Abstract
Integrated, data-driven criteria are necessary to evaluate delivery outcomes in pregnancies affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the ongoing COVID-19 pandemic. This study analyzed maternal demographics, clinical characteristics, treatments, and delivery outcomes of 85 ethnically diverse, adult pregnant women who tested positive for SARS-CoV-2 at the time of delivery. Median maternal and gestational ages were 27 years (interquartile range [IQR]: 23–31) and 39 weeks (IQR: 37.3–40.0), respectively. Of the 85 SARS-CoV-2–positive participants, 67 (79%) had no COVID-19 symptoms at the time of routine COVID-19 admission testing, 14 (16%) reported mild COVID-19 symptoms, and 4 (5%) presented severe COVID-19 symptoms that required hospitalization. Patients in the severe COVID-19 group had significantly longer hospitalizations than those with nonsevere COVID-19 (7 [IQR: 4.5–9.5] vs 2 [IQR: 2–3] days; P<0.01). Neonatal outcomes included 100% live births with a median 1-minute Apgar score of 8 and 15% preterm births. No neonatal deaths or vertical transmissions were reported, and all neonatal intensive care unit admissions were related to prematurity. Overall, maternal symptom prevalence and peripartum complication rates were low, suggesting a generally good prognosis for pregnant women with SARS-CoV-2 infections at the time of delivery.
Keywords: COVID-19 symptoms, pregnancy, maternal health, neonatal outcomes, SARS-CoV-2
The potential risks of becoming pregnant during the ongoing COVID-19 pandemic are concerning, as pregnancy confers increased susceptibility to respiratory infectious diseases1–9 and pregnant research subjects were not included in COVID-19 vaccine trials.10,11 Although most human coronavirus infections are mild, the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) viral epidemics of the past two decades have been especially grave, with high maternal mortality rates.1,12 In a recent morbidity and mortality report by the Centers for Disease Control and Prevention on 326,335 women of reproductive age (15–44 years) with COVID-19, pregnant women reported similar or milder symptoms than nonpregnant counterparts. Nonetheless, pregnant women were found to be more likely to be hospitalized and at increased risk for intensive care unit admission and receipt of mechanical ventilation (1.5%) than nonpregnant ones (0.9%), with similar risk of mortality.12,13
The timing of infection is a key factor to consider in the clinical management of pregnancies affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the viral strain responsible for COVID-19. Given the low occurrence of vertical transmission reported in the literature,1,3,14–20 it becomes important to understand the characteristics and outcomes of maternal COVID-19 in terms of pregnancies at the time of delivery. These unique challenges call for integrated, data-driven criteria to understand susceptibility to severe illness and minimize potential complications in the peripartum period.
This study sought to investigate maternal clinical characteristics and pregnancy outcomes in pregnant women infected with SARS-CoV-2 at the time of delivery to help inform patient management challenges in real inpatient settings.
METHODS
All consecutive deliveries from adult pregnant women with a positive reverse transcriptase-polymerase chain reaction nasopharyngeal swab test result for SARS-CoV-2 admitted to any of 14 Advocate Aurora Health medical centers in Wisconsin and Illinois up to October 3, 2020, were included in this study. The first study participant to meet inclusion criteria was admitted on March 23, 2020.
The Advocate Aurora institutional review board approved the abstraction of data generated for routine clinical practice and waived the requirement for informed patient consent. A comprehensive retrospective review of maternal demographics, clinical characteristics, treatments, and delivery outcomes was performed, and participants were divided according to COVID-19 severity into 3 groups: asymptomatic (no COVID-19 symptoms reported), mild COVID-19 (symptoms not requiring hospitalization), and severe COVID-19 (symptoms requiring hospitalization).
Descriptive statistics were calculated and presented as median and interquartile range (IQR) for continuous variables and as percentage for categorical variables.
RESULTS
A total of 85 deliveries from adult, ethnically diverse, pregnant women who tested positive for SARS-CoV-2 at hospital admission were investigated. Median maternal and gestational ages were 27 years (IQR: 23–31) and 39 weeks (IQR: 37.3–40.0], respectively. Participant characteristics are summarized in Table 1.
Table 1.
Characteristics of Hospitalized SARS-CoV-2–Positive Pregnant Women (N=85) at Delivery
| Characteristic | n (%) or median [IQR] |
|---|---|
| Maternal age | 27 [23–31] years |
| Gestational age | 39 [37.3–40.0] weeks |
| Ethnicity | |
| Hispanic | 33 (39%) |
| Caucasian | 28 (33%) |
| Black | 17 (20%) |
| Other | 7 (8%) |
| Signs and symptoms | |
| Fever | 7 (8%) |
| Dyspnea | 5 (6%) |
| Cough | 5 (6%) |
| Loss of sense of taste or smell | 5 (6%) |
| Headache | 4 (5%) |
| Congestion and rhinorrhea | 3 (4%) |
| Fatigue | 2 (2%) |
| Sore throat | 2 (2%) |
| Sputum production | 1 (1%) |
| Nausea/vomiting | 1 (1%) |
| Diarrhea | 1 (1%) |
| Muscle aches | 0 (0%) |
| Comorbidities | |
| Asthma | 9 (11%) |
| Hypertension | 6 (7%) |
| Dyslipidemia | 2 (2%) |
| Diabetes | 2 (2%) |
| Coronary artery disease | 1 (1%) |
| Management | |
| Oxygen required | 6 (7%) |
| Ventilator use | 3 (4%) |
| Acute respiratory distress syndrome | 1 (1%) |
| Outcomes | |
| Hospital stay | 2 [2–3] days |
| Live births | n=92* |
| Preterm births | 13 (15%)* |
| 1-minute Apgar score | 8 [8–9] |
| Neonatal SARS-CoV-2 detected at 24 hours | 0 (0%) |
| Neonatal death | 0 (0%) |
7 sets of twins were delivered, resulting in 92 live births; preterm birth percentage was calculated from total of 85.
IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
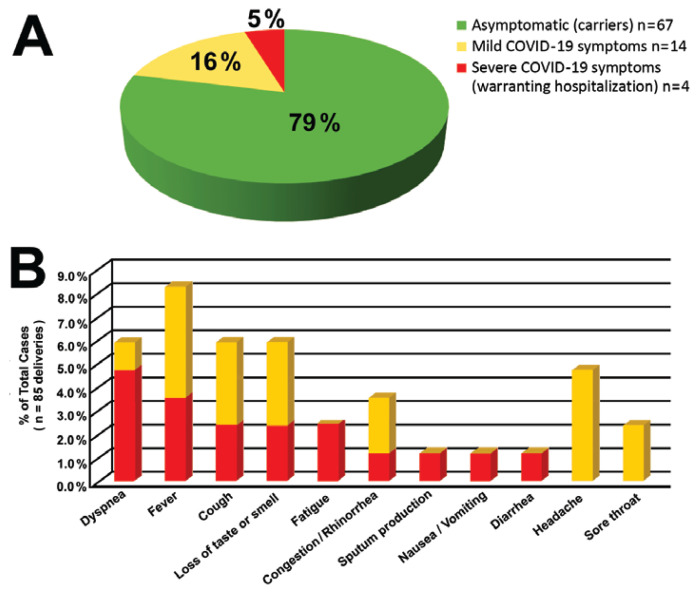
Overall, symptom prevalence was low, with fever being the most common COVID-19 symptom reported (8%), followed by dyspnea, cough, and loss of taste or smell (6% each). Maternal SARS-CoV-2–positive status was an incidental finding of routine COVID-19 admission testing in 79% of patients (asymptomatic group, n=67), mild COVID-19 symptoms were present in 16% of patients (mild COVID-19 group, n=14), and maternal COVID-19 symptoms were the primary reason for hospitalization in 5% of cases (severe COVID-19 group, n=4). Figure 1 illustrates symptom prevalence and distribution by group.
Figure 1.
Maternal symptoms in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive pregnancies at delivery. A: Patient distribution according to COVID-19 symptom severity. B: COVID-19 symptom prevalence in mildly and severely symptomatic patients.
Maternal comorbidity rates also were low, with few complications during hospitalization. Of 36 participants with baseline creatinine laboratory tests at admission, 4 asymptomatic patients and 2 patients with severe COVID-19 developed a creatinine increase of ≥25% during the course of the hospitalization, and 1 severe COVID-19 patient went on to develop acute respiratory distress syndrome necessitating mechanical ventilation. Overall median length of stay was 2 days [IQR: 2–3]. When compared using Wilcoxon rank-sum test, patients in the severe COVID-19 group were found to have had significantly longer hospitalizations than those with nonsevere COVID-19 (7 [IQR: 4.5–9.5] vs 2 [IQR: 2–3] days; P<0.01).
Neonatal outcomes included 100% live births with a median 1-minute Apgar score of 8 and 15% preterm births (ie, 13 of 85 total deliveries). Two deliveries (2.3% of the overall cohort of 85 and 50% of the severe COVID-19 group of 4) were induced in the 33rd week of pregnancy: one due to preeclampsia in a twin pregnancy and the other due to worsening of COVID-19 symptoms in a pregnancy complicated by asthma. Prematurity was the only indication for neonatal intensive care unit admission. All neonates tested negative for SARS-CoV-2 infection 24 hours after birth.
DISCUSSION
The vast health system interrogated for this study is uniquely positioned to report on large sample populations of COVID-19–positive pregnancies. National COVID-19 surveillance registries lack hospitalization data to distinguish admissions for COVID-19–related circumstances (eg, worsening respiratory status) from hospital admission for pregnancy-related treatment or procedures (eg, delivery). This limitation makes it very difficult to assess whether the outcomes reported are attributable to the pregnancy, the COVID-19 infection, both, or neither.
As clinical and epidemiological data become available, risk and management of COVID-19 pregnancies become increasingly well-informed.1,17,18,21–23 Since submission of our manuscript, several other descriptive reports on maternal SARS-CoV-2 and deliveries affected by COVID-19 in single U.S. health systems have been published.24–26 Consistent with our findings, these studies report largely asymptomatic COVID-19 presentation in this patient population and no increased feto-maternal risks compared to SARS-CoV-2–negative cohorts.
Our study timeframe captured the initial experience of COVID-19 in the midwestern United States. During the months of April and early May 2020, COVID-19 hospitalizations saw a positive trend that decreased and plateaued in July until the end of our study in October 2020. Given that all pregnant women were screened for SARS-CoV-2 at admission during this timeframe, we were able to determine that the vast majority (79%) of SARS-CoV-2 maternal infections at the time of delivery are asymptomatic and largely uncomplicated. Neonatal outcomes in our study were excellent; however, 2 of the 4 deliveries in pregnant women hospitalized for severe COVID-19 symptoms had to be induced prematurely.
Results from this retrospective study suggest overall good feto-maternal prognosis for pregnant women with SARS-CoV-2 infections at the time of delivery. Knowing that COVID-19 is unlikely to complicate deliveries endows physicians with data-driven criteria to prioritize minimizing the risk of contagion during lactation and neonatal care.
Patient-Friendly Recap.
To better inform care for women with peripartum COVID-19 and their newborns, the authors analyzed delivery outcomes for 85 adult women who tested positive for SARS-CoV-2 shortly prior to giving birth at a multihospital health system in the U.S. Midwest.
Most of the pregnant women testing positive for SARS-CoV-2 were asymptomatic, and none transmitted the virus to their babies.
While there were few maternal or neonatal complications overall, 2 of the 4 women with severe COVID-19 symptoms requiring hospitalization had to be induced prematurely.
Acknowledgments
The authors thank Jennifer Pfaff for editorial preparation of this brief report and Brian Schurrer for assistance with the figure.
Footnotes
Author Contributions
Study design: Zlochiver, Aziz, Jan. Data acquisition or analysis: all authors. Manuscript drafting: Zlochiver, Jan. Critical revision: Zlochiver, Aziz, Perez Moreno, Jan.
Conflict of interest
None.
References
- 1.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narang K, Enninga EAL, Gunaratne MD, et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95:1750–65. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynaecol Obstet. 2020;150:47–52. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrikopoulou M, Madden N, Wen T, et al. Symptoms and critical illness among obstetric patients with coronavirus disease 2019 (COVID-19) infection. Obstet Gynecol. 2020;136:291–9. doi: 10.1097/AOG.0000000000003996. [DOI] [PubMed] [Google Scholar]
- 5.Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2(2):100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro P, Matos AP, Werner H, Lopes FP, Tonni G, Araujo E., Júnior Covid-19 and pregnancy: an overview. Rev Bras Ginecol Obstet. 2020;42:420–6. doi: 10.1055/s-0040-1713408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertz D, Lo CK, Lytvyn L, Ortiz JR, Loeb M Flurisk-Investigators. Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC Infect Dis. 2019;19:683. doi: 10.1186/s12879-019-4318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panahi L, Amiri M, Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Arch Acad Emerg Med. 2020;8(1):e34. [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey PS, Ramin KD. Pneumonia in pregnancy. Obstet Gynecol Clin North Am. 2001;28:553–69. doi: 10.1016/s0889-8545(05)70217-5. [DOI] [PubMed] [Google Scholar]
- 10.Kaposy C, Lafferty L. Overcoming liability concerns in vaccine trials involving pregnant women. Account Res. 2012;19:156–74. doi: 10.1080/08989621.2012.678686. [DOI] [PubMed] [Google Scholar]
- 11.Brent RL. Risks and benefits of immunizing pregnant women: the risk of doing nothing. Reprod Toxicol. 2006;21:383–9. doi: 10.1016/j.reprotox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–75. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghema K, Lehlimi M, Toumi H, et al. Outcomes of newborns to mothers with COVID-19. Infect Dis Now. 2021;17(21):S2666–9919. 00065–8. doi: 10.1016/j.idnow.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37:861–5. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith V, Seo D, Warty R, et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15(6):e0234187. doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SH, Kang JM, Ahn JG. Clinical outcomes of 201 neonates born to mothers with COVID-19: a systematic review. Eur Rev Med Pharmacol Sci. 2020;24:7804–15. doi: 10.26355/eurrev_202007_22285. [DOI] [PubMed] [Google Scholar]
- 19.Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–64. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215:127–32. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 21.Khalil A, Kalafat E, Benlioglu C, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382:e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oltean I, Tran J, Lawrence S, et al. Impact of SARS-CoV-2 on the clinical outcomes and placental pathology of pregnant women and their infants: a systematic review. Heliyon. 2021;7(3):e06393. doi: 10.1016/j.heliyon.2021.e06393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Heyman T, Greechan M, et al. Maternal, neonatal and placental characteristics of SARS-CoV-2 positive mothers. J Matern Fetal Neonatal Med. 2021:1–9. doi: 10.1080/14767058.2021.1892637. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Bernstein P, Nair S, et al. A review of 92 obstetric patients with COVID-19 in the Bronx, New York and their peripartum anaesthetic management. Anaesthesiol Intensive Ther. 2021:1–11. doi: 10.5114/ait.2021.105120. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C, Andrusier M, Silver M, Applewhite L, Clare CA. Effect of SARS-CoV-2 infection on pregnancy outcomes in an inner-city black patient population. J Community Health. 2021:1–7. doi: 10.1007/s10900-021-00988-z. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]