Abstract
Peripheral artery disease (PAD) is characterized by impaired blood flow to the lower extremities, causing claudication and exercise intolerance. Exercise intolerance may result from reduced skeletal muscle capillary density and impaired muscle oxygen delivery. This cross-sectional study tested the hypothesis that capillary density is related to claudication times and anaerobic threshold (AT) in patients with PAD. A total of 37 patients with PAD and 29 control subjects performed cardiopulmonary exercise testing on a treadmill for AT and gastrocnemius muscle biopsies. Skeletal muscle capillary density was measured using immunofluorescence staining. PAD had decreased capillary density (278 ± 87 vs 331 ± 86 endothelial cells/mm2, p = 0.05), peak VO2 (15.7 ± 3.9 vs 24.3 ± 5.2 mL/kg/min, p ⩽ 0.001), and VO2 at AT (11.5 ± 2.6 vs 16.1 ± 2.8 mL/kg/min, p ⩽ 0.001) compared to control subjects. In patients with PAD, but not control subjects, capillary density was related to VO2 at AT (r = 0.343; p = 0.038), time to AT (r = 0.381; p = 0.020), and time after AT to test termination (r = 0.610; p ⩽ 0.001). Capillary density was also related to time to claudication (r = 0.332; p = 0.038) and time after claudication to test termination (r = 0.584; p ⩽ 0.001). In conclusion, relationships between capillary density, AT, and claudication symptoms indicate that, in PAD, exercise limitations are likely partially dependent on limited skeletal muscle capillary density and oxidative metabolism.
Keywords: anaerobic threshold, angiogenesis, capillary density, exercise, peripheral artery disease (PAD)
Introduction
Peripheral artery disease (PAD) affects approximately 8.5 million Americans and greater than 200 million people worldwide.1–4 The defining clinical characteristic of PAD is claudication, defined as a reproducible discomfort in skeletal muscle when walking. Because claudication is largely independent of limb hemodynamics,5–8 one explanation for the decreased exercise tolerance is low microvasculature blood flow in the skeletal muscle. There is reduced skeletal muscle capillary density in patients with PAD compared to control subjects.9–11 Furthermore, there is a positive relationship between capillary density and exercise tolerance in PAD, but not in control subjects.9,10 In addition, there is a positive relationship between capillary density and claudication onset time (COT) in PAD.9 Mechanistically, in patients with PAD, improvements in peak VO2 follow only after improvements in skeletal muscle capillary density.12 Taken together, these findings outline a growing acceptance that decreased skeletal muscle microvascularization is primarily responsible for exercise intolerance in PAD.
Most studies that have investigated exercise tolerance in PAD only measured peak walking time (PWT) and COT as functional outcome measures.13 By utilizing a cardiopulmonary exercise test (CPX), additional functional capacity measures such as peak VO2 and anaerobic threshold (AT) can be acquired. By definition, AT occurs when demand for energy metabolism is greater than supply, causing a change from aerobic to anaerobic metabolism. This results in compartmental pain and skeletal muscle fatigue. Oxygen consumption at AT depends on factors affecting oxygen delivery to the tissues. It is increased when oxygen flow is enhanced and decreased when oxygen flow is diminished.14 The reduction in skeletal muscle blood flow in PAD makes studying capillary density and AT attractive in this population. Although others have reported on AT in PAD, no study has examined the relationship between AT and capillary density. The primary hypothesis had two components: (1) capillary density relates to measures of AT in patients with PAD, but not in control subjects; (2) capillary density relates to measures of claudication in patients with PAD. Based on these hypotheses, the purpose of this cross-sectional study was to investigate the relationship between capillary density and functional capacity measurements in patients with PAD.
Methods
Subjects
Sixty-six subjects (29 control subjects and 37 patients with PAD) were recruited from the clinics and community at Duke University Medical Center. Recruitment was conducted from January 2004 to August 2013. All subjects were physically inactive at enrollment, defined as no physical activity outside of activities of daily living for the previous 3 months. Patients with PAD were selected based on both symptom-limiting claudication and an ankle–brachial index (lower leg ABI) ⩽ 0.90 at rest, or diagnostic angiographic evidence of PAD (50% occlusion of a conduit artery from a catheterization study). Patients were required to be on a stable indicated medical regimen of statin, anti-platelet, and anti-hypertensive medications, or to provide medical reasoning for a lack of indicated medications. Exclusion criteria included critical limb ischemia, severe peripheral neuropathy, diabetes mellitus, revascularization for PAD within 3 months of enrollment, unstable angina or severe coronary artery disease, or other conditions that would prohibit CPX testing. Control subjects, ages 40–75 years old, were recruited by newspaper advertisement and flyer postings. To document the absence of major chronic disease or risk factors, control subjects had an initial screening history and physical examination by a physician. All control subjects denied symptoms of claudication and were screened for undiagnosed PAD by lower leg ABI testing and a physical exam by a physician. All subjects were informed of testing protocols and the potential risks and benefits of participation. Each subject provided written informed consent before enrollment in the study. The Institutional Review Board of Duke University approved the research protocol.
Cardiopulmonary exercise testing (CPX)
Patients with PAD underwent a PAD-specific Gardner graded treadmill protocol (performed at a standard speed (2 miles/hour or 3.2 km/hour) with a 2% grade increase every 2 minutes)15 with a 12-lead electrocardiogram and expired gas analysis. All patients with PAD were encouraged to walk until claudication became intolerable. Control subjects were tested using a protocol of 2-minute stages, increasing the workload by approximately 1 metabolic equivalent (MET) per stage. Expired gases were analyzed continuously using a TrueMax 2400 ParvoMedics (Sandy, UT, USA) unit and averaged in 15-second intervals. All subjects were tested on the same metabolic cart with the same technicians. Peak VO2 (mL/kg/min) was measured in all patients. In addition, COT, time after claudication (time from COT to PWT), and PWT were recorded for patients with PAD.
Determination of anaerobic threshold (AT)
AT was determined using the V-slope method.16 Fifteen-second averages of VO2 and VCO2 were obtained from the metabolic cart. Two separate experienced readers were given, unidentified and in random order, the plots of VO2 versus VCO2 production for each CPX and asked to mark the point of AT. For a value to be considered valid, both readers had to agree within a variance of 150 mL/min of oxygen. If the two readers were in agreement, the values were averaged. If the two readers were not in agreement within 150 mL/min, a third experienced reader blindly read the plot. If no two of three readers agreed within 150 mL/min, the VO2 at AT was considered indeterminate. A third reader was required for seven plots (11%). Each of these were resolved by the third reader being in agreement with one of the two original readers within the parameter of 150 mL/min. In addition to reporting oxygen consumption at AT, time to AT and time after AT (time from AT to peak walking time) were recorded.
Skeletal muscle biopsy and histological analysis/indirect immunofluorescence
Skeletal muscle biopsies were taken from the medial aspect of the gastrocnemius muscle utilizing a modified Bergstrom needle technique.17 All PAD patient biopsies were performed on the leg that both had the lowest ABI and which caused exercise limiting claudication during the cardiopulmonary exercise test. Biopsies were performed approximately 1 week after the CPX test. A sample was embedded in cross-section using optical cutting temperature (OCT) tissue freezing medium (Tissue-Tek®; Sakura Finetek USA, Inc., Torrance, CA, USA), snap frozen in liquid nitrogen, and stored at −80°C for histological analyses.
Frozen muscle sections (7 μm thick) were cut using a Leica CM-1950 cryostat (Wetzlar, Germany) and placed on positively charged slides. The slides were stored at −80°C until needed. Sections were removed from the freezer and allowed to reach room temperature. Sections were fixed by immersion into 100% ice-cold acetone for 10 minutes, air dried for 10 minutes (room temperature), and then rehydrated in phosphate-buffered saline (PBS) for 5 minutes. Sections were blocked for 30 minutes with 10% normal goat serum in PBS containing 0.5% cold-water fish skin gelatin (Sigma, St. Louis, MO). To highlight endothelial cells (capillary density), the reagent mouse anti-human CD31 (clone 9G11, 20 μg/mL, catalog # BBA7; R&D, Inc., Minneapolis, MN, USA) was used, followed by the reagent goat anti-mouse Alexa-Fluor-488 (40 μg/mL, catalog # A31620; Invitrogen (ThermoFisher Scientific), Carlsbad, CA, USA) for fluorescence as instructed by the manufacturer and previously described.18 Hybridoma lines BA-D5 and SC-71 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA).19 Hybridomas were cultured and purified by the Lymphocyte Culture Center at the University of Virginia (Charlottesville, VA, USA). BA-D5 (8.9 μg/mL) and SC-71 (12.3 μg/mL) were co-incubated overnight at 4°C in blocking solution plus 5% normal mouse serum. Slides were washed twice in PBS and coverslips were applied using Prolong-Gold (Invitrogen (ThermoFisher Scientific)).
Images were captured using a Zeiss LSM 510-UV confocal microscope (Zeiss Microscopy, Oberkochen, Germany) at 100× magnification. A blinded observer analyzed the images using Image-Pro Plus 4.5.1. Capillary density for each sample was calculated by dividing the total number of CD31-positive capillaries by the muscle fiber area (mm2 of tissue, which was measured using Image-Pro Plus) per section. Examination of the capillaries-to-fiber ratio was performed on individual fibers that did not touch two pre-selected adjacent boundaries of the image and in which more than 75% of the circumference of the fiber was seen.
Statistical analysis
Differences in demographic and clinical characteristics between patients with PAD and control subjects were determined using a one-way ANOVA. For categorical variables, differences were determined by chi-squared analysis. Because age was different between groups, regression analyses for the priority measures of peak VO2, capillary density, and VO2 AT were performed controlling for age. Once determined different after controlling for age, bivariate correlations were performed to determine the relationships between capillary density and the functional variables for each group independently. Correlation coefficients for the two groups were compared and assessed for a significant difference using Fisher r-to-z transformations and the website http://vassarstats.net/rdiff.html. All tests were two-tailed; tabular data are presented as means ± SD; p ⩽ 0.05 was considered significant for all tests. Except for Fisher transformations, data analysis was performed using the Statistical Package for the Social Sciences (SPSS), Version 25 (IBM Corp., Armonk, NY, USA).
Results
Subject demographics
Table 1 shows subject demographics and clinical characteristics. Subjects were matched for race, sex, and body mass index between PAD and control groups. Patients with PAD were older than control subjects (68.8 ± 10.2 vs 52.8 ± 7.1, p ⩽ 0.001). Because age was different, an additional analysis was performed to determine if age correlated with capillary density. In an analysis of variance (ANOVA; regression analysis), capillary density was different between groups after controlling for age. Furthermore, correlations showed no relationship between capillary density and age when groups were combined or in either group separately (control: r = −0.291, p = 0.125; PAD: r = 0.151, p = 0.374). The PWT for control subjects was 623.3 seconds ± 201.3 and for PAD patients was 574.9 ± 284.8 seconds. We did not test differences in PWT between groups because different treadmill protocols were used.
Table 1.
Baseline demographics and clinical characteristics.
| Control (n = 29) | PAD (n = 37) | p-value | |
|---|---|---|---|
| Age, years | 52.8 ± 7.1 | 68.8 ± 10.2 | < 0.001 |
| Sex, male % | 31.0 | 51.4 | 0.100 |
| Weight, kg | 84.0 ± 18.2 | 80.5 ± 17.5 | 0.435 |
| Body mass index, kg/m2 | 29.5 ± 5.8 | 27.5 ± 4.8 | 0.150 |
| ABI | 1.06 ± 0.1 | 0.61 ± 0.1 | < 0.001 |
| Race, % | |||
| Non-Hispanic white | 65.5 | 73.0 | 0.516 |
| Black | 34.5 | 27.0 | 0.862 |
| Other | 0 | 0 | – |
| Risk factors and comorbid illnesses, % | |||
| Dyslipidemia | 13.8 | 67.6 | < 0.001 |
| Hypertension | 3.4 | 81.1 | < 0.001 |
| Obesity | 44.8 | 32.4 | 0.307 |
| Current or past smoker | 31.0 | 81.1 | < 0.001 |
| Coronary artery disease | 0 | 35.1 | < 0.001 |
| Cerebral vascular disease | 0 | 18.9 | 0.013 |
| Chronic obstructive pulmonary disease | 0 | 16.2 | 0.022 |
| Medications, % | |||
| Beta-blocker | 0 | 40.5 | < 0.001 |
| ACE inhibitor | 0 | 43.2 | < 0.001 |
| Statin | 10.3 | 67.6 | < 0.001 |
| Aspirin | 31.0 | 81.1 | < 0.001 |
| Diuretic | 3.4 | 37.8 | 0.001 |
| Platelet inhibitor clopidogrel | 0 | 35.1 | < 0.001 |
| Platelet inhibitor cilostazol | 0 | 5.4 | 0.201 |
Data given as mean ± SD or %.
ABI, ankle–brachial index; ACE, angiotensin-converting enzyme; PAD, peripheral artery disease.
Capillary density and cardiopulmonary exercise test measures between groups
Figure 1 depicts capillary density images of both a patient with PAD and a control subject. Without controlling for age, capillary density was lower in PAD compared to control subjects (p = 0.017). When compared to control subjects and controlling for age, patients with PAD maintained reduced capillary density by endothelial cells/mm2 (278.7 ± 87.5 vs 331.4 ± 86.4, p = 0.050). Although slightly higher in control subjects, no differences were detected between groups for the ratio of endothelial cells per fiber (2.2 ± 0.5 vs 2.0 ± 0.6, p = 0.669).
Figure 1.
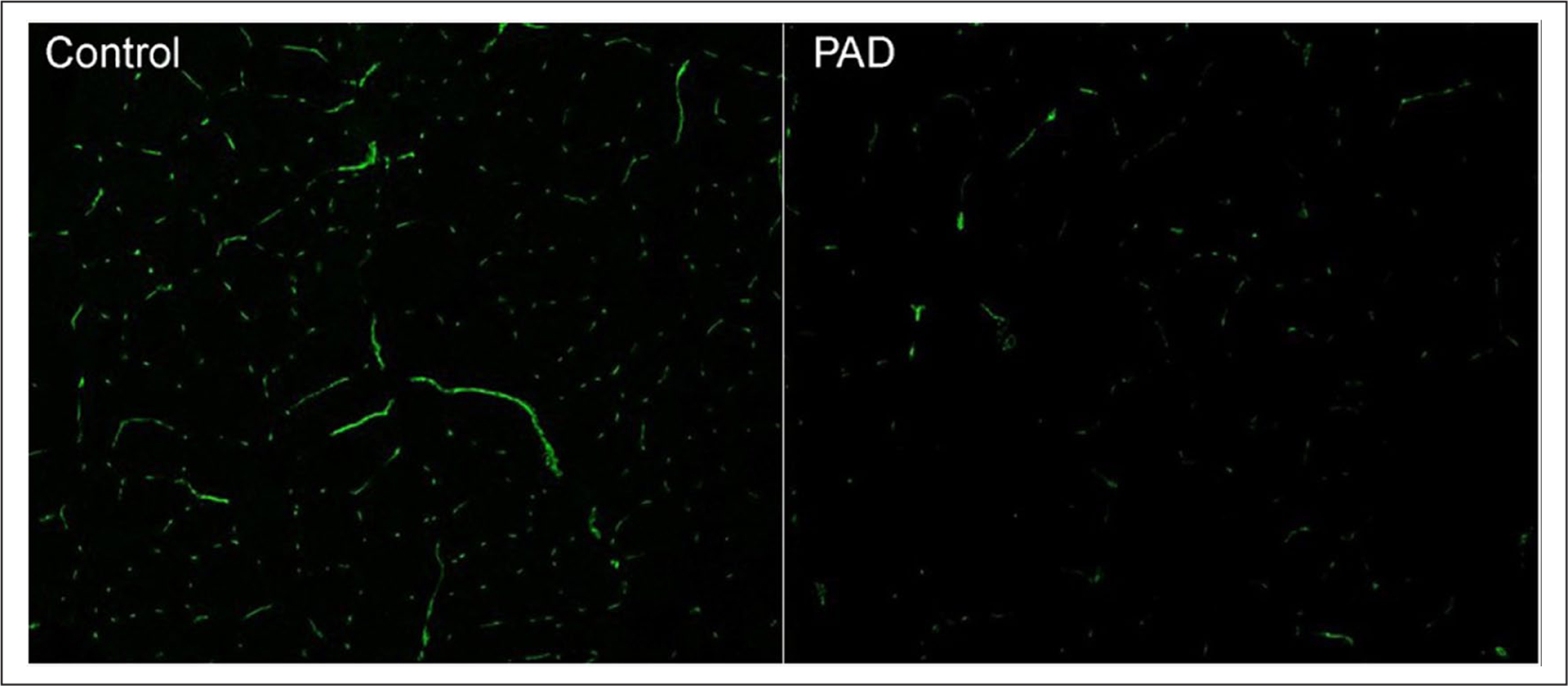
Representation of capillary density in a control subject and a patient with peripheral artery disease (PAD).
Endothelial cells (neon green) were identified by using reagents mouse anti-human CD31 (clone 9G11, 20 μg/mL [R&D, Inc.]) and goat anti-mouse Alexa-Fluor-488 (40 μg/mL [Invitrogen – ThermoFisher Scientific]) as instructed by the manufacturers.
After controlling for age, significant differences were also detected in peak VO2, percent predicted peak VO2, and VO2 at AT. These values are shown in Table 2. Although different treadmill protocols were used, peak walking times did not differ between control subjects and patients with PAD (623 ± 201 seconds for control subjects; 575 ± 284 seconds for PAD patients; p = 0.441).
Table 2.
Measures of oxygen consumption at peak exercise, anaerobic threshold, and COT in patients with PAD and control subjects.
| Control (n = 29) | PAD (n = 37) | p-value | |
|---|---|---|---|
| Peak VO2 (mL/kg/min) | 24.3 ± 5.2 | 15.7 ± 3.9 | < 0.001 |
| % Predicted peak VO2 | 106.0 ± 19.5 | 70.5 ± 17.4 | < 0.001 |
| VO2 at AT (mL/kg/min) | 16.1 ± 2.8 | 11.5 ± 2.6 | < 0.001 |
| % Peak VO2 at AT | 67.0 ± 8.1 | 73.3 ± 7.3 | 0.001 |
| % Peak heart rate at AT | 71.4 ± 6.4 | 83.1 ± 7.5 | < 0.001 |
| VO2 at COT (mL/kg/min) | – | 10.6 ± 2.5 | N/A |
| % Peak VO2 at COT | – | 67.7 ± 17.3 | N/A |
| Time to claudication (sec) | – | 215.1 ± 153.6 | N/A |
| Time after claudication (sec) | – | 359.9 ± 204.9 | N/A |
AT, anaerobic threshold; COT, claudication onset time; N/A, not applicable, PAD, peripheral artery disease; peak VO2, peak oxygen consumption.
Relationships between capillary density and cardiopulmonary exercise test measures between groups
For control subjects, there were no significant correlations between capillary density and cardiopulmonary exercise test measures (Figures 2 and 3). In contrast, in patients with PAD, significant correlations were detected at both maximal and submaximal measures. Figure 2 shows that in patients with PAD, capillary density was significantly related to peak VO2 (r = 0.567; p = 0.001) and peak walking time (r = 0.599; p ⩽ 0.001). Figure 3 shows PAD capillary density to be correlated with VO2 at AT (r = 0.343; p = 0.038), exercise time to AT (r = 0.381; p = 0.020), and exercise time after AT (r = 0.610; p ⩽ 0.001). Figures 2 and 3 overlay both control subjects and PAD patient relationships to demonstrate the discordant relationships. Relationships were significantly different between groups for capillary density versus peak VO2, p = 0.003; capillary density versus PWT, p = 0.011; and capillary density versus time after AT, p = 0.17. The relationships between capillary density versus VO2 at AT and time to AT both approached significance at p = 0.06.
Figure 2.
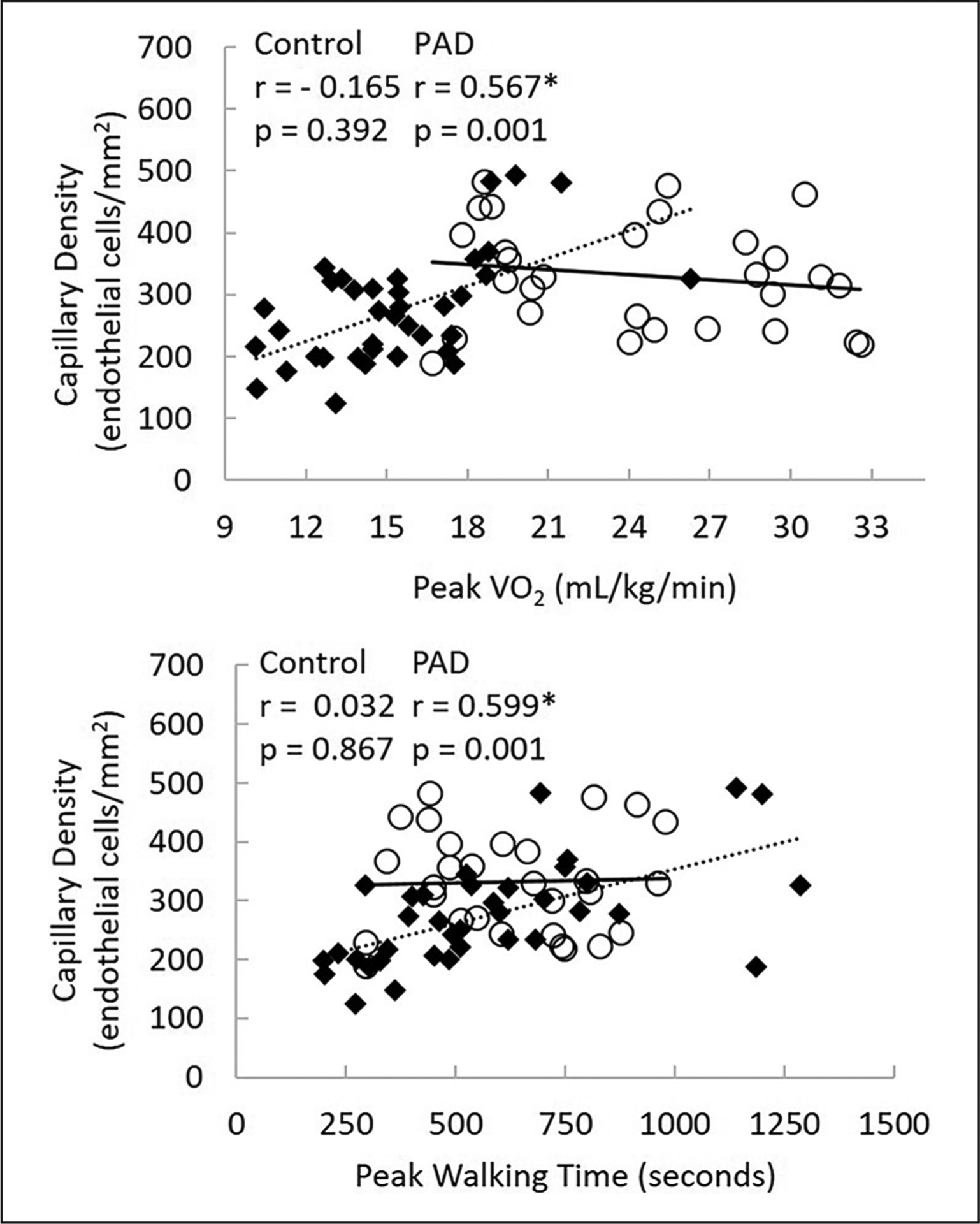
The relationship between capillary density and measures of peak exercise between groups.
○, Control subjects; ◆, patients with PAD; PAD, peripheral artery disease; peak VO2, peak oxygen consumption).
*Correlation coefficients are different between control subjects versus patients with PAD.
Figure 3.
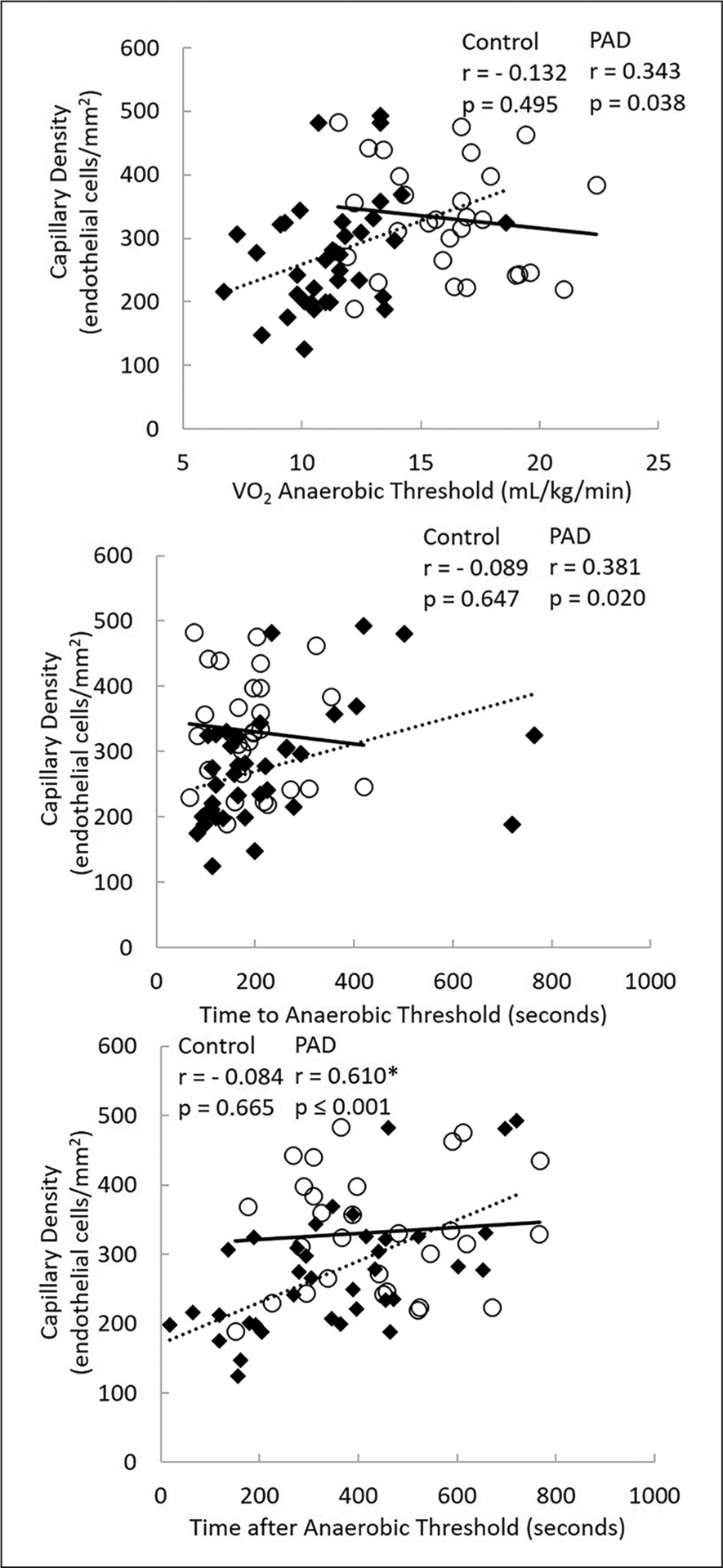
The relationship between capillary density and measures of anaerobic threshold between groups.
○, Control subjects; ◆, patients with PAD; PAD, peripheral artery disease; peak VO2, peak oxygen consumption).
*Correlation coefficients are different between control subjects versus patients with PAD.
The relationships of PAD capillary density and anaerobic threshold with claudication
Capillary density was significantly related to time to claudication (r = 0.332; p = 0.038) and time after claudication (r = 0.584; p ⩽ 0.001) (Figure 4). In addition, the VO2 at AT was related to the onset time of claudication (r = 0.407; p = 0.012) and the VO2 at claudication (r = 0.514; p = 0.001) (online Supplemental Figure 1). Last, the following were related: time to claudication, time to AT (r = 0.592; p ⩽ 0.001), time after claudication, and time after AT (r = 0.737; p ⩽ 0.001) (online Supplemental Figure 1).
Figure 4.
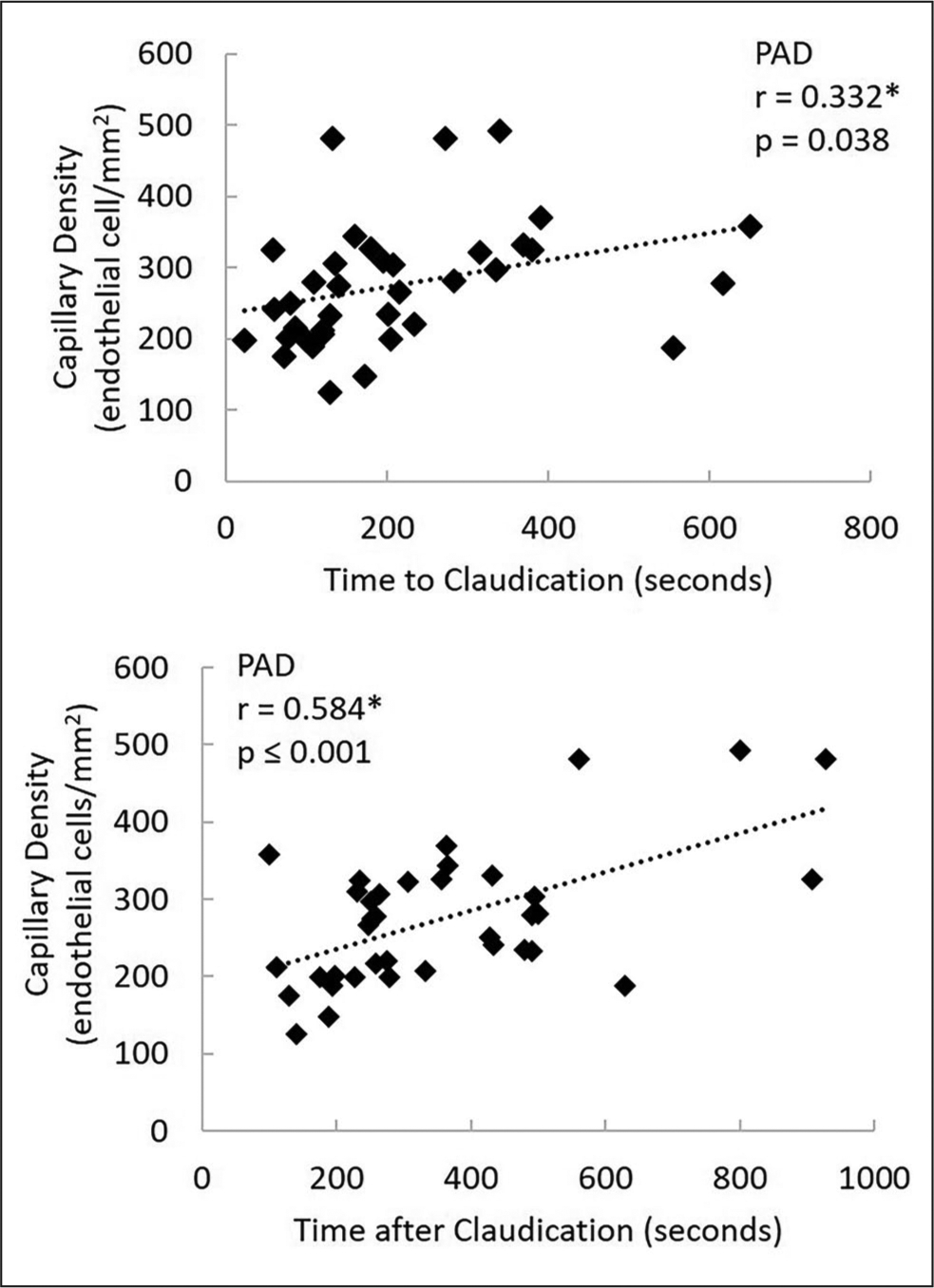
The relationship between capillary density and times before and after claudication in patients with peripheral artery disease (PAD).
*Significant correlation coefficient between measures, p ⩽ 0.05.
Discussion
To the best of our knowledge, no previous study has simultaneously measured skeletal muscle capillary density and multiple markers of exercise capacity from a CPX test (peak VO2, AT, and claudication) in both patients with PAD and control subjects. By comparing the relationships between these measures and groups, several interesting and novel findings extend current knowledge about exercise intolerance in PAD. In patients with PAD, capillary density was related to five measures of functional capacity, but not in the control group (Figures 2 and 3). Furthermore, capillary density was related to COT and time after claudication in patients with PAD (Figure 4). Last, there appears to be a relationship in both time and oxygen consumption in the presentation of symptoms and metabolic acidosis (online Supplemental Figure 1) in patients with PAD. Although the study design did not permit establishment of causal relationships, these findings add to the literature, suggesting capillary density of skeletal muscle may play a critical role in the functional capacity of PAD.
The current study also confirms earlier findings regarding a lack of relationship between capillary density and functional measures in control subjects. In fact, as shown in Figures 2 and 3, all correlation coefficients were at or near zero. Thus, although capillary density plays a key role in delivering microcirculation and removing waste in active muscles, there likely is no direct relationship between capillary density and exercise tolerance in control subjects. Recognition that capillary density and exercise tolerance are unrelated in healthy individuals emphasizes the contrasting physiology in patients with PAD.
Knowing from previous studies that capillary density is a primary determinant of maximal exercise (peak VO2 and peak walking time) in patients with PAD, but not in healthy subjects, we sought to examine possible capillary density implications to the submaximal index of AT. We reasoned that since increases of lactic acid play a role in muscle fatigue and pain, capillary density and AT would be related to claudication in patients with PAD. Several investigations describe AT measures in the PAD population.20–25 However, only one has reported a (positive) relationship between oxygen consumption at AT and initial claudication distance in patients with PAD.20 In the work of others, AT occurs at a greater relative workload in patients with PAD compared to control subjects; suggesting a metabolic abnormality in muscle oxygen kinetics during exercise.26,27
One of the primary findings of this study was that capillary density was related to oxygen consumption at AT, time to AT, and time after AT in patients with PAD, but not in control subjects. Confirming evidence is provided by the finding that capillary density was also related to COT and time after claudication. These data provide additional evidence that capillary density likely plays a primary role in AT and symptoms of claudication. If reduced capillary density drives early AT and the development of claudication symptoms, this should be reflected by temporal relationships between oxygen consumption at AT and claudication exercise times. During an acute bout of exercise, oxygen consumption at claudication onset is very similar to oxygen consumption at AT in the majority of patients with PAD.22 Online Supplemental Figure 1 supports this hypothesis by showing significantly correlated values. The mean time patients reported symptoms of claudication were slightly before the mean time that gas analysis of AT detection occurred (215 vs 224 seconds). Similarly, the corresponding oxygen consumption at the onset of claudication occurred slightly before the oxygen consumption of AT (10.6 vs 11.2 mL/kg/min). We believe this slight delay may represent the time needed to detect AT through ventilatory systems, thereby reflecting the gap in time between the physical lactate accumulation at the muscle cell level and the ability of ventilator detection of AT by metabolic gas analysis.
Recent studies, using contrast-enhanced ultrasound perfusion imaging of the calf before and after exercise, demonstrate patients with PAD have similar microvascular blood flow at rest, but are unable to increase perfusion in response to exercise, whereas controls increased perfusion significantly.28,29 Our data support the hypothesis that decreased capillary density of the working muscle contributes to attenuated perfusion, leading to early anaerobic metabolism and claudication. Future confirming investigations where both contrast-enhanced ultrasound and capillary density are measured are needed. In order to interpret conduit blood flow versus microvasculature on exercise tolerance, we analyzed correlations between ABI (marker of conduit blood flow) and capillary density (a marker of microvasculature) to exercise measurements. ABI was not related to any measurements investigated in Figures 2–4 and online Supplemental Figure 1.
In addition to traditional measures of functional capacity (VO2, AT, COT, PWT), we introduce two additional measures: time after AT and time after claudication. These are important because it is an indication of exercise tolerance after the onset of symptoms. Exercise training in a painful claudication zone improves exercise tolerance,13,29–31 and increased capillary density by stimulating angiogenesis may be the major mechanism accounting for this.12 These two new measurements support this by demonstrating increased capillary density relates to increased exercise time after AT and after COT. Although uncomfortable, many patients with PAD are willing to continue to walk with moderate levels pain for social or occupational reasons. Thus, patients with PAD are often willing to tolerate higher intensities of claudication pain for a longer time during exercise training to gain benefit, specifically in a clinical rehab setting.
It is important to briefly discuss the interpretation of different capillary density measures in this study. A strength of this study is that capillary density is measured by endothelial cells per mm2 and by the ratio of endothelial cells to fiber. A decreased muscle fiber diameter may lead to a false interpretation of capillary changes, as more capillaries can be observed in a field without actual changes in capillary number.32,33 If, however, fiber diameter remains unchanged or decreases with concomitant decreases in capillaries, a true rarefaction exists. Although fiber diameter is not available in this study, patients with PAD had fewer capillaries per mm2 concomitantly with similar capillary-to-fiber ratios compared to control subjects. The finding that the capillaries-to-fiber ratio is not significantly different from control subjects is consistent with the majority of previous studies reporting this measure.34–37 These two measurements of capillary density together suggest fiber atrophy was not the reason for capillary density differences between groups.
Strengths and limitations
A major strength of this investigation was the large number of subjects for which both capillary density and CPX data were collected. The large numbers allowed us to detect relationships not previously described. The exclusion of diabetic patients was both a strength and limitation to this investigation. It strengthened the design by eliminating any physiologic pathology in diabetic patients. However, eliminating this population limits the generalizability of results, as a large percentage of patients with PAD have diabetes. The use of two different treadmill protocols would not significantly affect measures of peak VO2 or VO2 AT, or the relationship with these measures to capillary density, but likely had a minor influence on the time components (e.g. PWT). However, aggressively increasing the treadmill workloads in the control subjects to elicit lower PWTs may have then compromised AT interpretation, subject safety, and extended the PWT to greater than 20 minutes. Another limitation was the failure to measure exercise lactates in order to determine whether lactate accumulations differed between groups and if, in PAD, lactate was related to capillary density and claudication symptoms. This would be a logical next step for further investigation.
Conclusion
In summary, in contrast to control subjects, there is evidence of multiple strong relationships between muscle capillary density and functional capacity measurements in patients with PAD. These data extend the known relationships between capillary density and exercise tolerance in PAD by demonstrating novel relationships between capillary density and VO2 at AT, time to AT, time after AT, and time after COT. These findings have both clinical relevance for exercise training and quality of life in the substantial population of patients with PAD in the US and worldwide.
Supplementary Material
Funding
This research was supported by R01-HL755752 (Brian H. Annex) from the National Institutes of Health, National Heart, Lung, and Blood Institute, and in part by internal Duke University research funds available to one of the authors (William E. Kraus).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
The supplementary material is available online with the article.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med 2007; 32: 328–333. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics 2020 update: A report from the American Heart Association. Circulation 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Pande RL, Perlstein TS, Beckman JA, et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011; 124: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob Health 2019; 7: e1020–e1030. [DOI] [PubMed] [Google Scholar]
- 5.Gardner AW, Skinner JS, Cantwell BW, et al. Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc 1992; 24: 163–170. [PubMed] [Google Scholar]
- 6.Pernow B, Zetterquist S. Metabolic evaluation of the leg blood flow in claudicating patients with arterial obstructions at different levels. Scand J Clin Lab Invest 1968; 21: 277–287. [DOI] [PubMed] [Google Scholar]
- 7.Szuba A, Oka RK, Harada R, et al. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc Med 2006; 11: 155–163. [DOI] [PubMed] [Google Scholar]
- 8.Permenter BJ, Raymond J, Fiatarone Singh MA. The effect of exercise on haemodynamics in intermittent claudication: A systematic review of randomized controlled trials. Sports Med 2010; 40: 433–447. [DOI] [PubMed] [Google Scholar]
- 9.Robbins JL, Jones WS, Duscha BD, et al. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol 2011; 111: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askew CD, Green S, Walker PJ, et al. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg 2005; 41: 802–807. [DOI] [PubMed] [Google Scholar]
- 11.Baum O, Torchetti E, Malik C, et al. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. Am J Physiol Regul Integr Comp Physiol 2016; 310: R943–951. [DOI] [PubMed] [Google Scholar]
- 12.Duscha BD, Robbins JL, Jones WS, et al. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler Thromb Vasc Biol 2011; 31: 2742–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev 2017; 12: CD000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman K. The anaerobic threshold: Definition, physiological significance and identification. Adv Cardiol 1986; 35: 1–23. [PubMed] [Google Scholar]
- 15.Gardner AW, Skinner JS, Cantwell BW, et al. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 1991; 23: 402–408. [PubMed] [Google Scholar]
- 16.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 1975; 35: 609–616. [PubMed] [Google Scholar]
- 18.Duscha BD, Kraus WE, Keteyian SJ, et al. Capillary density of skeletal muscle: A contributing mechanism of exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol 1999; 33: 1956–1963. [DOI] [PubMed] [Google Scholar]
- 19.Schiaffino S, Gorza L, Sartore S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 1989; 10: 197–205. [DOI] [PubMed] [Google Scholar]
- 20.Da Rocha Chehuen M, Cucato G, Dos Anjos Souza Barbosa J, et al. Ventilatory threshold is related to walking tolerance in patients with intermittent claudication. Vasa 2012; 41: 275–281. [DOI] [PubMed] [Google Scholar]
- 21.Farah BQ, Ritti-Dias RM, Cucato GG, et al. Clinical predictors of ventilatory threshold achievement in patients with claudication. Med Sci Sports Exerc 2015; 47: 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes Ritti-Dias RM, de Moraes Forjaz CL, Cucato GG, et al. Pain threshold is achieved at intensity above anaerobic threshold in patients with intermittent claudication. J Cardiopulm Rehabil Prev 2009; 29: 396–401. [DOI] [PubMed] [Google Scholar]
- 23.Tuner SL, Easton C, Wilson J, et al. Cardiopulmonary responses to treadmill and cycle ergometry exercise in patients with peripheral vascular disease. J Vasc Surg 2008; 47: 123–130. [DOI] [PubMed] [Google Scholar]
- 24.Cucato GG, Chehuen Mda R, Costa LA, et al. Exercise prescription using the heart of claudication pain onset in patients with intermittent claudication. Clinics (Sao Paulo) 2013; 68: 974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Da Rocha Chehuen M, Cucato GG, Saes GF, et al. Reproducibility of anaerobic and pain thresholds in male patients with intermittent claudication. J Cardiopulm Rehabil Prev 2016; 36: 358–367. [DOI] [PubMed] [Google Scholar]
- 26.Barker GA, Green S, Green AA, et al. Walking performance, oxygen uptake kinetics and resting muscle pyruvate dehydrogenase complex activity in peripheral arterial disease. Clin Sci (Lond) 2004; 106: 241–249. [DOI] [PubMed] [Google Scholar]
- 27.Bauer TA, Brass EP, Barstow TJ, et al. Skeletal muscle StO2 kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol 2007; 100: 143–151. [DOI] [PubMed] [Google Scholar]
- 28.Davidson BP, Belcik JT, Landry G, et al. Exercise versus vasodilator stress limb perfusion imaging for the assessment of peripheral artery disease. Echocardiography 2017; 34: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundi R, Prior SJ, Addison O, et al. Contrast-enhanced ultrasound reveals exercise-induced perfusion deficits in claudicants. J Vasc Endovasc Surg 2017; 2: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy TP, Cutlip DE, Regensteiner JG, et al. ; for the CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation 2012; 125: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott MM, Kibbe MR. Improving lower extremity functioning in peripheral artery disease: Exercise, endovascular revascularization, or both? JAMA 2017; 317: 689–690. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter S, Karpati G. Necrosis of capillaries in denervation atrophy of human skeletal muscle. Muscle Nerve 1982; 5: 250–254. [DOI] [PubMed] [Google Scholar]
- 33.Jozsa L, Balint J, Reffy A, et al. Capillary density of tenotomized skeletal muscles. II. Observations on human muscles after spontaneous rupture of tendon. Eur J Appl Physiol Occup Physiol 1980; 44: 183–188. [DOI] [PubMed] [Google Scholar]
- 34.Hammarsten J, Bylund-Fellenius AC, Holm J, et al. Capillary supply and muscle fibre types in patients with intermittent claudication: Relationships between morphology and metabolism. Eur J Clin Invest 1980; 10: 301–305. [DOI] [PubMed] [Google Scholar]
- 35.Clyne CA, Weller RO, Bradley WG, et al. Ultrastructural and capillary adaptation of gastrocnemius muscle to occlusive peripheral vascular disease. Surgery 1982; 92: 434–440. [PubMed] [Google Scholar]
- 36.Askew CD, Green S, Walker PJ, et al. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg 2005; 41: 802–807. [DOI] [PubMed] [Google Scholar]
- 37.White SH, McDermott MM, Sufit RL, et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: An observational study. J Transl Med 2016; 14: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


