Abstract
Objective
Carbonation as a sensory enhancement strategy for prevention of aspiration of thin liquids has not been thoroughly studied. The aim of our study was to examine the effect of carbonation on penetration–aspiration and pharyngeal residue in dysphagia patients using Fiber-Optic Endoscopic Evaluation of Swallowing (FEES) and to identify parameters associated with a response to carbonation.
Methods
A cross-sectional study of patients undergoing FEES in a dysphagia clinic. Patients were offered 100 cc of dyed water. Penetration–aspiration was scored using the penetration–aspiration scale (PAS). Residue was scored using the Yale Pharyngeal Residue Severity Rating Scale (YPR-SRS). Patients with a PAS ≥ 2 for water were subsequently offered 100 cc of carbonated water. PAS, YPR-SRS and residue clearance were compared between thin and carbonated liquids. Multivariate logistic regression analysis was used to identify predictors for good response to carbonation.
Results
84 patients were enrolled, 77.4% males, with diverse dysphagia etiologies (58.3% neurogenic, 11.9% radiation-induced, 23.8% deconditioning-induced, and 6% neck surgery induced). Median PAS was 7 (IQR 4–8) for thin liquids and 4.5 (IQR 2–8) for carbonated liquids (P = 0.0001). YPR-SRS was reduced for carbonated compared to thin liquids in the vallecula (1.58 ± 0.83 vs 1.76 ± 0.93, P = 0.001) and piriform sinuses (1.5 ± 0.87 vs 1.67 ± 0.9, P = 0.002). 31 patients had improvement in PAS with carbonation. Deconditioning as a dysphagia etiology was found to predict good response to carbonation on multivariate logistic regression analysis.
Conclusion
Carbonation may prevent aspiration and improve residue management for some patients with dysphagia for liquids.
Level of evidence
IV.
Keywords: Carbonation, Dysphagia, Deglutition, Aspiration, Residue, FEES
Sensory enhancement strategies (SES) are a known rehabilitation tool in dysphagia management [1]. The sensory input influences multiple neural pathways and modulates all phases of the swallowing reflex by effecting swallow initiation, timing and intensity [2–6]. SES can be achieved by thermal, tactile, gustatory and electrical stimulations [7–11]. These stimulations elicit wider recruitment of sensory receptive fields which in turn induce greater muscle recruitment and a stronger swallow [11].
Carbonation has been explored as a SES with effects on multiple sensory pathways, including mechanical perception of the CO2 bubbles, nociception and chemical perception of carbonic acid, a byproduct of CO2 metabolism [12–14]. Its palatability has made it an easily applicable SES in the clinical setting [9, 15–20]. Studies exploring the effects of carbonation on swallowing physiology have shown conflicting results, probably due to methodological variability [21]. Nonetheless, available data suggest that carbonation increases lingual-palatal pressure intensity and duration, reduces laryngeal elevation duration [22, 23], and modulates higher cortical swallowing behaviors [24, 25].
All clinical studies addressing the effects of carbonated liquids to date have utilized video-fluoroscopic swallow study (VFSS) in their methodology. However, barium does not share all the properties of thin liquids [17, 26, 27], and this is especially true for carbonated liquids, given the technical challenges of carbonizing barium [21]. This limitation can be overcome by evaluating effects of carbonized liquids on dysphagia using Fiberoptic Endoscopic Evaluation of Swallowing (FEES). However, to the best of our knowledge, no such studies were published.
Studies examining the effects of carbonation on dysphagia have shown that benefit from carbonation is not uniform for all patients [15–20]. Patients with neurogenic dysphagia are the main population which showed benefit from carbonation [17–20]. Only one study to date attempted to explore factors that can predict which patients will benefit from carbonation. Turkington et al. attempted to identify predictors for response to carbonation in a neurogenic dysphagia patient group and found no significant predicting factors [20]. We hypothesized that carbonation, being a SES, will be found most beneficiary in patients who have impairments in their sensory pathways. However, it is not clear exactly which elements of the aerodigestive tract are most excited by carbonation. While taste receptors exist mainly in the oral cavity, nociceptors and mechanoreceptors are abundant throughout the larynx and pharynx as well [28]. There are studies showing that the genetic tasting profile influenced response to chemotactic stimuli including carbonation [29, 30], suggesting the oral cavity is the main area that perceives carbonation. However, Turkington et al.’s study did not show patients’ genetic tasting profile predicted response to carbonation [20]. Whether impairment in laryngeal sensation effects response to carbonation has yet to be studied.
The aim of our study was to examine the effects of carbonation of thin liquids on penetration/aspiration, residue severity and residue clearance in patients with dysphagia and to identify clinical parameters associated with responses to carbonation using FEES.
We hypothesized that (1) carbonation will contribute to prevention of aspiration in patients with dysphagia and that (2) the effects of carbonation will be most prominent in populations with impaired laryngeal sensation.
Methods
This study was a cross-sectional study. Patients were recruited from the Sheba Medical Center Dysphagia Clinic during 2017–2018. Exclusion criteria were age < 18 years. The study was approved by the institutional review board. All patients signed an informed consent form. Data collected included age, sex, Functional Oral Intake Score [31], Eating Assessment Tool-10 (EAT-10) score [32] and etiology of dysphagia.
All patients underwent a full history, physical examination and a FEES. Laryngeal anatomical abnormalities, such as vocal cord paralysis and post-operative changes, were documented.
Dysphagia etiology was divided into 4 categories: (1) Neurogenic dysphagia, defined as dysphagia secondary to neurological insults, such as cerebrovascular accidents (CVA), traumatic brain injury, neuromuscular or neurodegenerative disorders; (2) dysphagia secondary to head and neck cancer and radiation, (3) dysphagia related to deconditioning, defined as dysphagia associated with reduced biological reserve and general deterioration without a direct insult to the swallowing mechanism [33]. Patients included in this group suffered from multiple and/or severe systemic morbidities, such as non-H&N oncological diseases, extensive surgery or prolonged intubation, frailty and old age, cognitive deterioration or prolonged immobility; (4) dysphagia related to non-oncologic surgery of the head and neck, such as vascular or orthopedic surgery.
A flexible digital video-rhinolaryngoscope (Pentax Fiber naso-pharyngo-laryngoscope FNL 15RP3, Japan, or Storz video-rhinolaryngoscope VP 11101, Germany) was passed through the most patent naris with administration of a trace amount of topical anesthetic (2% Lidocaine hydrochloride gel) to coat the laryngoscope, which has been shown not to significantly alter the FEES results [34, 35]. Laryngeal sensation was evaluated by gently touching both arytenoids up to two times and noting laryngeal adductor reflex (LAR) or cough. If no response was elicited after touching the arytenoids, the laryngoscope was advanced to lightly touch the true cords. A score of 1–3 was assigned to laryngeal sensation. Elicitation of the LAR with light touch of the arytenoids was scored 1, LAR elicitation with touch of the true cords was scored 2 and absence of LAR or cough after touching the true cords received a score of 3. A laryngeal sensation score of ≥ 2 was considered abnormal sensation.
Secretion stasis was scored using the Murray secretion scale (MSS) [36]. An MSS score of ≥ 1 was considered abnormal. Both laryngeal sensation and MSS were scored prior to initiation of bolus challenges.
Aspiration was evaluated using the Penetration–Aspiration Scale (PAS) [37]. The worst PAS observed during each liquid challenge was the PAS assigned to that challenge. PAS scores were categorized into normal/near normal (score 1 or 2), penetration (score 3–5), and aspiration (score 6–8).
Residue was scored using the Yale Pharyngeal Residue Severity Rating Scale (YPR-SRS) [38]. The worst YPR-SRS noted during the bolus challenge was the score assigned to that challenge. To represent the efficiency of the residue clearance, we used the previously published method [39, 40] of scoring the number of swallows the patient required to reach a 2 ≥ YPR-SRS in each site (vallecula/pyriform sinuses). If the patient reached the goal 2 ≥ YPR-SRS in 1–2 swallows, the bolus challenge received a score of 1, and if in 3–5 swallows, a score of 2. If more than 5 swallows were required to reach YPR-SRS ≥ 2, a score of 3 was given, and if the goal of 2 ≥ YPR-SRS was not reached despite multiple swallowing attempts, a score of 4 was given.
Each FEES was video-recorded and scored by two examiners simultaneously during or shortly after the completion of the FEES. The examiners included an Otolaryngologist and a speech language pathologist, both with over 5 years of experience in interpreting FEES. Any disagreement on the score for sensation, MSS, aspiration, residue severity or residue clearance was resolved by consensus after reviewing the FEES recordings.
Patients who were consuming thin liquids prior to FEES were presented with a 100 cc thin liquid in a cup as the first bolus challenge. If the PAS for thin liquids was 2 ≥, they were presented next with a 100 cc carbonated liquids in a cup as a second bolus challenge. Patients who were not consuming thin liquids prior to FEES were first presented with a purée consistency (½ and 1 tea spoon of puréed apples (IDDSI = 3), and only if they demonstrated safe swallows (PAS < 6) were then presented with thin liquids, followed by carbonated liquids. Patients who did not receive a thin liquid challenge were excluded from the study.
The thin liquids challenge was performed with filtered tap water colored with blue food dye in room temperature (approximately 23 °C). The carbonated liquids were made from water from the same tap which was inserted into a SodaStream© (SodaStrem International Ltd., Pepsico, Israel) machine set at maximum carbonation. Carbonated water was also dyed blue. Patients were offered the cups and were encouraged to drink as they liked, either continuous or single sips. Patients who demonstrated severe aspirations during the thin liquids challenge (PASthin ≥ 7) were instructed to stop drinking even if they did not completely empty the cup.
To explore for factors associated with response to carbonation, participants were classified as “responders” and “non-responders” based on their change in PAS with carbonation. There is debate in the literature on how to use and analyze the PAS to best represent clinical dysphagia [41, 42]. Categorization of PAS, as opposed to its use as an ordinal scale, makes less statistical assumptions on the scores of the PAS and may offer more clinically meaningful results, but this might come at the expense of sensitivity of detecting subtle differences between scores. We therefore utilized two forms of definition for response to carbonation. In the first, responders were defined as patients who demonstrated lower PAS with carbonation (PAScarb) compared to PAS for thin liquids (PASthin), i.e. PASthin > PAScarb. Non-responders were defined as patients demonstrating no change in PAS or worse PAS with carbonation (PASthin ≤ PAScarb). In the second definition method, responders were defined as a patient exhibiting improvement in PAS category. Non-responders were defined as patients showing no change in PAS category or had worsening of PAS category.
Statistical analysis was performed using SPSS version 21.1 (IBM, Armonk, NY, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC). Continuous variables were described as median and interquartile range or mean and standard deviation. Categorical variables were described using numbers and percentage. The differences of continuous variables on categorical variables were compared by Wilcoxon’s rank-sum test or Kruskal–Wallis test, and the association between categorical variables was evaluated by Fisher’s exact test. Paired continuous variables were compared using Wilcoxon’s signed rank test, and the agreement between paired categorical variables was compared using Stuart–Maxwell test or McNemar test, depending on the number of categories. Multivariate Logistic regression was performed to explore predictors for a dichotomized response to carbonation. All tests were two-tailed and a P value of 0.05 or less was considered statistically significant.
Results
Population
Eighty-seven patients were recruited to the study, 3 were excluded for aspiration of purée. Of the 84 patients remaining, 65 (77.4%) were males, with a mean age of 66.6 ± 13.7 years. Dysphagia etiology was diverse. Table 1 describes the characteristics of the study population. The group with deconditioning-induced dysphagia was significantly older than the other etiology groups (P = 0.02). There were no other significant differences between etiology groups for sex, FOIS, laryngeal sensation, MSS and EAT-10 scores.
Table 1.
Patient population characteristics
| No (%) | Age, years (mean ± SD) | Sex male (%) | FOIS (Median, IQR) (n = 84) |
Laryngeal anatomy [Normal] No (%) (n = 84) |
Laryngeal sensation No (%) (n = 83) |
MSS No (%) (n = 82) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | D | A | 0 | 1 | 2 | 3 | ||||||
| All patients | 84 (100%) | 66.6 ± 13.7 | 65 (77.4%) | 6 (5–6) | 70 (83.3) | 49 (59) | 30 (36.1) | 4 (4.8) | 15 (18.3) | 14 (17.1) | 23 (28) | 30 (36.6) |
| Neurogenic dysphagia | 49 (58.3%) | 64.2 ± 13.9 | 41 (83.7%) | 6 (5–7) | 44 (89.8) | 29 (59.2) | 17 (34.7) | 3 (6.1) | 7 (14.2) | 4 (8.2) | 13 (26.5) | 23 (46.9) |
| Head and neck oncology with radiation | 10 (11.9%) | 62.7 ± 13.6 | 8 (80%) | 5.5 (3.5–6) | 7 (70) | 5 (50) | 4 (40) | 1 (10) | 2 (20) | 4 (40) | 2 (20) | 2 (20) |
| Deconditioningb | 20 (23.8%) | 74.7 ± 11.2a | 12 (60%) | 6 (5–6) | 17 (85) | 11 (55) | 8 (40) | 0 (0) | 5 (25) | 5 (25) | 6 (30) | 4 (20) |
| Non-oncologic neck surgery | 5 (6%) | 66.6 ± 11.7 | 4 (80%) | 6 (6–6) | 2 (40) | 4 (80) | 1 (20) | 0 (0) | 1 (20) | 1 (20) | 2 (40) | 1 (20) |
MSS Murray secretion scale, 0 = no secretion, 1 = mild secretion not entering vestibule, 2 = moderate, secretions changing from 1 to 3 during exam, 3 = Severe secretions in the laryngeal vestibule
Laryngeal anatomy: normal or abnormal. Laryngeal sensation: N normal; D decreased; A absent; FOIS Functional Oral Intake Score; SD standard deviation
aAge in deconditioning group was significantly higher than other groups (P = 0.02, Kruskal–Wallis test)
bDeconditioning-induced dysphagia included the following pathologies: oncologic diseases other than H&N (n = 6), old age (n = 5), systemic diseases, such as COPD (n = 1), connective tissue disease (n = 1), severe myopathy (n = 1), mental deterioration due to severe depression or dementia (n = 3), prolonged intubation (n = 1) and extensive surgery, such as open heart cardiac surgery without recurrent laryngeal nerve injury (n = 2)
Carbonation effect on aspiration
Median PASthin was 7 (IQR 4–8) and PAScarb 4.5 (IQR 2–8). PASthin was significantly higher than PAScarb (P < 0.0002, Wilcoxon signed-rank test). Figure 1 presents the PASthin compared to PAScarb.
Fig. 1.
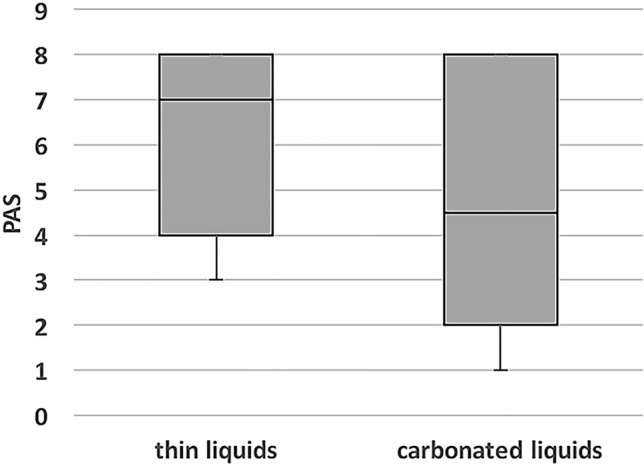
Comparison of Penetration–Aspiration Scale between thin and carbonated liquids. PAS Penetration–Aspiration Scale. Results are for the entire patient cohort (n = 84). P = 0.0002 (Wilcoxon rank test)
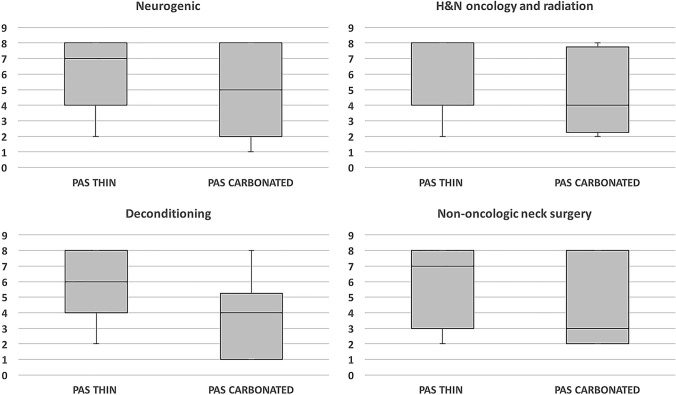
When comparing PAS of each dysphagia etiology, only patients with deconditioning showed significant reduction in PAS in response to carbonation. The neurogenic dysphagia group showed nearly significant reduction in PAS with carbonation. Patients with dysphagia secondary to H&N oncology and radiation or non-oncologic neck surgery did not show a significant reduction in PAS in response to carbonation. Table 2 and Fig. 2 present the PASthin and PAScarb for the different dysphagia etiology groups.
Table 2.
Change in PAS between thin liquids and carbonated liquids for different dysphagia etiologies
| PASthin | PAScarb | P-valuea | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| All patients | 7 (4–8) | 4.5 (2–8) | 0.0002 |
| Neurogenic dysphagia | 7 (4–8) | 5 (2–8) | 0.07 |
| Head and neck oncology with radiation | 4 (4–8) | 4 (2.2–7.8) | 0.10 |
| Deconditioning | 6 (4–8) | 4 (1–5.2) | 0.001 |
| Non-oncologic neck surgery | 7 (3–8) | 3 (2–8) | 0.66 |
PAS Penetration–Aspiration Scale; IQR interquartile range
aWilcoxon signed-rank test
Fig. 2.
Comparison of Penetration–Aspiration Scale between thin and carbonated liquids for different dysphagia etiologies. H&N Head and Neck; PAS Penetration–Aspiration Scale
Carbonation effect on pharyngeal residue severity and clearance
Mean YPR-SRS and clearance scores for the vallecula and pyriform sinuses were lower for carbonated compared to thin liquids (P = 0.002 and 0.003 for YPR-SRS and P = 0.02 and 0.008 for residue clearance scores, respectively, Wilcoxon signed-rank test). Analysis of each etiology group separately yielded significant reduction of YPR-SRS for the neurogenic dysphagia group in both the vallecula and piriform sinuses, and nearly significant reduction in piriform sinus residue severity for the deconditioning group. There was significant improvement in piriform sinus clearance scores for the deconditioning group and nearly significant improvement in vallecula clearance scores for the neurogenic group. Table 3 presents the comparison between residue severity and residue clearance scores between thin and carbonated liquids for the entire cohort and each etiology group.
Table 3.
Comparison of residue severity and clearance between thin and carbonated liquids
| Thin liquids | Carbonated liquids | |||||||
|---|---|---|---|---|---|---|---|---|
| Vallecula residue (n = 82) | Vallecula clearance (n = 71) | Piriform sinus residue (n = 82) | Piriform sinus clearance (n = 71) | Vallecula residue (n = 84) | Vallecula clearance (n = 72) | Piriform sinus residue (n = 83) | Piriform sinus clearance (n = 72) | |
| All patients | 1.77 ± 0.93a | 0.70 ± 0.79a | 1.67 ± 0.93a | 0.63 ± 0.77a | 1.58 ± 0.84a | 0.57 ± 0.70a | 1.52 ± 0.88a | 0.51 ± 0.78a |
| Neurogenic dysphagia | 1.8 ± 0.97a | 0.7 ± 0.79b | 1.72 ± 0.92a | 0.63 ± 0.67 | 1.55 ± 0.79a | 0.54 ± 0.59b | 1.58 ± 0.85a | 0.52 ± 0.67 |
| Head and neck oncology with radiation | 2 ± 1.05 | 0.7 ± 0.68 | 1.4 ± 0.7 | 0.33 ± 0.5 | 1.8 ± 1.14 | 0.56 ± 0.73 | 1.3 ± 0.67 | 0.22 ± 0.44 |
| Deconditioning | 1.7 ± 0.88 | 0.75 ± 1.0 | 1.5 ± 0.76b | 0.65 ± 1.0a | 1.6 ± 0.88 | 0.69 ± 1.0 | 1.35 ± 0.75b | 0.5 ± 1.0a |
| Non-oncologic neck surgery | 1.4 ± 0.55 | 0.6 ± 0.55 | 2.4 ± 1.67 | 1.2 ± 1.1 | 1.4 ± 0.55 | 0.6 ± 0.55 | 2 ± 1.73 | 1 ± 1.22 |
Values presented are mean ± standard deviation. Analysis performed with Wilcoxon signed-rank test
aP < 0.05
bP < 0.1
Response to carbonation
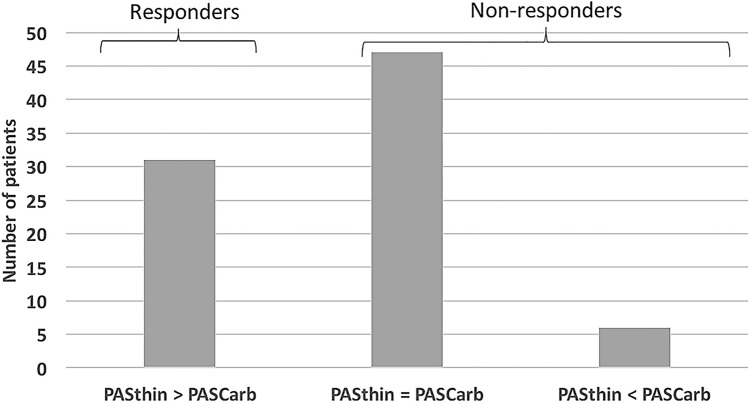
Applying the first definition for “responders”, 31 patients were “responders” (i.e. PASthin > PAScarb) and 53 were “non-responders” (6 patients with PASthin < PAScarb, and 47 with PASthin = PAScarb). Figure 3 presents the distribution of responses to carbonation in the study cohort.
Fig. 3.
Distribution of different responses to carbonation. PAS Penetration–Aspiration Scale. Carb Carbonated
There were significantly more subjects with deconditioning-induced dysphagia in the responders group (P = 0.03). There were no significant differences in the other characteristics compared between responders and non-responders, such as age, sex, laryngeal anatomy, laryngeal sensation, EAT-10, FOIS or MSS. Table 4 presents the characteristics of responders and non-responders. The mean age of the 6 patients with PASthin < PAScarb was 50.3 years. 5 of them had neurogenic causes for dysphagia (myotonic dystrophy, TBI, Parkinson’s disease and CVA) and one was recovering from a carotid body tumor resection with vagal paralysis.
Table 4.
Characteristics of responders and non-responders to carbonation
| Responders (N = 31) | Non-responders (N = 53) | P value | ||
|---|---|---|---|---|
| Age (mean ± STD) | 65.6 ± 14.2 | 67.3 ± 13.5 | 0.50b | |
| Sex (male) N (%) | 22 (70.9%) | 43 (81.1%) | 0.29a | |
| Etiology no. (%) | Neurogenic dysphagia |
14 (45.2% of responders) 28.6% of etiology group |
35 (58.5% of non-responders) 71.4% of etiology group |
0.03a |
| Head and neck oncology with radiation |
3 (9.7) 30% |
7 (13.2) 70% |
||
| Deconditioning |
13 (41.9) 65% |
7 (13.2) 35% |
||
| Non-oncologic neck surgery |
1 (3.2) 20% |
4 (7.5) 80% |
||
| FOIS | 6 (5–6.5) | 6 (5–6) | 0.84b | |
| EAT-10 (median, IQR) | 11 (9–27) | 16 (10–27) | 0.79b | |
| Laryngeal anatomy [normal] no. (%) | 25 (80.6) | 45 (84.9) | 0.76a | |
| Laryngeal sensation no. (%) | 1 | 17 (54.8) | 32 (60.3) | 0.63a |
| 2 | 13 (41.9) | 17 (32) | ||
| 3 | 1 (3.2) | 3 (5.6) | ||
| MSS | 0 | 5 (16.1) | 10 (18.8) | 0.88a |
| 1 | 5 (16.1) | 9 (16.9) | ||
| 2 | 10 (32.2) | 13 (24.5) | ||
| 3 | 10 (32.2) | 20 (37.7) |
N normal; PAS penetration aspiration scale; MSS Murray’s secretion scale; FOIS Functional Oral Intake Score. Etiology groups: (1) Neurogenic, (2) Head and Neck oncology- and radiation-related, (3) Deconditioning-related, (4) Non-oncologic neck surgery-related
aFisher’s exact test
bKruskal–Wallis test
When applying the second definition for “responders”, there were 22 responders and 62 non-responders. Of the latter, 4 patients changed PAS category for the worse.
We performed two multivariate logistic regression analyses in search of predictors for dichotomized response to carbonation, one for each definition of response to carbonation. Table 5 presents the results of the both logistic regression analyses. When utilizing the first definition of response to carbonation, age and deconditioning as a dysphagia etiology were found to be predictors for a favorable response to carbonation. Age as a predictive factor showed a CI reaching an OR of 1.00, and was therefore considered borderline significant. When utilizing the second definition, only deconditioning etiology was found to be a significant predictor for good response to carbonation.
Table 5.
Logistic regression analyses results for predictors to favorable response to carbonation
| OR 95% CI |
Responder: Penetration to normal, Aspiration to penetration/normal Non-responder: No change in category or penetration to aspiration, normal to penetration/aspiration |
Responder: PAScarb < PASthin Non-responder: PAScarb ≤ PASthin |
|---|---|---|
| Etiology 1 vs 2 |
0.54 (0.02, 4.80) |
1.05 (0.17, 5.55) |
| Etiology 1 vs 3 |
7.91a (1.68, 47.53) |
10.99a (2.57, 59.93) |
| Etiology 1 vs 4 |
1.00 (0.04, 11.14) |
0.53 (0.02, 5.11) |
| Age (years) |
0.98 (0.93, 1.02) |
0.96a (0.92, 1.00) |
| Sex |
0.90 (0.18, 4.04) |
1.31 (0.32,5.30) |
| Laryngeal anatomy (normal/abnormal) |
2.70 (0.51, 15.21) |
2.19 (0.46, 11.03) |
| Larynx sensation (2,3) |
1.16 (0.31, 4.28) |
1.74 (0.53, 5.95) |
|
3.23 (0.12, 45.67) |
1.80 (0.07, 20.67) |
|
| MSS (1,2,3) |
0.23 (0.01, 2.27) |
1.36 (0.21, 8.79) |
|
1.30 (0.23, 8.15) |
3.16 (0.59, 20.26) |
|
|
2.39 (0.45, 14.96) |
1.72 (0.34, 9.64) |
|
| FOIS |
1.00 (0.65, 1.56) |
1.09 (0.75, 1.64) |
| Constant |
0.61 (0.01, 28.81) |
1.40 (0.04, 53.10) |
| Observations | 81 | 81 |
PAS penetration aspiration scale; MSS Murray’s secretion scale; FOIS Functional Oral Intake Score. Etiology groups: (1) neurogenic, (2) head and neck oncology- and radiation-related, (3) deconditioning-related, (4) non-oncologic neck surgery-related. OR odds ratio; CI confidence interval
aP < 0.05
Discussion
Our study results support our first hypothesis that carbonation of thin liquids reduces aspiration and improves residue management. The novelty of our study lies with its FEES-based methodology and its large sample size, the largest in current literature so far. Our second hypothesis, that impaired laryngeal sensation will predict response to carbonation was disproved by the study results. Rather, the study showed an unexpected novel finding that dysphagia resulting from deconditioning is a predictive factor for a favorable response to carbonation.
The overall positive effect of carbonation on aspiration in our study is consistent with results from previous studies [16–20]. However, most of the available studies have focused on patients with neurogenic dysphagia [18–20]. In our study, neurogenic dysphagia patients showed significant improvement with residue severity and clearance but only near-significant improvement in aspiration prevention. The population which demonstrated a larger benefit from carbonation was the deconditioned group.
Deconditioning often occurs in frail patients facing acute morbidities which negatively impact their functional abilities, and challenge their depleted physiological reserves [43–47]. The underlying pathophysiology of dysphagia in deconditioned patients remains unclear. Decrease in muscle mass due to immobilization, sarcopenia, polypharmacy and disuse atrophy of pharyngeal musculature are some explanations offered as causes for dysphagia in this population [48–50]. The deconditioned group in our study was significantly older than other etiology groups, but otherwise did not show any significant differences regarding laryngeal sensation, secretion stasis, FOIS or EAT-10 results. One possible explanation for our results is that the deconditioned group has better preserved central sensory pathways, a key component involved in response to SES [11]. Deconditioned patients may be more sensitive to carbonation effects since their sensory pathways are intact, yet may be hypoactive, as opposed to neurogenic dysphagia patients who might suffer from central pathway deficits due to their underlying neuropathology. The hypoactive sensory pathways may respond more robustly to carbonation than the injured pathways. This hypothesis needs to be explored in future studies.
Literature pertaining to the effects of carbonation on H&N dysphagia patients is scarce and methodologically weak [16]. Our study results show that patients with H&N-related dysphagia, oncological and non-oncological, did not benefit from carbonation. Perhaps the mechanism of dysphagia in H&N patients is more mechanical in nature, due to radiation-induced fibrosis or post-operative anatomical deficits that are less improvable by SES. Moreover, mucosal scarring or post-radiation changes may render the mucosa less perceptive of carbonation, perhaps due to loss of noci- and chemoceptors in the tissue.
Literature suggests that carbonation enhances the swallowing process by exciting multiple sensory pathways, chemical, mechanical and nociceptive [12–14, 25, 51, 52]. Whether the oral and pharyngo-larygeal sensory pathways play an equal role in perception of carbonation is unclear. Our study results suggest that sensation in the larynx does not play a major role in response of carbonation. There are inherent limitations to testing laryngeal sensation during FEES, including debatable reliability of the “touch method” [53] and the fact that sensation, the afferent arm of the LAR, is inferred about by the function of the efferent arm of the reflex. Nonetheless, it is possible that the larynx is simply less sensitive to carbonation than the oral cavity, with reduced density of sensory receptors and nerve endings compared to the oral cavity [28].
Current literature examining the effects of carbonation on swallowing is based solely on VFSS [21]. Our study was the first to use a FEES-based methodology. This allowed for simulation of every-day drinking conditions, using the same liquids the patients consume daily, overcoming a major flaw of VFSS. Furthermore, liquid volume, temperature and drinking instructions, were also taken into consideration to simulate real-life conditions and challenge the effects of carbonation: A bolus volume four time larger than tested in the literature to date (100 cc) [17, 18, 22, 52, 54], since benefits of carbonation were shown to be reduced with larger volumes [18]; Bolus presentation at room temperature to isolate carbonation effects from the temperature effects on swallowing [22, 55] despite the fact that perception of carbonation is enhanced if the liquids are cold [56, 57]; and instructing patients to drink freely. Future studies employing FEES-based methodology are needed to optimize carbonated liquid presentation.
Our study has several limitations. First, since the patients were instructed to drink freely, sip sizes were not controlled for. Second, the H&N oncology and radiation as well as the non-oncologic surgery groups had a small sample size. Third, liquid presentation order was non-randomized leading to potential bias due to fatigue. Bias could also stem from the non-blinded rater methodology. Furthermore, inter- and intra-rater reliabilities were not calculated for this study, though all the scales that were utilized in this study are validated and reliable except for the residue clearance scale. Another possible bias stems from the possibility of the unknown pharyngeal wall-coating properties of carbonated liquids compared to thin liquids, which might alter the sensitivity of aspiration detection during FEES [58].
Conclusion
Carbonation aids in aspiration prevention and residue management in patients with dysphagia resulting from deconditioning and to a lesser degree for patients with neurogenic dysphagia.
Acknowledgements
We thank Ilan Brufman for his significant contribution to this manuscript.
Funding
The study did not receive funding.
Declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
The study took place in the Department of Otolaryngology Head and Neck Surgery in the Sheba Medical Center, Israel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robbins J, Butler SG, Daniels SK, Diez Gross R, Langmore S, Lazarus CL, Martin-Harris B, McCabe D, Musson N, Rosenbek J. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008;51(1):S276–S300. doi: 10.1044/1092-4388(2008/021). [DOI] [PubMed] [Google Scholar]
- 2.Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42:285–295. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hrycyshyn AW, Basmajian JV. Electromyography of the oral stage of swallowing in man. Am J Anat. 1972;133:333–340. doi: 10.1002/aja.1001330307. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Miyamoto T, Terao A, Yokoyama A. Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience. 2007;145:791–794. doi: 10.1016/j.neuroscience.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Doty R. Influence of stimulus pattern on reflex deglutition. Am J Physiol. 1951;166:142–155. doi: 10.1152/ajplegacy.1951.166.1.142. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Loomis CW, Bieger D. Vagal afferent input determines the volume dependence of rat esophageal motility patterns. Am J Physiol Gastrointest Liver Physiol. 2001;281:G44–G53. doi: 10.1152/ajpgi.2001.281.1.G44. [DOI] [PubMed] [Google Scholar]
- 7.Cola P, Gatto A, Da Silva R, Spadotto A, Schelp A, Henry M. The influence of sour taste and cold temperature in pharyngeal transit duration in pharyngeal transit duration in patients with stroke. Arq Gastroenterol. 2010;47:18–21. doi: 10.1590/S0004-28032010000100004. [DOI] [PubMed] [Google Scholar]
- 8.Logemann J. Preswallow sensory input: its potential importance to dysphagic patients and normal individuals. Dysphagia. 1996;11:9–10. doi: 10.1007/BF00385792. [DOI] [PubMed] [Google Scholar]
- 9.Miura Y, Morita Y, Koizumi H, Shingai T. Effects of taste solutions, carbonation, and cold stimulus on the power frequency content of swallowing submental surface electromyography. Chem Senses. 2009;34:325–331. doi: 10.1093/chemse/bjp005. [DOI] [PubMed] [Google Scholar]
- 10.Rofes L, Arreola V, Martin A, Clave P. Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut. 2013;62:1280–1287. doi: 10.1136/gutjnl-2011-300753. [DOI] [PubMed] [Google Scholar]
- 11.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25(4):323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrashekar J, Yarmolinsky D, Von Buchholtz L, et al. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarmolinsky D (2014) Mechanisms for taste sensation of carbonation [dissertation]. Columbia University, Columbia. Available from ProQuest LLC, Ann Arbor
- 14.Elshukri O (2012) The effects of carbonated fluids on the human cortical swallowing motor system [dissertation]. University of Manchester, Manchester. Available from https://www.escholar.manchester.ac.uk/uk-ac-man-scw:192229
- 15.Morishita M, Mori S, Yamagami S, Mizutani M. Effect of carbonated beverages on pharyngeal swallowing in young individuals and elderly inpatients. Dysphagia. 2014;29:213–222. doi: 10.1007/s00455-013-9493-6. [DOI] [PubMed] [Google Scholar]
- 16.Jennings K, Siroky D, Jackson C. Swallowing problems after excision of tumors of the skull base: diagnosis and management in 12 patients. Dysphagia. 1992;7:40–44. doi: 10.1007/BF02493420. [DOI] [PubMed] [Google Scholar]
- 17.Bülow M, Olsson R, Ekberg O. Videoradiographic analysis of how carbonated thin liquids and thickened liquids affect the physiology of swallowing in subjects with aspiration on thin liquids. Acta Radiol. 2003;44:366–372. doi: 10.1080/j.1600-0455.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 18.Sdravou K, Walshe M, Dagdielis L. Effects of carbonated liquids on oropharyngeal swallowing measures in people with neurogenic dysphagia. Dysphagia. 2011;27:1–11. doi: 10.1007/s00455-011-9359-8. [DOI] [PubMed] [Google Scholar]
- 19.Larsson V, Torisson G, Bülow M, Londos E. Effects of carbonated liquid on swallowing dysfunction in dementia with Lewy bodies and Parkinson’s disease dementia. Clin Interv Aging. 2017;12:1215–1222. doi: 10.2147/CIA.S140389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkington L, Ward EC, Farrell A, Porter L, Wall LR. Impact of carbonation on neurogenic dysphagia and an exploration of the clinical predictors of a response to carbonation. Int J Lang Commun Disord. 2019;54(3):499–513. doi: 10.1111/1460-6984.12458. [DOI] [PubMed] [Google Scholar]
- 21.Turkington L, Ward EC, Farrell A. Carbonation as a sensory enhancement strategy: a narrative synthesis of existing evidence. Disabil Rehabil. 2016;39(19):1958–1967. doi: 10.1080/09638288.2016.1213894. [DOI] [PubMed] [Google Scholar]
- 22.Krival K, Bates C. Effects of club soda and ginger brew on linguapalatal pressures in healthy swallowing. Dysphagia. 2011;27:228–239. doi: 10.1007/s00455-011-9358-9. [DOI] [PubMed] [Google Scholar]
- 23.Moritaka H, Kitadel M, Sawamura S, et al. Effect of carbon dioxide in carbonated drinks on linguapalatal swallowing pressure. Chem Senses. 2014;39:133–142. doi: 10.1093/chemse/bjt062. [DOI] [PubMed] [Google Scholar]
- 24.Michou E, Mastan A, Ahmed S, et al. Examining the role of carbonation and temperature on water swallowing performance: a swallowing reaction-time study. Chem Senses. 2012;61:799–807. doi: 10.1093/chemse/bjs061. [DOI] [PubMed] [Google Scholar]
- 25.Elshukri O, Michou E, Mentz H, et al. Brain and behavioral effects of swallowing carbonated water on the human pharyngeal motor system. J Appl Physiol. 2015;120:408–415. doi: 10.1152/japplphysiol.00653.2015. [DOI] [PubMed] [Google Scholar]
- 26.Fink TA, Ross JB. Are we testing a true thin liquid? Dysphagia. 2009;24:285–289. doi: 10.1007/s00455-008-9203-y. [DOI] [PubMed] [Google Scholar]
- 27.Dietsch A, Solomon N, Steele C, Pelletier C. The effect of barium on perceptions of taste intensity and palatability. Dysphagia. 2014;29:96–108. doi: 10.1007/s00455-013-9487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capra NF. Mechanisms of oral sensation. Dysphagia. 1995;10:235–247. doi: 10.1007/BF00431416. [DOI] [PubMed] [Google Scholar]
- 29.Plonk D, Butler SG, Grace-Martin K, et al. Effects of chemesthetic stimuli, age, and genetic taste groups on swallowing apnea duration. Otolaryngol Head Neck Surg. 2011;145:618–622. doi: 10.1177/0194599811407280. [DOI] [PubMed] [Google Scholar]
- 30.Todd JT, Butler SG, Plonk DP, et al. Effects of chemesthetic stimuli mixtures with barium on swallowing apnea duration. Laryngoscope. 2012;122:2248–2251. doi: 10.1002/lary.23511. [DOI] [PubMed] [Google Scholar]
- 31.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516–1520. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 32.Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the Eating Assessment Tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117(12):919–924. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 33.Jardine M, Miles A, Allen J. Dysphagia onset in older adults during unrelated hospital admission: quantitative videofluoroscopi measures. Geriatrics. 2018;3:66. doi: 10.3390/geriatrics3040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fife TA, Butler SG, Langmore SE, Lester S, Wright SC, Jr, Kemp S, Grace-Martin K, Lintzenich CR. Use of topical nasal anesthesia during flexible endoscopic evaluation of swallowing in dysphagic patients. Ann Otol Rhinol Laryngol. 2015;124(3):206–211. doi: 10.1177/0003489414550153. [DOI] [PubMed] [Google Scholar]
- 35.O'Dea MB, Langmore SE, Krisciunas GP, Walsh M, Zanchetti LL, Scheel R, McNally E, Kaneoka AS, Guarino AJ, Butler SG. Effect of lidocaine on swallowing during FEES in patients with dysphagia. Ann Otol Rhinol Laryngol. 2015;124(7):537–544. doi: 10.1177/0003489415570935. [DOI] [PubMed] [Google Scholar]
- 36.Murray J, Langmore SE, Ginsberg S, Dostie A. The significance of oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia. 1996;11:99–103. doi: 10.1007/BF00417898. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbek JC, Robbins JA, Roecker EB, Coyle JC, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 38.Neubauer PD, Rademaker AW, Leder SB. The Yale pharyngeal residue severity rating scale: an anatomically defined and image-based tool. Dysphagia. 2015;30(5):521–528. doi: 10.1007/s00455-015-9631-4. [DOI] [PubMed] [Google Scholar]
- 39.Shapira-Galitz Y, Shoffel-Havakuk H, Halperin D, Lahav Y. Association between laryngeal sensation, pre-swallow secretions and pharyngeal residue on fiberoptic endoscopic examination of swallowing. Dysphagia. 2019;34(4):548–555. doi: 10.1007/s00455-019-10001-4. [DOI] [PubMed] [Google Scholar]
- 40.Shapira-Galitz Y, Shoffel-Havakuk H, Halperin D, Lahav Y. Correlation between pharyngeal residue and aspiration in fiber-optic endoscopic evaluation of swallowing: an observational study. Arch Phys Med Rehabil. 2019;100(3):488–494. doi: 10.1016/j.apmr.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 41.Steele CM, Grace-Martin K. Reflections on clinical and statistical use of the penetration-aspiration scale. Dysphagia. 2017;32:601–616. doi: 10.1007/s00455-017-9809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borders JC, Brates D. Use of the penetration-aspiration scale in dysphagia research: a systematic review. Dysphagia. 2020;35:583–697. doi: 10.1007/s00455-019-10064-3. [DOI] [PubMed] [Google Scholar]
- 43.Hathaway B, Vaezi A, Egloff AM, Smith L, Wasserman-Wincko T, Johnson JT. Frailty measurements and dysphagia in the outpatient setting. Ann Otol Rhinol Laryngol. 2014;123(9):629–635. doi: 10.1177/0003489414528669. [DOI] [PubMed] [Google Scholar]
- 44.Fried LP, Walston JD, Ferrucci L. Frailty. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard’s geriatric medicine and gerontology. 6. New York, NY: McGraw-Hill; 2009. [Google Scholar]
- 45.Sanchez-Garcia S, García-Peña C, Salvà A, Sánchez-Arenas R, Granados-Garcia V, Cuadros-Moreno J, Velázquez-Olmedo LB, Cárdenas-Bahena Á. Frailty in community-dwelling older adults: association with adverse outcomes. Clin Interv Aging. 2017;12:1003–1011. doi: 10.2147/CIA.S139860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Incalzi RA, Capparella O, Gemma A, Landi F, Bruno E, Di Meo F, Carbonin P. The interaction between age and comorbidity contributes to predicting the mortality of geriatric patients in the acute-care hospital. J Intern Med. 1997;242:291–298. doi: 10.1046/j.1365-2796.1997.00132.x. [DOI] [PubMed] [Google Scholar]
- 47.Levers M, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs. 2006;56:282–291. doi: 10.1111/j.1365-2648.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- 48.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol Ser A Biol Sci Med Sci. 2008;63:1076–1081. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- 49.Maeda K, Koga T, Akagi J. Tentative nil per os leads to poor outcomes in older adults with aspiration pneumonia. Clin Nutr. 2016;35:1147–1152. doi: 10.1016/j.clnu.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Popman A, Richter M, Allen J, Wham C. High nutrition risk is associated with higher risk of dysphagia in advanced age adults newly admitted to hospital. Nutr Diet. 2018;75:52–58. doi: 10.1111/1747-0080.12385. [DOI] [PubMed] [Google Scholar]
- 51.Mastan A, Michou E, Mistry S, Elshukri O, Hamdy S. Evidence for an enhancing effect of carbonated liquids on complex human swallowing behavior. Gut. 2011;60:A162. doi: 10.1136/gut.2011.239301.343. [DOI] [Google Scholar]
- 52.Pelletier C, Steele C. Influence of the perceived taste intensity of chemesthetic stimuli on swallowing parameters given age and genetic taste differences in healthy adult woman. J Speech Hear Res. 2014;57:45–56. doi: 10.1044/1092-4388(2013/13-0005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneoka A, Pisegna JM, Krisciunas GP, Nito T, LaValley MP, Stepp CE, Langmore SE. Variability of the pressure measurements exerted by the tip of laryngoscope during laryngeal sensory testing: a clinical demonstration. Am J Speech Lang Pathol. 2017;26(3):729–736. doi: 10.1044/2017_AJSLP-16-0006. [DOI] [PubMed] [Google Scholar]
- 54.Ding R, Logemann JA, Larson CR, et al. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: a surface electromyographic study. J Speech Lang Hear Res. 2003;46:977–989. doi: 10.1044/1092-4388(2003/076). [DOI] [PubMed] [Google Scholar]
- 55.Selçuk B, Uysal H, Aydogdu I, Akyuz M, Ertekin C. Effect of temperature on electrophysiological parameters of swallowing. J Rehabil Res Dev. 2007;44(3):373–380. doi: 10.1682/JRRD.2006.08.0089. [DOI] [PubMed] [Google Scholar]
- 56.Yau NJ, McDaniel MR. The effect of temperature on carbonation perception. Chem Senses. 1991;16:337–348. doi: 10.1093/chemse/16.4.337. [DOI] [Google Scholar]
- 57.Michou E, Mastan A, Ahmed S, Mistry S, Hamdy S. Examining the role of carbonation and temperature on water swallowing performance: a swallowing reaction-time study. Chem Senses. 2012;61:799–807. doi: 10.1093/chemse/bjs061. [DOI] [PubMed] [Google Scholar]
- 58.Curtis JA, Skikaly ZN, Dakin AE, Troche MS (2020) Detection of aspiration, penetration and pharyngeal residue during flexible endoscopic evaluation of swallowing (FEES): Comparing the effects of color, coating and opacity. Dysphagia. 10.1007/s00455-020-10131-0 [DOI] [PubMed]