Abstract
Background:
The aims of this study were to 1) determine whether acute nicotine withdrawal increases the intake of junk food (high in salt, fat, and sugar) and 2) assess whether the endogenous opioid system is involved in junk food intake during nicotine withdrawal using naltrexone as a pharmacological probe.
Methods:
Smokers were randomly assigned to 24-hr withdrawal from tobacco products (n = 42) or smoking ad libitum (n = 34). A non-smoking group (n = 29) was included. Participants completed two laboratory sessions where a placebo or 50 mg of naltrexone was administered. At the end of each session, participants were given a tray of snack items that differed in high to low energy density and dimensions of salty, sweet, and fat. Self-reported mood and withdrawal measures were collected immediately before the snacks were offered. Generalized linear and logistic models were used to assess the effects of acute smoking withdrawal, drug, and sex on the intake of snack items and self-reported measures.
Results:
Choice and consumption of food items were impacted by smoking condition (withdrawal > ad lib smoking and non-smokers; p < .05), the opioid blockade (naltrexone < placebo; p < .05), and sex (male > female; p < .05). The effects were evidenced in high sweet and high fat foods. No differences were found in low sweet and fat foods.
Conclusions:
These findings extend earlier studies indicating impact of tobacco use on appetite, and identify the regulatory influence of the endogenous opioid system on appetite during nicotine withdrawal.
Keywords: Smoking, Tobacco use, Opioid blockade, Naltrexone, Sex difference, Food intake
1.0. Introduction
Tobacco use and a poor diet are major preventable risk factors for the development of cardiovascular disease and cancer (Pérez-Escamilla et al., 2012; Vernarelli et al., 2015). These risk factors often co-exist in the same individual. The combination of tobacco use and a poor diet is a major health concern as their combined effects can decrease life expectancy by approximately 13 years (Peeters et al., 2003).
The relationship between tobacco use and poor diet may be explained by smokers’ preference for energy dense, high caloric food (Heydari et al., 2014; Raatz et al., 2017; Sutter et al., 2016). Data from a national representative study (N = 5,293) indicated that smokers consumed significantly more high caloric foods compared to non-smokers (MacLean et al., 2018). Another study using food diaries found that the intake of key nutrients was lower among smokers than in non-smokers (Raatz et al., 2017). Intake of high caloric foods is further enhanced during abstinence from tobacco use (Hall et al., 1989; Rodin, 1987; Spring et al., 1991; Wurtman and Wurtman, 1995), which increases 100 to 300 kcal/day after smoking cessation (Klesges et al., 1989; Yannakoulia et al., 2018), and contributes to post-smoking weight gain (Leischow and Stitzer, 1991). The fear of weight gain is a major barrier to abstinence among smokers contemplating quitting (Germeroth and Levine, 2018). Key to removing this barrier is an understanding of the mechanisms that increase the penchant for high caloric foods during smoking abstinence.
One explanation is that the withdrawal experience during smoking abstinence increases the rewarding properties of high caloric foods and motivation to consume them (Lerman et al., 2004; Stojakovic et al., 2017). In one study, smokers who abstained from smoking for 24 hours worked harder to earn carbohydrate-rich snacks compared to non-smokers (Spring et al., 2003). In a preclinical study (Bunney et al., 2016), rats trained to lever press for nicotine, sucrose, or a lower caloric food chow exhibited a significantly greater increase in sucrose vs food intake once nicotine was withdrawn. A possible neurobiological mechanism underpinning withdrawal-induced motivation to consume high caloric foods may involve the endogenous opioid system
Motivational aspects of food intake (taste, liking, and wanting) and tobacco smoking (Billes et al., 2014; Criscitelli and Avena, 2016; King et al., 2013; Mason et al., 2015a; Mason et al., 2015b; Murray et al., 2014) are both regulated, in part, by opioid neurotransmission within mesocorticolimbic areas of the brain (opioid system) (Criscitelli and Avena, 2016). Endogenous opioid blockade with naltrexone modulates mesocorticolimbic neuroactivity in those with substance dependence (Kohno et al. 2018; Tambour and Quertemont, 2006) and attenuates motivational aspects of eating and smoking behaviors (Lagleben et al. 2012; Mason et al., 2015a; Mason et al., 2015b; Rukstalis et al. 2005). However, no study has directly examined whether endogenous opioid blockade attenuates the heightened caloric intake that characterizes acute tobacco withdrawal. Demonstrating this may have clinical implications in light of observations that indicated that fear of post-quit weight gain may be a barrier to prolonged abstinence (Germeroth and Levine, 2018).
This study’s aim was to examine the effect of endogenous opioid blockade (naltrexone) on withdrawal-induced food intake. Participants selected foods that differed in sweet, fat, and salt palatability, to approximate the foods frequently eaten by smokers and smokers during acute withdrawal. Also, this study included a group of non-smokers as a comparison. We hypothesized that acute withdrawal from cigarette smoking would result in elevation of food intake, especially foods high in fat and sugar, and that endogenous opioid blockade would attenuate this effect. Further, as sex is a major differentiating factor in post smoking cessation weight gain and emerging evidence suggests endogenous opioid blockade has sex-specific effects (al'Absi et al., 2004), we investigated these relationships in men and women.
2.0. Methods and Materials
2.1. Participants
Participants aged 18-75 years were recruited using fliers posted around the University of Minnesota campus community and online advertisements. This study was part of a larger study that examined relationships between psychobiological stress response and endogenous opioid system in smokers going through an acute tobacco withdrawal. Results related to stress and other factors that are beyond the scope of this study will be reported elsewhere. Eligibility criteria for the study included: 1) no current nor history of hypertension, renal or hepatic disease, nor cardiac or other chronic diseases (e.g., coronary heart disease, diabetes, neurological disorders, thyroid disorders, respiratory disorders); 2) no current nor history of major psychiatric disorders (e.g., depression, schizophrenia, alcohol and drug abuse); 3) no current opiate dependence, nor recent daily opiate use, nor use of any narcotic medication within 3 days before the study; 4) no regular use of prescribed medication except contraceptives; 5) no pregnancy; and 6) weight within ±30% of Metropolitan Life Insurance norms. In addition, smokers needed to smoke at least 10 cigarettes per day over the past 2 years and had to show no interest in quitting cigarette smoking. Non-smokers must have smoked no more than 100 cigarettes in their lifetime and must not have smoked during the previous 5 years. This study was approved by the Institutional Review Board (IRB) of the University of Minnesota.
2.2. Laboratory Procedure
Interested participants contacted the Stress and Resilience Research Laboratory (Duluth and Minneapolis campuses, University of Minnesota) for an initial phone screening. Participants who met preliminary inclusion criteria were scheduled for an on-site medical screening. After written consent was obtained, participants’ height and weight were measured and information related to health status and contraindications to administration of naltrexone was collected. Participants were then asked to complete demographic and smoking-related self-report measures. Participants were scheduled for two laboratory sessions. Randomization to tobacco-use condition (ad libitum or withdrawal) took place at the end of the medical screening. Smokers who were assigned to the ad libitum cigarette smoking condition were asked to smoke cigarettes at their own pace for 24 hours before each session. Also, they were given a smoking break during the sessions to minimize effects of tobacco withdrawal. Smokers assigned to the smoking withdrawal condition were instructed to abstain from all tobacco products for 24 hours before each session. They were not allowed to use any nicotine products until the session was over. Non-smokers completed the same protocol except they were not randomized to a cigarette smoking condition.
All lab sessions started at noon to control for diurnal variations in hormones (not reported here). The sessions lasted approximately 4 hours and participants were being tested individually. Prior to arriving to each session, participants were asked to abstain from any medications including analgesics for three days, alcohol for 24 hours, and caffeine for 4 hours. Reminder calls were provided to the participant before each session. To limit discomfort due to in hunger during laboratory sessions and facilitate the control of diet prior to each laboratory session, light meals were provided. Participants were asked to consume the meal at least two hours before each session. These consisted of commercially available microwave lunches. They provided adequate nutrition, allowed the participant a choice of menus, and were easy to prepare. Selection of lunch items were determined with a study dietician.
Upon reporting to the laboratory, all participants completed a urine test to screen for drug use (including opioids) and pregnancy (women only). Smokers were asked to provide a sample of expired carbon monoxide (CO) using a Bedfont Micro+ monitor (coVita, Haddonfield, NJ). Smokers in the withdrawal condition who had CO of 9ppm or higher were rescheduled. The participant was brought to a testing room and provided with a standard lunch (see above). The laboratory session included the following periods: 1) baseline [20 min], 2) absorption [naltrexone or placebo: 40 min], 3) stress and other behavioral tasks [not reported here: 90 min], 4) rest [20 min], and 5) snack. During the snack period, a basket containing snack items (see below) was offered. The order of the drug condition was randomized using a double-blind procedure. Self-reported mood and withdrawal measures were collected after rest before offering the snack basket.
2.3. Measures and Apparatus
2.3.1. Demographic information and smoking history
In the medical screening, participants were asked about their age, education, and marital status. Height and weight were also measured. Smokers were asked about tobacco use history (i.e., cigarettes per day, years of smoking, nicotine dependence as assessed by Fagerstrom Test for Nicotine Dependence (FTND)(Heatherton et al., 1991)).
2.3.2. Assessment of Food Intake
A dietitian was consulted to develop the snack intake protocol. The protocol consisted of allowing participants to select from 8 commercially available snack food items classified into 4 groups (2 items per group) based on fat, salt, and sugar content: High Fat Sweet (HFSW): Nabisco Oreo Double Stuff Cakesters and Nabisco Nutter Butter Cookies, Low Fat Sweet (LFSW): Kellogg’s Original Rice Krispie Treats and Nabisco 100 Cal Lorna Doone Cookies, High Fat Salty (HFSA): Frito Lay Lay’s Classic Potato Chips and Keebler Toast and Peanut Butter Crackers, and Low Fat Salty (LFSA): Nabisco 100 Calorie Ritz Snack Mix and Pepperidge Farm Goldfish Cheddar Crackers. Participants were allowed to select as many snacks as they wanted from a serving tray. The amount of consumption was calculated by subtracting the weight of a snack item after the task from the weight of the item before the task while accounting for the wrapper weight. Macronutrients such as calories, fat (gram), sodium (mg), cholesterol (mg) and protein (g) were used from nutrition facts labels and calculated based on the consumption. Participants completed the lab with exactly the same protocol twice (naltrexone and placebo).
2.3.3. Assessment of Smoking Urges and Mood
Questionnaire of Smoking Urges-Brief:
The Questionnaire of Smoking Urges-Brief (QSU-B)(Cox et al., 2001; Tiffany and Drobes, 1991) assessed craving in the abstinent and current smoker groups. The assessment consisted of 10 items that measured the extent participants wanted to smoke in that moment on a 10-point Likert scale (1=Strongly Disagree and 10= Strongly Agree). The QSU-B assesses 2 dimensions of smoking urges: an intention to smoke to draw positive effects (positive reinforcement) and an intention to smoke to obtain relief from negative affect (negative reinforcement).
Subjective State Scale:
The Subjective State Scale (SSS)(al'Absi et al., 2003) was used to assess negative and positive affect. The positive affect subscale consists of items that assess the extent participants feel cheerful, content, calm, in control, and interested. The distress subscale assesses the extent participants feel anxious, irritable, impatient, and restless. SSS included items of the Minnesota Nicotine Withdrawal Scale (MNWS). They were: irritability, restlessness, anxiety, anger, depressed or sad mood, difficulty concentrating, and hunger; (Hughes and Hatsukami, 1998; Hughes et al., 1991). Two additional items were assessed alone, one, craving, refers to the extent the participant felt desire to smoke, and the other item, hunger refers to the extent the participant felt hungry.
2.4. Statistical Analyses
Descriptive statistics were used to characterize the demographics of the sample (sex, age, marital status, years of education, and BMI). One-way ANOVAs and chi-square tests were performed to compare smoking conditions on the demographic variables. For the primary analyses assessing food consumption, data for macronutrients consumed were semi-continuous and included both zero values, indicating participants did not eat any of a specific food type, and continuous values, indicating the amount consumed if the participant choose to eat a specific food type. When analyzing semi-continuous data with true zeros, parametric mixture distributions known as two-part models are the most appropriate as they allow for unbiased analysis of both zero and the nonzero values (Boulton and Williford, 2018). Two-part models are used to minimize bias intrinsic to zero inflated data when analyzed with conventional statistical approaches and they help to uncover processes that may differ with respect to whether a zero value is present and the process that determines the outcome level a participant has if they experience any level of it (Neelon and O’Malley, 2019; Olsen and Schafer, 2001). In this study, this relates to whether someone choses to eat a food item (step 1), and the amount the food item is eaten if selected (step 2). In step one, a binary variable was created and it was analyzed by a generalized logistic regression model with the generalized estimating equation (GEE) method (logit link function, binomial distribution, unstructured within-subject correlation matrix). Analyses were conducted on each food type separately. In step two, a continuous variable was created containing only nonzero values associated with amount of food consumed. Data (macronutrients consumed) were analyzed using generalized linear models with GEE method (log link function, gamma distribution, and unstructured within-subject correlation matrix). For all models, treatment (naltrexone and placebo) served as the within-subjects/repeated measures variable while smoking status (non-smoker, ad lib smoker, withdrawal smoker) and sex served as the between-subject factors. Interactions between smoking X treatment and sex X treatment were entered into all models. BMI and age were entered as covariates due to their relationships with food intake (de Boer et al., 2013; Togo et al., 2001). Also, GEE models (identity link function, normal distribution, and unstructured within-subject correlation matrix) were used to assess the relationship between the above predictors on self-reported smoking withdrawal (MNWS) and craving, mood, and hunger. Smoking-related measures were analyzed among smokers only. For all analyses involving multiple comparisons, significance values were adjusted using Bonferroni corrections. A normal probability distribution was used as the self-reported data followed a normal distribution. The Wald Chi-Square statistic was reported and represented the significance (p < .05) of all model effects and interactions. SPSS version 24 was used for all statistical analyses.
3.0. Results
3.1. Sample Characteristics
Of 118 participants who enrolled in the study, 11 participants were removed from analyses due to missing data (failed to show up at their second laboratory appointment) and two participants were removed because they did not consume any snack in both sessions, resulting in a total of N=105 for final analyses. The final N for each smoking condition was as follows: non-smokers: N=29; ad lib smokers: N= 34; withdrawal smokers: N=42. Preliminary analysis found no differences in demographic variables between those who were included in the final analysis and those who were not (p > .50) except that drop outs were significantly younger (t (116) = 2.48, p = .02). Table 1 shows participants’ demographic characteristics of the final sample. Participants tended to be in their mid- to late-thirties and approximately half were female. Smoking conditions significantly differed with respect to years of education (F (2, 100) = 8.45, p < .001) and post-hoc analyses indicated that non-smokers had a significantly greater number of years of education than smokers in both conditions (ps < .001). On average, smokers smoked 15 (SD = 5.9) cigarettes per day for 11 (SD = 9.8) years with a FTND score of 5 (SD = 2.2). These smoking-related variables did not differ between ad lib and withdrawal smoking conditions (p > .30). As expected, smokers in the withdrawal condition had lower CO than those in the ad lib condition in both lab 1 (t (74) = 7.89, p < .001) and lab 2 (t (73) = 9.06, p < .001; see Table 1).
Table 1.
Sample characteristics.
| Non-Smoker (N=29) |
Ad Lib (N=34) |
Withdrawal (N=42) |
|
|---|---|---|---|
| Sex (% Female) | 51.7% | 41.2% | 40.5% |
| Marital Status | |||
| Single | 65.5% | 73.5% | 81.0% |
| Married | 20.7% | 5.9% | 11.9% |
| Divorced | 6.9% | 11.8% | 2.4% |
| Widowed/Separated | 3.4% | 2.9% | 2.4% |
| No Answer | 3.4% | 5.9% | 2.4% |
| Age | 35.79 (11.97) | 34.12 (12.0) | 33.36 (11.35) |
| Years of Education* | 15.15 (2.40) | 12.74 (2.44) | 13.36 (2.21) |
| BMI | 26.95 (4.97) | 28.08 (6.75) | 25.71 (4.82) |
| Cigarettes per day | n/a | 14.82 (5.97) | 15.07 (5.97) |
| Years of smoking | n/a | 10.03 (8.92) | 11.54 (10.59) |
| FTND | n/a | 5.41 (2.27) | 4.90 (2.18) |
| CO | |||
| Lab 1 | n/a | 16.39 (9.99) | 2.79 (1.21) |
| Lab 2 | n/a | 15.15 (7.95) | 2.65 (1.32) |
Notes. Otherwise indicated, entries show mean and standard deviation.
Non-Smoker vs. Current Smoker and Withdrawal (p > 0.01).
3.2. Step 1: Choice to Eat Type of Food Item
Figure 1 shows the percentage of times participants from each group selected the HFSW (Figure 1A), HFSA (Figure 1B), LFSW (Figure 1C), and LFSA (Figure 1D) foods following naltrexone or placebo during the free-choice food consumption task. With respect to the choice to select the HFSW food items, there was a significant drug main effect (p<.01), but not for smoking condition, or sex. As shown in Figure 1A, participants in all smoking groups were less likely to choose the HFSW food after naltrexone treatment compared to placebo treatment (B = −.66, SE = .25, 95% CI= −1.15 to −.17, Wald chi-square = 6.93, df = 1, Sig = .008, exp(B) = .52, 95% CI for exp(B)= .32 to .85). There was also a significant model effect for the choice to consume the LFSW foods in the form of an interaction between treatment and smoking condition (Wald Chi-square=8.04; df=2; p<.05), such that naltrexone was specifically associated with reduced LFWS intake in the smoking withdrawal condition compared to placebo (B = −.93, SE = .32, 95% CI: 1.56 to −.30, Wald chi-square: 8.35; df = 1, Sig = .004, exp(B) = .39, 95% CI for exp(B) = .21 to .74).There was a main model effect for gender for choice of the LFSA (Wald Chi-square=4.79, df=1, p<.05) – men were less likely to choose the LFSA food items compared to women (B = −.71, SE = .31, 95% CI: −1.32 to −.10, Wald chi-square: 5.24, df = 1, Sig = .02, exp(B) = .49, 95% CI for exp(B) = .27 to .90). There were no significant model effects for HFSA.
Figure 1.
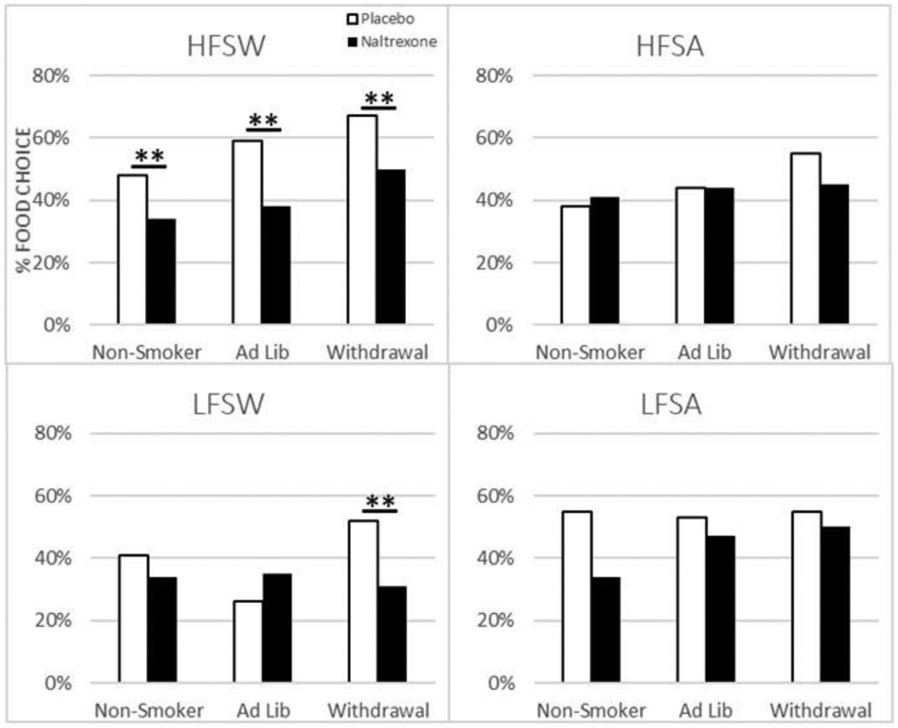
Percent Choice to Consume HFSW, HFSA, LFSW, and LFSA Food Items by smoking condition. White bars represent Placebo, black bars represent Naltrexone.
3.3. Step 2: Macronutrient Intake
For Step 2, assessing total macronutrient intake, food types were combined due to the close relationship between macronutrient content and the food type classification (e.g., HFSW foods have higher calories and fat compared to LFSA foods). Adjusted GEE models (controlling for age and BMI) indicated significant model main effects for drug (Wald Chi-square = 8.07; df = 1; p < .01) with fewer calories consumed following naltrexone vs. placebo (B = −.18; SE = .05; 95% CI= −.28 to −.07; Wald chi-square: 11.31; df = 1; Sig = .001, exp(B) = .84; 95% CI for exp(B) = .76 to .93), a smoking condition main effect (Wald Chi-square = 8.25; df = 2; p < .05) with participants in the ad lib smoking condition consuming fewer calories than smokers in the withdrawal condition (B = −.22, SE = .10, 95% CI: −.42 to −.011, Wald chi-square: 4.28, df = 1;,Sig = .04, exp(B) = .81, 95% CI for exp(B) = .66 to .98), and a significant gender effect (Wald Chi-square = 5.73; df = 1; p < .05) with men consuming more total calories than women (B = .20, SE = .09, 95% CI = .02 to .38, Wald chi-square = 4.53, df = 1, Sig = .03, exp(B) = 1.22; 95% CI for exp(B) = 1.02 to 1.46). Figure 2, shows the mean total calories consumed by non-smokers, ad lib smokers, and smokers undergoing withdrawal following administration of naltrexone and placebo. Compared to placebo (Mean = 455.10, SD = 274.40), treatment with naltrexone (Mean = 354.59, SD = 222.39) resulted in a decrease in total calories consumed. Smokers undergoing withdrawal consumed more calories (Mean = 457.58, SD = 272.19) than both non-smokers (Mean = 351.54, SD = 248.50) and ad lib smokers (Mean = 385.16, SD = 207.36). Males consumed more calories than females (p < .05, not shown). As would be expected owing to the relative proportion of macronutrient content remaining constant within the food items, the same model effects and direction were reported for the other macronutrients (fat, sodium, and carbs).
Figure 2.
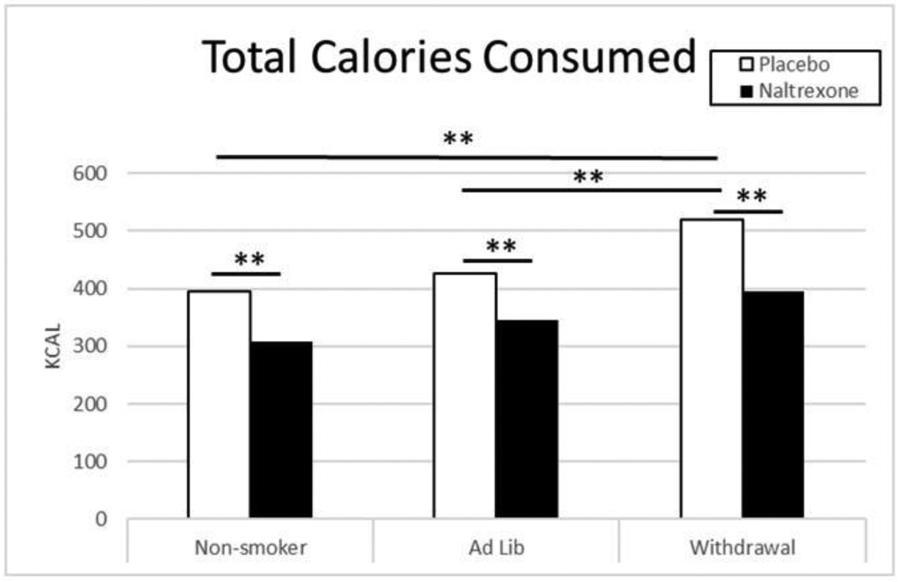
Total Calories Consumed (Food Types Combined) by drug and smoking condition. White bars represent Placebo, black bars represent Naltrexone.
3.4. Effects of Naltrexone and Smoking Status on Self-Reported Smoking Urges and Mood
Significant model effects were reported for withdrawal scores (Figure 3C) but not for craving (Figure 3A), positive reinforcement, nor negative reinforcement. For withdrawal, there was a significant main effect of smoking condition (Wald Chi-square = 5.33; df = 1; p<.05) only. Withdrawal symptoms (Figure 3C) were significantly higher among smokers in the smoking withdrawal condition compared to those in the ad lib smoking condition (B = 3.22, SE = 1.29, 95% CI: .69 to 5.75, Wald chi-square: 6.23, df = 1, Sig = .01, exp(B) = 25.03, 95% CI for exp(B) = 1.99 to 31.89;). With respect to the mood measures (Figure 3B), significant main effects were found for distress and hunger, but not for positive affect. For distress (Figure 3B), there was a main effect of smoking condition (Wald Chi-square = 14.88; df = 2; p < .01). In comparison to smokers in the withdrawal condition, ad-lib smokers (B = −3.51, SE = 1.14, 95% CI = −5.75 to −1.26, Wald chi-square = 9.39, df = 1, Sig. = .002, exp(B) = .03, 95% CI for exp(B) = .03 to .28) and non-smokers (B = −4.02, SE = 1.06, 95% CI = −6.11 to −1.94, Wald chi-square = 14.29, df = 1, Sig = .000, exp (B) = .018, 95% CI for exp(B) = .02 to .14) reported lower levels of For hunger there was a significant model effect of drug (Wald Chi-square = 9.10; df = 1; p < .01) and smoking condition (Wald Chi-square = 7.43; df = 2; p < .05). As shown in Figure 3D, treatment with naltrexone resulted in a significant decrease in hunger compared to placebo treatment (B = −.59, SE = .214, 95% CI: −1.01 to −.17, Wald chi-square = 7.51, df = 1, Sig = .006, exp(B) = .56, 95% CI for exp(B) = 0.37 to .85) and compared to hunger in non-smokers, hunger was higher among smokers in the ad-lib smoking condition (B = .93, SE = .38, 95% CI = .19 to 1.67, Wald chi-square = 6.14, df = 1, Sig = .013, exp(B) = 2.54, 95% CI for exp(B) = 1.22 to 5.32) and the withdrawal condition (B = .868, SE = .35, 95% CI = .18 to 1.60, Wald chi-square = 6.04, df = 1, Sig = .01, exp(B) = 2.38, 95% CI for exp(B) = 1.19 to 4.76).
Figure 3.
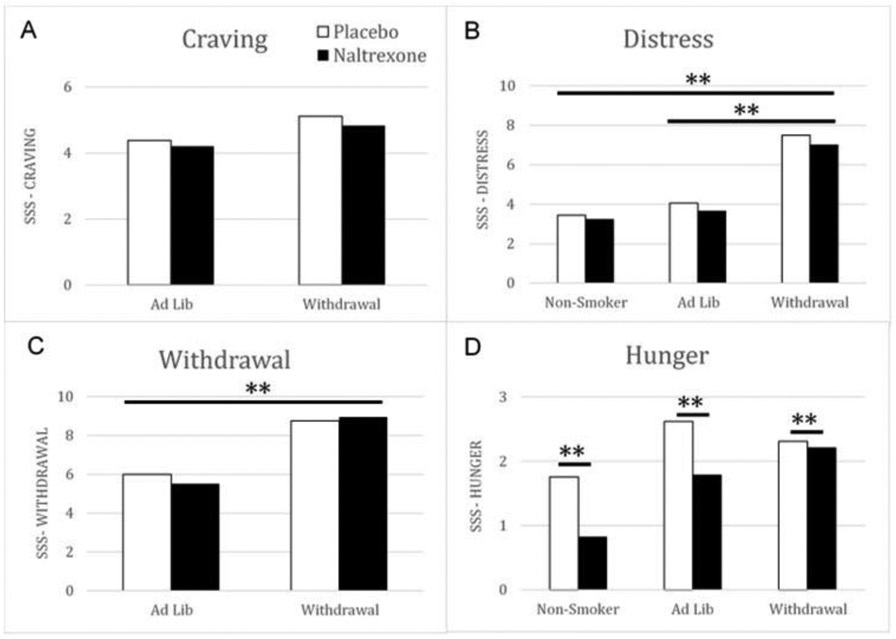
Effects of Naltrexone and Smoking Status on Self-Reported Smoking Urges and Mood. White bars represent Placebo, black bars represent Naltrexone.
4.0. Discussion
The results of this study shed light on the behavioral mechanisms that underlie the consumption of high caloric junk foods among cigarette smokers during states of ad lib smoking and states of acute withdrawal. In the present study, smokers undergoing acute tobacco withdrawal consumed significantly more calories and self-reported significantly higher levels of distress compared to ad lib smokers and non-smokers. Naltrexone administration resulted in a significant decrease in calories consumed across all smoking status groups. However, this decrease was greatest in the withdrawal group and generally restricted to foods with the highest fat and sugar content. Naltrexone administration also resulted in a reduction in self-reported hunger across all smoking status groups. Interestingly, this effect was stronger in the ad lib smoker and non-smoker groups. These results indicated that naltrexone did not influence self-reported hunger in the smoking withdrawal group, suggesting naltrexone decreased caloric intake through an alternative mechanism.
In the current study, acute tobacco withdrawal was characterized by higher levels of distress and increased total calorie intake relative to ad lib smokers and non-smokers. These results support a well-established literature (Hatsukami et al., 1993; Leischow and Stitzer, 1991; Moffatt and Owens, 1991; Stamford et al., 1986). Our findings may be related to the use of food, especially foods high in calories, to cope with the negative affect and distress that characterizes the smoking withdrawal state. Results from preclinical and clinical research support this and demonstrate that stress induction increases proclivity for high fat and high sugar foods (Epel et al., 2001; Steptoe et al., 1998). This has implications for smokers who likely experience acute withdrawal on a daily basis due to public smoking restrictions (e.g., hours go by during work where smoking is likely not permitted). It is possible that during these times of smoking restriction high caloric foods may be consumed in an attempt to “self-medicate” distress associated with acute withdrawal.
In the present study the mu opioid receptor agonist, opioid blockade using naltrexone significantly decreased food intake in all groups, especially with respect to smokers undergoing withdrawal who consumed approximately 110 fewer Kcal compared to placebo. This is in contrast to the approximate 60 Kcal decrease in non-smokers and ad lib smokers. The results from the subjective measures in the present study help to characterize the attenuating effects of endogenous opioid blockade on food consumption. Naltrexone suppressed self-reported hunger in line with previous work supporting naltrexone as a weight control agent (King et al., 2012; Maggio et al., 1985; Ornellas and Chavez, 2011). This suggests that opioid blockades attenuating effects on food intake may have more to do with increasing feelings of satiety. However, it is important to note this conclusion is drawn from a main effect largely associated with differences noted in non-smokers and ad lib smokers as is shown in Figure 3. While there was no significant interaction between treatment and smoking condition, it is clear this effect was greater among non-smokers and ad lib smokers, those in withdrawal showed little to now effect of naltrexone on self-reported hunger despite having a significant decrease in total calories consumed following naltrexone treatment. This suggests that a different process/mechanism may underlie the attenuating effects of endogenous opioid blockade on food intake among those experiencing smoking withdrawal.
5.0. Limitations
Results of this study are limited by the use of the cross-sectional approach. Whether chronic smoking caused changes in snack consumption or individuals who were predisposed to consume more snacks have an increased risk of tobacco use is not clear at this time. Since we recruited smokers who were not interested in cessation, results may not be generalizable. Further, our protocol assessed the influence of 24-hrs of smoking abstinence on food intake. Future work would benefit from extending the abstinence period to determine duration of withdrawal-induced increases in junk food intake. In addition, social desirability within the laboratory setting may have influenced food consumption behavior. A more systematic investigation involving treatment with extended period of naltexone and momentary assessment of withdrawal and food craving could provide a more naturalistic examination of the influence of opioid system function on withdrawal-induced hedonic aspects of junk foods. Snack intake in a laboratory setting with a limited choice may be different from snack intake patterns at home where there is likely a greater variety of food options including more vitamin rich foods such as fruit and vegetables. Future laboratory studies could enhance ecological validity by providing a greater variety of foods (e.g., fruits and vegetables). Finally, there was a significant difference between smokers and non-smokers in years of education, such that non-smokers had a significantly greater number of years of education compared to smokers. Thus, a potential influence of education level on the measures reported cannot be ruled out. Nevertheless, this study used a well-controlled design to systematically assess effects of acute smoking withdrawal and opioid blockade on food consumption and self-reported mood and withdrawal.
6.0. Conclusion
This study demonstrates that the smoking may be associated with heightened calorie intake during acute withdrawal experiences. Further, opioid blockade acutely normalized calorie intake to levels seen in non-smokers suggesting that the opioid system may be a mechanism of withdrawal-induced intake of calories. These results highlight a strong connection between acute smoking withdrawal and food intake that may contribute to the poor dietary choices of smokers undergoing acute withdrawal.
Highlights.
Tobacco use and a poor diet are risk factors for chronic diseases
We examined effects of endogenous opioid blockade on withdrawal-induced food intake
Participants were less likely to select high fat food after naltrexone than placebo
Smokers undergoing withdrawal consumed more calories than non-smokers
The opioid system may be a mechanism of withdrawal-induced intake of junk foods
Acknowledgment
We thank the following individuals for their help with collecting (Barbara Gay, Elizabeth Ford, Dayna Schleppenbach, Soni Rraklli Uccellini, Angie Forsberg) and managing (Jie Gooder) the data for this study.
Role of funding source
This research was supported in part by grants to the first author from the National Institute of Health (R01DA016351 and R01DA027232). The grant had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors of this manuscript do not have any conflict of interested related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al'Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE, 2004. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med 66(2), 198–206. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B, 2003. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior 74(2), 401–410. [DOI] [PubMed] [Google Scholar]
- Billes SK, Sinnayah P, Cowley MA, 2014. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res 84, 1–11. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Williford A, 2018. Analyzing Skewed Continuous Outcomes With Many Zeros: A Tutorial for Social Work and Youth Prevention Science Researchers. Journal of the Society for Social Work and Research 9(4), 721–740. [Google Scholar]
- Bunney PE, Burroughs D, Hernandez C, LeSage MG, 2016. The effects of nicotine self-administration and withdrawal on concurrently available chow and sucrose intake in adult male rats. Physiol Behav 154, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG, 2001. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Criscitelli K, Avena NM, 2016. The neurobiological and behavioral overlaps of nicotine and food addiction. Prev Med 92, 82–89. [DOI] [PubMed] [Google Scholar]
- de Boer A, Ter Horst GJ, Lorist MM, 2013. Physiological and psychosocial age-related changes associated with reduced food intake in older persons. Ageing Res Rev 12(1), 316–328. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K, 2001. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Germeroth LJ, Levine MD, 2018. Postcessation weight gain concern as a barrier to smoking cessation: Assessment considerations and future directions. Addict Behav 76, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield GS, Lumb A, 2008. Smoking, dietary restraint, gender, and the relative reinforcing value of snack food in a large university sample. Appetite 50(2-3), 278–289. [DOI] [PubMed] [Google Scholar]
- Hall SM, McGee R, Tunstall C, Duffy J, Benowitz N, 1989. Changes in food intake and activity after quitting smoking. J Consult Clin Psychol 57(1), 81–86. [DOI] [PubMed] [Google Scholar]
- Hall SM, Tunstall CD, Vila KL, Duffy J, 1992. Weight gain prevention and smoking cessation: cautionary findings. Am J Public Health 82(6), 799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, LaBounty L, Hughes J, Laine D, 1993. Effects of tobacco abstinence on food intake among cigarette smokers. Health Psychol 12, 499–502. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Heydari G, Heidari F, Yousefifard M, Hosseini M, 2014. Smoking and diet in healthy adults: a cross-sectional study in tehran, iran, 2010. Iran J Public Health 43(4), 485–491. [PMC free article] [PubMed] [Google Scholar]
- Hill DC, Moss RH, Sykes-Muskett B, Conner M, O'Connor DB, 2018. Stress and eating behaviors in children and adolescents: Systematic review and meta-analysis. Appetite 123, 14–22. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK, 1998. Errors in using tobacco withdrawal scale. Tob Control 7(1), 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW, 1991. Symptoms of tobacco withdrawal. Arch Gen Psychiatry 48, 52–59. [DOI] [PubMed] [Google Scholar]
- King AC, Cao D, O'Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stachoviak RJ, 2012. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol 32(5), 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Cao D, Zhang L, O'Malley SS, 2013. Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biol Psychiatry 73(9), 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, La Vasque ME, 1989. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull 106(2), 204–230. [DOI] [PubMed] [Google Scholar]
- Kohno M, Dennis LE, McCready H, Schwartz DL, Hoffman WF, Korthuis PT, 2018. A preliminary randomized clinical trial of naltrexone reduces striatal resting state functional connectivity in people with methamphetamine use disorder. Drug Alcohol Depend 192, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben DD, Busch EL, O'Brien CP, Elman I, 2012. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology (Berl) 220(3), 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischow SJ, Stitzer ML, 1991. Effects of smoking cessation on caloric intake and weight gain in an inpatient unit. Psychopharmacology (Berl) 104(4), 522–526. [DOI] [PubMed] [Google Scholar]
- Lerman C, Berrettini W, Pinto A, Patterson F, Crystal-Mansour S, Wileyto EP, Restine SL, Leonard DG, Shields PG, Epstein LH, 2004. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl) 174(4), 571–577. [DOI] [PubMed] [Google Scholar]
- MacLean RR, Cowan A, Vernarelli JA, 2018. More to gain: dietary energy density is related to smoking status in US adults. BMC Public Health 18(1), 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio CA, Presta E, Bracco EF, Vasselli JR, Kissileff HR, Pfohl DN, Hashim SA, 1985. Naltrexone and human eating behavior: a dose-ranging inpatient trial in moderately obese men. Brain Res Bull 14(6), 657–661. [DOI] [PubMed] [Google Scholar]
- Mason AE, Laraia B, Daubenmier J, Hecht FM, Lustig RH, Puterman E, Adler N, Dallman M, Kiernan M, Gearhardt AN, Epel ES, 2015a. Putting the brakes on the "drive to eat": Pilot effects of naltrexone and reward-based eating on food cravings among obese women. Eat Behav 19, 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AE, Lustig RH, Brown RR, Acree M, Bacchetti P, Moran PJ, Dallman M, Laraia B, Adler N, Hecht FM, Daubenmier J, Epel ES, 2015b. Acute responses to opioidergic blockade as a biomarker of hedonic eating among obese women enrolled in a mindfulness-based weight loss intervention trial. Appetite 91, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt RJ, Owens SG, 1991. Cessation from cigarette smoking: changes in body weight, body composition, resting metabolism, and energy consumption. Metabolism 40, 465–470. [DOI] [PubMed] [Google Scholar]
- Murray E, Brouwer S, McCutcheon R, Harmer CJ, Cowen PJ, McCabe C, 2014. Opposing neural effects of naltrexone on food reward and aversion: implications for the treatment of obesity. Psychopharmacology (Berl) 231(22), 4323–4335. [DOI] [PubMed] [Google Scholar]
- Neelon B, O’Malley AJ, 2019. Two-Part Models for Zero-Modified Count and Semicontinuous Data, in: Sobolev B, Gatsonis C (Eds.), Methods in Health Services Research. Springer US, New York, NY, pp. 1–23. [Google Scholar]
- Olsen MK, Schafer JL, 2001. A Two-Part Random-Effects Model for Semicontinuous Longitudinal Data. Journal of the American Statistical Association 96(454), 730–745. [Google Scholar]
- Ornellas T, Chavez B, 2011. Naltrexone SR/Bupropion SR (Contrave): A New Approach to Weight Loss in Obese Adults. P t 36(5), 255–262. [PMC free article] [PubMed] [Google Scholar]
- Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, 2003. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 138(1), 24–32. [DOI] [PubMed] [Google Scholar]
- Pérez-Escamilla R, Obbagy JE, Altman JM, Essery EV, McGrane MM, Wong YP, Spahn JM, Williams CL, 2012. Dietary energy density and body weight in adults and children: a systematic review. J Acad Nutr Diet 112(5), 671–684. [DOI] [PubMed] [Google Scholar]
- Raatz SK, Jahns L, Johnson LK, Scheett A, Carriquiry A, Lemieux A, Nakajima M, al'Absi M, 2017. Smokers report lower intake of key nutrients than nonsmokers, yet both fall short of meeting recommended intakes. Nutr Res 45, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin J, 1987. Weight change following smoking cessation: the role of food intake and exercise. Addict.Behav. 12, 303–317. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Strasser A, Lynch KG, Perkins K, Patterson F, Lerman C, 2005. Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology (Berl) 180(1), 41–48. [DOI] [PubMed] [Google Scholar]
- Spring B, Pagoto S, McChargue D, Hedeker D, Werth J, 2003. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Behav 76(2), 351–360. [DOI] [PubMed] [Google Scholar]
- Spring B, Wurtman J, Gleason R, Wurtman R, Kessler K, 1991. Weight gain and withdrawal symptoms after smoking cessation: a preventive intervention using d-fenfluramine. Health Psychol 10(3), 216–223. [DOI] [PubMed] [Google Scholar]
- Stamford BA, Matter S, Fell RD, Papanek P, 1986. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am.J Clin Nutr 43(4), 486–494. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Lipsey Z, Wardle J, 1998. Stress, hassles and variations in alcohol consumption, food choice and physical exercise: A diary study. British Journal of Health Psychology 3(1), 51–63. [Google Scholar]
- Stojakovic A, Espinosa EP, Farhad OT, Lutfy K, 2017. Effects of nicotine on homeostatic and hedonic components of food intake. J Endocrinol 235(1), R13–r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter ME, Nasim A, Veldheer S, Cobb CO, 2016. Associations between unhealthy dieting behaviors and tobacco use among adolescents. J Eat Disord 4, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Quertemont E, 2007. Preclinical and clinical pharmacology of alcohol dependence. Fundamental & Clinical Pharmacology 21(1), 9–28. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ, 1991. The development and initial validation of a questionnaire on smoking urges. Br J Addict 86(11), 1467–1476. [DOI] [PubMed] [Google Scholar]
- Togo P, Osler M, Sørensen TI, Heitmann BL, 2001. Food intake patterns and body mass index in observational studies. Int J Obes Relat Metab Disord 25(12), 1741–1751. [DOI] [PubMed] [Google Scholar]
- Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ, 2015. Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. Eur J Nutr 54(1), 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, Wurtman JJ, 1995. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res 3 Suppl 4, 477s–480s. [DOI] [PubMed] [Google Scholar]
- Yannakoulia M, Anastasiou CA, Zachari K, Sidiropoulou M, Katsaounou P, Tenta R, 2018. Acute effect of smoking and smoking abstinence on energy intake and appetite-related hormones blood concentrations. Physiol Behav 184, 78–82. [DOI] [PubMed] [Google Scholar]


