Abstract
Purpose
To explore the associations between macular choroidal and retinal thickness and axial elongation in non-myopic and myopic junior students.
Methods
In this school-based longitudinal observational study, axial length was measured by optical low-coherence reflectometry, and choroidal thickness and retinal thickness were measured by spectral-domain optical coherence tomography. Myopia was defined as non-cycloplegic objective spherical equivalent refraction ≤ −0.50 diopters. Structural equation modeling and multiple linear regression models were used to analyze the associations between baseline choroidal and retinal thickness with axial elongation.
Results
Out of 1307 students examined at baseline in 2017, 1197 (91.58%) returned for follow-up examination in 2018, with a median age of 12.00 years (interquartile range [IQR], 1.00) and included 667 boys (55.72%). Within a 1-year period, the median axial elongation of right eyes was 230 µm (IQR, 180) in boys and 200 µm (IQR, 160) in girls (P = 0.032). The thinner temporal choroidal thickness was associated with greater 1-year axial elongation only in myopic students (β, −0.20; 95% confidence interval [CI], −0.37, −0.03), the thinner temporal retinal thickness was associated with greater 1-year axial elongation in both non-myopic (β, −2.67; 95% CI, −4.52, −0.82) and myopic (β, −0.99; 95% CI, −1.68, −0.30) students, after adjustment for sex, age, and height. Subfoveal and nasal choroidal and retinal thickness were not significantly associated with axial elongation in either non-myopic or myopic students.
Conclusions
A thinner temporal choroid at age 12 years may predict greater 1-year axial elongation in myopic students, and a thinner temporal retina may predict greater 1-year axial elongation in both non-myopic and myopic students. This finding may help to identify children at risk and control axial elongation with potential preventive strategies.
Keywords: choroidal thickness, retinal thickness, axial elongation
The prevalence of myopia has been observed to increase markedly in recent decades, especially in China, and approximately half of the world population could be affected by the year 2050.1 Myopia may become one of the leading causes of irreversible vision impairment worldwide,2 due to myopia-related complications including myopic maculopathy and optic nerve damage.3 The mechanical stretching caused by axial elongation of the eye globe may play a major role in these ocular complications.4 Thus, knowledge about the physiologic mechanisms of axial elongation would allow better control of the development and progression of ocular complications related to myopia.
Macular choroidal thickness is correlated negatively with axial length, decreasing with increasing levels of myopia and decreasing with age in adults.5 During active accommodation, the choroid thins and axial length increases proportional to the accommodative effort.6 Young adults with thicker choroids and who exhibit longitudinal choroidal thickening show slower axial eye growth.7 In cross-sectional studies on children, thinner macular choroidal thickness was found to be associated with longer axial length and higher levels of myopia, as in adults.8,9 Longitudinal studies carried out on children have reported that the choroid increased in thickness during follow-up. This increase was smaller in eyes that added greater axial length and had relatively negative refractive development; in some cases, choroid thickness decreased.10,11 In a 1-year longitudinal study, choroidal thinning was found to occur early in myopic progression.12 These findings support a potential role for the choroid in the mechanisms regulating eye growth in childhood, and a thinner choroid may predict the onset or progression of myopia.11
In addition to choroidal thickness, retinal thickness may also correlate with refractive error and axial length. Subfoveal retinal thickness was found to increase with increasing axial length and myopia degree, whereas parafoveal retinal thickness decreased with increasing axial length.13,14 Some other studies found no association between macular retinal thickness and axial length.15,16 Few studies have described the association of both retinal and choroidal thickness with axial length in the same cohort. One cross-sectional study found that choroidal thickness but not retinal thickness correlated closely with axial length and refractive diopters in Chinese children.9 A longitudinal study indicated that choroidal thickness decreased in all subfields during myopic shift, whereas the thickness of the retinal layers increased or was unchanged in most subfields.12 Also, the sample sizes of previous longitudinal studies on choroidal and retinal thickness and axial length were relatively small. It has been suggested that the choroid and retina might thin during development of myopia and axial elongation, and an association between longer eye at baseline and greater subsequent axial eye elongation has been found.17 Whether macular choroid and retina thinning precedes axial elongation in non-myopic children and myopic children is still unclear. The purpose of this longitudinal study with a larger sample was to evaluate the association between baseline macular choroidal and retinal thickness and the rapidity of axial elongation in non-myopic and myopic junior students.
Methods
Study Population
A multistage cluster randomized sampling was used. Six districts were randomly selected from the original 16 districts in Beijing, then nine junior high schools were randomly selected, and grade 7 students from these schools were selected in 2017. All students provided verbal consent, and their parents provided informed consent before the ophthalmic examination. Approval for this study was obtained from the human research ethics committee of Capital Medical University and Beijing Tongren Hospital. Meanwhile, all procedures used in this study adhered to the tenets of the Declaration of Helsinki.
Data Collection
The participants underwent an ophthalmic examination at both the baseline survey and follow-up after 1 year. The ophthalmic examination included the measurement of biometry using optical low-coherence reflectometry (Lenstar 900 Optical Biometer; Haag-Streit, Koeniz, Switzerland); three measurements were automatically performed and then averaged. Axial elongation was calculated as the axial length at follow-up minus the axial length at baseline. Refractometry was performed by autorefractometry (KR-8900 autorefractor; Topcon, Tokyo, Japan) in a non-cycloplegic state; three measurements were automatically obtained and then averaged. Spherical equivalent (SE) was calculated as the spherical value plus half of the cylindrical value, and myopia was defined as SE ≤ −0.50 diopter (D).17
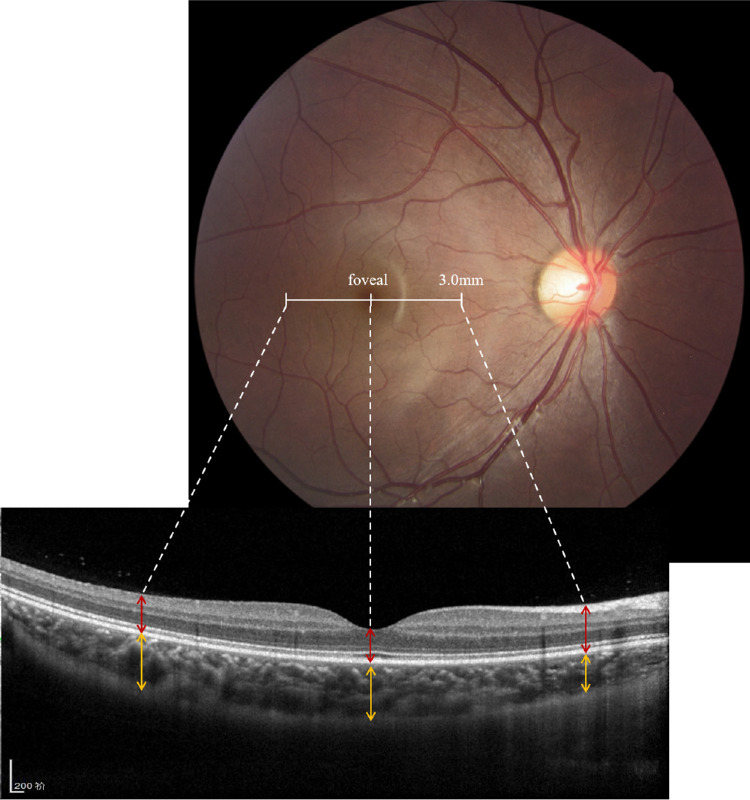
Optical coherence tomography (OCT) images of the choroid and retina were obtained from the right eyes of students through the use of spectral-domain OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany). An enhanced depth imaging system utilizing 9-mm line scans and an eye-tracking system was used to obtain horizontal sections through the fovea. The wavelength of the OCT instrument was 870 nm, with an axial resolution of 5 µm and a scanning speed of 40,000 A-scans per second. Each radial OCT image was the average of 30 B-scans. The ophthalmic examination was carried out by trained ophthalmologists and optometrists.
The OCT images at baseline were analyzed by a trained ophthalmologist (Y.G.) using Eye Explorer 5.3.3.0 (Heidelberg Engineering) in a masked manner. Choroidal thickness was measured manually from the outer portion of the hyperreflective line corresponding to the retinal pigment epithelium (RPE) to the inner surface of the sclera, and retinal thickness was measured from the internal limiting membrane to the interface between the photoreceptor outer segments and RPE. Eye Explorer software was used to segment layers, and if automatic segmentation errors occurred then manual segmentation by the trained ophthalmologist was performed. The thicknesses of the choroid and retina in the subfoveal region and at a distance of 3000 µm nasally and temporally to the foveola determined by the software were measured (Fig. 1). To determine intrameasurement variability, one ophthalmologist (Y.G.) randomly selected the images of 100 eyes of 100 participants and measured them twice in a masked manner with an interval of 2 weeks. The intrameasurement variability that resulted from variation in the measurements obtained by this measurer was greater than 0.93 (intragroup correlation coefficient [ICC]) for the measurements of choroidal and retinal thickness.
Figure 1.
Enhanced depth imaging OCT was used to measure the thicknesses of the choroid and retina in the subfoveal region and at a distance of 3000 µm nasally and temporally to the foveola on horizontal scans of right eyes were measured. Choroidal thickness (line) was defined as the vertical distance from the RPE to the inner surface of the sclera; retinal thickness was defined as the distance from the internal limiting membrane to the interface between photoreceptor outer segments and the RPE.
Height (cm) was measured by an ultrasonic height/weight survey meter (NHN-318; Omron, Kyoto, Japan). Age was the span between the survey date and the birth date of students.
Statistical Methods
Only the data of right eyes were included in the analysis because of the high correlation of refractive error between the two eyes (correlation coefficient r = 0.80). Kolmogorov–Smirnoff tests were used to examine the normal distribution of continuous variables. Continuous variables for normal distributions were expressed as mean ± SD; otherwise, they were expressed as median (interquartile range [IQR]). Categorical variables were expressed as number (percentage). Also, t-tests were used to compare continuous variables of normal distributions between two groups, Wilcoxon's rank-sum tests were used to compare continuous variables non-normally distributed between two groups, and the χ2 test was used to compare categorical variables. Analysis of variance (ANOVA) was used to evaluate intergroup differences in 1-year axial elongation.
Structural equation modeling was applied to measure latent choroidal and retinal thickness as the choroidal and retinal thicknesses at subfovea, at 3000 µm nasal to fovea, and at 3000 µm temporal to fovea. The structural equation modeling was performed using the SAS CALIS procedure (SAS Institute, Cary, NC, USA) based on the variance–covariance matrix and the maximum likelihood method, which encompassed the measurement model and regression analysis. The structural equation modeling used in this study, presented in Supplementary Figure S1, followed the standard conventions in which latent variables are portrayed as ovals and manifest variables are portrayed as rectangles. Associations of latent choroidal and retinal thickness with 1-year axial elongation were assessed using two structural equation models; model 1 was unadjusted, and model 2 was adjusted for sex, age, and height. The associations among choroidal and retinal thicknesses at subfovea, at 3000 µm nasal to fovea, and at 3000 µm temporal to fovea at baseline with 1-year axial elongation were assessed using two linear regression models, where model 1 was unadjusted, and model 2 was adjusted for sex, age, and height. In the sensitivity analysis, the choroidal and retinal thicknesses at subfovea, at 3000 µm nasal to fovea, and at 3000 µm temporal to fovea at baseline were separately divided into four categories according to quartiles (Q1, Q2, Q3, Q4). The differences in 1-year axial elongation among the quartiles of choroidal and retinal thickness were assessed using two models, where model 1 was an unadjusted ANOVA, and model 2 was adjusted for sex, age, and height in multiple regression analyses. The thickest quartile was used as reference in these two models. The association analyses are presented based on the presence of myopia at baseline.
Statistical analysis was performed using SAS 9.4 (SAS Institute). P < 0.05 was considered statistically significant, and all P values were two sided.
Results
In 2018, 1197 individuals (91.59% of the 1307 students examined for axial length at baseline) returned for the follow-up examination. There were no differences in sex, age, height, or baseline axial length between the students who attended both examinations and those participated only in the baseline examination. Of the 1197 participants, 667 were boys (55.72%) are and 530 were girls (44.28%), with a median age of 12.00 years (IQR, 1.00). The median 1-year increase in axial length that occurred from 12 years of age to 13 years was 220 µm (IQR, 180). The median SE at baseline was −2.07 D (IQR, 3.13); 984 students were myopic (SE ≤ −0.50 D). There were significant differences between boys and girls in axial length at baseline (median, 24.66 vs. 24.18 mm; P < 0.001), axial elongation (median, 230 vs. 200 µm; P = 0.032), nasal choroidal thickness (median, 151 vs. 159 µm; P = 0.045), subfoveal retinal thickness (median, 217 vs. 213 µm; P = 0.002), nasal retinal thickness (median, 298 vs. 294 µm; P < 0.001), and temporal retinal thickness (median, 262 vs. 257 µm; P < 0.001) (Table 1).
Table 1.
Characteristics of the Grade 7 Students According to Sex in Beijing, China
| Median (IQR) | ||||
|---|---|---|---|---|
| Parameters | Total (n = 1197) | Boys (n = 667) | Girls (n = 530) | P |
| Age (y) | 12.00 (1.00) | 12.00 (1.00) | 12.00 (1.00) | 0.038 |
| Height (cm) | 160.10 (10.25) | 163.00 (11.50) | 158.50 (7.20) | <0.001 |
| Spherical equivalent (D) | −2.07 (3.13) | −2.00 (3.00) | −2.19 (3.12) | 0.171 |
| Axial length at baseline (mm) | 24.43 (1.59) | 24.66 (1.55) | 24.18 (1.57) | <0.001 |
| Axial elongation (µm) | 220 (180) | 230 (180) | 200 (160) | 0.032 |
| Subfoveal choroidal thickness (µm) | 262 (97) | 263 (93) | 262 (102) | 0.946 |
| Nasal choroidal thickness (µm) | 155 (66) | 151 (59) | 159 (76) | 0.045 |
| Temporal choroidal thickness (µm) | 276 (80) | 279 (79) | 270 (82) | 0.093 |
| Subfoveal retinal thickness (µm) | 215 (20) | 217 (22) | 213 (20) | 0.002 |
| Nasal retinal thickness (µm) | 296 (25) | 298 (25) | 294 (24) | <0.001 |
| Temporal retinal thickness (µm) | 260 (18) | 262 (17) | 257 (16) | <0.001 |
Wilcoxon rank-sum tests were performed.
Bold indicates statistical significance.
The median choroidal thickness was greatest at the temporal region (subfoveal, 262 µm; nasal, 155 µm; temporal, 276 µm), and the median retinal thickness was greatest at the nasal region (subfoveal, 215 µm; nasal, 296 µm; temporal, 260 µm). Compared with non-myopic students, myopic students had greater median axial elongation (105 vs. 240 mm; P < 0.001) but thinner median subfoveal choroidal thickness (320 vs. 258 µm; P < 0.001), nasal choroidal thickness (186 vs. 151 µm; P < 0.001), temporal choroidal thickness (310 vs. 272 µm; P < 0.001), nasal retinal thickness (301 vs. 295 µm; P < 0.001), and temporal retinal thickness (264 vs. 259 µm; P < 0.001).
One-Year Axial Elongation in Non-Myopic Students at Baseline
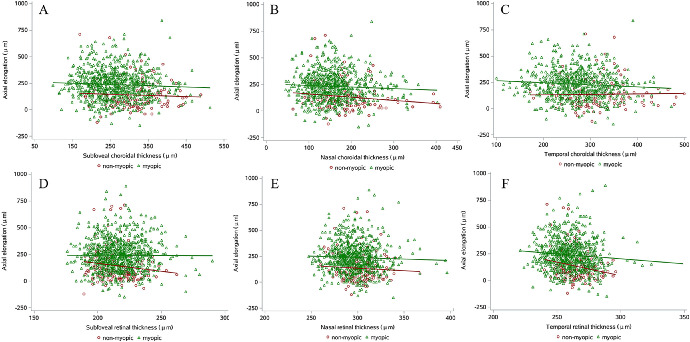
In the non-myopic students, the latent choroidal thickness at baseline measured by subfoveal, nasal, and temporal choroidal thickness was not associated significantly with axial elongation, as determine by the structural equation modeling (P > 0.05) (Table 2, Supplementary Fig. S1). Also, the subfoveal, nasal, and temporal choroidal thickness was not associated significantly with axial elongation, as determined by the linear regression models (P > 0.05) (Table 3, Fig. 2). In the sensitivity analysis (Supplementary Table S1, Supplementary Fig. S2), students who had the smallest nasal choroidal thickness had greater axial elongation compared with the group with the greatest nasal choroidal thickness when adjusted for age and height (difference, 93.49 µm; 95% CI, 5.26, 181.71). Among the different quartile groups of subfoveal or temporal choroidal thickness, there was no significant difference in axial elongation (P > 0.05).
Table 2.
Association of Choroidal and Retinal Thickness With One-Year Axial Elongation (µm)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Parameters | β (95% CI) | P | β (95% CI) | P |
| Non-myopic students | ||||
| Choroidal thickness | −0.10 (−0.32, 0.13) | 0.393 | −0.09 (−0.32, 0.14) | 0.452 |
| Retinal thickness | −0.17 (−0.35, 0.02) | 0.077 | −0.19 (−0.38, −0.003) | 0.046 |
| Myopic students | ||||
| Choroidal thickness | −0.10 (−0.18, −0.02) | 0.017 | −0.09 (−0.17, −0.01) | 0.022 |
| Retinal thickness | −0.08 (−0.18, 0.01) | 0.092 | −0.09 (−0.19, 0.001) | 0.052 |
Latent choroidal thickness and retinal thickness were measured as choroidal and retinal thickness at subfovea, at 3000 µm nasal to fovea, and at 3000 µm temporal to fovea. Structural equation modeling:
model 1, unadjusted structural equation model;
model 2, structural equation model adjusting for sex, age, and height.
Bold indicates statistical significance.
Table 3.
Associations of Choroidal and Retinal Thickness at Different Regions With One-Year Axial Elongation (µm)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Parameters | β (95% CI) | P | β (95% CI) | P |
| Non-myopic students | ||||
| Subfoveal choroidal thickness | −0.11 (−0.48, 0.26) | 0.565 | −0.07 (−0.45, 0.31) | 0.725 |
| Nasal choroidal thickness | −0.30 (−0.71, 0.11) | 0.145 | −0.30 (−0.73, 0.12) | 0.158 |
| Temporal choroidal thickness | 0.00 (−0.38, 0.38) | 0.999 | 0.02 (−0.37, 0.40) | 0.930 |
| Subfoveal retinal thickness | −1.45 (−2.94, 0.03) | 0.056 | −1.47 (−3.02, 0.08) | 0.063 |
| Nasal retinal thickness | −0.51 (−1.74, 0.72) | 0.415 | −0.70 (−1.96, 0.56) | 0.272 |
| Temporal retinal thickness | −2.59 (−4.44, −0.75) | 0.006 | −2.67 (−4.52, −0.82) | 0.005 |
| Myopic students | ||||
| Subfoveal choroidal thickness | −0.12 (−0.26, 0.02) | 0.098 | −0.11 (−0.25, 0.03) | 0.112 |
| Nasal choroidal thickness | −0.14 (−0.32, 0.04) | 0.127 | −0.12 (−0.30, 0.06) | 0.195 |
| Temporal choroidal thickness | −0.19 (−0.36, −0.02) | 0.027 | −0.20 (−0.37, −0.03) | 0.022 |
| Subfoveal retinal thickness | −0.03 (−0.59, 0.53) | 0.917 | −0.04 (−0.60, 0.53) | 0.896 |
| Nasal retinal thickness | −0.28 (−0.79, 0.22) | 0.274 | −0.34 (−0.85, 0.17) | 0.190 |
| Temporal retinal thickness | −0.89 (−1.57, −0.22) | 0.010 | −0.99 (−1.68, −0.30) | 0.005 |
Model 1, unadjusted linear regression model; model 2, linear regression model adjusting for sex, age, and height. Bold indicates statistical significance.
Figure 2.
Scatterplot of choroidal thickness and retinal thickness and 1-year axial elongation in non-myopic and myopic students. (A) Subfoveal choroidal thickness. (B) Nasal choroidal thickness. (C) Temporal choroidal thickness. (D) Subfoveal retinal thickness. (E) Nasal retinal thickness. (F) Temporal retinal thickness.
The latent retinal thickness at baseline measured by subfoveal, nasal, and temporal retinal thickness was not significantly associated with greater axial elongation based on unadjusted structural equation modeling (β, −0.17; 95% CI, −0.35, 0.02) but was significantly associated when adjusted for sex, age, and height (β, −0.19; 95% CI, −0.38, −0.003) in non-myopic students (Table 2, Supplementary Fig. S1). The thinner temporal retinal thickness at baseline was significantly associated with greater axial elongation in the crude linear regression model (β, −2.59; 95% CI, −4.44, −0.75) and when adjusted for sex, age, and height (β, −2.67; 95% CI, −4.52, −0.82) (Table 3, Fig. 2). Neither subfoveal thickness nor nasal retinal thickness was associated significantly with axial elongation (P > 0.05). In the sensitivity analysis (Supplementary Table S1, Supplementary Fig. S2), compared with the group of students with the greatest subfoveal retinal thickness, students in the second thinnest group had 81.02-µm higher axial elongation when adjusted for age and height (95% CI, 19.98, 142.41). With regard to temporal retinal thickness, compared with students with the greatest thickness, students in the thinnest group had 71.92-µm greater axial elongation when adjusted for age and height (95% CI, 0.19, 143.65). Students in the second thinnest group had 71.07-µm greater axial elongation when adjusted for age and height (95% CI, 15.50, 126.63). Among the different quartile groups of nasal retinal thickness, there was no significant difference in axial elongation (P > 0.05).
One-Year Axial Elongation in Myopic Students at Baseline
Among the myopic students, thinner latent choroidal thickness at baseline measured by subfoveal, nasal, and temporal choroidal thickness was significantly associated with greater axial elongation in crude structural equation model (β, −0.10; 95% CI, −0.18, −0.02) and when adjusted for sex, age, and height (β, −0.09; 95% CI, −0.17, −0.01) (Table 2, Supplementary Fig. S1). Thinner temporal choroidal thickness was significantly correlated with greater axial elongation in the crude linear regression model (β, −0.19; 95% CI, −0.36, −0.02) and when adjusted for sex, age, and height (β, −0.20; 95% CI, −0.37, −0.03) (Table 3, Fig. 2). Neither subfoveal nor nasal choroidal thickness was associated significantly with axial elongation (P > 0.05). In the sensitivity analysis (Supplementary Table S2, Supplementary Fig. S3), compared with students with the greatest temporal choroidal thickness, students with the thinnest had 34.75-µm greater axial elongation when adjusted for age and height (95% CI, 6.85, 62.66). Among the different quartile groups of subfoveal or nasal choroidal thickness, there was no significant difference in axial elongation (P > 0.05).
The latent retinal thickness at baseline, as measured by subfoveal, nasal, and temporal retinal thickness, was not significantly associated with axial elongation in crude structural equation modeling (β, −0.08; 95% CI, −0.18, 0.01) or when adjusted for sex, age, and height (β, −0.09; 95% CI, −0.19, 0.001) in myopic students (Table 2, Supplementary Fig. S1). Thinner temporal retinal thickness at baseline was significantly associated with greater axial elongation in the crude linear regression model (β, −0.89; 95% CI, −1.57, −0.22) and when adjusted for sex, age, and height (β, −0.99; 95% CI, −1.68, −0.30) (Table 3, Fig. 2). Subfoveal and nasal retinal thickness was not associated significantly with axial elongation (P > 0.05). In the sensitivity analysis (Supplementary Table S2, Supplementary Fig. S3), compared with students with the greatest temporal retinal thickness, students in the thinnest group had 46.44-µm greater axial elongation when adjusted for age and height (95% CI, 18.74, 74.14). Students in the second thinnest group had 29.67-µm greater axial elongation when adjusted for age and height (95% CI, 1.18, 58.15), and students in the third thinnest group had 43.35-µm greater axial elongation when adjusted for age and height (95% CI, 15.20, 71.50). Among the different quartile groups of nasal retinal thickness, there was no significant difference in axial elongation (P > 0.05).
Discussion
This prospective cohort study showed that a thinner temporal choroid at age 12 years was associated with greater subsequent 1-year axial elongation in myopic students, and a thinner temporal retina at age 12 years was associated with greater subsequent 1-year axial elongation in both non-myopic and myopic students.
The median axial length growth rate of 220 µm (IQR, 180) in the present study is markedly higher compared with previous studies. For example, the median 5-year increase in axial length for 714 Danish adolescents 11.5 years old was 248 µm (IQR, 225).17 The mean 18-month increase in axial length for 101 Australia children 13.1 ± 1.4 years old was 105 ± 155 µm,18 and the axial length growth rate of 2408 6-year-old Dutch children in a recent 3-year longitudinal study was 210 ± 80 µm/year.19 This difference may be due to differences in genetic composition, lifestyle patterns, a more competitive educational system in East and Southeast Asian countries,20 and higher myopia progression rates in Asian populations.21 In addition, the different axial elongation rates might be partly explained by the baseline characteristics of the children in this study, such as age, axial length (24.43 mm; IQR, 1.59), and SE (−2.07 D; IQR, 3.13) compared with 23.2 mm (IQR, 0.8) and 0.0 D (IQR, 0.6) for the 11.5-year-old Danish adolescents and 22.36 mm (IQR, 0.75) in the 6-year-old European children.17,19 As axial eye growth speed decreases with increasing age,19 the longer baseline axial length and greater myopic SE were predicators for larger subsequent eye length growth.17
Axial length increased more in boys than girls in this study. Wang and coworkers22 suggested that changes in axial length and height with age are concomitant, and children with earlier peak height velocity experience earlier peak SE and axial length velocity and age of myopia onset.23 Boys are usually taller, grow faster, and have larger eye size than girls.24 Sex and hormonal status have been indicated to influence choroidal blood flow25 and retinal thickness.26
In the present study, 1-year axial elongation increased by 0.20 µm for every decrease of 1 µm in temporal choroidal thickness in myopic students 12 years of age. Compared with students with the greatest temporal choroidal thickness, myopic students in the thinnest group had 34.75-µm greater axial elongation. Previous cross-sectional studies in children or young adults have found that subfoveal27,28 and parafoveal choroids27 are thinner in longer eyes, and it has been suggested that the thinning of ocular tissues is caused by axial eye elongation. A study in children 10 to 15 years of age indicated that the magnitude of difference in choroidal thickness associated with myopia appears greater than would be predicted by simple passive choroidal thinning with axial elongation.29 In addition, several longitudinal studies have demonstrated a thickening of choroid during the follow-up periods, with the subfoveal10,11,18 and parafoveal choroid10,18 thickening being less or thinning among children undergoing faster axial eye growth. A recent 1-year longitudinal study of 118 Chinese children 7 to 12 years of age found that increases in axial length are not related to decreases in subfoveal or parafoveal choroidal thickness.12 These findings suggest that reduced choroidal thickness may not be simply explained by the secondary stretching effects of eye elongation and support a potential role for the choroid in the mechanisms regulating eye growth in childhood. The 5-year longitudinal study in Danish adolescents found that a thin subfoveal choroid at age 11 years did not predict axial eye elongation from ages 11 to 16 years, although 5-year axial elongation increased by 0.17 µm for every decrease of 1 µm in subfoveal choroidal thickness at baseline when not adjusted in myopic students.17 Consistent with this finding, the present study also observed no association between subfoveal choroidal thickness and 1-year axial elongation in either non-myopic or myopic students, whereas 1-year axial elongation was found to increase by 0.20 µm for every decrease of 1 µm in temporal choroidal thickness in myopic students 12 years of age. It has been suggested that the choroidal changes may represent a balance between a thickening of the choroid related to normal ocular growth and a thinning of the choroid related to the rapid axial eye growth.18 This may explain why no association between baseline choroidal thickness and axial elongation was found in non-myopic students in this study. The association between thinner choroidal thickness and greater axial elongation found in this study may contribute to the control of excessive axial elongation, as axial elongation is accompanied by ocular complications.4
Animal studies of experimental myopia have discovered that transient alterations in choroidal thickness precede changes in axial length.30,31 The choroid has been implicated in the modulation of eye growth due to its rapid thinning in response to hyperopic defocus (image plane behind the retina), in order to adjust the position of the retina (axial length) to optimize image quality.32 Similarly, in human eyes, the choroid thins rapidly in response to hyperopic defocus imposed by a negative-powered lens33 or sustained accommodation.34 The choroid may have a direct influence on eye growth by secreting growth factors that act on the sclera.35 Or, it may act as a barrier to the diffusion of growth factors or signaling molecules from retina36 or as a mechanical buffer to expansion of the eye globe.37 Obviously, the exact role of the choroid in the control of eye growth is still a matter of speculation that should be investigated in more longitudinal studies.
Similar to this study, a previous study in a pediatric population reported that choroidal thickness was highest in the temporal choroid. Ruiz-Moreno and colleagues38 suggested that the higher metabolic needs of the foveal retina compared with the surrounding environment may cause a reduction in the temporal choroidal thickness while sparing the subfoveal choroid. The thinning of the choroid is most evident on the temporal side during myopia progression, possibly due to stretching of the temporal choroid and sclera.39 Jin and coworkers found that, after orthokeratology treatment, the increase in choroid thickness was greatest in the temporal zones.40 It has been hypothesized that the nonvascular smooth muscle cells and intrinsic choroidal neurons are involved in the modulation of choroidal thickness to stabilize the foveal position during accommodation.41 Because they are most numerous within the central–temporal quadrant of the choroid,42,43 the resultant choroidal thinning during accommodation would be most pronounced within the temporal region.6 Such observations suggest that temporal choroidal thickness may be more sensitive and may partly explain our finding that a thinner temporal choroid but not subfoveal choroid was associated with greater axial elongation.
In this study, 1-year axial elongation increased by 2.67 µm in non-myopic students and by 0.99 µm in myopic students for every decrease of 1 µm in temporal retinal thickness at baseline. Compared with students with the greatest temporal retinal thickness, non-myopic students had 71.92-µm and myopic students in the thinnest group had 46.44-µm greater axial elongation. Consistently, some cross-sectional studies have shown myopic axial elongation of the globe to be associated with thinning of the parafoveal retina, whereas central foveal retinal thickness was not found to decrease with longer axial length.14,44 Some other studies have reported that the retina was thicker in the central fovea but thinner in the parafoveal regions in participants with myopia.13,45 In contrast, several studies have found no relationship between subfoveal or parafoveal retinal thickness and axial length.15,16 Vincent and colleagues46 observed no significant differences between more and less myopic eyes for measures of retinal thickness at any locations. A 1-year longitudinal study in Chinese children found that the thickness of the retinal layers increased or was unchanged in most subfields during myopic shift, and the changes in the parafoveal or perifoveal retinal layers were not related to changes in axial length.12 Such inconsistency may be due to the population differences and different study designs. As the thinner temporal retina was associated with greater axial elongation in both non-myopic and myopic students in the present study, the retina might play some role in the axial elongation. Additional longitudinal studies on the mechanism would contribute to our ability to address ocular complications related to axial elongation.
Foveal retinal thickness did not decrease with longer axial length in previous studies, suggesting that there may be a mechanism to maintain the stability of foveal retinal thickness, which would be important for the development of normal visual function.44 This suggestion is in agreement with the finding that best-corrected visual acuity was independent of axial length in eyes without myopic retinopathy,47 which could explain our finding that thinner foveal retinal thickness was not associated with greater axial elongation in junior students. Studies have reported that the refractive state of the peripheral retina can affect eye growth.48,49 Also, it has been presumed that the equatorial to retroequatorial region is the sensory part of the mechanism for emmetropization.14 Thus, the temporal retina might play a bigger role in regulating axial growth than the subfoveal retina.
The strengths of this study include its school-based prospective cohort design and large sample size, which provided greater insights into axial elongation compared with cross-sectional studies. There are also some limitations of this study. First, the extremely narrow age range of the children might influence the generalization of our findings to other populations with different ages, although it allowed for robust analysis. Second, the follow-up duration was 1 year; thus, only short-term associations between choroidal and retinal thickness and axial elongation were observed. Longer follow-up is needed in future prospective cohort studies. Third, refractometry was performed in a non-cycloplegic state, which could have reduced the reliability of refractive error determination in the children because of accommodation, thus overestimating negative refractive power.50 Some of the students in the myopic group might have been emmetropic or hyperopic, which could limit the power to detect associations in the subgroup of myopic students, and such associations should be verified. The association between choroidal and retinal thickness and axial elongation in non-myopic students should be further studied. In addition, there is evidence that cycloplegia might influence choroidal thickness. One study found a decrease of choroidal thickness with cycloplegia,51 and another demonstrated an increase.52 Finally, no provision was made to perform the measurements of choroidal and retinal thickness at the same time of day for all participants. Diurnal variations in choroidal thickness have been reported, although retinal thickness was not found to exhibit significant diurnal variation in normal individuals.53 However, the time of day when such measurements are made should be taken into account in future studies.
Conclusions
A thinner temporal choroid may predict greater axial elongation over the subsequent year in myopic students, and a thinner temporal retina at 12 years of age may predict greater axial elongation in both non-myopic and myopic students. Subfoveal and nasal choroidal and retinal thickness was not associated with axial elongation in either non-myopic or myopic students. Knowledge regarding the mechanisms involved will lead to better management and control of axial elongation. Recognizing thinner temporal choroidal and retinal thickness as being a predictive factor for axial elongation could allow earlier identification of children at risk and who would benefit from potential preventive strategies.
Supplementary Material
Acknowledgments
The authors thank support and participation of the schools, the students, and their parents, and the contribution of ophthalmologists and optometrists.
Supported by a grant from the National Natural Science Foundation of China (81602909).
Disclosure: F. Tian, None; D. Zheng, None; J. Zhang, None; L. Liu, None; J. Duan, None; Y. Guo, None; Y. Wang, None; S. Wang, None; Y. Sang, None; X. Zhang, None; W. Cao, None; J. Zhang, None; M. Sun, None; Q. Tian, None; X. Meng, None; X. Guo, None; L. Wu, None
References
- 1. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology . 2016; 123(5): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 2. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet . 2012; 379(9827): 1739–1748. [DOI] [PubMed] [Google Scholar]
- 3. Ohno-Matsui K, Kawasaki R, Jonas JB, et al.. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol . 2015; 159(5): 877–883.e7. [DOI] [PubMed] [Google Scholar]
- 4. Ohno-Matsui K, Lai TY, Lai CC, Cheung CM.. Updates of pathologic myopia. Prog Retin Eye Res . 2016; 52: 156–187. [DOI] [PubMed] [Google Scholar]
- 5. Wei WB, Xu L, Jonas JB, et al.. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology . 2013; 120(1): 175–180. [DOI] [PubMed] [Google Scholar]
- 6. Woodman-Pieterse EC, Read SA, Collins MJ, Alonso-Caneiro D. Regional changes in choroidal thickness associated with accommodation. Invest Ophthalmol Vis Sci . 2015; 56(11): 6414–6422. [DOI] [PubMed] [Google Scholar]
- 7. Ulaganathan S, Read SA, Collins MJ, Vincent SJ.. Daily axial length and choroidal thickness variations in young adults: associations with light exposure and longitudinal axial length and choroid changes. Exp Eye Res . 2019; 189: 107850. [DOI] [PubMed] [Google Scholar]
- 8. Zhang JM, Wu JF, Chen JH, et al.. Macular choroidal thickness in children: the Shandong Children Eye Study. Invest Ophthalmol Vis Sci . 2015; 56(13): 7646–7652. [DOI] [PubMed] [Google Scholar]
- 9. Jin P, Zou H, Zhu J, et al.. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol . 2016; 168: 164–176. [DOI] [PubMed] [Google Scholar]
- 10. Hansen MH, Li XQ, Larsen M, et al.. Five-year change in choroidal thickness in relation to body development and axial eye elongation: the CCC2000 Eye Study. Invest Ophthalmol Vis Sci . 2019; 60(12): 3930–3936. [DOI] [PubMed] [Google Scholar]
- 11. Fontaine M, Gaucher D, Sauer A, Speeg-Schatz C.. Choroidal thickness and ametropia in children: a longitudinal study. Eur J Ophthalmol . 2017; 27(6): 730–734. [DOI] [PubMed] [Google Scholar]
- 12. Jin P, Zou H, Xu X, et al.. Longitudinal changes in choroidal and retinal thicknesses in children with myopic shift. Retina . 2019; 39(6): 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim MC, Hoh ST, Foster PJ, et al.. Use of optical coherence tomography to assess variations in macular retinal thickness in myopia. Invest Ophthalmol Vis Sci . 2005; 46(3): 974–978. [DOI] [PubMed] [Google Scholar]
- 14. Jonas JB, Xu L, Wei WB, et al.. Retinal thickness and axial length. Invest Ophthalmol Vis Sci . 2016; 57(4): 1791–1797. [DOI] [PubMed] [Google Scholar]
- 15. Ooto S, Hangai M, Tomidokoro A, et al.. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Invest Ophthalmol Vis Sci . 2011; 52(12): 8769–8779. [DOI] [PubMed] [Google Scholar]
- 16. Ooto S, Hangai M, Sakamoto A, et al.. Three-dimensional profile of macular retinal thickness in normal Japanese eyes. Invest Ophthalmol Vis Sci . 2010; 51(1): 465–473. [DOI] [PubMed] [Google Scholar]
- 17. Hansen MH, Kessel L, Li XQ, Skovgaard AM, Larsen M, Munch IC.. Axial length change and its relationship with baseline choroidal thickness - a five-year longitudinal study in Danish adolescents: the CCC2000 Eye Study. BMC Ophthalmol . 2020; 20(1): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ.. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci . 2015; 56(5): 3103–3112. [DOI] [PubMed] [Google Scholar]
- 19. Tideman JWL, Polling JR, Vingerling JR, et al.. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol . 2018; 96(3): 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgan IG, French AN, Ashby RS, et al.. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res . 2018; 62: 134–149. [DOI] [PubMed] [Google Scholar]
- 21. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL 3rd, Holden BA.. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci . 2012; 89(1): 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Ding X, Liu B, Zhang J, He M.. Longitudinal changes of axial length and height are associated and concomitant in children. Invest Ophthalmol Vis Sci . 2011; 52(11): 7949–7953. [DOI] [PubMed] [Google Scholar]
- 23. Yip VC, Pan CW, Lin XY, et al.. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci . 2012; 53(13): 7961–7966. [DOI] [PubMed] [Google Scholar]
- 24. Miglior S, Brigatti L, Velati P, et al.. Relationship between morphometric optic disc parameters, sex and axial length. Curr Eye Res . 1994; 13 (2): 119–124. [DOI] [PubMed] [Google Scholar]
- 25. Centofanti M, Bonini S, Manni G, Guinetti-Neuschüler C, Bucci MG, Harris A.. Do sex and hormonal status influence choroidal circulation? Br J Ophthalmol . 2000; 84(7): 786–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wexler A, Sand T, Elsås TB.. Macular thickness measurements in healthy Norwegian volunteers: an optical coherence tomography study. BMC Ophthalmol . 2010; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harb E, Hyman L, Gwiazda J, et al.. Choroidal thickness profiles in myopic eyes of young adults in the correction of myopia evaluation trial cohort. Am J Ophthalmol . 2015; 160(1): 62–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li XQ, Jeppesen P, Larsen M, Munch IC.. Subfoveal choroidal thickness in 1323 children aged 11 to 12 years and association with puberty: the Copenhagen Child Cohort 2000 Eye Study. Invest Ophthalmol Vis Sci . 2014; 55 (1): 550–555. [DOI] [PubMed] [Google Scholar]
- 29. Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci . 2013; 54(12): 7578–7586. [DOI] [PubMed] [Google Scholar]
- 30. Wallman J, Wildsoet C, Xu A, et al.. Moving the retina: choroidal modulation of refractive state. Vision Res . 1995; 35(1): 37–50. [DOI] [PubMed] [Google Scholar]
- 31. Wildsoet C, Wallman J.. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res . 1995; 35(9): 1175–1194. [DOI] [PubMed] [Google Scholar]
- 32. Hung LF, Wallman J, Smith EL 3rd. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci . 2000; 41(6): 1259–1269. [PubMed] [Google Scholar]
- 33. Chakraborty R, Read SA, Collins MJ.. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci . 2013; 90(11): 1187–1198. [DOI] [PubMed] [Google Scholar]
- 34. Woodman EC, Read SA, Collins MJ.. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vision Res . 2012; 72: 34–41. [DOI] [PubMed] [Google Scholar]
- 35. Summers JA. The choroid as a sclera growth regulator. Exp Eye Res . 2013; 114: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nickla DL, Wallman J.. The multifunctional choroid. Prog Retin Eye Res . 2010; 29(2): 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Alphen GW. Choroidal stress and emmetropization. Vision Res . 1986; 26(5): 723–734. [DOI] [PubMed] [Google Scholar]
- 38. Ruiz-Moreno JM, Flores-Moreno I, Lugo F, Ruiz-Medrano J, Montero JA, Akiba M.. Macular choroidal thickness in normal pediatric population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci . 2013; 54(1): 353–359. [DOI] [PubMed] [Google Scholar]
- 39. Lee K, Lee J, Lee CS, Park SY, Lee SC, Lee T.. Topographical variation of macular choroidal thickness with myopia. Acta Ophthalmol . 2015; 93(6): e469–e474. [DOI] [PubMed] [Google Scholar]
- 40. Jin WQ, Huang SH, Jiang J, Mao XJ, Shen MX, Lian Y.. Short term effect of choroid thickness in the horizontal meridian detected by spectral domain optical coherence tomography in myopic children after orthokeratology. Int J Ophthalmol . 2018; 11(6): 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schrödl F, De Laet A, Tassignon MJ, et al.. Intrinsic choroidal neurons in the human eye: projections, targets, and basic electrophysiological data. Invest Ophthalmol Vis Sci . 2003; 44(9): 3705–3712. [DOI] [PubMed] [Google Scholar]
- 42. May CA. Non-vascular smooth muscle cells in the human choroid: distribution, development and further characterization. J Anat . 2005; 207(4): 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flügel C, Tamm ER, Mayer B, Lütjen-Drecoll E. Species differences in choroidal vasodilative innervation: evidence for specific intrinsic nitrergic and VIP-positive neurons in the human eye. Invest Ophthalmol Vis Sci . 1994; 35(2): 592–599. [PubMed] [Google Scholar]
- 44. Yamashita T, Tanaka M, Kii Y, Nakao K, Sakamoto T.. Association between retinal thickness of 64 sectors in posterior pole determined by optical coherence tomography and axial length and body height. Invest Ophthalmol Vis Sci . 2013; 54(12): 7478–7482. [DOI] [PubMed] [Google Scholar]
- 45. Chen S, Wang B, Dong N, Ren X, Zhang T, Xiao L.. Macular measurements using spectral-domain optical coherence tomography in Chinese myopic children. Invest Ophthalmol Vis Sci . 2014; 55(11): 7410–7416. [DOI] [PubMed] [Google Scholar]
- 46. Vincent SJ, Collins MJ, Read SA, Carney LG.. Retinal and choroidal thickness in myopic anisometropia. Invest Ophthalmol Vis Sci . 2013; 54(4): 2445–2456. [DOI] [PubMed] [Google Scholar]
- 47. Yin G, Wang YX, Zheng ZY, Yang H, Xu L, Jonas JB.. Ocular axial length and its associations in Chinese: the Beijing Eye Study. PLoS One . 2012; 7(8): e43172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith EL 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH.. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci . 2010; 51(8): 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosén R, Lundström L, Unsbo P.. Influence of optical defocus on peripheral vision. Invest Ophthalmol Vis Sci . 2011; 52(1): 318–323. [DOI] [PubMed] [Google Scholar]
- 50. Sankaridurg P, He X, Naduvilath T, et al.. Comparison of noncycloplegic and cycloplegic autorefraction in categorizing refractive error data in children. Acta Ophthalmol . 2017; 95(7): e633–e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yuvacı İ, Pangal E, Yuvacı S, et al.. An evaluation of effects of different mydriatics on choroidal thickness by examining anterior chamber parameters: the Scheimpflug imaging and enhanced depth imaging-OCT study. J Ophthalmol . 2015; 2015: 981274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Öner V, Bulut A, Öter K.. The effect of topical anti-muscarinic agents on subfoveal choroidal thickness in healthy adults. Eye (Lond) . 2016; 30(7): 925–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tan CS, Ouyang Y, Ruiz H, Sadda SR.. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci . 2012; 53(1): 261–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.