Abstract
Background
Current treatments for several corneal lesions show limited efficacy. Here we report the clinical evaluation of the efficacy of a novel eye drop preparation produced in a public cord blood (CB) bank.
Materials and methods
In a multicentre, retrospective, consecutive case study we evaluated 33 patients (46 eyes) unresponsive to conventional treatments who required urgent intervention. The patients were given allogeneic eye drops obtained from cord blood platelet lysate (CBED) to treat severe ocular surface lesions under a compassionate use protocol. The CBED were prepared from CB units donated for haematopoietic stem cell transplantation that did not contain the minimum stem cell dose required for this use. Patients were grouped by acute conditions (neurotrophic ulcers: group I; other corneal ulcers: group II; corneal burns: group III), and chronic conditions (ocular graft-versus-host disease: group IV; severe dry eye syndrome: group V). The patients received one or two drops of the product to the affected eye four to six times per day for 19 days. A further 19-day cycle of treatment could be repeated according to the initial clinical response.
Results
Patients received a median of 19 CBED vials (interquartile range 19–57, range 19–442) to complete the therapy. Group I–II–III patients showed full and partial ulcer recovery in 25 (78%) and six (19%) eyes respectively. One eye (3%) did not respond to treatment. For groups IV–V improvement was reported for 12 (85%) eyes and lesions worsened on treatment in both eyes (15%) of one patient. No severe adverse events were directly attributed to CBED.
Discussion
Promptly available CBED resulted in a well-tolerated allogeneic treatment that showed evidence of efficacy in this cohort of patients. These positive results support further studies on CBED from platelet lysate as a novel product of CB banks. A prospective clinical trial in neurotrophic keratitis (NCT03084861) is ongoing to confirm these preliminary data.
Keywords: cord blood, eye drops, corneal ulcers, neurotrophic keratitis, dry eye
INTRODUCTION
Serum eye drops, most frequently an autologous product obtained from the patient’s own blood, have been used for the treatment of severe ocular surface lesions1. This use is based on the evidence that blood serum and natural tears show similar pH and osmolarity values, and share a number of constituents including growth factors and vitamin A2. This helps to restore an environment that promotes re-epithelialisation and supports ocular surface health. These factors are likely to be responsible for the therapeutic benefits observed for serum eye drops in comparison to those afforded by conventional commercial ocular lubricants3.
However, the availability of autologous serum is usually delayed by as much as 2 weeks, due to completion of microbiological controls. Moreover, autologous serum eye-drop preparations may not be feasible in patients who are positive for serological markers of infectious diseases, in the very young or the elderly and in patients requiring intensive care treatment, for whom autologous blood collection may be difficult or impossible. In these circumstances, an allogeneic source could be considered. There are studies suggesting the use of healthy blood donors as a source to obtain allogeneic eye drops, as reviewed by Giannaccare et al.1. An attractive alternative to this readily available source is the use of umbilical cord blood (CB), which has unique biological characteristics including key growth factors and anti-inflammatory molecules4,5. Previous studies have described the therapeutic effects of CB serum-derived eye drops6,7.
We investigated the novel approach of using CB platelet lysate rather than serum as the source material for eye drops. This source is particularly convenient for public CB banks, where large numbers of anticoagulated CB units can be repurposed for this use from those unsuitable for haemopoietic stem cell transplantation8–10. These units have routinely controlled quality and safety profiles11 and are ideal for generating allogeneic, off-the-shelf, CB eye drops (CBED).
This article reports a preliminary clinical evaluation of CBED prepared in our CB bank that were used to treat a consecutive case series of patients with severe ocular surface lesions unresponsive to conventional treatments.
MATERIALS AND METHODS
Study design and patient selection
This was a consecutive case series study analysing the clinical outcomes of patients treated with CBED for severe refractory surface ocular lesions. Some of these patients were treated after failing to comply with strict inclusion criteria of an ongoing randomised clinical trial to evaluate the safety and efficacy of CBED for neurotrophic keratitis (NCT03084861), and others were included as part of a compassionate use scheme. The patients’ physicians proposed the use of CBED if a patient: (i) had a severe pathology of the ocular surface; (ii) was unresponsive to conventional treatment (artificial tears, lubricant gels, therapeutic contact lenses) or to other blood derivatives such as autologous or, if possible, allogeneic serum; (iii) required urgent intervention, which precluded the production of autologous serum eye drops; (iv) showed full understanding of the therapy conditions and agreed to adhere to the treatment protocol and comply with the scheduled control visits.
The series included 33 consecutive patients (46 eyes) treated between November 2015 and April 2019 in five ophthalmological centres that constituted the Barcelona CBED study group (see Appendix I). Following informed consent, a request was submitted to obtain compassionate use authorisation from the Spanish Drug Agency (AEMPS) for each case.
After approval, a batch of frozen CBED was released from the CB bank and sent to the patient’s ward for immediate start of the treatment.
Preparation of cord blood eye drops
In Spain, human plasma for non-substitutive (transfusional) use is regulated as a “special medicine” and its manipulation requires compliance with specific norms issued by the AEMPS. In this regard, the Blood and Tissue Bank (BST) holds Investigational Drug Approval for the therapeutic use of CBED (PEI 16-116). In addition, BST obtained a non-exclusive license from Episkey Srl (Lovero, Italy) to manufacture, use and distribute CB-derived plasma and platelet components.
The CBED manufacturing process requires a stock of stored CB platelet concentrates (CBPC) as the starting material. Full manufacturing validation of CBPC has been published elsewhere10. The CBPC used for manufacturing CBED contained 1,000×109/L platelets in 5–30 mL and were negative for infectious disease markers including human immunodeficiency virus (HIV)-1/2, hepatitis C virus (HCV), hepatitis B core, cytomegalovirus and human T-lymphotropric virus I–II antibodies, hepatitis B virus (HBV) surface antigen, nucleic acid testing for HIV, HBV and HCV (triple NAT), antibodies to Trypanosoma cruzi (Chagas disease), Treponema pallidum (syphilis); and negative cultures for aerobic and anaerobic bacteria and fungi. The CBED vials were produced under Good Manufacturing Practice conditions, in class C clean rooms of the BST facilities.
The CBPC underwent three freeze/thawing cycles (frozen without cryoprotectant at −80 °C and thawed in a water bath at 37 °C)12 to obtain a platelet lysate (CBPL) rich in anti-inflammatory and tissue regenerative factors. Finally, the CBPL was centrifuged at 5,000 g for 15 min (Allegra® X-15R centrifuge, equipped with a SX4750A ARIES swinging bucket rotor, Beckman Coulter Inc, Indianapolis, IL, USA) to sediment the platelet stroma and the CBED were obtained by vol/vol dilution of the supernatant with Plasmalyte (Baxter SL, Valencia, Spain). The volume of CBED obtained from a single CB unit (defined as “one batch”) was dispensed into a commercial kit of 20 vials (COL20, BioMed Device Srl, Modena, Italy) which were frozen without antimicrobials or other preservatives. The volume of CBED after Plasmalyte dilution defined the number of vials that could be generated in each batch. Each individual vial contained between 350–500 μL. Assuming that one drop is equal to 30 μL (COL-20 guide), each vial contained 11–16 drops. A treatment of four to six applications per day in both eyes required 8–12 drops/day. This means that one vial contained the necessary quantity for 1 day of therapy. Each CBPC-derived batch could include up to 40 vials. Finally, the vials were packed in boxes each containing 19 vials and stored frozen until release. One vial per batch was stored long-term for regulatory purposes. Specification for CBED batch release were: platelets ≤15×109/L, leucocytes ≤0.5×109/L, erythrocytes ≤0.01×1012/L and proven sterility. A total of 119 CBPC were used for preparing the required CBED. Of them, 20 (13%) did not comply with acceptance criteria (10% due to microbiology-positive results and 3% due to platelet count exceeding the limit) and were discarded. From the CBED processes that met acceptance criteria, 161 individual packages of 19 vials each were finally shipped during the study period. The cost of manufacturing the CBED amounted to € 202.5 per batch of 19 vials.
Clinical protocol
After treatment approval from the AEMPS, a CBED pack was shipped from the CB bank to the patient’s ward and stored in a hospital freezer. Alternatively, if the patient’s condition indicated that CBED could be self-administered at home, instructions were given to the patient or a companion on frozen storage of the CBED in a domestic freezer. To start treatment, one vial was thawed at room temperature. During the day of use, the thawed vial was capped and stored in a domestic refrigerator in a sterile plastic bottle to prevent any contamination and maintain growth factor stability13. Health personnel or patients were instructed to administer one or two drops into each affected eye four to six times along a day, with a minimum of at least 2 hours between applications. Each morning a new vial was thawed for use. The duration of the initial treatment period was 19 days, and the treatment course could be repeated if some degree of improvement was observed at clinical evaluation. Improvement was defined as a positive variation of clinical symptoms, ulcer size or keratopathy reduction after treatment, compared to before administration. Used vials were returned to the CB bank for verification.
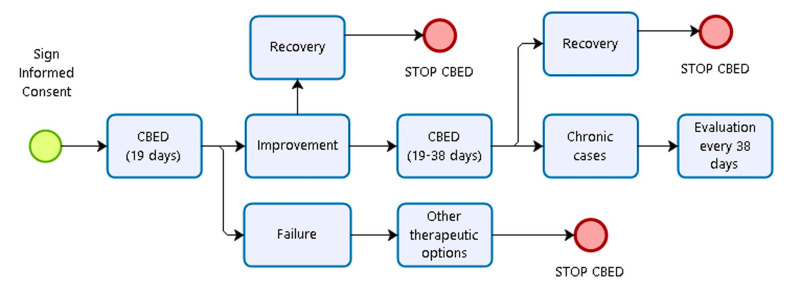
A common protocol for clinical follow-up was established with all ophthalmology clinics (Figure 1). First, an ophthalmological examination was carried out within 2–3 days after starting application to check tolerance of the treatment and to detect any adverse events. Follow-up visits were carried out weekly during the first treatment course and once monthly if the treatment was repeated.
Figure 1. Algorithm of the decision to administer or stop treatment with cord blood-derived eye drops.
CBED: cord blood-derived eye drops.
Clinical data collection and statistical analysis
Treated patients were grouped by condition: neurotrophic ulcers (group I), other corneal ulcers (group II), corneal burns (group III), ocular graft-versus-host disease (GVHD) (group IV), and severe dry eye syndrome (group V).
For the retrospective data collection, a purpose-designed form with instructions to uniform data interpretation was sent to each participant clinician to recover the following information: (i) visual acuity (Snellen chart) where possible; (ii) qualitative corneal aesthesiometry (normal, hypoaesthesia or anaesthesia), according to the investigator’s criteria; (iii) evaluation of corneal ulcer/de-epithelialised area (in mm2) and keratopathy (in affected quadrants) by positive staining with fluorescein using slit lamp biomicroscopy; (iv) clinical variables (corneal inflammation, conjunctivalisation, corneal neovascularisation, pain) assessed according to semi-qualitative or semi-quantitative scales; (v) presence of complications: thinning, perforation, melting, calcifications, infections, and vascularisation; (vi) presence of adverse events associated with the treatment, and (vii) subjective self-reports. In addition, clinical data were collected using information obtained during programmed visits weekly during the first treatment course, and then monthly during the extension of the therapy if applicable. If available, ocular surface images were also collected.
The clinical outcomes were evaluated during and after treatment. For groups I, II and III, outcomes were arbitrarily defined in terms of ulcer closure as recovery (100% ulcer closure), improvement (some degree of reduction), or failure (if no change or worsening was appreciated). For patients with chronic conditions in groups IV and V, the outcomes were only defined as improvement or failure according to changes in clinical evaluation observed before and after CBED therapy.
Descriptive statistics are reported using the mean and standard deviation or the median and range.
RESULTS
The consecutive case series comprised 33 patients (46 eyes), 20 males and 13 females, aged 1 month to 92 years (mean 61±23). A total of 1,897 CBED vials obtained from 99 CB units were used during the study period. Patients received a median of 19 CBED vials (interquartile range 19–57; range 19–96 for groups I–II–III and 38–442 for groups IV–V).
Clinical outcomes divided by aetiological group are reported in Table I.
Table I.
Clinical outcomes after treatment with cord blood-derived eye drops of patients divided by aetiological group
| Group | Aetiology | Patients n (%) |
Eyes n (%) |
Recovery n (%) |
Improvement n (%) |
Failure n (%) |
|---|---|---|---|---|---|---|
| I | Neurotrophic ulcers | 18 (69.2) | 19 (59.4) | 14 (73.7) | 5 (26.3) | 0 (0) |
| II | Corneal ulcers (others) | 4 (15.4) | 6 (18.7) | 5 (83) | 1 (17) | 0 (0) |
| III | Corneal burns | 4 (15.4) | 7 (21.9) | 6 (80) | 0 (0) | 1 (20) |
| Total for groups I–II–III | 26 (100) | 32 (100) | 25 (78) | 6 (19) | 1 (3) | |
| IV | Ocular GVHD | 3 (42.9) | 6 (42.9) | na | 4 (67) | 2 (33) |
| V | Severe DES | 4 (57.1) | 8 (57.1) | na | 8 (100) | 0 (0) |
| Total for groups IV–V | 7 (100) | 14 (100) | na | 12 (85) | 2 (15) | |
GVHD: graft-versus-host disease; DES: dry eye syndrome; na: not applicable.
In groups I–II–III, full and partial ocular surface ulcer recovery was observed in 25 (78%) and six (19%) of 32 eyes, respectively. One eye (3%) did not respond to treatment. In groups IV and V, improvement and failure were reported in 12 (85%) and two (15%) eyes respectively. Overall (groups I–V), no response to CBED treatment was reported in three of 46 eyes (6.5%).
Individual data and outcomes from patients enrolled in groups I–II–III and IV–V are reported in Tables II and III, respectively. Pictures from selected patients’ eyes before and after CBED treatment are shown in Figures 2 and 3. Additional observations from selected cases are reported below by aetiological group.
Table II.
Patients’ general data and clinical follow-up: groups I–II–III
| Group | CCU, gender, age | Eye | Pre-CBED ineffective treatments added to conventional therapy*; comments on lesions before CBED use; infectious pathologies | Applied vials | Clinical follow-up (pre / post CBED) | Outcome | Notes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | CN | CO | Pain | Ulcer % reduction | |||||||
| I | 1, M, 80 | L | ASED, allogeneic serum ED | 40 | +++/+ | +/++ | ++/+++ | +++/− | 100 | R | - |
| I | 3, F, 59 | L R |
ASED | 60 | −/− | −/− | −/− | −/− | 100 | R R |
- |
| I | 5, F, 91 | L | No pre-CBED treatment added to CT | 19 | −/− | −/− | ++/+++ | −/− | 100 | R | 1 |
| I | 6, M, 69 | L | No pre-CBED treatment added to CT | 19 | −/− | −/− | +++/++ | −/− | 100 | R | - |
| I | 7, M, 34 | L | No pre-CBED treatment added to CT | 19 | −/− | −/− | +++/− | −/− | 100 | R | - |
| I | 8, M, 51 | R | No pre-CBED treatment added to CT | 19 | −/− | −/− | −/+++ | −/− | 100 | R | 2 |
| I | 9, M, 27 | L | AM graft. Corneal recurrent erosions | 19 | +/++ | ++/+++ | +/++ | +/+ | 65 | I | 3 |
| I | 13, M, 32 | R | No pre-CBED treatment added to CT | 38 | +/− | −/− | ++/+ | +++/+ | 93 | I | 4 |
| I | 16, M, 66 | L | Temporary tarsorrhaphy | 38 | −/− | −/− | ++/+ | −/− | 100 | R | |
| I | 18, F, 91 | L | No pre-CBED treatment added to CT. Persistent epithelial defect secondary to corneal surgery | 19 | −/− | −/− | ++/+ | +/+ | 100 | R | - |
| I | 19, M, 56 | L | AM graft. Bilateral herpetic keratitis with corneal abscess and hypopyon after keratoplasty; HIV+, HCV+ | 38 | ++/++ | −/− | ++/++ | −/− | 81 | I | - |
| I | 20, F, 63 | L | ASED. Temporary lateral tarsorrhaphy | 19 | −/− | −/− | +++/+ | −/− | 100 | R | - |
| I | 22, M, 77 | R | AM graft. Temporal lateral tarsorrhaphy; Syphilis+ | 19 | −/− | −/− | ++/+ | −/− | 83 | I | - |
| I | 24, F, 85 | R | AM graft | 19 | −/− | −/− | −/− | +++/− | 100 | R | - |
| I | 28, M, 41 | R | No pre-CBED treatment added to CT; HIV+, Syphilis+ | 19 | −/− | −/− | −/− | −/− | 100 | R | 5 |
| I | 29, M, 86 | L | No pre-CBED treatment added to CT; HIV+ | 19 | −/− | −/+ | −/− | −/− | 67 | I | - |
| I | 30, F, 85 | R | No pre-CBED treatment added to CT | 19 | −/− | −/+ | −/− | +++/− | 100 | R | - |
| I | 31, M, 55 | L | No pre-CBED treatment added to CT | 19 | ++/− | +/+ | ++/+ | ++/− | 100 | R | - |
| II | 4, M, 75 | L R |
Temporary lateral tarsorrhaphy (LE); intensive care patient | 19 | +++/+ | L −/++ R −/− |
L ++/++ R −/− |
nav | 100 | R R |
6 |
| II | 10, M, 51 | L R |
GVHD. Topical cyclosporine and topic tacrolimus; corneal erosion with calcification complicated by corneal ulcer | 76 | +/− | −/− | +/+ | +/+ | L= 96 R=100 |
I R |
- |
| II | 27, F, 1mo | L | Topical ciprofloxacin and acyclovir | 38 | −/− | −/− | −/− | nav | 100 | R | - |
| II | 32, F, 76 | L | No pre-CBED treatment added to CT | 19 | −/− | −/− | +++/++ | −/− | 100 | R | - |
| III | 23, M, 3 | L R |
AM graft | 96 | +++/− | −/− | L +/− R +++/++ |
nav | 100 | R R |
- |
| III | 25, M, 62 | R | AM graft | 57 | ++/++ | −/− | +/+ | −/− | na | F | 7 |
| III | 26, M, 50 | L R |
AM graft | 19 | ++/− | −/− | L −/− R +/− |
+/− | 100 | R R |
- |
| III | 33, M, 44 | L R |
AM graft | 95 | +/− | −/− | ++/+ | nav | L=100 R=100 |
R R |
- |
conventional treatment: artificial tears, lubricant gels, therapeutic contact lenses.
CCU: unique patient number; CBED: cord blood-derived eye drops; ASED: autologous serum eye drops; Gender: M: male, F: female: ED: eye drops; Eye: L: left, R: right; CT: conventional treatment; Outcome: R: recovery; I: improvement; F: failure; AM: amniotic membrane; CI: conjunctival inflammation; − absent; + mild; ++ moderate; +++ severe; CN: corneal neovascularisation; − absent; + 1 quadrant; ++ 2 quadrants; +++ 3 quadrants; CO: corneal opacity; − absent; + mild; ++ moderate; +++ severe; Pain: − absent; + mild; ++ moderate; +++ severe; na: not applicable; nav: not available. Notes. 1. Bacterial keratitis reported at 3 weeks, resolved with antibiotic treatment. 2. Infection reported at 3 weeks; mild persistent epithelial defect. 3. Clear improvement during days 1–10, then no changes. 4. Patient with Down’s syndrome. CBED treatment requested as a bridge to ASED availability. 5. Enduring treatment in a patient with glaucoma with maintenance of ulcer closure. 6. Suspension of treatment at 10 days due to infection and re-institution after application of an antibiotic. Pain evaluation not available (intensive care unit patient). 7. Ulcer size not available (intensive care unit patient).
Table III.
Patients’ general data and clinical follow-up: groups IV–V
| Group | CCU, gender, age | Eye | Pre-CBED ineffective treatments added to conventional therapy*; infectious pathologies | Applied vials | Clinical follow-up (pre / post CBED) | Outcome | Notes | ||
|---|---|---|---|---|---|---|---|---|---|
| Pain | Photophobia | Visual acuity | |||||||
| IV | 2, F, 46 | L R |
Allogeneic serum ED; topic cyclosporine and topic tracrolimus | 20 | +/+++ | +++/++ | HM/HM HM/HM |
F F |
1 |
| IV | 11, M, 36 | L R |
No pre-CBED treatment added to CT | 249 | +++/+ | +++/+ | 0.5/0.5 0.5/0.5 |
I I |
2 |
| IV | 15, M, 63 | L R |
No pre-CBED treatment added to CT | 57 | ++/+ | ++/+ | L: 0.3/0.6 R: 0.5/0.8 |
I I |
3 |
| V | 12, F, 92 | L R |
No pre-CBED treatment added to CT | 442 | ++/+ | ++/+ | 0.3/0.45 0.3/0.45 |
I I |
4 |
| V | 14, F, 65 | L R |
No pre-CBED treatment added to CT; HCV+ | 154 | ++/+ | +++/+ | L: 0.7/0.8 R: 0.7/0.8 |
I I |
- |
| V | 17, F, 58 | L R |
ASED and autologous platelet concentrate ED | 38 | ++/+ | ++/+ | L: 0.9/1.0 R: 0.8/0.7 |
I I |
- |
| V | 21, F, 70 | L R |
ASED; topic cyclosporine | 57 | +++/++ | nav | L: 0.6/0.9 R: 0.4/0.6 |
I I |
- |
Conventional treatment: artificial tears, lubricant gels, therapeutic contact lenses.
CCU: unique patient number; CBED: cord blood-derived eye drops; ASED: autologous serum eye drops; Gender: M: male, F: female: ED: eye drops; Eye: L: left, R: right; Pain and Photophobia: + mild, ++ moderate, +++ severe; Outcome: I: improvement, F: failure; ; nav: not available; HM: visual acuity determined by hand movement.
Notes. 1. A severe case with calcification of ocular surface in whom corneal transplantation failed. Fungal infection during treatment with corneal perforation at 3 weeks. Finally operated to place keratoprosthesis (Boston) as last option. 2. Currently on treatment. 3. No improvement with first batch. After stopping treatment, started new course with 38 vials with improvement. 4. Currently on treatment. Photophobia decreasing.
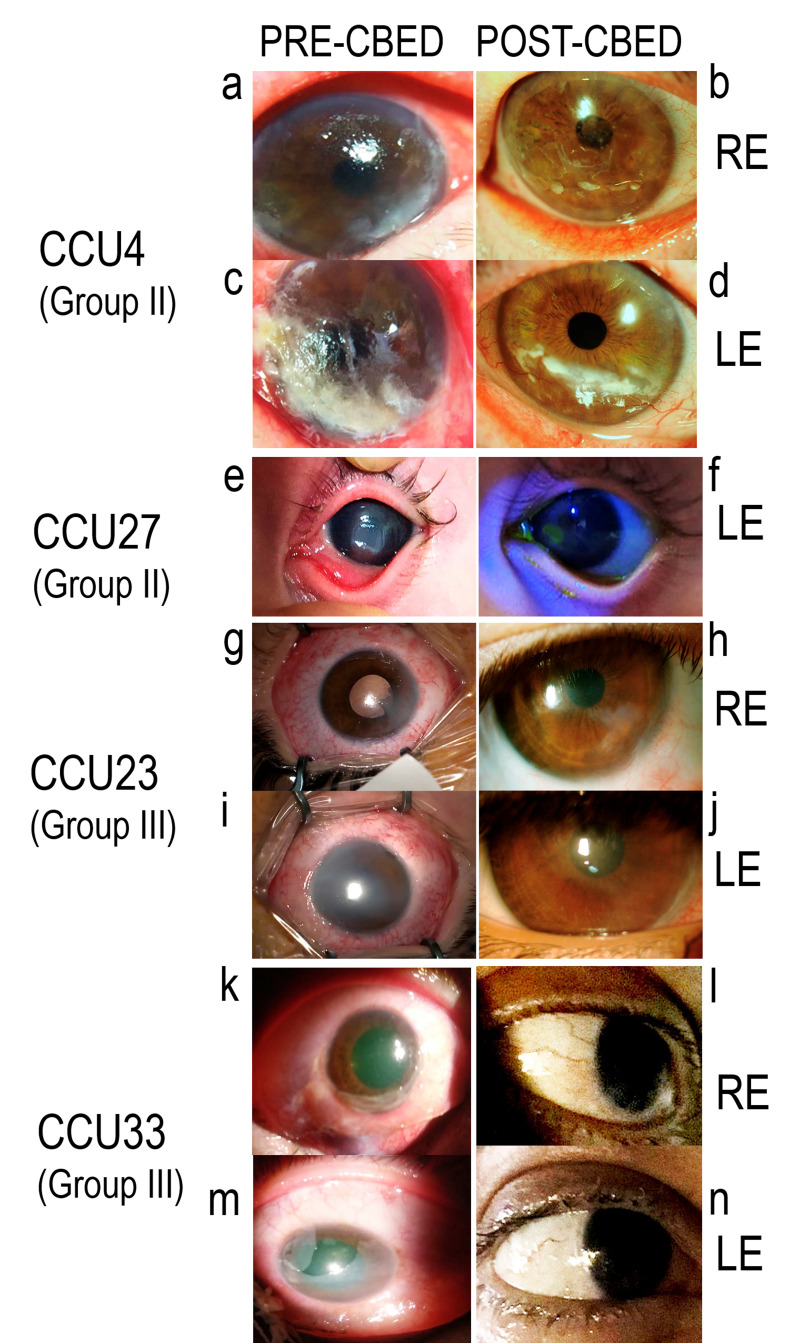
Figure 2. Clinical outcomes.
CCU4, a 75-year old patient with exposure-induced corneal ulcers in both eyes before (a and c) and after (b and d) treatment with cord blood-derived eye drops (CBED). CCU27, a 1-month old patient with corneal ulcer caused by trauma before (e) and after (f) application of CBED. CCU23, a 3-year old patient who presented with acute bilateral corneal burns before (g and i) and after (h and j) CBED treatment to the right eye (RE) and left eye (LE). CCU33, a 44-year old presenting with acute bilateral corneal burns before (k and m) and after (l and n) CBED treatment to the RE and LE. CCU: unique patient’s number.
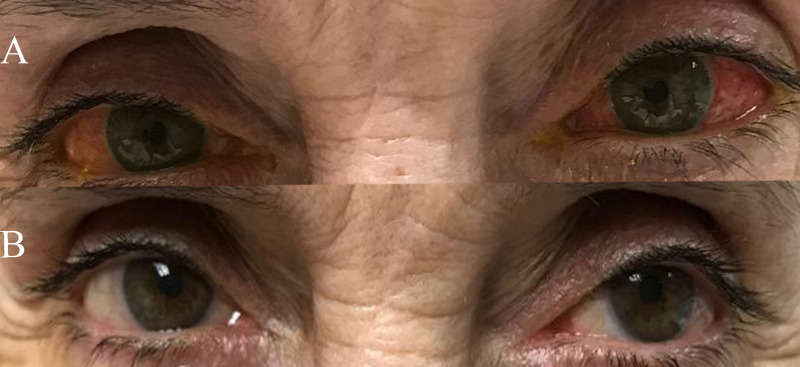
Figure 3. Clinical outcomes.
CCU14, a patient with severe dry eye syndrome, hypovitaminosis A and hepatitis C virus infection, 3 weeks after discontinuing treatment (A) and 2 days after resuming treatment with cord blood-derived eye drops (B). CCU: unique patient’s number.
In group I, patient CCU8 showed corneal stromal infiltrates 21 days after starting CBED treatment, but achieved complete healing after topical antibiotic treatment. CCU20 presented with a peripheral corneal ulcer that was persistent in spite of intensive therapy with topical tobramycin, ciprof loxacin, autologous serum eye drops, poly-carboxymethylglucose sulphate (Cacicol®, Théa Farma SpA, Milan, Italy) and tarsorrhaphy. Application of CBED showed efficacy within 5 days with full ulcer closure. The epithelial defect did not reappear at follow-up.
In group II, CCU4, with exposure ulcers in both eyes, demonstrated substantial structural improvement and reduction of inflammation; the left eye had severe corneal infiltration with melting in the lower temporal quadrant of the cornea which healed after 19 days of CBED treatment (Figure 2c–f). CCU27 had corneal trauma with wide surface de-epithelialisation, which was unresponsive to conventional treatment (Figure 2a–b). After 2 weeks of intensive CBED topical administration, complete recovery of ocular surface integrity and corneal transparency was noted, with no recurrence at follow-up.
In group III, CCU23 showed bilateral damage (Figure 2g–j) and received a combination of amniotic membrane transplantation every 5 days and an hourly application of CBED. The right eye had 360° limbic ischaemia, notable eyelid oedema, associated with intense conjunctival oedema, and loss of corneal transparency. Improvement of both ocular surfaces was observed with this combined therapeutic approach. CCU25, in whom CBED treatment failed, showed de-epithelialisation of the whole cornea, limbus and part of the conjunctiva. CCU33 had an ocular burn of grade IV–V (Dua scale) with bilateral epithelial defects which did not respond to amniotic membrane transplantation. After application of CBED, 100% recovery was achieved in both eyes (Figure 2k–n).
In group IV, CCU2 presented with bilateral severe limbic deficiency with corneal neovascularisation and conjunctivalisation affecting both eyes. After CBED treatment the disease progressed and ended up in a corneal perforation with fungal infection. The adverse event in CCU2 was severe and was resolved after antifungal treatment and amniotic membrane transplantation. This event was considered not related to the CBED, but due to disease progression.
In group V, CCU14 had severe dry eye syndrome due to hypovitaminosis A which was unresponsive to conventional therapy. This patient was also unable to use autologous serum because of HCV infection. Improvement was evident after 19 days of CBED treatment but the condition recurred after CBED withdrawal. Treatment with CBED was resumed and controlled the recurrence during the follow-up (Figure 3).
Patients’ self-reports included positive outcome (general improvement) in all cases, except CCU2 and CCU25.
DISCUSSION
This study showed encouraging outcomes from the compassionate use of novel allogeneic CBED prepared from platelet lysate for the treatment of a consecutive series of 33 patients (46 eyes) with severe ocular surface lesions refractory to conventional therapies. Full ulcer healing was observed in 78% of the treated eyes in 26 patients with neurotrophic keratitis, corneal ulcers of different aetiology and corneal burns. Clinical improvement was noted in 85% of the treated eyes from seven patients with GVHD and severe dry eye syndrome.
The compassionate use of CBED was approved by the Spanish national drug agency AEMPS based on the patients’ urgent need for effective treatment, which prevented the use of autologous serum in 19 patients who could not wait until the completion of blood collection and manipulation procedures and the sterility testing required before serum release14. Moreover, autologous serum could not be used in nine additional cases due to practical reasons (positive infectious markers, elderly age, severe comorbidities) or concern that autologous serum from patients with GVHD could contain noxious pro-inflammatory factors. This concern was also supported by the failure of previous treatment with autologous serum in two and three additional patients with severe dry eye syndrome secondary to Sjögren’s syndrome, as reported in other studies15, and neurotrophic keratitis, respectively. A number of studies compared allogeneic eye drops obtained from different serum and plasma sources and evaluated their immune modulatory properties14,16. Interestingly, previous studies showed that adult peripheral blood is richer in inflammatory factors than CB plasma5 and that CB plasma contains unique molecules not present in peripheral blood, such as NKG2D ligands (MIC, ULBP1), which play an important role in immune suppression17. This feature supports the preferential use of CB in conditions associated with abnormal inflammation. Our clinical observations corroborate previous investigations16 showing that the administration of 20% CBED prepared from CB serum to 14 patients with persistent corneal defects was effective in 86% of the cases, with no significant complications. Significant improvements after 1 month of treatment with 20% CBED from CB serum of 30 patients with severe corneal epithelial damage were also reported7. CBED at 20% serum dilution were also used in 33 eyes with chemical burns, demonstrating the safety and lack of toxicity of this treatment. Complete epithelialisation was achieved in 12 of 18 cases in shorter times compared to the times taken by artificial tears and autologous serum18. Additional positive results with CB serum were reported from patients with severe dry eye syndrome associated with neurotrophic keratitis18 and GVHD19. A reduction in the frequency of recurrences from 2.24±1.09 to 0.5±0.79 was also reported in patients treated with artificial tears and CB serum, during a follow-up of 14.7±2.5 months20. Studies comparing autologous and CB serum in 92 eyes demonstrated higher therapeutic effectiveness in recipients of the CB serum eye drops21. Thus, there is scientific evidence to support the use of topical CB serum in severe ocular surface diseases. The clinical outcomes from our trial are aligned with those described in the literature2,7,16,22. Operationally, the selection of platelet lysate as the source material for the preparation of CBED offers the advantage of using a large proportion of anticoagulated CB donations containing too few stem cells to make their long term banking convenient for public CB banks10.
There are several limitations in our study, including the small number of patients tested with each of the conditions, a variable degree of clinical symptoms at presentation, and the lack of concurrent controls treated with conventional therapy only. In spite of these limitations, it is encouraging to note that positive outcomes were obtained in a large proportion of this cohort of patients in whom all applicable therapeutic options had failed.
While the currently available data suggest that CB serum and platelet lysate could be provisionally considered complementary sources for the production of CBED, additional studies should be performed to test different dosages and administration schedules in different conditions. Moreover, blinded randomised clinical trials are necessary to conclusively determine the respective clinical efficacy of biological eye drops prepared from different blood sources. These comparative studies should also include the very expensive recombinant molecules that have recently became available on the market23.
CONCLUSIONS
This study provides preliminary evidence on the safety and efficacy of CBED prepared from platelet lysate as a new therapeutic blood component manufactured in a public CB bank. These preliminary data support the development of further studies to obtain regulatory approval for routine CBED clinical use. A prospective, randomised clinical trial is currently ongoing in patients suffering from neurotrophic keratitis (NCT03084861).
ACKNOWLEDGEMENTS
We thank the mothers who donate cord blood units to help patients in need. We also acknowledge the health care providers at the maternity units and personnel involved in transportation, cell processing and CBED manufacturing and quality testing for their invaluable help. We also thank participating patients, physicians, nurses, pharmacists and other health care personnel in hospitals that contributed to the use of CBED for treating patients in need. Finally, we thank Dr. Richard Duggleby and Ms. Pauline Dodi (ANRI, UK) for their help with reviewing the English of this manuscript.
APPENDIX I.
The Barcelona Cord Blood Eye Drop (CBED) Study Group
This is a multicentre retrospective case series study and the following people are considered as co-authors representing the Barcelona CBED study group:
| Marta Torrabadella | Banc de Sang i Teixits (BST), Barcelona, Spain |
| Zoraida del Campo | Hospital Santa Creu i Sant Pau, Barcelona, Spain |
| Antonio Sabala | Hospital Universitari German Trias y Pujol, Badalona, Spain |
| Alexandra Arango | Hospital Universitari German Trias y Pujol, Badalona, Spain |
| Nevena Romanic | Hospital Universitari German Trias y Pujol, Badalona, Spain |
| Anna Mones | Hospital Universitari German Trias y Pujol, Badalona, Spain |
| Silvia Bover | Hospital Santa Caterina, Salt, Girona, Spain |
| Teresa Torrent | Hospital Dr Josep Trueta, Girona, Spain |
| Veronica Mas | Hospital Dr Josep Trueta, Girona, Spain |
| Miriam Barbany | Hospital Mutua de Terrassa, Terrassa, Spain |
| Irene Sassot | Hospital Mutua de Terrassa, Terrassa, Spain |
| Daniela Ortiz | Hospital Universitari Joan XXIII, Tarragona, Spain |
Footnotes
FUNDING
All expenses were sustained out by the Blood and Tissue Bank (BST) as a sponsor of the study.
AUTHORSHIP CONTRIBUTIONS
SQ, DS, LR, RC: designed the study; SM, LB, JP and the Study group (Appendix I): recruited and followed up patients; SM, LB, JP, RC-M, PR, DS: analysed data; AM, PR, RC: discussion of the results; SQ, PR, LR, SM, RC-M, DS: wrote the manuscript. All authors discussed and revised the manuscript.
CONFLICT OF INTEREST
PR is a co-inventor of a patent on platelet fractions from cord blood and holds shares of Episkey, a start-up company aimed at developing novel reagents and therapeutics from human blood.
REFERENCES
- 1.Giannaccare G, Versura P, Buzzi M, et al. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci. 2017;56:595–604. doi: 10.1016/j.transci.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Anitua E, Muruzabal F, Tayebba A, et al. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 2015;93:e605–14. doi: 10.1111/aos.12710. [DOI] [PubMed] [Google Scholar]
- 3.Rauz S, Koay S-Y, Foot B, et al. The Royal College of Ophthalmologists guidelines on serum eye drops for the treatment of severe ocular surface disease: full report. Eye. 2017;32:44–8. doi: 10.1038/eye.2017.209. [DOI] [PubMed] [Google Scholar]
- 4.Parazzi V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets. 2010;21:549–54. doi: 10.3109/09537104.2010.514626. [DOI] [PubMed] [Google Scholar]
- 5.Parazzi V, Lavazza C, Boldrin V, et al. Extensive characterization of platelet gel releasate from cord blood in regenerative medicine. Cell Transplant. 2015;24:2573–84. doi: 10.3727/096368915X687471. [DOI] [PubMed] [Google Scholar]
- 6.Giannaccare G, Carnevali A, Senni C, et al. Umbilical cord blood and serum for the treatment of ocular diseases: a comprehensive review. Ophthalmol Ther. 2020;9:235–48. doi: 10.1007/s40123-020-00239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versura P, Profazio V, Buzzi M, et al. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. 2013;32:412–8. doi: 10.1097/ICO.0b013e3182580762. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E. History of cord blood transplantation. Bone Marrow Transplant. 2009;44:621–6. doi: 10.1038/bmt.2009.280. [DOI] [PubMed] [Google Scholar]
- 9.Rebulla P, Pupella S, Santodirocco M, et al. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016;14:73–9. doi: 10.2450/2015.0122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samarkanova D, Rodríguez L, Vives J, et al. Cord blood-derived platelet concentrates as starting material for new therapeutic blood components prepared in a public cord blood bank: from product development to clinical application. Blood Transfus. 2020;18:208–16. doi: 10.2450/2020.0305-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warwick R, Armitage S. Cord blood banking. Best Pract Res Clin Obstet Gynaecol. 2004;18:995–1011. doi: 10.1016/j.bpobgyn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Strandberg G, Sellberg F, Sommar P, et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57:1058–65. doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 13.López-Garciá JS, Garciá-Lozano I, Rivas L, et al. Stability of growth factors in autologous serum eyedrops after long-term storage. Curr Eye Res. 2016;41:292–8. doi: 10.3109/02713683.2015.1016180. [DOI] [PubMed] [Google Scholar]
- 14.Thanathanee O, Phanphruk W, Anutarapongpan O, et al. Contamination risk of 100% autologous serum eye drops in management of ocular surface diseases. Cornea. 2013;32:1116–9. doi: 10.1097/ICO.0b013e3182910036. [DOI] [PubMed] [Google Scholar]
- 15.Stern M, Schaumburg C, Dana R, et al. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3:425–42. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon KC. Use of umbilical cord serum in ophthalmology. Chonnam Med J. 2014;50:82–5. doi: 10.4068/cmj.2014.50.3.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox ST, Laza-Briviesca R, Pearson H, et al. Umbilical cord blood plasma contains soluble NKG2D ligands that mediate loss of natural killer cell function and cytotoxicity. Eur J Immunol. 2015;45:2324–34. doi: 10.1002/eji.201444990. [DOI] [PubMed] [Google Scholar]
- 18.Yoon K-C, You I-C, Im S-K, et al. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Yoon K-C, Jeong I-Y, Im S-K, et al. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007;39:231–5. doi: 10.1038/sj.bmt.1705566. [DOI] [PubMed] [Google Scholar]
- 20.Yoon K-C, Choi W, You I-C, Choi J. Application of umbilical cord serum eyedrops for recurrent corneal erosions. Cornea. 2011;30:744–8. doi: 10.1097/ICO.0b013e31820d850f. [DOI] [PubMed] [Google Scholar]
- 21.Yoon K-C, Heo H, Im S-K, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144:86–92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 22.López-Plandolit S, Morales M-C, Freire V, et al. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea. 2010;29:843–8. doi: 10.1097/ICO.0b013e3181a81820. [DOI] [PubMed] [Google Scholar]
- 23.Fleeman N, Mahon J, Nevitt S, et al. Cenegermin for treating neurotrophic keratitis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoecon Open. 2019;3:453–61. doi: 10.1007/s41669-019-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]