Abstract
Introduction:
Surgical complications resulting from breast reconstruction may delay the initiation of PMRT, which can impact breast cancer outcomes. We hypothesized that immediate breast reconstruction may be associated with delays in the initiation of PMRT, but that this delay would not significantly impact overall patient survival.
Methods:
Using the National Cancer Database, we identified women with breast cancer who underwent mastectomy and received PMRT within 1 year of surgery. Delayed PMRT was defined as treatment initiated ≥6 months after surgery in patients who received adjuvant chemotherapy, or as ≥12 weeks after surgery in patients who received neoadjuvant or no chemotherapy.
Results:
Overall, 171,136 patients were identified. Women undergoing breast reconstruction had an increased median time to PMRT, 154 (IQR, 64–196) vs. 132 days (IQR, 52–188.5) (p<0.001), and on logistic regression were more likely to experience a delay in initiating PMRT (OR, 1.25; 95% CI, 1.188–1.314). Other factors associated with delayed PMRT included: increased Charlson-Deyo scores, neoadjuvant chemotherapy, non-private insurance, and black race. Cox Proportional Hazards models revealed no evidence of a reduced adjusted overall survival in the immediate breast reconstruction group (HR, 0.836; 95% CI 0.802–0.871, p<0.001). Restricted cubic spline analysis identified the threshold number of days at which the start of PMRT began to impact survival at 169 (95% CI, 160–190), 75 (95% CI, 42–90), and 71 (95% CI, 41–90) days in patients undergoing adjuvant, neoadjuvant, and no chemotherapy, respectively.
Conclusion:
Immediate breast reconstruction is associated with a modest delay in initiating PMRT but does not impact overall survival.
INTRODUCTION
Breast cancer is a significant medical and public health issue in the United States, affecting one in eight women during their lifetime.1 Contemporary breast cancer care involves a multidisciplinary approach to optimize treatment planning, and for many women undergoing mastectomy, includes systemic therapy, radiotherapy, and breast reconstruction.2–5 Complex and tailored multimodal breast cancer care necessitates continued collaboration amongst subspecialties to ensure the timeliness of patient-centered, evidence-based cancer treatment.
The timing of breast reconstruction (i.e. immediate–at the time of mastectomy vs. delayed–after mastectomy) can vary, and often depends on a variety of patient and provider-level factors, in addition to the anticipated components of cancer treatment. Immediate breast reconstruction offers several advantages for women including improved psychosocial benefits and potential reductions in health care utilization and cost.6–9 Nevertheless, women requiring post-mastectomy radiation therapy (PMRT) can be at a higher risk for reconstruction-related complications,9–13 that may prompt recommendations to delay breast reconstruction. Despite this, the utilization of immediate breast reconstruction has increased in recent years, and many centers advocate that PMRT is not an absolute contraindication for reconstruction at the time of mastectomy.14,15
In the immediate setting, a primary concern regarding breast reconstruction is the potential for post-operative complications to delay or interfere with the delivery of adjuvant treatment. Studies have shown that while immediate breast reconstruction may delay the initiation of chemotherapy, this delay is not clinically significant and does not lead to inferior oncologic outcomes.16–18 With respect to PMRT, it has been suggested that reconstruction can limit the ability of the radiation oncologist to achieve adequate coverage of the chest wall and internal mammary chain, or place the heart and lungs at risk of excess exposure.19–21 Evidence has shown that PMRT decreases the risk of locoregional recurrence while also improving overall survival, and significant delays in the administration of PMRT can negatively impact patient outcomes.22,23 Thus, current guidelines from the American Society of Radiation Oncology and the National Comprehensive Cancer Network recommend PMRT for T1–2 tumors and one to three positive lymph nodes, as well as select high-risk node negative patients. Therefore, the number of breast reconstructive patients who receive PMRT continues to rise.24
The impact of immediate breast reconstruction on the timely initiation of PMRT has not been fully explored. Thus, using the National Cancer Database (NCDB), we examined whether immediate breast reconstruction after mastectomy delays the initiation of PMRT, and ultimately impacts overall patient survival. Our hypothesis was that immediate breast reconstruction may be associated with delays in the initiation of PMRT, but that this delay would not significantly impact overall patient survival. Given the paucity of data regarding the impact of delayed PMRT, we also sought to determine what length of delay to the initiation of PMRT may begin to impact overall survival.
METHODS
Data Source
The data source for this study was the National Cancer Database (NCDB). The data obtained from the NCDB is derived from over 1500 Commission on Cancer-accredited centers, and represents more than 70% of newly diagnosed cancers in the United States and more than 34 million historical records.25
Study Design
All adult female patients who were diagnosed with breast cancer from 2004–2014 and received PMRT within 1 year of mastectomy were identified for inclusion in this study. Patients with missing radiotherapy or chemotherapy dosing or timing data, or metastatic disease (cM1 or pM1) were excluded from this study. In addition, patients who received PMRT greater than 1 year after mastectomy or prior to mastectomy, or who received radiation treatment to areas other than the breast, chest wall, or lymph nodes were excluded from this study. The patient cohort was first divided into two groups based on reconstruction status after mastectomy, and then into three subgroups based on chemotherapy type (i.e. neoadjuvant, adjuvant, and no chemotherapy). The consort diagram detailing patient exclusion and inclusion criteria is included as Figure 1.
Figure 1:
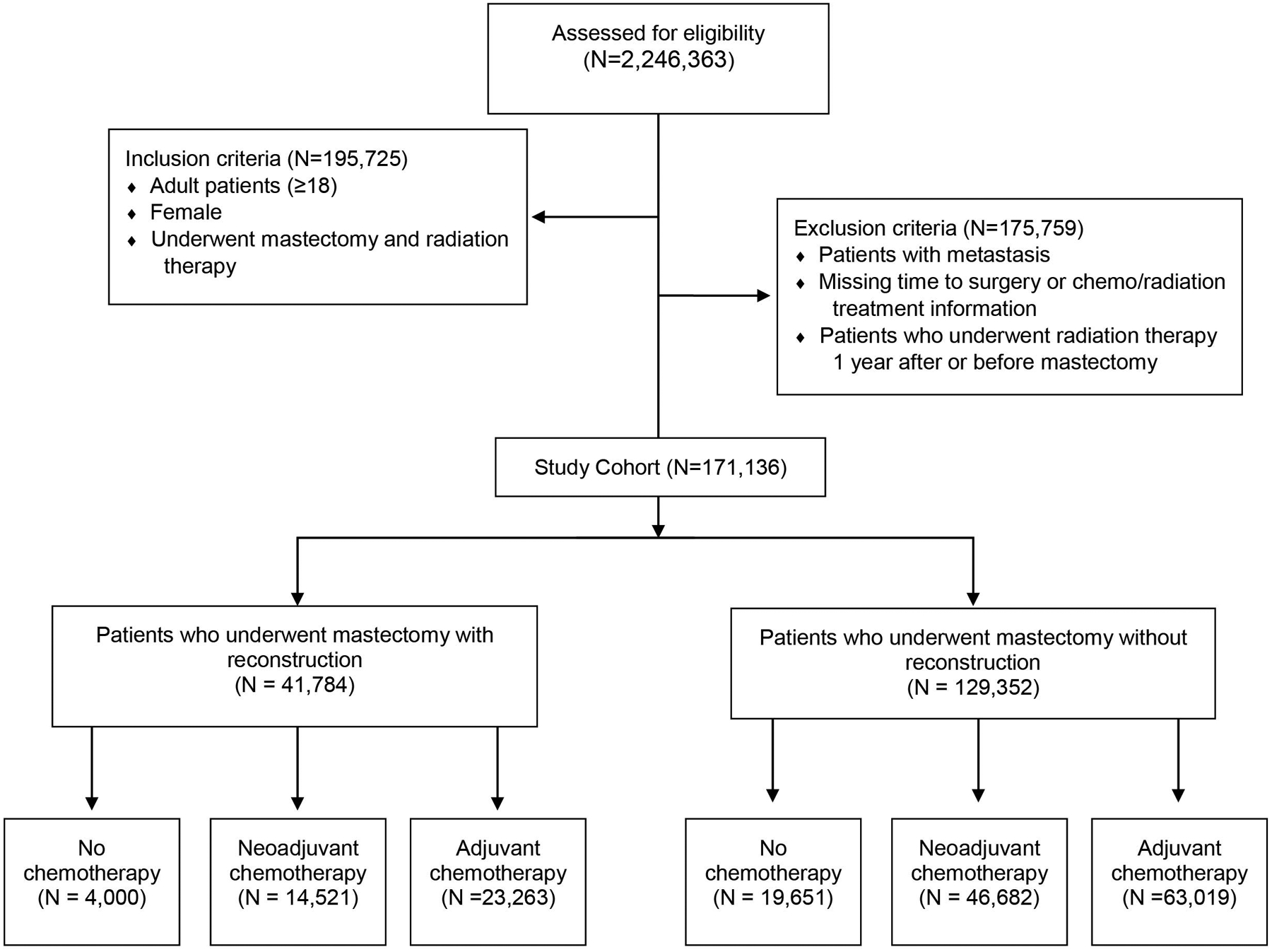
Key inclusion and exclusion criteria
Definition of Variables
Independent variables analyzed in this study included: age at diagnosis, gender, race, ethnicity, comorbidity score, income level, insurance status, facility type, facility location, hormone receptor status, human epidermal growth factor recptor-2 (HER-2) status, clinical and pathological TNM staging, tumor grade, number of positive lymph nodes, number of lymph nodes examined, tumor size, radiation volume, regional dose of radiation therapy, days to radiation from surgery, use of chemotherapy, chemotherapy type (i.e. adjuvant only, neoadjuvant, no chemotherapy), use of hormone therapy, surgery type, and surgical laterality.
Time to PMRT was calculated as the time from mastectomy to start of radiation therapy. Post-mastectomy radiation therapy was defined as delayed if: 1) It occurred ≥ 6 months after surgery if the patient received adjuvant chemotherapy, or 2) It occurred ≥ 12 weeks after surgery if the patient received neoadjuvant or no chemotherapy. These time points were chosen based on institutional experience and were examined in the patient data and our restricted cubic spline analysis for validation as reasonable intervals to define delayed PMRT.
Study Outcomes
The primary endpoint for this study was overall survival (OS). OS was defined as the time from diagnosis to death or the last follow-up. Mortality was defined as all-cause mortality and was not limited to breast cancer related causes. Secondary endpoints included odds of a patient experiencing a delay in PMRT and odds of receipt of reconstruction following mastectomy.
Statistical Analysis
Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables, stratified by reconstruction after mastectomy. Differences between groups were tested using the chi-square test for categorical variables, and analysis of variance (ANOVA), as appropriate, for continuous variables. Logistic regression was used to estimate the association of reconstruction with the odds of delayed PMRT, after adjustment for the covariates. An additional logistic regression model was used to identify factors associated with the odds of reconstruction following mastectomy. All logistic regression models were built in the generalized estimating equations framework and included an exchangeable correlation structure. Kaplan-Meier curves were used to visualize unadjusted overall survival and log-rank tests were used to test for differences between groups. Median OS, and 5- and 10-year survival rates were estimated using the Kaplan-Meier method. Cox Proportional Hazards models were used to estimate the association of delayed PMRT and reconstruction on OS after adjustment for age, race, insurance type, facility type and location, Charlson/Deyo score, clinical T and N stage, ER and PR status, laterality, tumor grade, and chemotherapy type. An additional Cox Proportional Hazards model that included a delay*reconstruction interaction term was utilized to determine if the effect of a delay in PMRT differs for patients who underwent reconstruction vs. no reconstruction, and pairwise hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated from this model for each combination of delay and reconstruction. To account for the correlation of patients treated at the same facility, a robust sandwich covariance estimator was included in all adjusted survival models.
To determine what length of delay in PMRT begins to impact survival, three multivariable Cox proportional hazards models with restricted cubic splines (RCS) were created to identify the optimal days to radiation from surgery for adjuvant, neoadjuvant and no chemotherapy patients. Covariates in the models included age, race, insurance status, comorbidity score, T/N stage at diagnosis, ER-status, PR-status, tumor grade, hospital type, hospital location, and laterality. The use of RCS allows for a flexible multivariable model that accounts for the nonlinear relationship between time to radiation from surgery and survival without assuming the existence of potential cut points. Three, 4-, and 5-knot models were examined and 4-knot models, with knots placed at the 5th, 35th, 65th, and 95th percentiles were chosen based on the Akaike Information Criteria (AIC).26,27 Results from each model identify a “days to radiation value” corresponding to the critical point of the log hazard ratio function. To estimate a threshold value more representative of the true patient population, a bootstrap simulation with a Monte Carlo Markov Chain procedure with 1000 iterations was used for each chemotherapy group. The mean of all estimated thresholds was used as the days to radiation threshold for each chemotherapy group, and a 95% confidence interval (CI) was estimated as the 2.5th-97.5th percentile of the distribution of bootstrapped thresholds. No adjustments were made for multiple comparisons. Only patients with available data for all covariates were included in each analysis. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary NC).
RESULTS
Patient Characteristics
There were 171,136 patients analyzed after all exclusions. Of these, 24.4% (n=41,784) underwent mastectomy with reconstruction, and 75.6% (n=129,352) underwent mastectomy without reconstruction. The baseline unadjusted comparison of patient demographics and oncologic characteristics of patients who did and did not undergo breast reconstruction are shown in Table, Supplemental Digital Content 1. Overall, the median age of the patient cohort was 54 (IQR 46–64). Patients undergoing breast reconstruction predominately displayed the following characteristics: white race, fewer comorbidities, median incomes ≥$48,000, from a large metropolitan setting, private insurance, positive hormone (ER/PR) receptor status, and negative HER-2 receptor status. Chemotherapy was received by 90.4% of patients in the reconstruction cohort and 84.8% of patients in the non-reconstruction cohort.
Association of Immediate Breast Reconstruction and Time to PMRT
Unadjusted baseline comparisons revealed that patients who underwent breast reconstruction received PMRT at a greater time interval from surgery as compared to those who did not undergo reconstruction [median, 154 days (IQR, 64–196) vs. 132 (IQR, 52–188.5), p<0.001] Figure, Supplemental Digital Content 2. Given the observed difference in reconstruction and non-reconstruction cohorts, we then assessed if breast reconstruction was associated with a delay in PMRT, as defined in our study, after adjustment for other covariates. As shown in Table 1, multivariable logistic regression revealed that patients who underwent breast reconstruction were more likely to experience a delay in PMRT (OR, 1.25; 95% CI, 1.188–1.314; p<0.001). Other factors associated with a delay in PMRT included: increased Charlson-Deyo scores, neoadjuvant chemotherapy, non-private insurance, and black race. The receiver operating characteristic curve (ROC) which corresponds with the regression model utilized in Table 1 is included as Figure, Supplemental Digital Content 3.
Table 1:
Predictors of experiencing a delay in post-mastectomy radiation therapy
| OR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Group | <0.001 | ||
| No Reconstruction | -REF- | ||
| Reconstruction | 1.25 (1.188 – 1.314) | <0.001 | |
| Age (years) | 0.995 (0.993 – 0.996) | <0.001 | |
| Race | <0.001 | ||
| White | -REF- | ||
| Asian | 0.804 (0.729 – 0.888) | <0.001 | |
| Black | 1.215 (1.159 – 1.275) | <0.001 | |
| Other | 1.122 (0.979 – 1.287) | 0.10 | |
| Insurance Status | <0.001 | ||
| Private | -REF- | ||
| Government | 1.265 (1.214 – 1.318) | <0.001 | |
| Not Insured | 1.273 (1.15 – 1.408) | <0.001 | |
| Facility Location | 0.007 | ||
| West | -REF- | ||
| Midwest | 0.833 (0.751 – 0.925) | <0.001 | |
| Northeast | 0.906 (0.806 – 1.019) | 0.10 | |
| South | 0.933 (0.842 – 1.033) | 0.18 | |
| Facility Type | <0.001 | ||
| Integrated Network | -REF- | ||
| Academic | 1.055 (0.894 – 1.246) | 0.52 | |
| Community | 1.284 (1.096 – 1.505) | 0.002 | |
| Comprehensive | 1.166 (1.001 – 1.359) | 0.05 | |
| Grade | 0.07 | ||
| 1 | -REF- | ||
| 2 | 1.036 (0.981 – 1.095) | 0.20 | |
| 3 | 1.068 (1.007 – 1.133) | 0.03 | |
| Clinical N | <0.001 | ||
| cNX | -REF- | ||
| cN0 | 0.867 (0.806 – 0.932) | <0.001 | |
| cN1 | 0.781 (0.723 – 0.844) | <0.001 | |
| cN2 | 0.852 (0.784 – 0.925) | <0.001 | |
| cN3 | 0.712 (0.642 – 0.789) | <0.001 | |
| Clinical T | <0.001 | ||
| cTX | -REF- | ||
| cT0 | 0.988 (0.749 – 1.302) | 0.93 | |
| cT1 | 0.892 (0.824 – 0.965) | 0.005 | |
| cT2 | 0.971 (0.9 – 1.048) | 0.45 | |
| cT3 | 0.874 (0.805 – 0.948) | 0.001 | |
| cT4 | 0.828 (0.763 – 0.898) | <0.001 | |
| CDCC Score | <0.001 | ||
| 0 | -REF- | ||
| 1 | 1.216 (1.158 – 1.276) | <0.001 | |
| ≥ 2 | 1.504 (1.37 – 1.651) | <0.001 | |
| Chemotherapy Type | <0.001 | ||
| No Chemotherapy | -REF- | ||
| Adjuvant Only | 0.178 (0.166 – 0.19) | <0.001 | |
| Neoadjuvant | 1.162 (1.084 – 1.246) | <0.001 | |
| ER | 0.84 | ||
| Positive | -REF- | ||
| Negative | 0.995 (0.945 – 1.047) | 0.84 | |
| PR Status | 0.81 | ||
| Positive | -REF- | ||
| Negative | 1.006 (0.96 – 1.054) | 0.81 | |
| Laterality | <0.001 | ||
| Unilateral | -REF- | ||
| Bilateral | 0.847 (0.814 – 0.881) | <0.001 |
The model has accounted the correlation of patients treated at same hospital.
Impact of Breast Reconstruction and Delayed PMRT on Overall Survival
Despite immediate breast reconstruction increasing the risk for experiencing a delay in PMRT, an adjusted Cox Proportional Hazard model demonstrated no evidence of reduced adjusted overall survival in this cohort (HR, 0.836; 95% CI, 0.802–0.871; p<0.001). Factors associated with a reduced overall survival were tumor stage, increased Charlson-Deyo score, negative hormone receptor status, higher tumor grade, non-private insurance, treatment in the South or Midwest, and black race (Table 2). The model with delay*reconstruction interaction yielded similar results. None of the pairwise hazard ratio indicates a significant reduction in adjusted overall survival (Table, Supplemental Digital Content 4). Kaplan-Meier curves which were used to estimate the overall survival rates are shown in Figure, Supplemental Digital Content 5.
Table 2:
Overall survival of the patient cohort
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Group | |||
| No Reconstruction | -REF- | <0.001 | |
| Reconstruction | 0.836 (0.802 – 0.871) | <0.001 | |
| Radiation Delay | 0.74 | ||
| No | -REF- | ||
| Yes | 1.006 (0.971 – 1.043) | 0.74 | |
| Age (years) | 1.019 (1.018 – 1.021) | <0.001 | <0.001 |
| Race | <0.001 | ||
| White | -REF- | ||
| Asian | 0.751 (0.68 – 0.83) | <0.001 | |
| Black | 1.225 (1.178 – 1.274) | <0.001 | |
| Other | 0.958 (0.837 – 1.096) | 0.53 | |
| Insurance | <0.001 | ||
| Private | -REF- | ||
| Not Insured | 1.254 (1.148 – 1.37) | <0.001 | |
| Government | 1.271 (1.229 – 1.313) | <0.001 | |
| Facility Type | <0.001 | ||
| Integrated Network | -REF- | ||
| Academic | 0.959 (0.893 – 1.03) | 0.25 | |
| Comprehensive | 1.052 (0.989 – 1.119) | 0.11 | |
| Community | 1.117 (1.04 – 1.2) | 0.002 | |
| Facility Location | <0.001 | ||
| West | -REF- | ||
| Northeast | 1.087 (1.022 – 1.156) | 0.008 | |
| South | 1.138 (1.078 – 1.201) | <0.001 | |
| Midwest | 1.156 (1.091 – 1.226) | <0.001 | |
| Grade | <0.001 | ||
| 1 | -REF- | ||
| 2 | 1.347 (1.273 – 1.425) | <0.001 | |
| 3 | 1.848 (1.743 – 1.961) | <0.001 | |
| CDCC Score | <0.001 | ||
| 0 | -REF- | ||
| 1 | 1.261 (1.216 – 1.307) | <0.001 | |
| ≥ 2 | 1.714 (1.601 – 1.835) | <0.001 | |
| Clinical N | <0.001 | ||
| cNX | -REF- | ||
| cN0 | 0.812 (0.766 – 0.86) | <0.001 | |
| cN1 | 0.997 (0.941 – 1.057) | 0.93 | |
| cN2 | 1.179 (1.106 – 1.257) | <0.001 | |
| cN3 | 1.534 (1.425 – 1.652) | <0.001 | |
| Clinical T | <0.001 | ||
| cTX | -REF- | ||
| cT0 | 1.022 (0.764 – 1.368) | 0.88 | |
| cT1 | 0.74 (0.693 – 0.789) | <0.001 | |
| cT2 | 0.954 (0.901 – 1.01) | 0.11 | |
| cT3 | 1.13 (1.061 – 1.204) | <0.001 | |
| cT4 | 1.314 (1.234 – 1.399) | <0.001 | |
| ER Status | <0.001 | ||
| Positive | -REF- | ||
| Negative | 1.306 (1.257 – 1.357) | <0.001 | |
| PR Status | <0.001 | ||
| Positive | -REF- | ||
| Negative | 1.373 (1.325 – 1.424) | <0.001 | |
| Laterality | <0.001 | ||
| Unilateral | -REF- | ||
| Bilateral | 0.897 (0.866 – 0.93) | <0.001 | |
| Chemotherapy Type | <0.001 | ||
| No Chemotherapy | -REF- | ||
| Adjuvant Only | 0.566 (0.544 – 0.589) | <0.001 | |
| Neoadjuvant | 0.826 (0.789 – 0.866) | <0.001 |
The model has accounted the correlation of patients treated at same hospital.
Further evaluation of the relationship between the timing of PMRT and survival with restricted cubic spline analysis identified the threshold number of days at which the start of PMRT began to impact survival at 169 (95% CI, 160–190), 75 (95% CI, 42–90), and 71 (95% CI, 41–90) days in patients undergoing adjuvant, neoadjuvant, and no chemotherapy, respectively (Figure 2). These results, in addition to the finding that within our cohort 94.7% of patients who underwent adjuvant chemotherapy received PMRT within 6 months of surgery, support our defined intervals for delayed PMRT in the reconstruction and non-reconstruction cohorts as reasonable cut points.
Figure 2:
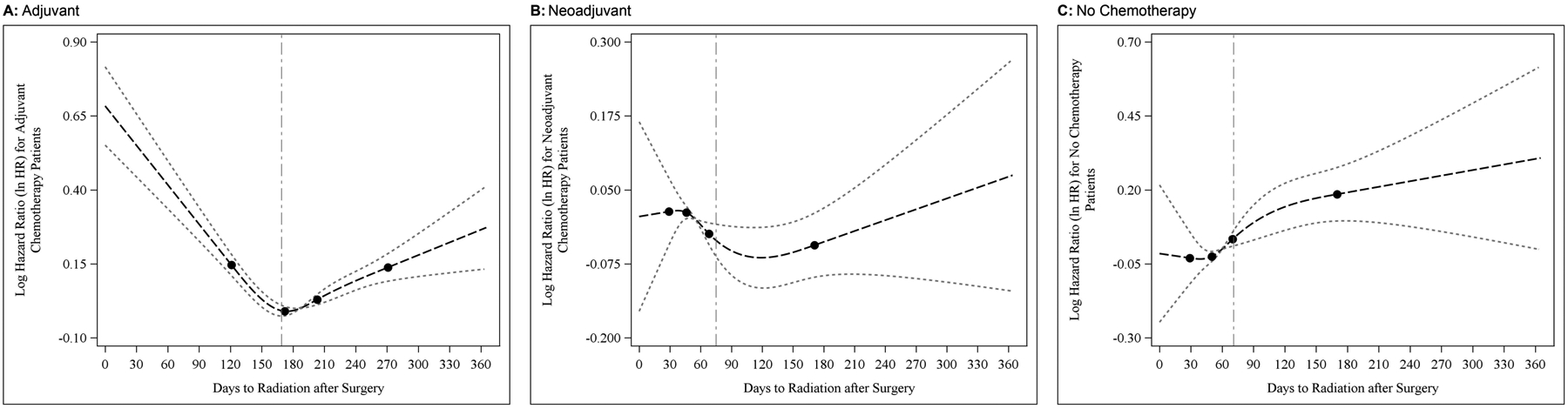
Smoothed restricted cubic spline plot of the adjusted log hazard ratio of death in patients receiving adjuvant (A), neoadjuvant (B), and no chemotherapy (C). The predicted hazards ratios were estimated from a proportional hazards model adjusting for covariates. The light dotted curves represent the 95% confidence intervals about the predicted odds ratio. The black dots correspond to the location of 4 knots used in the model. The dashed vertical line indicates the bootstrapped minimum.
Predictors of Undergoing Breast Reconstruction
To understand the factors that influence the receipt of breast reconstruction, a multivariate logistic regression was performed to identify predictors of breast reconstructive surgery (Table 3). Decreased age, reduced tumor stage, reduced comorbidities, increased income, private insurance, bilateral breast cancer, treatment in the Northeast or Midwest, and white race were factors associated with an increased likelihood of undergoing breast reconstruction. The ROC utilized in this analysis is included as Figure, Supplemental Digital Content 6.
Table 3:
Predictors of patients undergoing breast reconstruction
| OR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Age (years) | 0.961 (0.96 – 0.963) | <0.001 | <0.001 |
| Race | <0.001 | ||
| White | -REF- | ||
| Asian | 0.658 (0.605 – 0.715) | <0.001 | |
| Black | 0.864 (0.825 – 0.905) | <0.001 | |
| Other | 0.851 (0.744 – 0.974) | 0.02 | |
| Income Level | <0.001 | ||
| < 48,000 | -REF- | ||
| >= 48,000 | 1.226 (1.184 – 1.27) | <0.001 | |
| Area Rurality | 0.01 | ||
| Rural | -REF- | ||
| Metro | 1.179 (1.044 – 1.331) | 0.008 | |
| Urban | 1.113 (0.978 – 1.267) | 0.11 | |
| Insurance Status | <0.001 | ||
| Private | -REF- | ||
| Government | 0.606 (0.582 – 0.63) | <0.001 | |
| Not Insured | 0.467 (0.427 – 0.512) | <0.001 | |
| Facility Type | <0.001 | ||
| Integrated Network | -REF- | ||
| Academic | 0.701 (0.507 – 0.97) | 0.03 | |
| Community | 0.366 (0.27 – 0.496) | <0.001 | |
| Comprehensive | 0.582 (0.429 – 0.788) | <0.001 | |
| Facility Location | <0.001 | ||
| West | -REF- | ||
| Midwest | 1.284 (1.025 – 1.608) | 0.03 | |
| Northeast | 2.152 (1.683 – 2.753) | <0.001 | |
| South | 1.07 (0.852 – 1.344) | 0.56 | |
| CDCC Score | <0.001 | ||
| 0 | -REF- | ||
| 1 | 0.877 (0.837 – 0.918) | <0.001 | |
| ≥ 2 | 0.721 (0.646 – 0.805) | <0.001 | |
| Grade | <0.001 | ||
| 1 | -REF- | ||
| 2 | 0.958 (0.916 – 1.002) | 0.06 | |
| 3 | 0.853 (0.813 – 0.895) | <0.001 | |
| Clinical N | <0.001 | ||
| cNX | -REF- | ||
| cN0 | 1.609 (1.49 – 1.736) | <0.001 | |
| cN1 | 1.294 (1.195 – 1.401) | <0.001 | |
| cN2 | 1.079 (0.991 – 1.174) | 0.08 | |
| cN3 | 1.057 (0.96 – 1.163) | 0.26 | |
| Clinical T | <0.001 | ||
| cTX | -REF- | . | |
| cT0 | 1.027 (0.804 – 1.311) | 0.83 | |
| cT1 | 1.485 (1.382 – 1.596) | <0.001 | |
| cT2 | 1.272 (1.184 – 1.367) | <0.001 | |
| cT3 | 0.992 (0.919 – 1.072) | 0.85 | |
| cT4 | 0.51 (0.466 – 0.559) | <0.001 | |
| ER Status | |||
| Positive | -REF- | <0.001 | |
| Negative | 0.861 (0.822 – 0.902) | <0.001 | |
| PR Status | -REF- | <0.001 | |
| Positive | 0.922 (0.886 – 0.96) | <0.001 | |
| Negative | |||
| Laterality | <0.001 | ||
| Unilateral | -REF- | ||
| Bilateral | 2.219 (2.123 – 2.319) | <0.001 |
The model has accounted the correlation of patients treated at same hospital.
DISCUSSION
In this national evaluation of women undergoing PMRT for breast cancer, we found that although immediate breast reconstruction increased the risk for delayed initiation of PMRT, the delay was clinically insignificant and did not impact overall survival among women undergoing breast reconstruction. The rates of patients undergoing immediate breast reconstruction have continued to steadily increase, causing physicians to question the impact of immediate breast reconstruction on the timely administration of adjuvant treatment for breast cancer.24 Our findings suggest that women with comorbid conditions, those who received neoadjuvant chemotherapy, patients with non-private insurance, and those of black race are at higher risk of experiencing delays in PMRT after breast reconstruction, but that for most women, including these vulnerable patients, these delays do not impact overall survival. Furthermore, the restricted cubic spline analysis utilized in this study identified the threshold number of days at which the start of PMRT began to impact survival at 169 (95% CI, 160–190), 75 (95% CI, 42–90), and 71 (95% CI, 41–90) days in patients undergoing adjuvant, neoadjuvant, and no chemotherapy, respectively. Ultimately, we found that immediate breast reconstruction should remain an option in appropriately selected patients who require radiotherapy after mastectomy.
Other authors have reported variable association of breast reconstruction with post-operative complications that have the potential to delay adjuvant therapy for breast cancer. Zhong et al. and Hamahata et al. reported that patients undergoing immediate breast reconstruction were at a higher risk for experiencing surgical-related complications; however, immediate breast reconstruction was not independently predictive of a higher complication rate following adjustment for confounders.28,29 However, in 14,894 women undergoing mastectomy for breast cancer, and who underwent immediate autologous reconstruction, immediate implant-based reconstruction, or no reconstruction within the first 2 postoperative years, Jagsi et al. reported significantly increased odds of 30-day rehospitalization, wound complications, and infections amongst patients undergoing breast reconstruction.30
Current National Comprehensive Cancer Network guidelines recommend consideration of PMRT for individuals with 1–3 positive lymph nodes, as well as some node-negative patients with large or high-risk tumors, resulting in an increasing number of patients undergoing radiotherapy after mastectomy.24,31 The potentially deleterious interaction of PMRT and breast reconstruction, including increased rates of infection, implant loss, wound dehiscence, and fat necrosis,30 have left some multidisciplinary teams deferring immediate reconstruction in favor of a delayed approach, with hopes of avoiding these complications and interruptions in cancer treatment. Our study suggests that these concerns are not unfounded; women undergoing immediate breast reconstruction were more likely to experience a delay in the initiation of PMRT; however, importantly, we found that this delay did not impact overall survival. Although we were unable to determine the reasons behind delays in PMRT, they may be due to increased complications in the immediate post-operative period following breast reconstruction or possibly, radiation oncologists may allow longer healing times due to concerns about increased risks for complications with radiotherapy. Furthermore, this study identified patients with comorbid conditions, those who received neoadjuvant chemotherapy, patients with non-private insurance, and those of black race as groups who may be at an increased risk for experiencing delayed PMRT. Careful consideration should be given to the additional delay of PMRT that is associated with immediate breast reconstruction in these patient populations. These findings may help guide providers in identifying those women who are at greatest risk of experiencing delays in their PMRT, and in whom these delays have the potential to impact oncologic outcomes in the setting of planned PMRT and immediate breast reconstruction. Overall, the results of our study suggest that appropriately selected patients requiring PMRT may consider immediate breast reconstruction as part of their multidisciplinary care.
This study used national patient data to examine the influence of immediate breast reconstruction on the time to PMRT. Importantly, we acknowledge that there are no nationally defined intervals within which a patient is recommended to receive PMRT, and variations in institutional practices may exist. However, the results from the restricted cubic spline analysis, support our defined intervals for delayed PMRT. While data that adequately evaluates the optimal time from surgery to PMRT is limited, our results are in line with previously published reports that have helped to guide radiotherapy treatment timelines following mastectomy.32,33
Our study has several limitations that deserve to be acknowledged. First, we were unable to determine the specific causes for delays in the initiation of PMRT, which may include factors related to specific chemotherapy regimens, surgical complications, or methods of breast reconstruction. In addition, while we found that factors such as increased Charlson-Deyo scores, neoadjuvant chemotherapy, non-private insurance, and black race demonstrated an association with an increased time to PMRT, it is important to emphasize that because this is an observational study from a large administrative database, we cannot assess causation. Second, our cohort included a unique population of women with breast cancer who were healthy enough to undergo breast reconstruction, but who also had advanced disease requiring PMRT. It is unclear how these findings might apply to the general population of older women with early-stage breast cancer and underlying comorbidities that place them at a higher risk for experiencing delays in their adjuvant therapy. There also remains no nationwide consensus of when to offer breast reconstruction or exactly when to initiate PMRT following reconstructive surgery. Therefore, these decisions are often individualized based on provider and patient preferences.
CONCLUSION
Immediate breast reconstruction is an important component of contemporary multidisciplinary breast cancer care. Many factors influence reconstructive decisions and their timing, including the risk of delaying adjuvant breast cancer treatment. Recent studies have demonstrated that despite the expanded indications for PMRT, rates of breast reconstruction have not declined.24 Therefore, this study asks an important question: Does immediate breast reconstruction lead to a delay of PMRT, and if so, does this affect overall patient survival? Our study reveals that immediate breast reconstruction is associated with an increased risk of delayed PMRT but does not affect overall patient survival. Overall, these findings emphasize that immediate breast reconstruction should remain an option for appropriately selected patients who will benefit from radiation therapy after mastectomy for breast cancer. We hope this study will help inform decisions for breast reconstruction among women requiring post-mastectomy radiotherapy and guide appropriate patient selection for immediate breast reconstruction in this setting.
Supplementary Material
Figure, Supplemental Digital Content 2: Time to post-mastectomy radiation therapy after mastectomy
Figure, Supplemental Digital Content 3: Receiver Operating Characteristic Curve Associated with Time to PMRT
Figure, Supplemental Digital Content 5: Kaplan-Meier Curves Estimating Overall Patient Survival. (A) Represents the comparison between reconstruction and non-reconstruction cohorts. (B) Represents the comparison between the delay and no-delay PMRT cohorts. (C) Represents the comparison between reconstruction without a delay in therapy vs. reconstruction with a delay in therapy vs. no reconstruction without a delay in a therapy vs. no reconstruction with a delay in therapy.
Figure, Supplemental Digital Content 6: Receiver Operating Characteristic Curve Associated with Predictors of Undergoing Breast Reconstruction
Table, Supplemental Digital Content 1: Patient characteristics
Table, Supplemental Digital Content 4: Pairwise Hazard Ratio with the interaction model
Declaration of Funding:
Dr. RA Greenup is supported by the National Institutes of Health Office of Research in Women’s Health and National Institute of Child Health and Human Development Building Interdisciplinary Research Careers in Women’s Health K12HD043446 (PI: Andrews).
Footnotes
NCDB Data Use Statement: The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Cocquyt VF, Blondeel PN, Depypere HT, et al. Better cosmetic results and comparable quality of life after skin-sparing mastectomy and immediate autologous breast reconstruction compared to breast conservative treatment. British journal of plastic surgery 2003;56:462–470. [DOI] [PubMed] [Google Scholar]
- 3.Harcourt DM, Rumsey NJ, Ambler NR, et al. The psychological effect of mastectomy with or without breast reconstruction: a prospective, multicenter study. Plastic and reconstructive surgery 2003;111:1060–1068. [DOI] [PubMed] [Google Scholar]
- 4.Jagsi R, Jiang J, Momoh AO, et al. Trends and Variation in Use of Breast Reconstruction in Patients With Breast Cancer Undergoing Mastectomy in the United States. Journal of Clinical Oncology 2014;32:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plastic and reconstructive surgery 2000;106:1014–1025; discussion 1026–1017. [DOI] [PubMed] [Google Scholar]
- 6.Neyt MJ, Blondeel PN, Morrison CM, Albrecht JA Comparing the cost of delayed and immediate autologous breast reconstruction in Belgium. British journal of plastic surgery 2005;58:493–497. [DOI] [PubMed] [Google Scholar]
- 7.Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW The psychological impact of immediate rather than delayed breast reconstruction. European Journal of Surgical Oncology (EJSO) 2000;26:17–19. [DOI] [PubMed] [Google Scholar]
- 8.Khoo A, Kroll SS, Reece GP, et al. A comparison of resource costs of immediate and delayed breast reconstruction. Plastic and reconstructive surgery 1998;101:964–968; discussion 969–970. [DOI] [PubMed] [Google Scholar]
- 9.Lee BT, A. A. T,, Colakoglu S, et al. Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Annals of plastic surgery 2010;64:679–683. [DOI] [PubMed] [Google Scholar]
- 10.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plastic and reconstructive surgery 2004;113:1617–1628. [DOI] [PubMed] [Google Scholar]
- 11.Kronowitz SJ, Robb GL Radiation therapy and breast reconstruction: a critical review of the literature. Plastic and reconstructive surgery 2009;124:395–408. [DOI] [PubMed] [Google Scholar]
- 12.Spear SL, Ducic I, Low M, Cuoco F The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plastic and reconstructive surgery 2005;115:84–95. [PubMed] [Google Scholar]
- 13.Thomson HJ, Potter S, Greenwood RJ, et al. A prospective longitudinal study of cosmetic outcome in immediate latissimus dorsi breast reconstruction and the influence of radiotherapy. Annals of surgical oncology 2008;15:1081–1091. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plastic and reconstructive surgery 2004;113:877–881. [DOI] [PubMed] [Google Scholar]
- 15.Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer 2012;118:2552–2559. [DOI] [PubMed] [Google Scholar]
- 16.Alderman AK, Collins ED, Schott A, et al. The Impact of Breast Reconstruction on the Delivery of Chemotherapy. Cancer 2010;116:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Lee SK, Kim S, et al. Does Immediate Breast Reconstruction after Mastectomy affect the Initiation of Adjuvant Chemotherapy? Journal of Breast Cancer 2011;14:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xavier Harmeling J, Kouwenberg CAE, Bijlard E, Burger KNJ, Jager A, Mureau MAM The effect of immediate breast reconstruction on the timing of adjuvant chemotherapy: a systematic review. Breast Cancer Research and Treatment 2015;153:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SA, Hiley C, Nickleach D, et al. Breast reconstruction and post-mastectomy radiation practice. Radiation oncology (London, England) 2013;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. International Journal of Radiation Oncology*Biology*Physics 2006;66:76–82. [DOI] [PubMed] [Google Scholar]
- 21.Schechter NR, Strom EA, Perkins GH, et al. Immediate breast reconstruction can impact postmastectomy irradiation. American journal of clinical oncology 2005;28:485–494. [DOI] [PubMed] [Google Scholar]
- 22.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. The New England journal of medicine 1997;337:956–962. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet (London, England) 1999;353:1641–1648. [DOI] [PubMed] [Google Scholar]
- 24.Frasier LL, Holden S, Holden T, et al. Temporal Trends in Post-Mastectomy Radiation Therapy and Breast Reconstruction. JAMA oncology 2016;2:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Database, N. C. National Cancer Database.
- 26.Harrell FE Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. Cham: Springer; 2015. [Google Scholar]
- 27.Desquilbet L, Mariotti F Dose-response analyses using restricted cubic spline functions in public health research. Statistics in medicine 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 28.Hamahata A, Kubo K, Takei H, et al. Impact of immediate breast reconstruction on postoperative adjuvant chemotherapy: a single center study. Breast cancer (Tokyo, Japan) 2015;22:287–291. [DOI] [PubMed] [Google Scholar]
- 29.Zhong T, Hofer SO, McCready DR, Jacks LM, Cook FE, Baxter N A comparison of surgical complications between immediate breast reconstruction and mastectomy: the impact on delivery of chemotherapy--an analysis of 391 procedures. Annals of surgical oncology 2012;19:560–566. [DOI] [PubMed] [Google Scholar]
- 30.Jagsi R, Jiang J, Momoh AO, et al. Complications After Mastectomy and Immediate Breast Reconstruction for Breast Cancer: A Claims-Based Analysis. Annals of surgery 2016;263:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network : JNCCN 2009;7:122–192. [DOI] [PubMed] [Google Scholar]
- 32.Tsoutsou PG, Koukourakis MI, Azria D, Belkacemi Y Optimal timing for adjuvant radiation therapy in breast cancer: a comprehensive review and perspectives. Critical reviews in oncology/hematology 2009;71:102–116. [DOI] [PubMed] [Google Scholar]
- 33.Hickey BE, Francis DP, Lehman M Sequencing of chemotherapy and radiotherapy for early breast cancer. The Cochrane database of systematic reviews 2013:Cd005212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 2: Time to post-mastectomy radiation therapy after mastectomy
Figure, Supplemental Digital Content 3: Receiver Operating Characteristic Curve Associated with Time to PMRT
Figure, Supplemental Digital Content 5: Kaplan-Meier Curves Estimating Overall Patient Survival. (A) Represents the comparison between reconstruction and non-reconstruction cohorts. (B) Represents the comparison between the delay and no-delay PMRT cohorts. (C) Represents the comparison between reconstruction without a delay in therapy vs. reconstruction with a delay in therapy vs. no reconstruction without a delay in a therapy vs. no reconstruction with a delay in therapy.
Figure, Supplemental Digital Content 6: Receiver Operating Characteristic Curve Associated with Predictors of Undergoing Breast Reconstruction
Table, Supplemental Digital Content 1: Patient characteristics
Table, Supplemental Digital Content 4: Pairwise Hazard Ratio with the interaction model


