Abstract
Purpose
To evaluate whether choroidal thickness (CT) using arm-mounted optical coherence tomography (OCT) in infants screened for retinopathy of prematurity (ROP) correlates with oxygen exposure in neonates.
Methods
OCT images were obtained in infants screened for ROP in a single level IV neonatal intensive care unit. CT was measured at three different locations: the subfoveal center and 1.5 mm from the fovea center in each direction. Correlation and regression analyses were performed to determine the relationship between clinical factors and CT. Clinical factors included gestational age, birth weight, presence of bronchopulmonary dysplasia (BPD), and fraction of inspired oxygen (FiO2) at defined time points: 30 weeks postmenstrual age (PMA), 36 weeks PMA, and on day of imaging.
Results
Mean subfoveal, nasal, and temporal choroidal thicknesses CT (SFCT, NCT, and TCT, respectively) were 228.0 ± 51.4 µm, 179.7 ± 50.3 µm, and 186.4 ± 43.8 µm, respectively. SFCT was found to be significantly thicker than NCT and TCT (P < 0.0001 and P = 0.0002, respectively), but no significant difference was found between NCT and TCT (P = 0.547). Compared with infants without BPD, infants with BPD had thinner SFCT and NCT (P = 0.01 and P = 0.0008, respectively). Birth weight was positively correlated with SFCT (r = 0.39, P = 0.01) and NCT (r = 0.33, P = 0.045) but not TCT. Gestational age and ROP stage were not significantly associated with CT. SFCT was found to be significantly thinner with higher average FiO2 supplementation levels at 30 weeks PMA (r = –0.51, P = 0.01) but not at 36 weeks PMA. Regression analysis revealed that FiO2 at 30 weeks PMA was an independent predictor of SFCT in infants screened for ROP (P = 0.01).
Conclusions
Early postnatal exposure (<32 weeks PMA) to higher oxygen supplementation in premature neonates statistically predicts choroidal thinning.
Keywords: retinopathy of prematurity, oxygen, choroidal thickness, bronchopulmonary dysplasia
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness and has become more prevalent worldwide.1 Advancements in neonatal care have increased the survival rate of prematurely born neonates and have also led to more ROP.1 In humans, ROP is thought to occur when the immaturely developed retinal vasculature is exposed to relative hyperoxia (relative to in utero oxygen concentrations), leading to downregulation of growth factors such as vascular endothelial growth factor (VEGF) which results in early ROP, characterized by delayed retinal physiologic vascular development; this generally occurs at <32 weeks postmenstrual age (PMA). This vascular attenuation, generally seen as clinical stage 1 or 2 ROP, then leads to local hypoxia, which results in local upregulation of VEGF and subsequent aberrant neovascularization seen in the higher stages of ROP. The mechanisms underlying ROP have been studied using animal models of oxygen-induced retinopathy (OIR), in which high oxygen exposure (75% oxygen) induces a vaso-obliterative phase and then return of the animals to room air (21% oxygen) results in a relative hypoxic phase, fueling vasoproliferation.2,3
Lower birth weight and younger gestational age at birth are the two best-described risk factors for the development of ROP, and both of those risk factors are also well known to play a significant role in the risk for developing bronchopulmonary dysplasia (BPD).4–7 BPD results from an aberrant response to antenatal and postnatal injury to the developing lungs of infants born extremely premature.8 The management of BPD postnatally includes measures such as oxygen provision and mechanical ventilation, and infants with BPD require these postnatal interventions to varying degrees, as the spectrum of BPD can range from mild to extremely severe. As such, infants with BPD are at increased risk for developing ROP and worse stages of ROP.9–12 Oxygen supplementation (oxygen concentration, duration, and fluctuation), which is life saving especially in the weeks after birth, has been identified as a risk factor for ROP, making continuous oxygen saturation monitoring with pulse oximetry crucial to titrate oxygen supplementation.7 Importantly, hyperoxia exposure can also have significant damaging effects on other vascular beds and organs in the developing neonate such as the lungs.13 Infants with less severe BPD typically have decreasing supplemental oxygen requirements with appropriate nutrition and growth as they get older.
Studies have shown the choroid to be thinner in eyes of premature infants, including those with ROP, and this may affect visual acuity.14 Given how prematurity affects both BPD and ROP risk, the purpose of this study was to explore the relationship between these clinical factors and choroidal thickness (CT). We hypothesized that decreased CT is correlated with clinical factors that proxy hyperoxia exposure.
Methods
Subjects
A prospective cohort study was performed at the Mattel Children's Hospital, University of California–Los Angeles, with institutional review board approval and parental consent. Infants were eligible for the study if undergoing ROP screening (i.e., <30 weeks gestational age, birth weight <1500 g, per 2013 AAPOS (American Association for Pediatric Ophthalmology and Strabismus)/American Academy of Ophthalmology guidelines,15 or if deemed as having clinical instability per the clinical care team) or if undergoing a sedated procedure in the neonatal intensive care unit. Exclusion criteria included genetic abnormalities or congenital infections known to affect eye structure or development. Infants who received ROP treatment before imaging were excluded.
Clinical Characteristics of the Study Infants
Demographic and clinical data were prospectively collected for each subject, including gestational age (GA) at birth, birth weight (BW), sex, race, PMA at the time of imaging, and the fraction of inspired oxygen (FiO2) at 30 weeks PMA, at 36 weeks PMA, and on the day of imaging. FiO2 was determined as the average supplemental oxygen needed over the 24-hour period on the day when the infant was 30 + 0 weeks PMA, 36 + 0 weeks PMA, and on the day of optical coherence tomography (OCT) imaging. Effective FiO2 was calculated for infants receiving non-invasive modes of respiratory support, as per published studies.16,17 Briefly, effective FiO2 is calculated based on weight and effective flow of non-invasive (non-intubated patients) respiratory support in liters per minute, as was defined in the Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP) trial.16,17 At higher flow rates, effectively received FiO2 is closer to the oxygen delivered (less oxygen lost to the ambient environment), and as weight increases effective FiO2 decreases (more oxygen lost to the ambient environment). FiO2 supplementation was adjusted (between 21% and 100% oxygen) by the clinical care team to maintain oxygen saturations, as measured by continuous pulse oximetry, between 90% and 95% per unit guidelines. Infants on room air were categorized as being on 21% oxygen. Neonatal outcomes included the presence of bronchopulmonary dysplasia, defined as the need for supplemental oxygen at 36 weeks PMA in preterm infants.18 Although newer proposed guidelines suggest incorporating both oxygen and need for respiratory support, as well as further development and utilization of severity scales, the traditional diagnosis of BPD as a yes-or-no need for supplemental oxygen at 36 weeks PMA was utilized in this study.8 We also collected each infant's stage of ROP. Indirect ophthalmoscopy for ROP screening was performed. ROP zone, stage, and presence or absence of plus disease were documented according to the International Classification of Retinopathy of Prematurity.15 Infants were divided into two groups by ROP stage (stage 0 vs. stage 1, 2, or 3) for data analysis.
Optical Coherence Tomography Imaging
Pupillary dilation was achieved with administration of cyclopentolate 0.1% and phenylephrine 2.5% ophthalmic solution 1 hour prior to examination. Oral feeds were held for 4 hours prior to dilation to prevent reflux from gastroparesis. Infants in the neonatal intensive care unit (NICU) were imaged without sedation. Subjects were placed in a supine position, immobilized using a blanket wrapped in a standard swaddle, and given 10% sucrose with a pacifier. Vital signs (heart rate and oxygen saturations) were continuously monitored using pulse oximetry. Tetracaine eye drops were administered for topical anesthesia. Infants who had been discharged from the NICU were imaged under general anesthesia prior to undergoing a laser procedure/examination for clinical care indications.
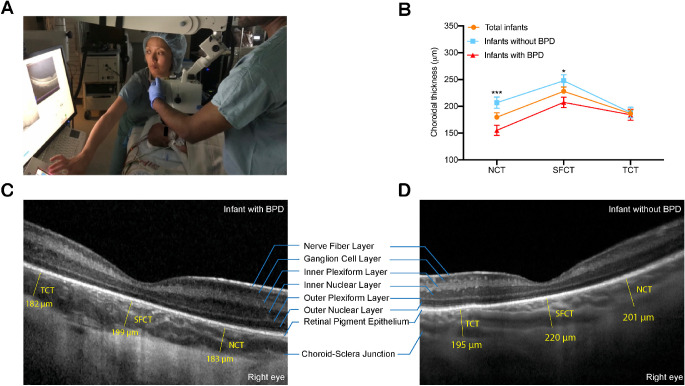
A disposable eyelid speculum was used to keep the eyelids retracted. Ocular lubrication was applied throughout the procedure to maintain a clear cornea. An arm-mounted Spectralis Flex Module (Heidelberg Engineering, Heidelberg, Germany) was used to obtain OCT images. The images were taken under the supervision of I.T. (ophthalmologist). At least two people were required to take images—one person to position the infant and camera in line with each other, and a second person to run the computer to capture the images (Fig. 1A). A seven-line scan 60 to 120 µm apart was taken through the macula to capture the fovea, defined as the most shallow point captured. A single foveal scan averaging up to 100 B-scans was obtained for better visualization of the subfoveal choroidal thickness (SFCT) in select patients who were able to tolerate further imaging.
Figure 1.
(A) OCT imaging was performed in supine pediatric patients during examination under anesthesia using the Heidelberg Spectralis Flex Module. (B) Mean choroidal thickness at the nasal (NCT), subfoveal (SFCT), and temporal (TCT) locations by BPD status. Infants with BPD had thinner SFCT and NCT when compared with infants without BPD (*P < 0.05 and ***P < 0.001, respectively). (C, D) Representative foveal B-scans of infants with and without BPD.
Image Analysis
Images were reviewed by two masked, independent graders (Y.H. and M.G.N.). A single B-scan through the foveal center, as defined by the foveal pit, was selected for analysis. When a foveal pit was absent, the foveal bouquet was used to determine the foveal center. CT was measured from Bruch's membrane to the inner part of the hyperreflective choroidoscleral interface. CT was measured at the subfoveal center (SFCT) and 1.5 mm from the fovea center in both directions to determine the nasal choroidal thickness (NCT) and temporal choroidal thickness (TCT) (Figs. 1C, 1D). Images in which the choroidoscleral interface could not be visualized were excluded from further analysis. Scan quality for all images was >15 dB.
Statistical Analyses
Statistical analyses were performed using SPSS Statistics (IBM, Armonk, NY, USA). Descriptive statistics are reported as mean ± SD. We randomly chose 20 cases to evaluate the intergrader reproducibility of CT measurements via Bland–Altman plots and intraclass correlation coefficients. Student's t-test was performed to compare SFCT in infants with BPD compared with those without BPD. To determine clinical predictors of CT, Pearson's or Spearman's correlation analysis was used to assess relationships between the risk factors of ROP and CT. The risk factors of ROP included GA, BW, and FiO2 at the defined time points. We then performed univariate regression of SFCT with the presence of BPD and significant factors in correlation analysis. Finally, we included parameters that were significantly associated with SFCT in univariate analysis in the multivariate regression analysis by stepwise selection to identify independent factors correlating with SFCT. The variance inflation factor (VIF) was used to assess the existence of collinearity among the variables in the multivariate regression model. VIF < 5 was defined as an acceptable degree of collinearity. Data were considered significant when P < 0.05.
Results
Infants’ Characteristics
Of the 25 infants initially enrolled, two eyes of two subjects were excluded due to images being captured after anti-VEGF intravitreal injection or laser treatment for ROP. Six eyes of five infants were excluded due to motion artifact, inability to find the foveal center, or inability to visualize the choroidoscleral junction at the nasal, subfoveal, and temporal locations. One baby only had one eye imaged. In total, 41 eyes of 22 infants were of sufficient quality to analyze CT. Of the 41 eyes included in analysis, three eyes were excluded from TCT analysis and two eyes were excluded from NCT analysis due to an inability to visualize the choroidoscleral junction.
The demographic and clinical characteristics of the included infants are shown in Table 1. The mean gestational age at birth was 28 weeks (range, 22–39), mean birth weight was 1281 g (range, 360–3501), and mean PMA at the time of imaging was 43 weeks (range, 32–79). Three infants did not meet ROP screening criteria. Of the 22 infants, 11 infants were diagnosed with BPD based on their need for supplemental oxygen at 36 weeks corrected GA. Eleven infants were diagnosed with ROP. Thirteen infants were receiving respiratory support at 30 weeks PMA, Eleven infants at 36 weeks PMA, and twelve infants on the day of imaging. Oxygen data were not available at all time points for 10 infants who were transferred to or from a different hospital before or after imaging. The range of supplemental oxygen required by infants in our cohort ranged from 21% to 81% (FiO2, 0.21–0.81). Infants with BPD were more likely to have stage 2 or 3 ROP than infants without BPD.
Table 1.
Demographic and Clinical Data (N = 22 Infants)
| Characteristic | |
|---|---|
| Male patients, n (%) | 12 (54.5) |
| Race, n (%) | |
| White | 10 (45.5) |
| Hispanic | 6 (27.3) |
| Other | 6 (27.3) |
| Gestational age (wk), mean ± SD (range) | 28.8 ± 5.2 (22–39) |
| Birth weight (g), mean ± SD (range) | 1281.5 ± 945.5 (360–3501) |
| PMA on day of imaging (wk), mean ± SD (range) | 43.1 ± 13.0 (32–79) |
| ROP stage (eyes), n | |
| Stage 0/vascularized | 21 |
| Stage 1 | 5 |
| Stage 2 | 7 |
| Stage 3 | 8 |
| Oxygen data | |
| Diagnosis of BPD, n (%) | 11 (50) |
| FiO2 at 30 wk (%), mean ± SD | 34.6 ± 12.8 |
| FiO2 at 36 wk (%), mean ± SD | 30.5 ± 18.3 |
| FiO2 at imaging (%), mean ± SD | 28.5 ± 14.6 |
In the total cohort, mean SFCT was significantly thicker than NCT (228.0 ± 51.35 µm vs. 179.71 ± 50.27 µm; P < 0.0001) and TCT (228.0 ± 51.35 µm vs. 186.4 ± 43.77 µm; P = 0.0002). NCT and TCT were not significantly different from one another (P = 0.620) (Fig. 1B).
Intergrader Reproducibility
Overall, we found excellent intergrader reproducibility of CT. The mean SFCT, NCT, and TCT had mean absolute differences of 9.88 µm, 15.29 µm, and 9.35 µm, respectively. The interclass correlations of SFCT, NCT, and TCT were 0.96 (95% confidence interval [CI], 0.88–0.99), 0.95 (95% CI, 0.85–0.98), and 0.94 (95% CI, 0.83–0.98), respectively.
Clinical Factors Associated With CT
Birth weight was positively correlated with SFCT (r = 0.39, P = 0.01) and NCT (r = 0.33, P = 0.045) but not TCT (P = 0.86) (Table 2, Fig. 2A). No significant relationship was found between gestational age at birth and ROP stage for SFCT, NCT, or TCT. Supplemental FiO2 at 30 weeks PMA was inversely correlated with SFCT (r = –0.51, P = 0.01) but not NCT and TCT (Table 2, Fig. 2B). However, neither FiO2 at 36 weeks postmenstrual age nor FiO2 at the day of imaging was correlated with SFCT, NCT, or TCT. Compared with infants without BPD, infants with BPD had significantly thinner SFCT (247.7 ± 51.62 µm vs. 207.5 ± 43.29 µm; P = 0.01) and NCT (206.8 ± 44.24 µm vs. 155.3 ± 42.96 µm; P = 0.0008) (Figs. 1B, 2C). Representative foveal B-scans of infants with and without BPD are shown in Figures 1C and 1D and in Supplementary Figure S1. FiO2 variability at 30 and 36 weeks PMA for all infants, infants with BPD, and infants without BPD is shown in Supplementary Figure S2.
Table 2.
Correlation Analysis for Clinical Features and Choroidal Thicknesses
| Variable | SFCT (r; P) | NCT (r; P) | TCT (r; P) |
|---|---|---|---|
| Gestational age* | 0.29; 0.07 | 0.29; 0.08 | –0.10; 0.57 |
| Birth weight* | 0.39; 0.01 | 0.33; 0.045 | –0.03; 0.86 |
| ROP stage* | –0.25; 0.12 | –0.25; 0.14 | 0.22; 0.19 |
| FiO2 (%) at 30 wk* | –0.51; 0.01 | –0.23; 0.30 | 0.18; 0.43 |
| FiO2 (%) at 36 wk† | –0.19; 0.31 | –0.09; 0.65 | 0.06; 0.77 |
| FiO2 (%) at imaging† | –0.19; 0.26 | –0.18; 0.32 | –0.09; 0.63 |
Pearson's correlation analysis was performed.
Spearman's correlation analysis was performed.
Figure 2.
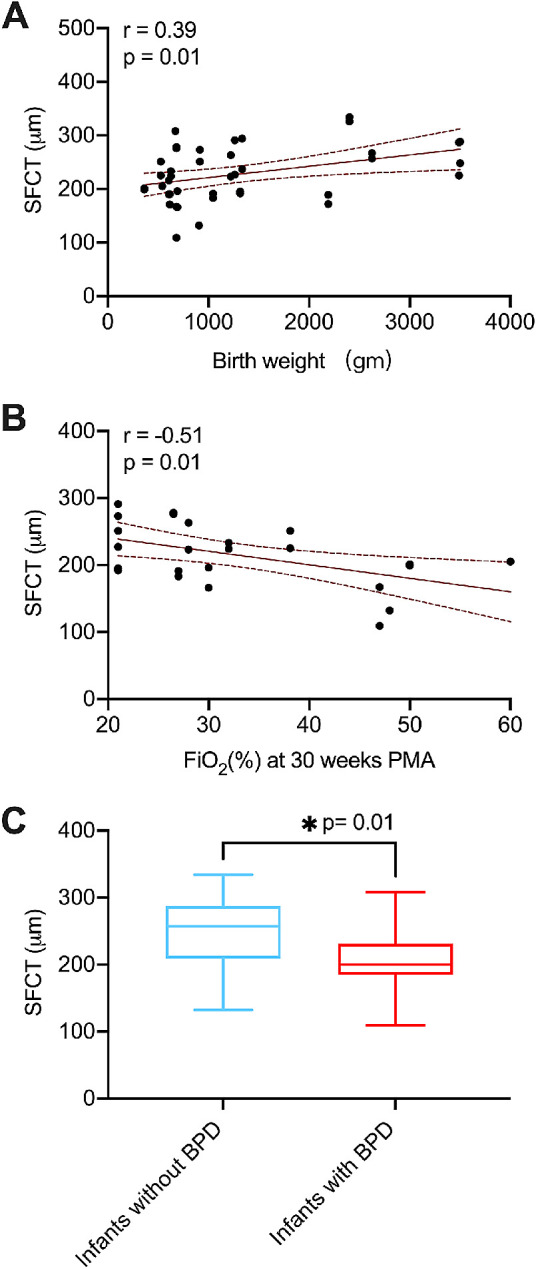
Clinical factors associated with SFCT. (A) Scatterplot shows correlation of SFCT thickness with birth weight and fraction of inspired oxygen (FiO2) at 30 weeks PMA. The curved dotted lines represent the 95% CIs for the trend line. SFCT was positively correlated with birth weight. (B) SFCT was inversely correlated with FiO2 at 30 weeks PMA. (C) Infants with BPD had thinner SFCT when compared with infants without BPD.
Regression Analysis for the Factors Associated With SFCT
Regression analysis was performed using the presence of BPD and the significant factors obtained from the correlation analysis to identify associated factors in SFCT (Table 3). The univariate regression analysis showed that birth weight was positively correlated with SFCT (β = 0.02, P = 0.01). In addition, infants diagnosed with BPD (β = –40.22, P = 0.01) and higher FiO2 requirement at 30 weeks postmenstrual age (β = –2.02, P = 0.01) were correlated with thinner SFCT. Multivariate regression analysis revealed that only FiO2 at 30 weeks postmenstrual age was an independent predictor of SFCT in infants screened for ROP (β = –2.02, P = 0.01). The VIFs of the multivariate regression model were less than 5, indicating an acceptable degree of collinearity between variables.
Table 3.
Univariate and Multivariate Analysis of SFCT
| Univariate Analysis | Multivariate Analysis* | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | |
| Birth weight | 0.02 | 0.01 to 0.04 | 0.01 | — | — | 0.08 |
| BPD | –40.22 | (–70.39 to –10.04) | 0.01 | — | — | 0.71 |
| FiO2 (%) | –2.02 | –3.54 to –0.50 | 0.01 | –2.02 | –3.534 to –0.50) | 0.01 |
By stepwise regression model.
Discussion
OCT imaging can be performed non-invasively in neonates, sometimes without the need for sedation. Moreno et al.19 demonstrated that the choroidoscleral junction can be visualized in 96% of premature infants imaged from 30 to 36 weeks PMA, and, in the same study, in 78% of premature infants imaged from 37 to 42 weeks PMA without the need for enhanced-depth imaging or swept-source OCT because immature retinal pigment epithelial cells allow improved visibility of the choroidoscleral junction. Although our study used spectral-domain OCT images obtained from an arm-mounted Spectralis Flex Module (Heidelberg Engineering), we were also able to successfully grade CT in 88% of infants from 32 to 79 weeks PMA.
The major finding in our study was the correlation of lower birth weight, presence of BPD (defined by exogenous oxygen use at 36 weeks), and higher FiO2 need at 30 weeks PMA with CT thinning. Multivariate regression analysis revealed that only FiO2 at 30 weeks PMA remained significant, implying that SFCT is most affected by early postnatal oxygen supplementation during the vascular attenuation phase, which generally occurs at <32 weeks PMA. Importantly, we separately considered the diagnosis of BPD and the discrete FiO2 need at 30 and 36 weeks PMA. The diagnosis of BPD implies a long-standing exposure to supplemental oxygen in preterm infants. In the context of our study, the diagnosis of BPD is associated with choroidal thinning. By considering FiO2 at discrete time points, we found that this relationship to CT thinning exists only at the earlier time point of 30 weeks PMA. We hypothesize that, just as retinal vessel growth is inhibited during this time of hyperoxia-induced vascular attenuation, a similar process is occurring in the choroid, causing thinner SFCT. This time period may also represent an important developmental window during which the choroid is sensitive to abnormal exposures. Animal studies in an OIR model of ROP have also demonstrated choroidal dysregulation and choroidal involution or thinning in young mice.20,21 However, there are important differences in animal models of OIR compared with human disease—notably, the levels of oxygen exposure (much higher oxygen levels are used in OIR models than in the typical preterm infant) and the timing and duration of oxygen exposure are different, which makes extrapolation from animal studies difficult.
Our study correlated birth weight to NCT and SFCT. Two other neonatal studies reported that SFCT was positively correlated with birth weight in infants screened for ROP imaged from 36 to 42 weeks PMA,22,23 whereas another demonstrated that in preterm infants imaged from 30 to 42 weeks PMA, CT was not correlated with birth weight.19 None of the prior studies reported on oxygen use or the potential association with oxygen exposure. The discrepancies among study findings may be due to variations in study populations, the use of different OCT devices, and/or the lack of oxygen consideration. Based on our findings, we recommend that oxygen exposure be considered as a variable that is associated with CT in infants; however, the mechanisms underlying the relationship between oxygen and CT remain unknown, as our study demonstrates an association, not causality. Oxygen-mediated effects on choroidal thinning may occur via the release of inflammatory factors such as interleukins, which have been shown to mediate neuroinflammation in neonates.24,25
Limitations of our study include a relatively small number of subjects and a wide range of PMAs at the time of imaging. However, strengths of the study include standardized NICU protocols on oxygen use, carefully acquired oxygen data across a range of PMAs spanning early and later stages of ROP, and high-quality images on an arm-mounted spectral-domain OCT device. Another limitation is that we did not have ocular biometry to standardize image size, so the nasal and temporal measurements may not be exactly at the same point.26
In summary, the current study demonstrated that SFCT is positively correlated with birth weight and inversely correlated with BPD and FiO2 need at early postnatal ages (30 weeks PMA) in premature infants. Most significantly, higher FiO2 exposure during early postnatal ages is a statistically independent predictor of thinner CT. Future work should consider the long-term implications of oxygen-related SFCT thinning on refractive error, visual function, and preventive measures to improve visual outcomes in premature infants.
Supplementary Material
Acknowledgments
Supported by an unrestricted grant from Research to Prevent Blindness to the Stein Eye Institute and the Norton Foundation.
Disclosure: Y. He, None; M. Pettenkofer, None; M.G. Nittala, None; S.R. Sadda, Heidelberg Engineering (F), Optos (F), Centervue (F), Carl Zeiss Meditec (R, S), Nidek (F, S), Topcon (F, S); I. Tsui, None; A. Chu, None
References
- 1. Shah PK, Prabhu V, Karandikar SS, et al.. Retinopathy of prematurity: past, present and future. World J Clin Pediatr. 2016; 5(1): 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartnett ME, Penn JS.. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012; 367(12): 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015; 122(1): 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SJ, Port AD, Swan R, et al.. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018; 63(5): 618–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ying GS, Bell EF, Donohue P, Tomlinson LA, Binenbaum G.. Perinatal risk factors for the retinopathy of prematurity in postnatal growth and ROP study. Ophthalmic Epidemiol. 2019; 26(4): 270–278. [DOI] [PubMed] [Google Scholar]
- 6. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med. 2017; 132: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hellström A, Smith LE, Dammann O.. Retinopathy of prematurity. Lancet. 2013; 382(9902): 1445–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thébaud B, Goss KN, Laughon M, et al.. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019; 5(1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang JW. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS One. 2019; 14(7): e0219934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giapros V, Drougia A, Asproudis I, Theocharis P, Andronikou S.. Low gestational age and chronic lung disease are synergistic risk factors for retinopathy of prematurity. Early Hum Dev. 2011; 87(10): 653–657. [DOI] [PubMed] [Google Scholar]
- 11. Singh JK, Wymore EM, Wagner BD, et al.. Relationship between severe bronchopulmonary dysplasia and severe retinopathy of prematurity in premature newborns. J AAPOS. 2019; 23(4): 209.e201–209.e204. [DOI] [PubMed] [Google Scholar]
- 12. Podraza W, Michalczuk B, Jezierska K, et al.. Correlation of retinopathy of prematurity with bronchopulmonary dysplasia. Open Med (Wars). 2018; 13: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kayton A, Timoney P, Vargo L, Perez JA.. A review of oxygen physiology and appropriate management of oxygen levels in premature neonates. Adv Neonatal Care. 2018; 18(2): 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu WC, Shih CP, Wang NK, et al.. Choroidal thickness in patients with a history of retinopathy of prematurity. JAMA Ophthalmol. 2013; 131(11): 1451–1458. [DOI] [PubMed] [Google Scholar]
- 15. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123(7): 991–999. [DOI] [PubMed] [Google Scholar]
- 16. STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000; 105(2): 295–310. [DOI] [PubMed] [Google Scholar]
- 17. Benaron DA, Benitz WE.. Maximizing the stability of oxygen delivered via nasal cannula. Arch Pediatr Adolesc Med. 1994; 148(3): 294–300. [DOI] [PubMed] [Google Scholar]
- 18. Baraldi E, Filippone M.. Chronic lung disease after premature birth. N Engl J Med. 2007; 357(19): 1946–1955. [DOI] [PubMed] [Google Scholar]
- 19. Moreno TA, O'Connell RV, Chiu SJ, et al.. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013; 54(6): 4140–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shao Z, Dorfman AL, Seshadri S, et al.. Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011; 52(9): 6238–6248. [DOI] [PubMed] [Google Scholar]
- 21. Kim Y, Hong HK, Park JR, et al.. Oxygen-induced retinopathy and choroidopathy: in vivo longitudinal observation of vascular changes using OCTA. Invest Ophthalmol Vis Sci. 2018; 59(10): 3932–3942. [DOI] [PubMed] [Google Scholar]
- 22. Erol MK, Coban DT, Ozdemir O, et al.. Choroidal thickness in infants with retinopathy of prematurity. Retina. 2016; 36(6): 1191–1198. [DOI] [PubMed] [Google Scholar]
- 23. Mangalesh S, McGeehan B, Tai V, et al.. Macular OCT characteristics at 36 weeks’ postmenstrual age in infants examined for retinopathy of prematurity. Ophthalmol Retina. 2021; 5(6): 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou TE, Rivera JC, Bhosle VK, et al.. Choroidal involution is associated with a progressive degeneration of the outer retinal function in a model of retinopathy of prematurity: early role for IL-1β. Am J Pathol. 2016; 186(12): 3100–3116. [DOI] [PubMed] [Google Scholar]
- 25. Omer M, Melo AM, Kelly L, et al.. Emerging role of the NLRP3 inflammasome and interleukin-1β in neonates. Neonatology. 2020; 117(5): 545–554. [DOI] [PubMed] [Google Scholar]
- 26. Llanas S, Linderman RE, Chen FK, Carroll J.. Assessing the use of incorrectly scaled optical coherence tomography angiography images in peer-reviewed studies: a systematic review. JAMA Ophthalmol. 2020; 138(1): 86–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.