 | ||
| Donť push hard for adolescents to be vaccinated with HPV vaccine if they are not engaging in risky sexual behaviors | 8 | 23 | 8 | 20 | ||||
| 92 | 77 | 92 | 80 | |||||
| Strongly/Somewhat agree | <.0001 | 0.0001 | ||||||
| Strongly/Somewhat disagree | ||||||||
| Proportion of patient population that are adolescents 11-18 years old | ||||||||
| 0-9% | 20 | 31 | 21 | 29 | ||||
| 10% or more | 80 | 69 | 0.12 | 79 | 71 | 0.05 | ||
| Proportion of patient population that has private insurance | ||||||||
| 0-24% | 26 | 74 | 25 | 75 | ||||
| 25% or more | 16 | 84 | 0.03 | 22 | 78 | 0.59 | ||
| Median (IQR) number of providers in practice | 6 (4-11) | 5 (3-9) | 0.03* | 6 (4-11) | 5 (3-10) | 0.03* | ||
Wilcoxon test
HPV = Human Papilloma Virus; Biv. = bivariable analysis; MV = multivariable analysis; RR = risk ratio; CI = confidence interval; Ref. = reference; FP = family physicians; Peds = Pediatricians; Freq = frequently; Occ = occasionally; IQR = interquartile range
Perceived Barriers to HPV Vaccine Delivery
Perceived barriers to HPV vaccination reported by ≥20% of physicians in either specialty as major were: misinformation parents receive from the Internet or social media, parental concerns about safety of HPV vaccine, parents not thinking HPV vaccine was necessary for their daughters or sons, and opposition to vaccination for moral or religious reasons (Figure 4).
Knowledge, Practices and Attitudes Regarding 2-Dose Schedules
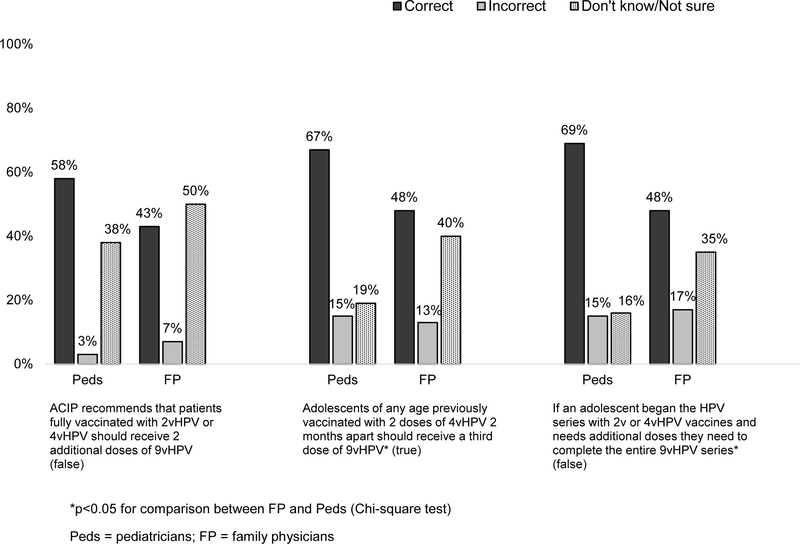
Physicians, especially FPs, frequently were incorrect or reported not knowing about the number of doses recommended in different scenarios or whether additional vaccination with 9vHPV should be offered adolescents who were fully immunized previously with 2vHPV or 4vHPV (Figure 5). Regarding dosing intervals, among Peds, 74% reported routinely recommending the second HPV dose 6 months after the first and 25% at 12 months; among FPs, corresponding percentages were 88% at 6–months and 12% at a 12–months (p<.0001).
Figure 5: Physician Knowledge Regarding 2-dose HPV Vaccine Schedules (Peds n=302, FP n=228).
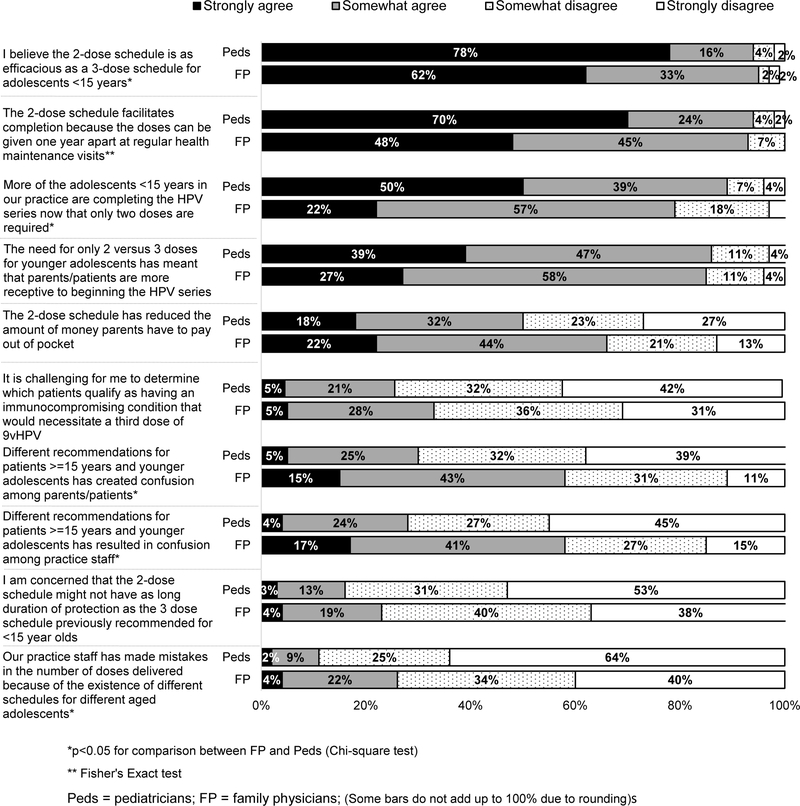
The vast majority of physicians strongly or somewhat agreed the 2-dose schedule is as efficacious as a 3-dose schedule for adolescents <15 years, and that the new schedule facilitated completion and initiation of the series among adolescents in this age group, with Peds being more likely to strongly agree with all of these statements than FPs (Figure 6). However, over half of FPs and close to one-third of Peds strongly or somewhat agreed that having different recommendations for adolescents ≥15 years and younger adolescents had created confusion among patients/parents or practice staff.
Figure 6: Primary Care Physicians’ Attitudes Regarding 2-dose HPV Vaccine (Peds n=302, FM n=228).
DISCUSSION
Despite the existence of highly effective and safe HPV vaccines, national vaccination coverage remains suboptimal. This is the first national survey of physician attitudes regarding the new ACIP recommendations for a 2-dose schedule in younger adolescents and offers a glimpse of physician practices with respect to current HPV vaccine recommendations and delivery practices. Our data show a high proportion of physicians are recommending HPV vaccine, although more are recommending for older adolescents than for 11–12 year-olds. A lower proportion of FP compared with Peds strongly recommend HPV vaccine to patients of all ages evaluated. Slightly more than half of Peds and less than half of FPs reported using a presumptive recommendation style, and less than half of both specialties used standing orders or electronic alerts in HPV delivery. Physicians reported high rates of refusal, especially by 11–12 year olds, who are the target population for vaccination according to national guidelines. Physicians were more likely to report high refusal rates by 11–12 year-olds if physicians did not use a presumptive style or strongly recommend vaccination to young adolescents, or if physicians anticipated less resistance to vaccination from patients older than 11–12 years. Although physicians demonstrated some knowledge gaps about the 2-dose recommendations, the majority believed the revised schedule was facilitating both initiation and completion of the HPV vaccine series among adolescents <15 years of age.
A physician recommendation has been shown to be one of the most important factors in parental vaccine acceptance,27–29 and the lack of a strong recommendation has been identified as an important barrier to HPV vaccination.22,45–48 Our data show substantial increases in the strength of recommendation compared with a survey conducted approximately five years ago using similar methodology.49 Percentages of Peds strongly recommending HPV vaccine have increased from 60% in the 2013 survey to 85% in the 2018 survey for 11–12 year-old females, and from 52% to 83% for 11–12 year-old males. Smaller increases have occurred among FPs during the same period (from 59% to 72% for 11–12 year-old females, and from 41% to 66% for 11–12 year-old males), although the disparity in strength of recommendations between specialties persists.49 Although the overall percentage of physicians strongly recommending for patients at 11–12 years was high in the current study, it was 13–15 absolute percentage points lower than for 13–14 year olds in both specialties.
Lower rates of strong recommendations for HPV vaccination among FPs have important implications, given their prominent role in adolescent health care delivery. A recent national analysis reported that the proportion of adolescents seen by FPs increases from 25% among 11 year-olds to 57% among 18 year-old males and 31% among 11 year-olds to 41% among 18 year-old females.50 The differences we observed by specialty are reflective of prior literature regarding childhood51–56 and adolescent49,57–62 vaccines and highlight the fact that FPs need to remain a major focus of vaccine education and practice improvement strategies to improve HPV vaccine delivery.
Reported rates of deferrals/refusals were high among our respondents, especially for patients ages 11–12 years and among FPs, and appear relatively stable from 5 years ago.49 Physicians, especially FPs, reported higher rates of deferral or refusal for their male patients compared with female patients in every age group. This may be related to the longer duration of recommendations for females or to a higher perceived benefit of HPV vaccine for females. The finding that physicians who perceive higher refusal/deferral rates among 11–12 year-olds more frequently were those who 1) do not strongly recommend vaccination in this age group, 2) anticipate uncomfortable discussions with parents of 11–12 year olds, and 3) expect to encounter more resistance from parents of 11–12 year-olds than older adolescents, is consistent with previous studies demonstrating that physicians are less likely to discuss HPV vaccine with patients aged 11–12 years if they believe parents are likely to defer.49,63,64 However, other literature has demonstrated that physicians may considerably overestimate the amount of resistance to vaccination that they are likely to encounter.65 Our data also demonstrate that physicians who report high levels of refusal/deferral are also less likely to use a presumptive recommendation style. To our knowledge this has not previously been reported. The circular nature of provider anticipation of refusal/deferral potentially leading to a weaker recommendation style and less persistence in responding to parental hesitancy66 could be creating a self-perpetuating cycle within a subgroup of physicians.
Our data suggest that improvements are needed in how HPV vaccine is being recommended. A “presumptive”41 style of initiating HPV discussions uses words that convey an assumption of vaccination and does not discuss HPV in a different manner than other adolescent vaccines; whereas, a “conversational”30 style engages parents in an open-ended discussion about HPV vaccine, without linguistic presupposition of vaccination. A presumptive approach has been shown to be associated with higher HPV acceptance compared with a conversational approach in multiple studies30–33 Updated implementation guidance from the AAP includes the use of a strong pediatrician recommendation with wording consistent with a presumptive style,67 and the AAFP website refers family physicians to CDC talking points that included recommendations for “same way” as for other adolescent vaccines and “same day” recommendations. Our data indicate that large percentages of physicians, including a majority of Peds, have adopted the presumptive communication style in initiating HPV discussion, but there are still substantial proportions who have not. In addition to improving physician communication styles, HPV delivery could also be optimized by increased use of evidence-based methods including standing orders and alert systems in the medical record to remind providers of the need for vaccination at the point of care, both recommended by the Community Preventive Services Task Force68.
Factors reported by physicians as important barriers to HPV vaccination, including perceived parental concerns about safety or effects of vaccination on sexual behavior, parents thinking HPV vaccine was not necessary or that there are too many vaccines at one visit, are consistent with previously identified barriers.22 However, the barrier most frequently reported by physicians in both specialties--the effect of misinformation parents receive from the Internet or social media--is one that has not been frequently highlighted. Although social media has been recognized as a significant source of vaccine-critical content,69–72 its contribution to low HPV vaccination acceptance has not been well-studied. A recent survey of 1263 parents of U.S. adolescents found that stories of HPV vaccine harms were more commonly found in social media than in traditional media or conversations and were associated with lack of initiation, delay, or refusal of HPV vaccination.73 Along with these data, our findings highlight the importance of developing effective public health communication strategies to counter misinformation and highlight benefits of HPV vaccination via social media.
Given the relative recency of recommendations for the 2-dose schedule for adolescents initiating HPV vaccination before age 15 years, it is not surprising that there were knowledge gaps for proportions of physicians about when a third dose is recommended, the acceptability of using different HPV vaccines to complete the series, and the need for additional 9vHPV vaccination in those already fully vaccinated with either 2vHPV or 4vHPV. Physician knowledge should increase over time, as will, presumably, the ability of electronic medical record or immunization registry-based systems to forecast the need for HPV vaccination. Interestingly, the majority of physicians in both specialties reported recommending the second dose of the 2-dose schedule at a 6 month interval rather than 12 months later at the next annual well child visit, even though national guidelines allow for either. This may reflect an understanding of the importance of series completion as soon as possible. Importantly, primary care physicians strongly endorsed the belief that the 2-dose schedule was facilitating both initiation and completion of the HPV series.
There are important strengths and limitations to our data. We surveyed large, nationally representative samples of Peds and FPs and achieved high response rates. Responses of our sentinel physicians may not be fully generalizable, although previous work has demonstrated the survey methods described yield similar responses to the most commonly employed method of sampling physicians nationally24. Non-respondents might have different views than respondents, although our high response rates helps to mitigate against this source of bias. Most importantly, our data are based on self-report rather than direct observation and may not entirely reflect actual physician practice.
This snapshot of HPV vaccine delivery in primary care demonstrates room for improvement in the way physicians are communicating about HPV vaccine and in their delivery practices. Most important in this regard is the continued finding that physicians who experience or expect to experience high rates of deferral/refusal may be anticipating and accommodating refusals by altering their recommendation strength and style. Such accommodations by physicians may perpetuate a lack of acceptance of HPV vaccine among parents. Greater physician awareness about the potential of overestimating the degree of parental resistance to HPV vaccination and about the effectiveness of a strong recommendation for HPV vaccine, delivered in the same way and same day as for other adolescent vaccines, may be key to increasing acceptance among parents of 11–12 year-olds. Increased use of available communication training materials and apps as well as further development of evidence-based messages for parents may be helpful in improving the way HPV vaccination is introduced74–78. Our data are very encouraging in showing substantial increases over the past 5 years in the percent of physicians who report strongly recommending HPV vaccine to 11–12 year-olds. The findings also suggest that according to primary care physicians, the 2-dose schedule could result in meaningful increases in HPV vaccination initiation and completion among adolescents, leading to greater protection against HPV-associated cancers in the United States.
What’s Known on This Subject:
Although diseases caused by HPV are responsible for major morbidity and mortality in the U.S., HPV vaccination rates remain low. Primary care physicians’ current HPV vaccine delivery practices and their experiences with HPV vaccine delivery are not well described.
What This Study Adds:
Although most physicians recommend HPV vaccine at 11–12 y.o., many are not using a presumptive style when introducing HPV vaccine, standing orders or electronic alerts for HPV delivery. Most perceive the 2-dose schedule is resulting in higher HPV completion rates.
Acknowledgements:
The authors would like to thank Lynn Olson, PhD, and Karen O’Connor from the Department of Research, AAP, and Bellinda Schoof, MHA from the AAFP, Arlene Weissman, PhD, and the leaders of the AAP and Jennifer Frost, MD, and the leaders of the AAFP for collaborating in the establishment of the sentinel network in pediatrics and family medicine. We would also like to thank all pediatricians and family physicians in the networks for participating and responding to this survey.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding Resource: This investigation was funded by the Centers for Disease Control and Prevention (cooperative agreement #1U1IP001072).
Abbreviations:
- American Academy of Family Physicians
AAFP
- American Academy of Pediatrics
AAP
- Centers for Disease Control and Prevention
CDC
- Advisory Committee on Immunization Practices
ACIP
- Pediatricians
Peds
- Family Physicians
FPs
Footnotes
Disclosures: Portions of this article were first presented at the annual Pediatric Academic Societies Meeting; Baltimore, MD, April 24 – May 1, 2019.
Financial Disclosure:The authors have no financial disclosures.
Conflict of Interest:The authors have no conflicts of interest.
References
- 1.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute. 2013;105(3):175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zandberg DP, Bhargava R, Badin S, Cullen KJ. The role of human papillomavirus in nongenital cancers. CA: a cancer journal for clinicians. 2013;63(1):57–81. [DOI] [PubMed] [Google Scholar]
- 3.Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bulletin of the World Health Organization. 2007;85(9):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3:S3/11–25. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. How Many Cancers Are Linked with HPV Each Year? HPV and Cancer 2018; https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed April 3, 2019. [Google Scholar]
- 6.Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus-Associated Cancers-United States, 2008–2012. MMWR Morbidity and mortality weekly report. 2016;65(26):661–666. [DOI] [PubMed] [Google Scholar]
- 7.Huskins WC, Sullivan CD, Wang J, et al. Tracking the impact of the National Institutes of Health Clinical and Translational Science Awards on child health research: developing and evaluating a measurement strategy. Pediatric research. 2012;71(5):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schottenfeld D, Fraumeni J, JF. Cancer Epidemiology and Preventsion. Third ed. New York, NY: Oxford University Press, Inc.; 2006. [Google Scholar]
- 10.Centers for Disease Control and Prevention. Human papillomavirus-associated cancers-United States, 2004–2008. MMWR Morbidity and Mortality Weekly Reports 2012; April 20, 2012:258–261. Available at. Accessed 15, 61. [PubMed] [Google Scholar]
- 11.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2018; www.cdc.gov/cancer/dataviz. Accessed April 24, 2019. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morbitiy and Mortality Weekly Reports 2011; 2011/December/23:1705–1708. Available at. Accessed 50, 60. [PubMed] [Google Scholar]
- 13.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and Mortality Weekly Reports. 2007;56(Rr-2):1–24. [PubMed] [Google Scholar]
- 14.McClung NM, Gargano JW, Bennett NM, et al. Trends in Human Papillomavirus Vaccine Types 16 and 18 in Cervical Precancers, 2008–2014. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2019;28(3):602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. IID-12.11 Increase the percentage of children aged 6 months through 17 years who are vaccinated annually against seasonal influenza. https://www.healthypeople.gov/node/6359/data_details#revision_history_header.
- 16.Walker TY, Elam-Evans LD, Yankey D, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2017. MMWR Morbidity and Mortality Weekly Reports. 2018;67(33):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammed KA, Geneus CJ, Osazuwa-Peters N, Adjei Boakye E, Tobo BB, Burroughs TE. Disparities in Provider Recommendation of Human Papillomavirus Vaccination for U.S. Adolescents. Journal of Adolescent Health. 2016;59(5):592–598. [DOI] [PubMed] [Google Scholar]
- 18.Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR Morbidity and Mortality Weekly Reports. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 19.Rutten LJ, St Sauver JL, Beebe TJ, et al. Clinician knowledge, clinician barriers, and perceived parental barriers regarding human papillomavirus vaccination: Association with initiation and completion rates. Vaccine. 2017;35(1):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farias AJ, Savas LS, Fernandez ME, et al. Association of physicians perceived barriers with human papillomavirus vaccination initiation. Preventive medicine. 2017;105:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine. 2016;34(9):1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA pediatrics. 2014;168(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination — Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and Mortality Weekly Reports. 2016;65:1405–1408. [DOI] [PubMed] [Google Scholar]
- 24.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. Evaluation & the health professions. 2008;31(1):43–64. [DOI] [PubMed] [Google Scholar]
- 25.Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(11):1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey AF, Pyrzanowski J, Lockhart S, Campagna E, Barnard J, O’Leary ST. Parents’ perceptions of provider communication regarding adolescent vaccines. Human vaccines & immunotherapeutics. 2016;12(6):1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor JA, Newman RD. Parental attitudes toward varicella vaccination. The Puget Sound Pediatric Research Network. Archives of pediatrics & adolescent medicine. 2000;154(3):302–306. [DOI] [PubMed] [Google Scholar]
- 28.Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. Association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics. 2006;118(5):e1287–1292. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy A, Basket M, Sheedy K. Vaccine attitudes, concerns, and information sources reported by parents of young children: results from the 2009 HealthStyles survey. Pediatrics. 2011;127 Suppl 1:S92–99. [DOI] [PubMed] [Google Scholar]
- 30.Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements Versus Conversations to Improve HPV Vaccination Coverage: A Randomized Trial. Pediatrics. 2017;139(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dempsey AF, Pyrznawoski J, Lockhart S, et al. Effect of a Health Care Professional Communication Training Intervention on Adolescent Human Papillomavirus Vaccination: A Cluster Randomized Clinical Trial. JAMA pediatrics. 2018;172(5):e180016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturm L, Donahue K, Kasting M, Kulkarni A, Brewer NT, Zimet GD. Pediatrician-Parent Conversations About Human Papillomavirus Vaccination: An Analysis of Audio Recordings. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2017;61(2):246–251. [DOI] [PubMed] [Google Scholar]
- 33.Goff SL, Mazor KM, Gagne SJ, Corey KC, Blake DR. Vaccine counseling: a content analysis of patient-physician discussions regarding human papilloma virus vaccine. Vaccine. 2011;29(43):7343–7349. [DOI] [PubMed] [Google Scholar]
- 34.Shah PD, Calo WA, Gilkey MB, et al. Questions and Concerns About HPV Vaccine: A Communication Experiment. Pediatrics. 2019;143(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rand CM, Schaffer SJ, Humiston SG, et al. Patient-provider communication and human papillomavirus vaccine acceptance. Clinical pediatrics. 2011;50(2):106–113. [DOI] [PubMed] [Google Scholar]
- 36.Malo TL, Gilkey MB, Hall ME, Shah PD, Brewer NT. Messages to Motivate Human Papillomavirus Vaccination: National Studies of Parents and Physicians. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(10):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Talking to Parents about Vaccines. Provider Resources for Vaccine Conversations with Parents 2015; https://www.cdc.gov/vaccines/hcp/conversations/conv-materials.html. [Google Scholar]
- 38.American Academy of Pediatrics. HPV Champion Toolkit. HPV Vaccine is Cancer Prevention https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/immunizations/HPV-Champion-Toolkit/Pages/HPV-Champion-Toolkit.aspx. [Google Scholar]
- 39.American Academy of Pediatrics. HPV Vaccine Implementation Guidance 2017; https://www.aap.org/en-us/Documents/immunization_hpvimplementationguidance.pdf.
- 40.American Academy of Family Physicians. Human Papillomavirus Vaccine (HPV). Disease-and Population-specific Immunizations https://www.aafp.org/patient-care/public-health/immunizations/disease-population/hpv.html. [Google Scholar]
- 41.Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dillman DA, Smyth J, Christian LM. Internet, Mail and Mixed-Mode Surveys: The Tailored Desgin Method. Vol 3rd. 3rd Edition ed. New York, NY: John Wiley Co.; 2009. [Google Scholar]
- 43.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–303. [DOI] [PubMed] [Google Scholar]
- 44.Atkeson LR, Adams AN, Bryant LA, Zilberman L, Saunders KL. Considering Mixed Mode Surveys for Questions in Political Behavior: Using the Internet and Mail to Get Quality Data at Reasonable Costs. Political Behavior. 2011;33(1):161–178. [Google Scholar]
- 45.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128(5):830–839. [DOI] [PubMed] [Google Scholar]
- 46.Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Maternal and child health journal. 2013;17(5):879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilkey MB, Moss JL, McRee AL, Brewer NT. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30(41):5928–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laz TH, Rahman M, Berenson AB. An update on human papillomavirus vaccine uptake among 11–17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30(24):3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allison MA, Hurley LP, Markowitz L, et al. Primary Care Physicians’ Perspectives About HPV Vaccine. Pediatrics. 2016;137(2):e20152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rand CM, Goldstein NPN. Patterns of Primary Care Physician Visits for US Adolescents in 2014: Implications for Vaccination. Academic Pediatrics. 2018;18(2):S72–S78. [DOI] [PubMed] [Google Scholar]
- 51.Kempe A, Babbel C, Wallace GS, et al. Knowledge of interim recommendations and use of Hib vaccine during vaccine shortages. Pediatrics. 2010;125(5):914–920. [DOI] [PubMed] [Google Scholar]
- 52.Kempe A, Patel MM, Daley MF, et al. Adoption of rotavirus vaccination by pediatricians and family medicine physicians in the United States. Pediatrics. 2009;124(5):e809–816. [DOI] [PubMed] [Google Scholar]
- 53.Nelson NP, Allison MA, Lindley MC, et al. Physician Knowledge and Attitudes About Hepatitis A and Current Practices Regarding Hepatitis A Vaccination Delivery. Acad Pediatr. 2017;17(5):562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis MM, Ndiaye SM, Freed GL, Clark SJ. One-year uptake of pneumococcal conjugate vaccine: a national survey of family physicians and pediatricians. The Journal of the American Board of Family Practice. 2003;16(5):363–371. [DOI] [PubMed] [Google Scholar]
- 55.Freed GL, Freeman VA, Clark SJ, Konrad TR, Pathman DE. Pediatrician and family physician agreement with and adoption of universal hepatitis B immunization. The Journal of family practice. 1996;42(6):587–592. [PubMed] [Google Scholar]
- 56.Schaffer SJ, Szilagyi PG, Shone LP, et al. Physician perspectives regarding pneumococcal conjugate vaccine. Pediatrics. 2002;110(6):e68. [DOI] [PubMed] [Google Scholar]
- 57.Allison MA, Cohn AC, Stokley S, et al. Timing of adolescent meningococcal conjugate vaccination attitudes and practices of pediatricians and family medicine physicians. American journal of preventive medicine. 2011;41(6):581–587. [DOI] [PubMed] [Google Scholar]
- 58.Schaffer SJ, Humiston SG, Shone LP, Averhoff FM, Szilagyi PG. Adolescent immunization practices: a national survey of US physicians. Archives of pediatrics & adolescent medicine. 2001;155(5):566–571. [DOI] [PubMed] [Google Scholar]
- 59.Dempsey AF, Cowan AE, Broder KR, Kretsinger K, Stokley S, Clark SJ. Adolescent Tdap vaccine use among primary care physicians. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2009;44(4):387–393. [DOI] [PubMed] [Google Scholar]
- 60.Oster NV, McPhillips-Tangum CA, Averhoff F, Howell K. Barriers to adolescent immunization: a survey of family physicians and pediatricians. The Journal of the American Board of Family Practice. 2005;18(1):13–19. [DOI] [PubMed] [Google Scholar]
- 61.Davis MM, Broder KR, Cowan AE, et al. Physician attitudes and preferences about combined Tdap vaccines for adolescents. American journal of preventive medicine. 2006;31(2):176–180. [DOI] [PubMed] [Google Scholar]
- 62.Kempe A, Allison MA, MacNeil JR, et al. Adoption of Serogroup B Meningococcal Vaccine Recommendations. Pediatrics. 2018;142(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC pediatrics. 2011;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McRee AL, Gilkey MB, Dempsey AF. HPV vaccine hesitancy: findings from a statewide survey of health care providers. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2014;28(6):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Healy CM, Montesinos DP, Middleman AB. Parent and provider perspectives on immunization: are providers overestimating parental concerns? Vaccine. 2014;32(5):579–584. [DOI] [PubMed] [Google Scholar]
- 66.Shay LA, Baldwin AS, Betts AC, et al. Parent-Provider Communication of HPV Vaccine Hesitancy. Pediatrics. 2018;141(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.American Academy of Pediatrics. HPV Vaccine Implementation Guidance Updated February 2017. 2017; https://www.aap.org/en-us/Documents/immunization_hpvimplementationguidance.pdf.
- 68.The Guide to Community Preventive Services. https://www.thecommunityguide.org/. Accessed Abril 3, 2019.
- 69.Briones R, Nan X, Madden K, Waks L. When vaccines go viral: an analysis of HPV vaccine coverage on YouTube. Health communication. 2012;27(5):478–485. [DOI] [PubMed] [Google Scholar]
- 70.Dunn AG, Leask J, Zhou X, Mandl KD, Coiera E. Associations Between Exposure to and Expression of Negative Opinions About Human Papillomavirus Vaccines on Social Media: An Observational Study. Journal of medical Internet research. 2015;17(6):e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keelan J, Pavri V, Balakrishnan R, Wilson K. An analysis of the Human Papilloma Virus vaccine debate on MySpace blogs. Vaccine. 2010;28(6):1535–1540. [DOI] [PubMed] [Google Scholar]
- 72.Broniatowski David A., SiHua Qi SM, Lulwah AlKulaib SM, Chen Tao, Benton Adrian, Quinn Sandra C., and Dredze Mark. Weaponized Health Communication: Twitter Bots and Russian Trolls Amplify the Vaccine Debate. American Public Health Association 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Margolis MA, Brewer NT, Shah PD, Calo WA, Gilkey MB. Stories about HPV vaccine in social media, traditional media, and conversations. Preventive medicine. 2019;118:251–256. [DOI] [PubMed] [Google Scholar]
- 74.LLC KS. HPV Vaccine: Same Way Same Day. 2018; https://apps.apple.com/us/app/hpv-vaccine-same-way-same-day/id1356847181. [Google Scholar]
- 75.Centers for Disease Control and Prevention. Talking to Parents about HPV Vaccine. Human Papillomavirus (HPV) 2016; https://www.cdc.gov/hpv/hcp/for-hcp-tipsheet-hpv.html. [Google Scholar]
- 76.Centers for Disease Control and Prevention. PreteenVaxScene Webinar Series. Human Papillomavirus (HPV) 2019; https://www.cdc.gov/hpv/preteenvaxscene-webinar.html. [Google Scholar]
- 77.American Academy of Pediatrics. AAP-CA3’S HPV Vaccine Provider Education And Promotion Webinar. 2016; https://aapca3.org/aap-ca3s-hpv-vaccine-provider-education-and-promotion-webinar/. [Google Scholar]
- 78.WAC Parth D. Shah, Gilkey Melissa B., Boynton Marcella H., Dailey Susan Alton, Todd Karen G., Robichaud Meagan O., Margolis Marjorie A., Brewer Noel T.. Questions and Concerns About HPV Vaccine: A Communication Experiment. 2019; https://pediatrics.aappublications.org/content/143/2/e20181872. [DOI] [PMC free article] [PubMed] [Google Scholar]