Abstract
The use of primary care databases has been integral in pharmacoepidemiological studies and pharmacovigilance. Primary care databases derive from electronic health records and offer a comprehensive description of aggregate patient data, from demography to medication history, and good sample sizes. Studies using these databases improve our understanding of prescribing characteristics and associated risk factors to facilitate better patient care, but there are limitations. We describe eight key scenarios where study data outcomes can be affected by absent prescriptions in UK primary care databases: (1) out-of-hours, urgent care and acute care prescriptions; (2) specialist-only prescriptions; (3) alternative community prescribing, such as pharmacy, family planning clinic or sexual health clinic medication prescriptions; (4) newly licensed medication prescriptions; (5) medications that do not require prescriptions; (6) hospital inpatient and outpatient prescriptions; (7) handwritten prescriptions; and (8) private pharmacy and private doctor prescriptions. The significance of each scenario is dependent on the type of medication under investigation, nature of the study and expected outcome measures. We recommend that all researchers using primary care databases be aware of the potential for missing prescribing data and be sensitive to how this can vary substantially between items, drug classes, patient groups and over time. Close liaison with practising primary care clinicians in the UK is often essential to ensure awareness of nuances in clinical practice.
Key Points
| Databases derived from digital patient records can provide valuable information about patient care. |
| Since primary care accounts for a majority of patient contacts in the healthcare setting, prescribing data from this setting can improve patient care in a number of ways, such as monitoring prescribing practice to improve drug safety. |
| Best use of primary care prescribing data requires an appreciation of clinical practice, standards and culture within primary care. |
Introduction
Routinely collated primary care data from electronic health records (EHRs) are the most frequently used data source for research into medical prescribing practices and pharmacovigilance and pharmacoepidemiological studies [1]. The use of EHRs in the United Kingdom (UK) was initiated in primary care, with 96% of all general practices using them by 1996 [2]. Currently, all UK-wide general practice consultations are recorded within EHRs [3]. Hence, we have a vast repository of health data that spans decades and has enormous value for patient safety, quality improvement and cost-effectiveness research [4]. Primary care databases such as those managed by the Secure Anonymised Information Linkage (SAIL) Databank [5], The Health Improvement Network (THIN) [6] and the Clinical Practice Research Datalink (CPRD) are a rich source of patient data. For example, CPRD is a UK-wide network of over 2000 general practices and includes over 60 million patients, of which 16 million were actively registered as of May 2021. It provides longitudinal data, with 20 years of follow-up for 25% of patients [7]. In June 2020, there were over 2700 publications investigating drug safety, use of medicines, effectiveness of health policy, healthcare delivery and disease risk factors using CPRD [8]. The wealth of prescribing information from such routinely collated primary care datasets is undeniable, but there are several scenarios to consider where the data can be susceptible to misinterpretation due to absent prescriptions. This review will describe eight key scenarios resulting in absent prescription data that can significantly affect outcomes and interpretation of research findings. The relevance of each scenario will vary based on the type of research and drug(s) under investigation.
Common Scenarios Where Prescription Data are Absent
Scenario I: Out-of-Hours, Urgent Care and Acute Care Prescriptions
Out-of-hours primary care services are a fundamental part of primary care provision in the UK. These providers manage cases that would have been seen by their general practice had they presented in normal hours, which is usually between 0800 hours and 1830 hours on weekdays, excluding bank holidays [9]. Demand for out-of-hours and urgent care services has risen consistently over the last 5 years, possibly due to increased patient numbers, organisational restructuring and funding issues within the National Health Service (NHS) [10]. Out-of-hours and urgent care prescriptions are rarely included in primary care databases as these settings may not have authorization to update patients’ primary care EHR. Potential absence of prescriptions due to out-of-hours prescribing is particularly relevant in studies investigating the management of acute infections and medication adherence for chronic disease. A study found that 23.7% of all out-of-hours encounters resulted in antibiotic prescriptions [11]. Another study found that, up to 30% of all calls to out-of-hours weekend services over a single day were documented as urgent requests for repeat medication for chronic disease management [8]. Since out-of-hours prescribing is not routinely recorded in the primary care EHR prescribing data, the clinical concern here is disruption of medication monitoring for prescribing safety due to interruptions in the general practice prescribing audit cycle.
Scenario II: Specialist-only Prescriptions
Primary care provides the majority of long-term repeat prescriptions for chronic conditions [12, 13], but there are some medications, particularly medications that require close safety monitoring, that are prescribed by specialist clinicians. These prescriptions will not appear in primary care databases. Over time, prescribing of these medications may be transferred to primary care under a “shared-care” agreement, whereby the general practice may provide prescriptions while sharing the care of the patient with the specialist team. In this scenario, a proportion of prescriptions will not appear in primary care databases, as a specialist may have prescribed the initial prescriptions. These agreements vary geographically based on locally agreed policy [14]. Moreover, general practitioners are not obliged to agree to shared-care prescribing, in which case, the specialist team would continue providing medication prescriptions. This scenario can affect primary care prescribing data and pharmacovigilance.
Examples of medications that are commonly prescribed under shared-care agreements include (1) disease-modifying anti-rheumatic drugs (DMARDs), (2) atypical antipsychotic medications and (3) cancer treatments.
Some DMARDS used to treat autoimmune conditions such as inflammatory bowel disease, rheumatic disease and psoriasis require regular monitoring. Examples include DMARDs such as azathioprine and methotrexate, which can be prescribed in general practice under shared-care arrangements after specialist initiation [12, 13].
Atypical antipsychotic medications such as clozapine used to treat resistant schizophrenia are often initiated by a specialist psychiatrist in a secondary care setting. Close monitoring is required because there is a risk of serious side effects such as arrhythmias and agranulocytosis [15]. As with DMARDs, once stable, clozapine can be prescribed and monitored by general practitioners within local shared-care prescribing agreements [16].
Cancer treatments such as long-term chemoprophylaxis after initial treatment in specialist centres can be prescribed in general practice. Examples include tamoxifen when prescribed after breast cancer [17] and gonadotrophin-releasing hormone prescribed as part of prostate cancer treatment.
Further examples of medications that may be prescribed within a shared-care agreement between specialist and primary care are tuberculosis and human immunodeficiency virus medications, in vitro fertilisation treatment and injectable medications such as prescribing for insulin pumps, desferrioxamine and total parenteral nutrition. This is not an exhaustive list of medications prescribed under shared-care agreements, but it does highlight the importance of considering these cases and their absence from primary care databases. Electronic records for secondary or tertiary care dispensing data are available for individual, patient-level cases in most hospitals, but unlike primary care data, they are not yet widely accessible for research purposes to establish evidenced-based understanding of prescribing practices in these settings [18].
Scenario III: Alternative Community Prescribing Such as Pharmacy, Family Planning Clinic or Sexual Health Clinic Medication Prescriptions
Health service users in the UK can access family planning clinic (FPC) and sexual health clinic (SHC) services directly without referrals, and any medications prescribed in these settings are not routinely represented in primary care databases. An example is long-acting reversible contraception (LARC) prescribing; this contraception can be prescribed in both primary care and FPC/SHC, and the latter is not represented in primary care records. The number of prescriptions issued in primary care versus FPC/SHC is variable. It can be influenced by external factors such as government-backed incentives awarded to general practices, such as the Quality Outcomes Framework (QOF), where financial incentives are awarded to encourage quality care. Financial incentives for LARC prescribing in general practice led to more than 100,000 additional primary care prescriptions in 600 practices, 3 years after these QOF incentives were introduced [19, 20]. Studies that utilise primary care databases to investigate medications that can be prescribed in alternative community settings must consider the prescribing trends in general practice and how these can be influenced by external factors such as incentive payments and health campaigns.
In this scenario, we can also consider prescribing by community pharmacies. Since 2015/2016, in addition to influenza vaccination in primary care settings, the NHS has funded the provision of influenza vaccinations to at-risk groups by community pharmacies [21, 22]. During the 2015/2016 flu season, September to March, community pharmacies prescribed 486,897 influenza vaccines compared with 10,447,651 prescribed in general practice. Those numbers increase to 1,524,753 prescribed by community pharmacies and a slight decrease to 10,326,783 prescribed in general practice during the 2019/2020 flu season, based on data from PharmaOutcomes®, Sonar informatics and NHS Business Services Authority (NHSBSA) data published by the Pharmaceutical Services Negotiating Committee (PSNC) and OpenPrescribing.net, EBM DataLab, University of Oxford, 2020 [23, 24]. Details of vaccinations provided outside general practice in the past have not routinely been included in primary care databases. This may have represented a subset of absent prescriptions in the past, but as of December 2020, there have been significant changes in the recording and management of immunisation vaccination data, due to the current coronavirus disease 2019 (COVID-19) pandemic. Roll out of the COVID-19 vaccination programme has facilitated changes in the recording and management of immunisation vaccination data in England. The centralised service for the management of seasonal influenza vaccination programmes has been upgraded to include the National Immunisation Vaccination System (NIVS), a web-based app that is accessible at the point of delivery for real-time recordings. As a result, COVID-19 vaccinations are recorded in the NIVS app or Outcomes4Health (known as Pinnacle) web-based system in all community sites, including pharmacies and pop-up vaccination sites. An equivalent app is also available for secondary care settings, the National Immunisation Management System (NIMS). Therefore, all vaccinations can be recorded at the point of delivery; these data are then sent directly to the general practice EHR or the Data Processing Service (DPS) and subsequently recorded in primary care databases. This upgraded process is still subject to some degree of missingness, but it is expected to improve the accessibility, versatility and connectivity of primary care databases, this process will capture vaccination data more effectively than the previous IT systems [25]. System changes of this type can influence whether community prescribing outside of general practice is recorded in primary care prescribing datasets.
Scenario IV: Newly Licensed Medication Prescriptions
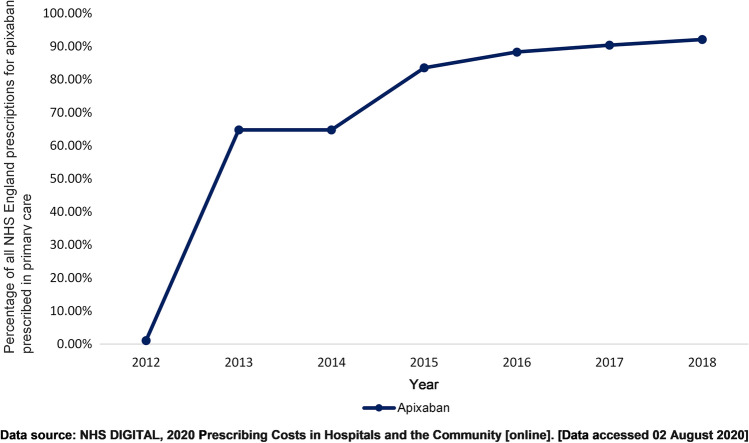
New medications may be prescribed by specialist clinicians initially, before being prescribed in primary care. During this initial period, a proportion of prescriptions will be absent from primary care EHR. Novel oral anticoagulants (NOACs), medications used to prevent stroke and thrombosis, provide an example of this scenario. On first introduction to clinical practice in 2008, NOAC prescribing took place in secondary care as post-operative prophylaxis [26]. Data show a delay in NOAC prescribing within primary care until 2012, when National Institute for Health and Care Excellence (NICE) guidelines highlighted the benefits of NOAC use in the management of non-valvular atrial fibrillation (AF) [26]. In addition, the ARISTOTLE study demonstrated that the NOAC apixaban was superior to warfarin at preventing strokes in patients with AF [27]. NHS Digital data demonstrate that in 2011/2012, 1.10% of all apixaban prescribing by the NHS in England was in primary care. By 2013/2014, 65% of apixaban prescribing occurred in primary care, and further increased to 92% by 2017/2018 (Fig. 1) [3]. This trend in NOAC prescribing in primary care has been described in various studies [28, 29] and has implications for pharmacovigilance and pharmacoepidemiology studies using these data. Research studies assessing newly licensed medication prescribing using primary care databases should consider associated prescribing guidance and implementation of guidance by primary care clinicians. NHS Digital prescribing data are derived from the NHSBSA through the electronic Prescribing Analysis and Cost Tool 2 (ePACT2), which covers all NHS general practice prescribing and dispensing in the UK. There is no central NHS collection of information on all prescribing in NHS hospitals in England, but NHS Digital does have limited access to some hospital prescribing data through organisations such as IQVIA. Hence, NHS Digital prescribing data for primary and secondary care are not available for all medications [30, 31].
Fig. 1.
The increasing trend in prescribing of apixaban, a novel oral anticoagulant, by primary care clinicians in England between 2012 and 2018 [30]. NHS National Health Service
Scenario V: Medications that Do Not Require Prescriptions
Medications that are deemed safe, efficacious and less amenable to misuse can sometimes be available to purchase over the counter (OTC) without a prescription. These will not appear in primary care databases, and the proportion of any particular medication or item that is prescribed versus purchased OTC can change over time. In 2018, the cost of medicines prescribed and dispensed in primary care in England reached £9.2 billion [32]. Subsequently, NHS England and NHS Clinical Commissioners (NHSCC) devised changes in primary care prescribing practices to support a more cost-effective approach [33]. From April 2019, in accordance with national recommendations from NHS England and NHSCC, to reduce prescribing for self-limiting conditions and encourage people to manage their conditions with self-care when appropriate, general practices were strongly advised to stop routinely prescribing medications that are available OTC, such as ibuprofen for minor conditions presenting with pain or fever [34]. Therefore, any study using primary care databases involving such medications would underestimate their use and potentially be limited by misclassification bias.
Prescribing studies that do not include any OTC use of non-steroidal anti-inflammatory drugs (NSAIDs) may underestimate the risk of adverse events associated with these drugs [35–37]. For example, data from the nationwide Danish Cardiac Arrest Registry showed that in 11.66% of 28,947 identified cases of out-of-hospital cardiac arrest (OHCA), patients had taken either ibuprofen or an alternative NSAID within 30 days prior to their OHCA. However, since ibuprofen is also available without prescription OTC in Denmark, this figure is likely an underestimation of the associated risk [36].
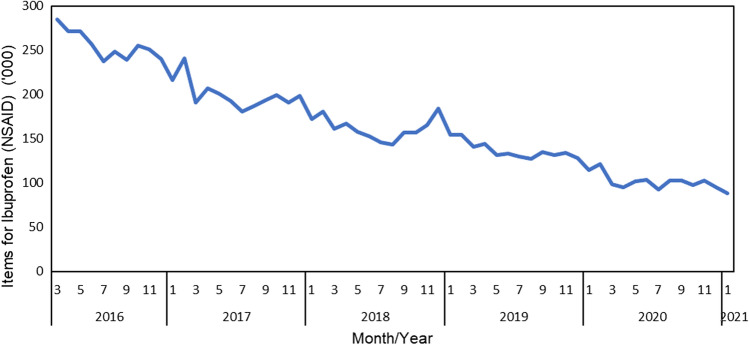
Every month, NHS Digital provides anonymised prescribing data from NHS England, which are summarised on the open prescribing website https://openprescribing.net/. Figure 2, this website shows a decrease in primary care prescriptions for ibuprofen in England over the last 5 years, an overall decline of 52.85 % [24]. This may be indicative of increased OTC use of ibuprofen rather than an actual decline in use.
Fig. 2.
Trends in prescribing ibuprofen as a product or combination product in all primary care servicesa in England between March 2016 and February 2021 [24]. Data source: OpenPrescribing.net, EBM DataLab, Department of Primary Care Health Sciences, University of Oxford, 2021. NSAID non-steroidal anti-inflammatory drug. aExcludes data from non-standard settings such as prisons, the military, out-of-hours services, etc.
Scenario VI: Hospital Inpatient and Outpatient Prescriptions
During hospital admissions and outpatient clinic attendance, patients will have their prescriptions provided by their hospital team. These include prescriptions issued in the emergency department. One implication is that acute hospital prescriptions such as intravenous antibiotic prescriptions for severe infections are missing from primary care databases. Another implication is that the first record of important long-term medications, such as secondary prevention medications associated with cardiac events or stroke, e.g. clopidogrel prescribed to treat acute presentation of stroke, will not appear in primary care databases until up to 90 days after the acute event [38].
Scenario VII: Handwritten Prescriptions
Electronic prescribing systems (EPS) are first-line primary care prescribing tools that are integrated with EHRs used in England. Handwritten prescriptions are not integrated and are used only in exceptional circumstances. There are two notable reasons for preferential use of EPS: (1) patient safety and medicines monitoring, as there is a clear audit trail for root cause analysis [39], and (2) security of the prescribing process to ensure that prescription details outlined by the prescriber cannot be modified or accessed by any unauthorized individuals. All general practices should have a standard operating procedure for the use and storage of handwritten prescriptions [40]. Electronic prescriptions sent via EPS represent 85% of all dispensed prescriptions in primary care [41]. Instances where general practices may issue handwritten prescriptions include home visits to housebound patients or EPS failure. Although prescribers are advised to update the details of any handwritten prescriptions in EPS retrospectively, this may not always occur. In scenarios where handwritten prescriptions have not been updated in EPS, the data will not be available in primary care databases.
Scenario VIII: Private Pharmacy and Private Doctor Prescriptions
Private prescriptions are not a component of routinely collated data, but there are an increasing number of medical prescriptions issued in these settings. Since 2010, a growing number of internet-based services supported by private doctors have been providing patient care, from acute care (e.g. antibiotics) to lifestyle (e.g. recreational use of a phosphodiesterase type 5 inhibitor such as sildenafil) medications. Patients who can afford to pay and require a service that is not available on the NHS or want to avoid NHS waiting times may use this service.
In the UK, the NHS provides a routine immunisation schedule for endemic infections, but travel vaccines are not provided on the NHS. As a result, travel vaccines given privately may not always be recorded in EHRs. It is important to consider private prescriptions in any studies where they can affect research study outcomes and conclusions.
Implications for Pharmacoepidemiological Research, Benefits and Challenges
Primary care databases provide an invaluable resource for pharmacoepidemiological studies to inform clinical guidelines, pharmacovigilance, public health policy and improve the quality of patient care [1, 42]. However, this review describes eight key scenarios, summarised in Table 1, where absent prescriptions and missing data from primary care databases can cause errors in interpretation of study outcomes. In descriptive studies that aim to investigate incidence, prevalence and trends in prescribing or drug utilisation using primary care prescribing data, there will be a clear underestimate in the described scenarios. Thus researchers should make it clear in their publications that their estimates are only applicable to prescribing patterns in general practice, and should demonstrate awareness of the limitations of the data and their implications.
Table 1.
Summary of the scenarios where primary care prescribing data are subject to absent prescriptions
| Define the prescribing scenario for item of interest to address absent prescriptions (eight key scenarios) | Examples of items |
|---|---|
| (i) Out-of-hours, urgent care and acute care prescriptions | Antibiotics, acute painkillers, emergency contraception |
| (ii) Specialist-only prescriptions | DMARDs, cancer treatments, clozapine |
| (iii) Alternative community prescribing, such as pharmacy, family planning clinic or sexual health clinic medication prescriptions | Long-acting reversible contraception, emergency contraception, influenza vaccination prescriptions (2015/2016–2020) |
| (iv) Newly licensed medications prescriptions | Novel oral anticoagulants in the early 2010s |
| (v) Medications that do not require prescriptions | Ibuprofen, paracetamol, laxatives, topical antifungals, emollients |
| (vi) Hospital inpatient and outpatient prescriptions | Anticoagulants, antibiotics, clopidogrel |
| (vii) Handwritten prescriptions | Antibiotics, acute painkillers |
| (viii) Private pharmacy and private doctor prescriptions | Antibiotics, hair loss treatments, erectile dysfunction treatments |
DMARD disease-modifying anti-rheumatic drug
There is no broad overarching solution for addressing this missingness of data, and while the described scenarios are not an exhaustive list, they cover the more common scenarios that may result in misinterpretation of findings.
The statistical method used to ascertain missingness will depend on the drug or item, objectives, study design and expected outcome. Multiple imputation of missing data is not an option in these scenarios where the prescription data missingness can be classified as ‘missing not at random’ because we have already explained that there are systematic differences between the missing and observed values. On the other hand, missing data on the prescription categories we have highlighted in these scenarios will mostly lead to non-differential misclassification of prescription status and could lead to a shifting of the relative risk/odds towards null, i.e. the observed estimate of an association between an exposure and outcome may be an underestimate in hypothesis-testing studies investigating the association between prescriptions and outcomes. As long as researchers take this into account in their interpretation, that should be sufficient, but possible solutions for addressing missingness include:
Linkage to prescribing data from other healthcare settings where possible. Currently, prescribing data from hospitals, out-of-hours services and specialist units are not available at a national level. However, data from the NHSBSA collated in NHS Digital can provide data on the level of prescribing in primary and secondary care for only some items, these data are publicly available [30, 31]. There is also the Hospital Treatment Insights (HTI) dataset, which links Hospital Episode Statistics to pharmacy records for 28.10% of hospital trusts in England [43]. This is a partial dataset mostly used to understand patient outcomes after treatment, but it could be used to estimate prescribing trends when linked to primary care datasets. In some instances, it may be beneficial to review open-access prescribing datasets for primary care prescribing trends [23, 24]. To date, there is no database available for aggregate primary and secondary care prescribing for all prescribed items in England.
Supplementing prescribing data from the primary care data using study-specific general practitioner and patient questionnaires could provide patient-level data on item prescribing in all settings, although this may have issues regarding anonymity and would require the appropriate ethical considerations and general data protection regulations.
An understanding of the strengths and limitations of primary care prescribing data is required for all studies and investigations using this data for research purposes.
Conclusion
The NHS is a UK-wide universal healthcare system, and > 95% of UK residents are registered with a general practice [44]. EHRs from general practice used to generate primary care databases are likely to remain an important research resource, driving quality improvement at the patient and population level. Although the scenarios described in this review highlight limitations of using primary care databases for pharmacovigilance and pharmacoepidemiological studies involving some medications in the UK, they also provide insights to inform study design and interpretation of data to yield more reliable and useful conclusions.
Declarations
Funding
GO is funded by the National Institute for Health Research (NIHR). PM, TMT and HS are employees of CPRD. CPRD is jointly sponsored by the UK government’s Medicines and Healthcare products Regulatory Agency and the NIHR. As a not-for-profit UK government body, CPRD seeks to recoup the cost of delivering its research services to academic, industry and government researchers through research user licence fees. For the writing of this manuscript, the authors received no funding.
Conflict of interest
GO, IW and DE are members of the Independent Scientific Advisory Committee of the Medicines and Healthcare Products Regulatory Agency database research group, which is a non-statutory expert advisory body giving advice on research-related requests to access data from the CPRD. PM, TMT and HS are employed by CPRD.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Ethics approval
Not applicable to this type of study.
Consent to participate
Not applicable to this type of study.
Consent for publication
Not applicable to this type of study.
Code availability
Not applicable.
Authors' contributions
GO contributed to conception, planning, identification of data sources, presentation of data and writing. PM contributed to conception, planning and writing. HS contributed to the presentation of data. TMT contributed to conception, planning and identification of data sources. IW contributed to conception. DE contributed to conception, planning and writing. All authors contributed to editing and approved the final version of the manuscript.
References
- 1.Ghosh RE, Crellin E, Beatty S, Donegan K, Myles P, Williams R. How Clinical Practice Research Datalink data are used to support pharmacovigilance. Ther Adv Drug Saf. 2019;10:2042098619854010. doi: 10.1177/2042098619854010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson T. Why general practitioners use computers and hospital doctors do not—part 1: incentives. BMJ. 2002;325(7372):1086–1089. doi: 10.1136/bmj.325.7372.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NHS Digital. GP Systems of Choice. National Health Service. https://digital.nhs.uk/services/gp-systems-of-choice#more-information. Accessed 21 Jan 2020.
- 4.Bradley SH, Lawrence NR, Carder P. Using primary care data for health research in England—an overview. Future Healthc J. 2018;5(3):207–212. doi: 10.7861/futurehosp.5-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secure Anonymised Information Linkage Databank. Overview of SAIL Databank. https://saildatabank.com/about-us/overview/. Accessed 1 June 2021.
- 6.The Health Improvement Network. What is THIN® data? https://www.the-health-improvement-network.com/en/. Accessed 1 June 2021.
- 7.The Clinical Practice Research Datalink CPRD. www.cprd.com. Accessed 10 Feb 2020.
- 8.The Clinical Practice Research Datalink. CPRD UK data driving real-world evidence. https://www.cprd.com/bibliography. Accessed 10 May 2021.
- 9.National-Audit-Office. Out‐of‐hours GP services in England. In.: Press Office, National Audit Office, England London, UK; 2014: 1–66.
- 10.Foster H, Moffat KR, Burns N, Gannon M, Macdonald S, O'Donnell CA. What do we know about demand, use and outcomes in primary care out-of-hours services? A systematic scoping review of international literature. BMJ Open. 2020;10(1):e033481. doi: 10.1136/bmjopen-2019-033481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colliers A, Adriaenssens N, Anthierens S, Bartholomeeusen S, Philips H, Remmen R, Coenen S. Antibiotic prescribing quality in out-of-hours primary care and critical appraisal of disease-specific quality indicators. Antibiotics. 2019;8(2):79. doi: 10.3390/antibiotics8020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NHS Executive Responsibility for prescribing between hospitals and GPs. EL. 2019;127(91):1–18. [Google Scholar]
- 13.NHS England. Responsibility for prescribing between primary and secondary/tertiary care. In.; 2018.
- 14.NHS England. The interface between primary and secondary care: Key messages for NHS clinicians and managers. NHS 2017. In.
- 15.Filia SL, Wheelhouse A, Lee SJ, Main M, de Castella A, Wilkins S, Kulkarni J. Transitioning patients taking clozapine from the public to private/GP shared-care setting: barriers and criteria. Aust N Z J Psychiatry. 2012;46(3):225–231. doi: 10.1177/0004867411433210. [DOI] [PubMed] [Google Scholar]
- 16.Winckel K, Siskind D. Clozapine in primary care. Aust Prescr. 2017;40(6):231–236. doi: 10.18773/austprescr.2017.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SG, Foy R, McGowan JA, Kobayashi LC, de Censi A, DeCensi A, Brown K, Side L, Cuzick J. Prescribing tamoxifen in primary care for the prevention of breast cancer: a national online survey of GPs' attitudes. Br J Gen Pract J R Coll Gen Pract. 2017;67(659):e414–e427. doi: 10.3399/bjgp17X689377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldacre B, MacKenna B. The NHS deserves better use of hospital medicines data. BMJ. 2020;370–374. [DOI] [PubMed]
- 19.Arrowsmith ME, Majeed A, Lee JT, Saxena S. Impact of pay for performance on prescribing of long-acting reversible contraception in primary care: an interrupted time series study. PLoS ONE. 2014;9(4):e92205. doi: 10.1371/journal.pone.0092205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan CR, Liu H. The relationship between area deprivation and prescription of long-acting reversible contraception in women of reproductive age in Lothian, Scotland, UK. J Fam Plan Reprod Health Care. 2017;43(4):281–288. doi: 10.1136/jfprhc-2016-101553. [DOI] [PubMed] [Google Scholar]
- 21.Warner JG, Portlock J, Smith J, Rutter P. Increasing seasonal influenza vaccination uptake using community pharmacies: experience from the Isle of Wight, England. Int J Pharm Pract. 2013;21(6):362–367. doi: 10.1111/ijpp.12037. [DOI] [PubMed] [Google Scholar]
- 22.Anderson C, Thornley T. Who uses pharmacy for flu vaccinations? Population profiling through a UK pharmacy chain. Int J Clin Pharm. 2016;38(2):218–222. doi: 10.1007/s11096-016-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pharmaceutical Services Negotiating Committee. Flu Vaccination—Statistics. Pharmaceutical Services Negotiating Committee. https://psnc.org.uk/services-commissioning/advanced-services/flu-vaccination-service/flu-vaccination-statistics/. Accessed 07 Aug 2020.
- 24.OpenPrescribing.net, DataLab E, University of Oxford 2020. https://openprescribing.net/analyse/#org=CCG&numIds=1001010J0AA&denom=nothing&selectedTab=chart. Accessed 19 Feb 2021.
- 25.N H S Digital. COVID-19 vaccination record queries. https://digital.nhs.uk/coronavirus/vaccinations/covid-19-vaccination-record-queries. Accessed 17 May 2021.
- 26.Loo SY, Dell'Aniello S, Huiart L, Renoux C. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol. 2017;83(9):2096–2106. doi: 10.1111/bcp.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 28.Medlinskiene K, Fay M, Petty D. Uptake of oral anticoagulants for stroke prevention in patients with atrial fibrillation in a single clinical commissioning group in England without restrictions to their use. Clin Drug Investig. 2019;39(4):401–405. doi: 10.1007/s40261-019-00763-y. [DOI] [PubMed] [Google Scholar]
- 29.Sabouret P, Bricard M, Hermann M-A, Cotté F-E, Deret-Bixio L, Rushton-Smith S. Discrepancy between guidelines for stroke prevention in atrial fibrillation and practice patterns in primary care. The nationwide French AFIGP survey. Arch Cardiovasc Dis. 2015;108(11):544–553. doi: 10.1016/j.acvd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 30.NHS Digital. Prescribing Costs in Hospitals and the Community. https://digital.nhs.uk/data-and-information/publications/statistical/prescribing-costs-in-hospitals-and-the-community. Accessed 02 Aug 2020.
- 31.NHS Digital. Practice level prescribing in England: a summary. 2020.
- 32.NHS England. Items which should not be routinely prescribed in primary care. https://www.england.nhs.uk/medicines-2/items-which-should-not-be-routinely-prescribed/. Accessed 17 June 2020.
- 33.NHS England and NHS Clinical Commissioning Group N. Conditions for which over the counter items should not routinely be prescribed in primary care: Guidance for CCGs. In.; 2018.
- 34.NHS England. Conditions for which over the counter items should not routinely be prescribed in primary care: guidance for CCGs. In.; 2019.
- 35.Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, Brophy JM. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357–370. [DOI] [PMC free article] [PubMed]
- 36.Schmidt M, Hallas J, Friis S. Potential of prescription registries to capture individual-level use of aspirin and other nonsteroidal anti-inflammatory drugs in Denmark: trends in utilization 1999–2012. Clin Epidemiol. 2014;6:155–168. doi: 10.2147/CLEP.S59156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sondergaard KB, Gislason G. NSAIDs and cardiac arrest: Non-steroidal anti-inflammatory drug use is associated with increased risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Eur Heart J. 2017;38(23):1788–1789. doi: 10.1093/eurheartj/ehx267. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Edwards D, Massou E, Saunders C, Brayne C, Mant J. Statin use and high-dose statin use after ischemic stroke in the UK: a retrospective cohort study. Clin Epidemiol. 2019 doi: 10.2147/CLEPS;201983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avery AA, Barber N, Ghaleb M, Dean Franklin B, Armstrong S, Crowe S, Dhillon S, Freyer A, Howard R, Pezzolesi C. Investigating the prevalence and causes of prescribing errors in general practice: the PRACtICe study. 2012.
- 40.BMJ Group and the Royal Pharmaceutical Society. British National Formulary. https://www.medicinescomplete.com/#/content/bnf/PHP97235?hspl=fp10ss2Ffp10mdass#content2Fbnf2FPHP9723523chapter-PHP97235-1. Accessed 17 June 2020.
- 41.Pharmaceutical Services Negotiating Committee. EPS prescriptions now over 85% of total items. https://psnc.org.uk/our-news/electronic-prescriptions-reach-more-than-85/. Accessed 1 July 2021.
- 42.Booth HP, Gallagher AM, Mullett D, Carty L, Padmanabhan S, Myles PR, Welburn SJ, Hoghton M, Rafi I, Valentine J. Quality improvement of prescribing safety: a pilot study in primary care using UK electronic health records. Br J Gen Pract. 2019;69(686):e605–e611. doi: 10.3399/bjgp19X704597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rockenschaub P, Ansell D, Shallcross L. Linking individual-level data on diagnoses and dispensing for research on antibiotic use: evaluation of a novel data source from English secondary care. Pharmacoepidemiol Drug Saf. 2018;27(2):206–212. doi: 10.1002/pds.4367. [DOI] [PubMed] [Google Scholar]
- 44.NHS Digital. Patients Registered at a GP Practice June 2020. https://digital.nhs.uk/data-and-information/publications/statistical/patients-registered-at-a-gp-practice/april-2019. Accessed 19 June 2020.