Abstract
Background
Following the first reports in the literature, the association between the ABO blood group and SARS-CoV-2 infection has been investigated by a number of studies, although with varying results. The main object of this systematic review was to assess the relationship between the ABO blood group and the occurrence and severity of COVID-19.
Materials and methods
A systematic literature search using appropriate MeSH terms was performed through Medline and PubMed. The outcomes considered were the prevalence of the blood group O vs non-O types in SARS-CoV-2 infected and non-infected subjects, and the severity of SARS-CoV-2 infection according to ABO group. The methodological quality of the studies included in the analysis was assessed with the Newcastle-Ottawa Scale, and the overall quality of the available evidence using the GRADE system. Benchmarks used to evaluate the effect size were odd ratios (ORs) for case control studies and risk ratios (RRs) for cohort studies.
Results
Twenty-one studies were included in the analysis. Overall, individuals with group O had a lower infection rate compared to individuals of non-O group (OR: 0.81; 95% CI: 0.75, 0.86). However, the difference in the effect size was significantly lower in cohort studies compared to case control studies. No evidence was found indicating an effect of the O type on the disease severity in the infected patients.
Discussion
We have found low/very low evidence that group O individuals are less susceptible to SARS-CoV-2 infection compared to those in the non-O group. No evidence was found indicating an effect of the O type on disease severity in SARS-CoV-2 infection.
Keywords: ABO blood group, COVID-19, disease, systematic review
INTRODUCTION
The ABO blood group is the most important among human blood group systems and consists of complex carbohydrate moieties at the extracellular surface of red blood cell (RBC) membrane1,2. While the A and B alleles of the ABO locus encode A and B glycosyltransferase activities, which convert precursor H antigen into either A or B determinants by adding an extra saccharide unit, group O individuals lack such transferase enzymes and express basic, unchanged H-antigen3. Along with their expression on RBCs, ABO antigens (namely A, B, AB and O) are also highly expressed on the surface of a variety of human cells and tissues4. Although the physiological role of ABO antigens and their related anti-A and anti-B natural isoagglutinins is still largely unknown, they play a prominent role in blood transfusion and cell, tissue, and organ transplantation4. In addition, several studies over the last 50 years have documented a close link between ABO blood groups and a wide array of diseases, including cancers and cardiovascular disorders5. The latter association is particularly relevant, considering the profound influence of ABO antigens on haemostasis, particularly in modulating von Willebrand factor (VWF) and factor VIII (FVIII) circulating levels6–10. In addition, the ABO blood group-related susceptibility to various types of viral infections, including HIV, hepatitis B, dengue and influenza viruses, has been consistently reported by several investigators over the last 20 years11–14. This has recently gained renewed interest thanks to the observation on the association between ABO blood type and the pandemic Coronavirus Disease 2019 (COVID-19)15. In particular, it has been hypothesised that individuals belonging to O blood type are less susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection than those belonging to non-O blood groups, or that they have a milder disease16. The hypothesis for this phenomenon lies in the presence in O blood group subjects of IgG anti-A isoagglutinins which would prevent the binding of SARS-CoV-2 to its receptor thereby stopping the virus entering the targeted human cells17. In this review, we will show and critically discuss the results of a systematic literature review and meta-analysis on the correlation between ABO blood groups and SARS-CoV-2 infection and severity, along with its possible implications for future health policies.
MATERIALS AND METHODS
Search methods
For this systematic review, we analysed the medical literature for published articles on the association between ABO blood type and SARS-CoV-2 infection. The Medline and PubMed electronic databases were searched for English language articles published from 1st January 2020 to 30th December 2020. Only those articles that had been subjected to peer review were included in the final analysis. The Medical Subject Heading and key words used were: “novel coronavirus disease”, “COVID-19”, “SARS-CoV-2”, “acute respiratory distress syndrome”, “ABO blood groups”, and “ABO blood type”. We also screened the reference lists of the most relevant review articles for additional studies that had not been captured in our initial literature search. Studies were selected independently by two reviewers (M.F. and M.C.), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (C.M.).
Criteria for study selection
Inclusion criteria were: 1) studies that reported ABO blood group prevalence among SARS-CoV-2 infected subjects and in non-infected subjects; 2) studies that reported severity of SARS-CoV-2 according to ABO group. Both cohort studies and case control studies were included; case reports were excluded.
Outcomes
The outcomes were: i) prevalence of the blood group O vs non-O types in SARS-CoV-2 infected subjects and in non-infected subjects; and ii) the severity of SARS-CoV-2 infection according to ABO group. The severity of SARS-CoV-2 infection we have considered were the endpoints used to define the severity reported in the selected studies.
Quality assessment
We evaluated both the quality of reporting and the methodological quality of the studies included in the analysis. For this purpose, we used the Newcastle-Ottawa Scale (NOS) checklist. The NOS is a 9-point scale that assigns points on the basis of the process of selection of the cohorts or of the case and of the controls (0–4 points), of the comparability of the cohorts or of the case and of the controls (0–2 points), and of the identification of the exposure and of the outcomes of study participants (0–3 points). The NOS was developed to assess the quality of non-randomised studies for the purpose of incorporating quality assessments in the interpretation of meta-analytic results. This scale is recommended by the Cochrane non-randomised studies methods18,19. This quality assessment was performed independently in duplicate (M.C., M.F.) and any disagreement was resolved by consensus. Using the NOS, a study can be awarded a maximum of 4 stars for selection, a maximum of 2 stars for comparability, and a maximum of 3 stars for outcome. Since some of the studies reporting prevalence of ABO group among SARS-CoV-2 infected and non-infected subjects also reported severity of SARS-CoV-2 infection, and as such, in this context, can be regarded as cohort studies, the quality assessment was performed separately for the two pre-specified outcomes. We considered a study which scored ≥7 a high-quality study, and the remaining as non-high quality studies. The publication bias was investigated by the funnel plot and the Egger test for funnel plot asymmetry in meta-analysis.
Summary of findings
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes, and constructed “Summary of findings” tables (Tables I and II). These tables present key information concerning the certainty of the evidence, the magnitude of the effects in the groups of subjects examined, and the sum of available data for the main outcomes. The “Summary of findings” tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. Outcomes in terms of occurrence of infection and severity of infection are presented in Table II.
Table I.
Main characteristics and results of the studies on the association between ABO blood groups and COVID-19
| Author, year | Country | Study design | Sample1 (n) | Mean age (years) | Gender (M/F) | ABO blood group prevalence (O vs non-O) | Main results |
|---|---|---|---|---|---|---|---|
| Abdollahi, 202023 | Iran | Case-control | Cases: 397 Controls: 500 |
Cases: 58.8 Controls: 48.5 |
Cases: 252/145 Controls: 231/269 |
Cases: 28 vs 72%1 Controls: 38 vs 62% |
Group O subjects have a reduced vulnerability to COVID-19. No association between ABO blood types and COVID-19 severity was observed. |
| Boudin, 202024 | France | Cross-sectional cohort, retrospective | Cases: 1,279 Controls: 409 |
Cases: 282 Controls: 272 |
Cases: 1,112/167 Controls: 354/55 |
Cases: 43.2 vs 56.8% Controls: 46.2 vs 53.8% |
In a large population confined to an aircraft carrier, ABO blood groups were not associated with increase/decrease in risk of SARS-CoV-2. |
| Dzik, 202025 | USA | Case-control | Cases: 957 Controls: 5,840 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: 48.6 vs 51.4% Controls: 46.6 vs 53.4% |
No association between ABO distribution and SARS-CoV-2 infection or mortality was observed. |
| Ellinghaus, 202022 | Italy, Spain | Case-control | Cases: 1,610 Controls: 2,205 |
Cases: NR Controls: NR |
Cases: 1,126/484 Controls: NR |
Cases: 37.5 vs 62.5% Controls: 47.8 vs 52.2% |
A protective effect in blood group O as compared with other blood groups was observed. |
| Fan, 202026 | China | Case-control | Cases: 105 Controls: 103 |
Cases: 56.8 Controls: 54.0 |
Cases: 55/50 Controls: 56/47 |
Cases: 21.9 vs 78.1% Controls: 29.1 vs 70.9% |
Females with blood type A were more susceptible to COVID-19. |
| Franchini, 202027 | Italy | Case-control | Cases: 447 Controls: 16,911 |
Cases: 47.7 Controls: 47.1 |
Cases: 385/62 Controls:10,321/6,590 |
Cases: 36.2 vs 63.8% Controls: 43.6 vs 56.4% |
The prevalence of O blood type in convalescent plasma donors recovered from COVID-19 was significantly lower than that observed in healthy blood donors. |
| Gallian, 202028 | France | Cross-sectional cohort, prospective | Cases: 27 Controls: 971 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: 22.2 vs 78.2% Controls: 46.1 vs 53.9% |
A lower prevalence of anti-SARS-CoV-2 neutralising antibodies was found in French group O blood donors. |
| Göker 202029 | Turkey | Case-control | Cases: 186 Controls: 1,881 |
Cases: 42 Controls: NR |
Cases: 100/86 Controls: NR |
Cases: 24.8 vs 75.2% Controls: 37.2 vs 62.8% |
The frequency of O blood group was significantly lower in COVID-19 patients compared to controls. Blood group types did not affect clinical outcomes. |
| Latz 202030 | USA | Cross-sectional cohort, retrospective | Cases: 1,289 Controls: 6,359 |
Cases: NR Controls: NR |
Cases: 427/862 Controls: NR |
Cases: 45.5 vs 54.5% Controls: 48.3 vs 51.7% |
ABO blood type was not associated with disease severity. O blood group subjects were less likely to test positive for COVID-19 than AB and B groups. |
| Leaf, 202031 | USA | Case-control | Cases: 2,033 Controls: 3.1 m |
Cases: 622 Controls: NR |
Cases: 1,297/736 Controls: NR |
Cases: 46.7 vs 53.3% Controls: NR |
O blood type was a protective risk factor for severe COVID-19 in white race individuals. No association was found with the risk of death. |
| Li, 202032 | China | Case-control | Cases: 2,153 Controls: 3,694 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: 25.7 vs 74.3% Controls: 33.8 vs 66.2% |
People with blood group O had a significantly lower risk of SARS-CoV-2 infection. |
| Wu, 202033 | China | Case-control | Cases: 187 Controls: 1,991 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: 21.9 vs 78.1% Controls: 30.2 vs 69.8% |
Individuals with group O had a lower risk of COVID-19 than non-O blood group subjects. |
| Yaylaci, 202034 | Turkey | Cross-sectional cohort, prospective | Cases: 397 Controls: NR |
Cases: 47.2 Controls: NR |
Cases: 176/221 Controls: NR |
Cases: 27.5 vs 72.5% Controls: NR |
No relationship was found between blood groups and mortality or ICU admission. |
| Zhang, 202035 | China | Cross-sectional cohort, retrospective | Cases: 134 Controls: 3,694 |
Cases: 60.8 Controls: NR |
Cases: 87/47 Controls: NR |
Cases: 19.2 vs 71.8% Controls: 33.8 vs 66.2% |
A lower infection rate was observed among group O subjects. There was no significant difference in ABO blood type distribution between survivors and non-survivors. |
| Zhao, 202036 | China | Case-control | Cases: 1,775 Controls: 3,694 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: 25.8 vs 74.2% Controls: 32.2 vs 67.8% |
Individuals with group O had a higher risk and those with group A a lower risk for SARS-CoV-2 infection. |
| Ray, 202038 | Canada | Population-based cohort, retrospective | Cases: 225,556 Controls: NR |
Cases: NR Controls: NR |
Cases: 65,566/159,820 Controls: NR |
Cases: NR Controls: NR |
The O and Rh- blood groups may be associated with a slightly lower risk for SARS-CoV-2 infection and severe COVID-19 illness. |
| Ziezt, 202039 | USA | Cross-sectional cohort, retrospective | Cases: 2,394 Controls: 10,657 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
A slightly increased infection prevalence among non-O types was found. Risk of intubation was decreased among A and increased among AB and B types, compared with type O. |
| Muniz-Diaz, 202041 | Spain | Case-control | Cases: 854 Controls:75,870 |
Cases: 452 Controls: 452 |
Cases: 338/516 Controls:39,014/36,856 |
Cases: 41.5 vs 48.5% Controls: 47.3 vs 42.7% |
ABO blood group is associated with susceptibility to acquire SARS-CoV-2 infection and with COVID-19 severity and mortality. |
| May, 202040 | USA | Cohort, retrospective | Cases: 165 Controls: NR |
Cases: 57 Controls: NR |
Cases: 61%/39% Controls: NR |
Cases: 43 vs 57% Controls: NR |
ABO blood group did not influence outcomes of patients with COVID-19. |
| Levi, 202037 | Brail | Cross-sectional cohort, retrospective | Cases: 2,037 Controls:1,813,237 |
Cases: NR Controls: NR |
Cases: NR Controls: NR |
Cases: 44.8 vs 55.2% Controls: 46.5 vs 53.5% |
ABO blood group types did not significantly impact the risk for SARS-CoV-2 infection. |
| Barnkob, 202042 | Denmark | Cohort, retrospective | Cases: 7,422 Controls:466,232 |
Cases: 522 Controls: 502 |
Cases: 32.9% men Controls: 32% men |
Cases: 38.4 vs 61.6% Controls: 41.7 vs 58.3% |
ABO blood group is a risk factor for SARS-CoV-2 infection but not for hospitalisation or death from COVID-19 |
Table II.
Summary of findings table. Relationship between ABO blood group and occurrence and severity of COVID-19
| Patient or population: COVID-19 infected subjects and uninfected controls. Settings: Inpatients and Outpatients. Comparison: ABO prevalence among COVID-19 infected and non-infected individuals; ABO prevalence in patients with severe or non-severe COVID-19 infections. | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | N. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non-O group | O group | |||||
| Overall comparison | 382,537/892,496 (42.8%) | 34.6% (32.1/36.8%) | OR, 0.81 (0.75/0.86) | 922,145 (16; 18 cohorts) | ⊕⊝⊝⊝ very low1 |
There was evidence that individuals with blood group 0 had a decreased risk of SARS-COV-2 infection |
| Case-control studies | 81,183/184,966 (43.8%) | 31.9% (28.0/36.3%) | OR, 0.73 (0.64/0.83) | 193,112 (10; 12 cohorts) | ⊕⊝⊝⊝ very low1 |
There was evidence that individuals with blood group 0 had a decreased risk of SARS-COV-2 infection |
| Cohort studies | 301,354/707,530 (42.5%) | 37.8% (36.1/39.9%) | OR, 0.89 (0.85/0.94) | 729,033 (7) | ⊕⊕⊝⊝ low2 |
There was evidence that individuals with blood group 0 had a decreased risk of SARS-COV-2 infection. However, compared to case-control studies, the magnitude of the effect size in cohort studies was significantly lower |
| Severity of infections (endpoint severe infection/ICU admission) | 1,083/5,541 (19.5%) | 19.5% (17.7/21.2%) | RR, 1.00 (0.91/1.09) | 9,147 (7) | ⊕⊕⊝⊝ low3 |
Overall, individuals with blood group 0 had the same risk of severe SARS-CoV-2 infection compared to individuals with non-0 blood group |
The assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded for inconsistency due to heterogeneity, and twice for risk of bias in case-control studies (confounding, selection, ascertainment);
downgraded twice for inconsistency, and because not all the studies performed matching or adjustment of plausible prognostic variables;
downgraded for imprecision (95% CI includes line of no effect), and because not all the studies adjusted for prognostic factors
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. CI: confidence interval; RR: risk ratio.
Statistical analysis
The role of the O-type blood group in SARS-CoV-2 infection was evaluated comparing the prevalence of O type in infected patients (cases) and in non-infected subjects (controls). The meta-analysis was performed using the inverse variance (IV) method for study weighting, pooling odd ratios (ORs) and/or risk ratios (RRs) at study level. A random-effects approach was followed, with DerSimonian-Laird estimator for tau. The I-squared index for inconsistence was calculated to address the heterogeneity between studies.
The effect size calculation for case-control studies is based on the prevalence of the exposure in the diseased and in the not diseased, and should be calculated with an OR ratio20. In any case, often the OR is a good approximation of RR, especially if the incidence in both exposed and not exposed is low (<10%) and the true RR remains close to 1. For cohort studies, we evaluated the mean relative risk (RR) for the infection as this represents a more understandable quantification of effect size and preventable fraction in the exposed population (PFE).
Subgroup analyses
Subgroup analysis was carried out according to the study design (case control or cohort). Differences in effect size between case control and cohort studies were evaluated with a test for subgroup difference. p<0.1 was considered to be a statistically significant subgroup effect21. Stata 16.1 and package “meta” with R version 4.0.3 software were used to perform calculations.
RESULTS
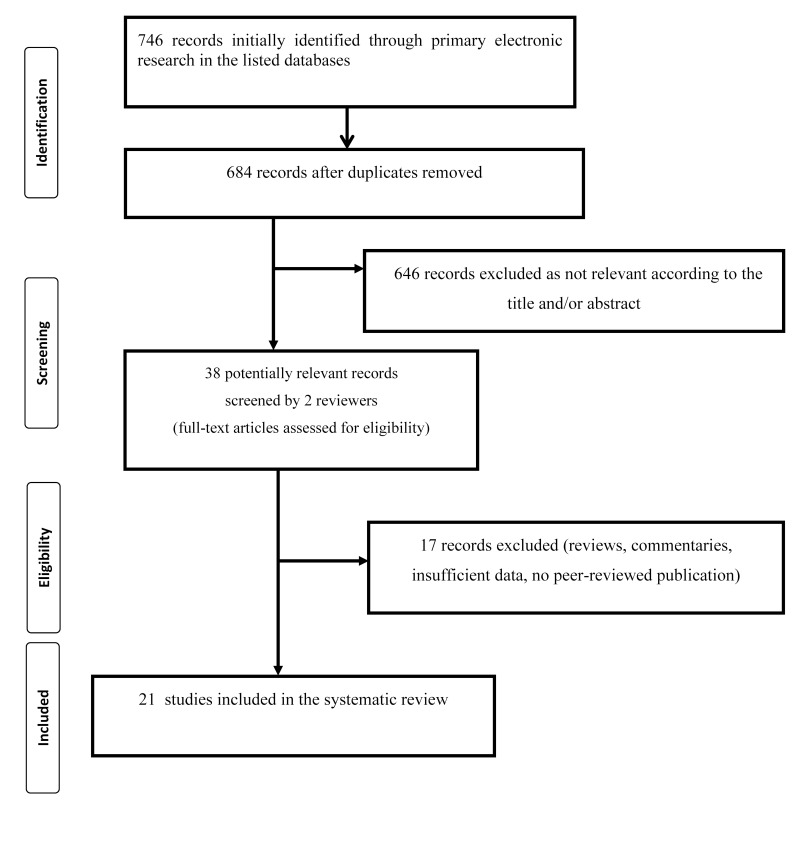
Overall, we identified 746 references through electronic and manual searches (Figure 1). Seven hundred and eight studies were excluded as duplicates or as not relevant to this review according to the title and/or abstract. After reading the full text of the remaining 38 potentially eligible studies, 17 were excluded (reviews, commentaries, non-peer reviewed publication, insufficient data). Only those studies fulfilling the selection criteria were included in the final analysis. Thus, for this systematic review, we considered 21 studies fulfilling our pre-specified criteria22–42. Table I summarises the main characteristics and results of these studies. Some studies included different cohorts of patients and, where 2×2 data were available, we calculated separate ORs for each cohort. Ellinghaus et al. provided data from Italian and Spanish hospitals22. Leaf et al.31 stratified the analysis according to race/ethnicity (White non-Hispanic, Black non-Hispanic, Asian non-Hispanic, and Hispanic). Zhao et al.36 collected patients and controls at the Jinyintan Hospital in Wuhan, and at Shenzhen Hospital, Guangdong Province, in the People’s Republic of China. In a Spanish study, Muñiz-Diaz et al.41 included a cohort of blood donors recruited for convalescent plasma donation after recovering from a mild SARS-CoV-2 infection, and a cohort of patients with severe SARS-CoV-2 infection who were transfused during hospitalisation (donors and transfused).
Figure 1.
Flow chart of the selection of studies
Bias assessment
The NOS checklists for individual studies are presented in the Online Supplementary Content (Tables SI–SIII). In cohort studies, the quality was judged high for both the outcomes analysed since all studies achieved from 7 to 9 stars. For the outcome prevalence of COVID-19 in case control studies, the NOS score was <7 in 8 of the 11 case control studies (Online Supplementary Table SII).
Publication bias was evaluated for the outcome prevalence of infection. On the whole, the asymmetric aspect of the funnel plot seems to be at least partly due to the different distributions of the study effect sizes according to the study designs (Online Supplementary Figures S1 and S2). When the two designs were examined jointly, the Egger test was significant (p=0.025); however, when the two designs were examined separately the significance was lost.
Quantitative analysis
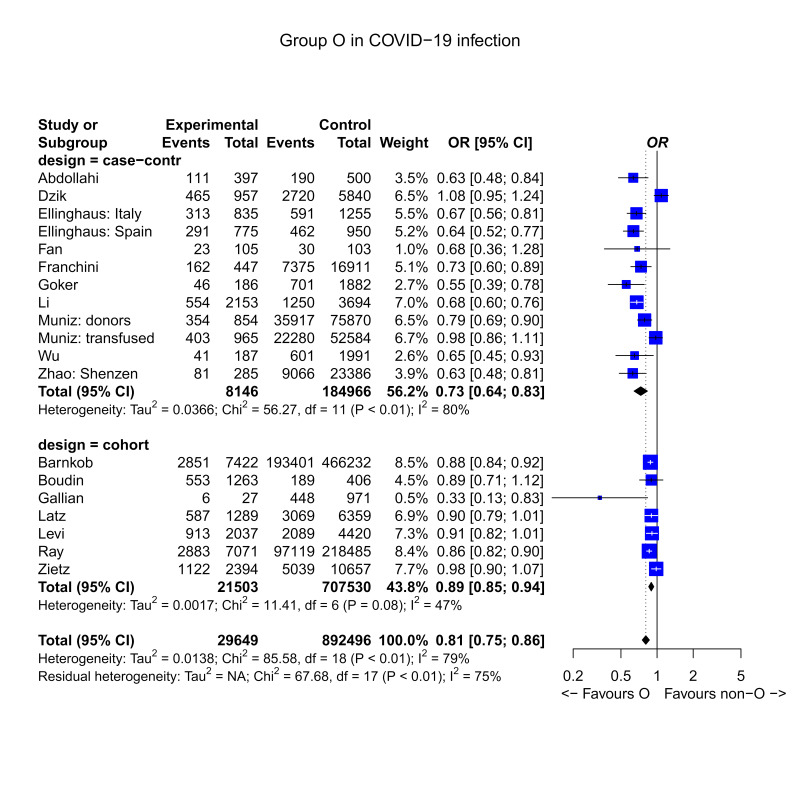
Outcome: prevalence of O type vs non-O blood types in SARS-CoV-2 infected patients
The outcome prevalence of ABO groups in COVID-19 infected or non-infected individuals was reported in 17 studies, including 7 cohort studies24,28,30,37–39,42 and 10 case control studies22,23,25–27,29,32,33,36,41. Figure 2 reports the forest plot of the prevalence of the blood group O vs non-O types in cases (infected SARS-CoV-2 patients) and control (non-infected) subjects. The role of the O-type blood group in SARS-CoV-2 infection was evaluated in 17 studies, accounting for 19 2×2 tables. Overall mean OR was 0.81 (95% confidence interval [CI]: 0.75, 0.86). The effect was significantly different from the null hypothesis of absence of effect by O type on the probability of SARS-CoV-2 infection (z=6.19, p<0.0001). The effect was protective, suggesting a lower risk in subjects of O type. The quality of the evidence was graded as very low for inconsistency due to heterogeneity, and for risk of biases in case control studies (confounding, selection, ascertainment) (Table II). The mean OR of case control studies was 0.73 (95% CI: 0.64, 0.83), whereas the mean OR of cohort studies was 0.89 (95% CI: 0.85, 0.94). Thus, the null hypothesis of OR=1 was rejected in both cases, but the difference in the effect size was significantly lower in cohort studies compared to case control studies (test for subgroup difference: Q=8.31, degree of freedom: 1, p=0.0039).
Figure 2.
Forest plot of the prevalence of the blood group 0 vs non-0 types in cases (infected SARS-CoV-2 patients) and control (uninfected) subjects
Mean RR, evaluated in cohort studies, was 0.92 (95% CI: 0.87, 0.97), with z-score=3.21, p=0.001, and substantial heterogeneity (I-squared=72.7%). PFE was 8.1% (95% CI: 3.2%, 12.7%). The quality of the evidence in cohort studies was graded as low due to inconsistency, and because not all the studies performed matching or adjustment of plausible prognostic variables.
Outcome: severity of SARS-CoV-2 infection
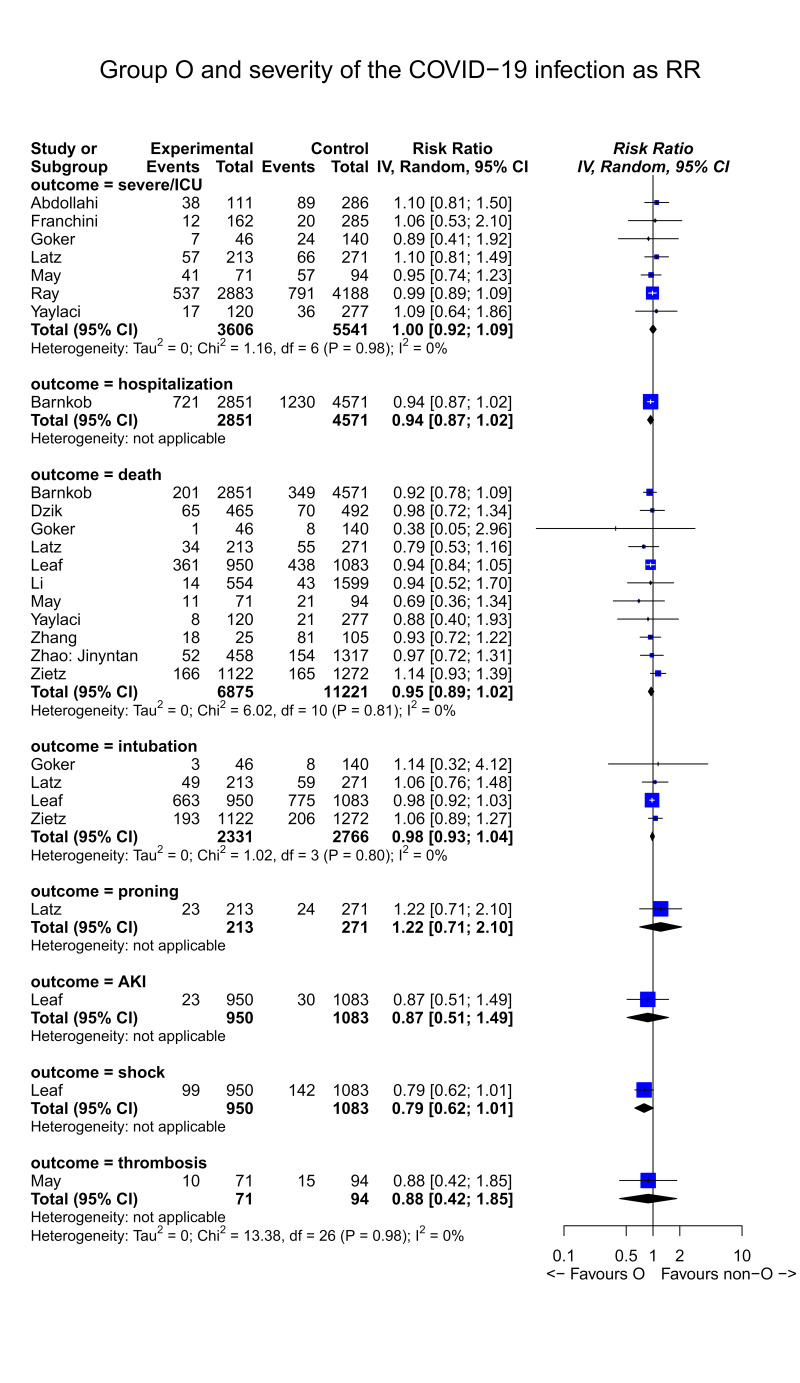
The differential exposure to O blood group in cases (severe infection) and controls (non-severe infection) was expressed as RR, and the RRs were then pooled using a random-effect model. The outcome severity of SARS-CoV-2 infection was reported in 14 studies23,25,27,29,30–32,34–36,38–40,42. The severity of the disease was not uniformly defined in the cohorts of infected patients, and 8 endpoints of severity were assessed, each vs the opposite condition. The endpoints included in the selected studies were: i) severe vs non-severe clinical disease (7 studies)23,27,29,30,34,38,40; ii) death (11 studies)25,29–32,34–36,39,40; iii) hospitalisation vs non-hospitalisation (1 study)42; iv) need for intubation (4 studies)29–31,39; v) requirement of proning in the treatment of respiratory insufficiency (1 study)30; vi) acute kidney injury at admission (1 study)31; vii) shock (1 study)31; viii) thrombosis (1 study)40.
Endpoints indicating clinical severity and related O type distribution are summarised in Figure 3; none were significantly associated to the O type blood group. In other words, no evidence was found indicating an effect of the O type on the disease severity in the infected patients. The RR was 1.0 (95% CI: 0.92–1.09) for the endpoint severe infection, and 0.95 (95% CI: 0.89–1.02) for death. The quality of the evidence was graded as low due to imprecision (95% CI includes line of no effect), and because not all the studies adjusted for prognostic factors
Figure 3.
Forest plot of the severity of SARS-CoV-2 infection according to blood group (O vs non-O blood group)
DISCUSSION
Due to the severity of the disease, mostly unpredictable, the identification of risk factors associated with SARS-CoV-2 infection and outcomes has become a research priority. Thus, following the first reports in literature on the association between ABO blood group and COVID-19, this has been the focus of attention in a number of investigative studies and, in particular, whether ABO blood type was associated not only with COVID-19 onset but also with its severity and disease-related mortality43. This growing interest is not surprising considering that the study of the interaction between ABO blood system and infections has a long history and extensive literature12. Individuals with blood group O were reported to be more susceptible to Norovirus, and also had a significantly higher prevalence of Helicobacter pylori, but were less susceptible to SARS and hepatitis B virus11,44–47. In another study, blood group A was associated with an increased risk of acute respiratory distress syndrome (ARDS) in trauma and sepsis patients48. A study by Lebiush et al. on influenza A (H1N1) observed a higher seroconversion rate in blood groups A and B49. Elnady et al. found that individuals with blood type A were more susceptible to rotavirus gastroenteritis than those with blood type B50. Among patients infected with dengue virus, Murugananthan et al. found that individuals with AB blood type had a 2.5 times higher risk of developing dengue haemorrhagic fever than those with other blood types51. Finally, there is strong evidence that ABO phenotype modulates severity of Plasmodium falciparum-associated malaria, with group A associated with severe disease and blood group O with milder disease52.
Regarding the issue of this systematic review, the first reports on the evidence of a relationship between SARS-CoV-2 infection and ABO blood groups were published early in China and so far 21 articles have been published worldwide, covering a large number of cases. The pathogenic mechanism underlying this association is quite complex and encompasses several molecular pathways. Further experimental studies are needed to better characterise the role of anti-SARS-CoV-2 neutralising anti-A IgG antibodies in COVID-19 onset, and the importance of plasma VWF/FVIII levels and endothelial cell activation in COVID-19-induced coagulopathy and pulmonary microvascular occlusion17,53. Whatever the underlying mechanism, this correlation is quite intriguing as it allows us to make some considerations that could have potentially important implications. First, the ABO-driven COVID-19 susceptibility could account for the inter-ethnic epidemiological difference in SARS-CoV-2 infection. Indeed, Africa is the continent with the lowest number of cases and deaths (1,044,513 confirmed cases with 21,722 deaths at 30th August 2020; data available at: https://covid19.who.int/). Curiously, among the different ethnicities, Africans have the highest percentage of O blood type (up to 60%). Another interesting observation regards the distribution of COVID-19 across different ages and genders. In fact, it is equally well known that men and the elderly are more affected by SARS-CoV-2 infection than women and young people. Previous studies had demonstrated that women with O blood type have higher anti-A IgG antibody levels than males, and that the titer of anti-A and anti-B isoagglutinins declines with age54,55. Although these findings do not constitute the definitive proof of the causal association between ABO blood type and gender-, age- and ethnic-related differences in the epidemiological distribution of COVID-19, these considerations are quite curious and deserve further investigation.
The results of this systematic review, which are in agreement with those published in another recent meta-analysis15, indicate the lower susceptibility of O blood type individuals to being infected by SARS-CoV-2 than non-O subjects. The investigational hypothesis of a protective effect exerted by the O type on the risk of the SARS-CoV-2 infection was confirmed by both study designs, case control and cohort. However, the effect size was lower in cohort studies compared to case control studies. This is not surprising since, compared to cohort design, case control studies are more susceptible to bias due to confounding co-variates with different distributions in cases (infected patients) and the control population. Sample size for case control studies is based on prevalence of exposure, not on incidence of outcome. Because the prevalence of the exposure is usually larger than the incidence of outcome, in most practical situations a case control study is more powerful than a cohort study for the same problem. On the other hand, case control studies are very likely to suffer from bias. Controls should be drawn from the same general population that gave rise to the cases. However, in practice, the comparability of cases and controls is difficult to achieve, making this aspect the Achilles heel of case control design. In the present systematic review, no evidence was found indicating an effect of the O blood type on the disease severity in the infected patients. Larger, well-designed epidemiological trials are, however, needed to clarify the relationship between the ABO blood group system and the risk of developing COVID-19 or a more severe disease.
Supplementary Information
Footnotes
The Authors declare no conflicts of interest.
REFERENCES
- 1.Storry JR, Olsson ML. The ABO blood group system revisited: a review and update. Immunohematology. 2009;25:48–59. [PubMed] [Google Scholar]
- 2.Yamamoto F, Clausen H, White T, et al. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–33. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 3.Lowe J. The blood group-specific human glycosyltransferases. Baillieres Clin Haematol. 1993;6:465–90. doi: 10.1016/s0950-3536(05)80155-6. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M, Liumbruno GM. ABO blood group: old dogma, new perspectives. Clin Chem Lab Med. 2013;51:1545–53. doi: 10.1515/cclm-2013-0168. [DOI] [PubMed] [Google Scholar]
- 5.Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus. 2013;11:491–9. doi: 10.2450/2013.0152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchini M, Mannucci PM. ABO blood group and thrombotic vascular disease. Thromb Haemost. 2014;112:1103–9. doi: 10.1160/TH14-05-0457. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Favaloro EJ, Targher G, Lippi G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit Rev Clin Lab Sci. 2012;49:137–49. doi: 10.3109/10408363.2012.708647. [DOI] [PubMed] [Google Scholar]
- 8.Franchini M, Lippi G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015;13:7. doi: 10.1186/s12916-014-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Crestani C, Frattini F, et al. ABO blood group and von Willebrand factor: biological implications. Clin Chem Lab Med. 2014;52:1273–6. doi: 10.1515/cclm-2014-0564. [DOI] [PubMed] [Google Scholar]
- 10.Ward S, O’Sullivan J, O’Donnell JS. The relationship between ABO blood group, von Willebrand factor and primary hemostasis. Blood. 2020;136:2864–74. doi: 10.1182/blood.2020005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Cheng G, Chui CH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450–1. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 12.Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28:801–70. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaidarova Z, Bravo MD, Kamel HT, et al. Blood group A and D negativity are associated with symptomatic West Nile virus infection. Transfusion. 2016;56:1699–706. doi: 10.1111/trf.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Zhang S, Liu M, et al. Distribution of ABO/Rh blood groups and their association with hepatitis B virus infection in 3.8 million Chinese adults: a population-based cross-sectional study. J Viral Hepat. 2018;25:401–11. doi: 10.1111/jvh.12829. [DOI] [PubMed] [Google Scholar]
- 15.Golinelli D, Boetto E, Maietti E, Fantini MP. The association between ABO blood group and SARS-CoV-2 infection: a meta-analysis. PLoS One. 2020;15:e0239508. doi: 10.1371/journal.pone.0239508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu BB, Gu DZ, Yu JN, et al. Association between ABO blood groups and COVID-19 infection, severity and demise: a systematic review and meta-analysis. Infect Genet Evol. 2020;84:104485. doi: 10.1016/j.meegid.2020.104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Focosi D. Anti-A isohaemagglutinin titres and SARS-CoV-2 neutralization: implications for children and convalescent plasma selection. Br J Haematol. 2020;190:e148–50. doi: 10.1111/bjh.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. [Accessed on 10/02/2021]. Available from: wwwohrica/programs/clinical_epidemiology/oxford.asp.
- 19.Reeves BC, Deeks JJ, Higgins JPT, et al. Higgins JPT, Green S, editors. on behalf of the Cochrane Non-Randomised Studies Methods Group. Chapter 13: Including non-randomised studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) [Accessed on 10/02/2021]. Available at: https://handbook-5-1.cochrane.org/chapter_13/13_including_non_randomized_studies.htm.
- 20.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Ment Health. 2019;22:153–60. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodward M. Epidemiology: study design and data analysis. 3rd Ed. Boca Raton, FL, USA: CRC Press; 2013. [Google Scholar]
- 22.Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383:1522–34. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdollahi A, Mahmoudi-Aliabadi M, Mehrtash V, et al. The Novel Coronavirus SARS-CoV-2 Vulnerability Association with ABO/Rh Blood Types. Iran J Pathol. 2020;15:156–160. doi: 10.30699/ijp.2020.125135.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudin L, Janvier F, Bylicki O, Dutasta F. ABO blood groups are not associated with risk of acquiring the SARS-CoV-2 infection in young adults. Haematologica. 2020;105:2841–3. doi: 10.3324/haematol.2020.265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzik S, Eliason K, Morris EB, et al. COVID-19 and ABO blood groups. Transfusion. 2020;60:1883–4. doi: 10.1111/trf.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Q, Zhang W, Li B, et al. Association between ABO blood group system and COVID-19 susceptibility in Wuhan. Front Cell Infect Microbiol. 2020;10:404. doi: 10.3389/fcimb.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franchini M, Glingani C, Del Fante C, et al. The protective effect of O blood type against SARS-CoV-2 infection. Vox Sang. 2021;116:249–50. doi: 10.1111/vox.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallian P, Pastorino B, Morel P, et al. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res. 2020;181:104880. doi: 10.1016/j.antiviral.2020.104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göker H, Aladağ Karakulak E, Demiroğlu H, et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci. 2020;50:679–83. doi: 10.3906/sag-2005-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latz CA, De Carlo C, Boitano L, et al. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99:2113–8. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leaf RK, Al-Samkari H, Brenner SK, et al. ABO phenotype and death in critically ill patients with COVID-19. Br J Haematol. 2020;190:e204–e8. doi: 10.1111/bjh.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Wang X, Chen J, et al. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol. 2020;190:24–7. doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Feng Z, Li P, Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin Chim Acta. 2020;509:220–3. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaylacı S, Dheir H, İşever K, et al. The effect of abo and rh blood group antigens on admission to intensive care unit and mortality in patients with COVID-19 infection. Rev Assoc Med Bras. 2020;66(Suppl 2):86–90. doi: 10.1590/1806-9282.66.S2.86. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiol Infect. 2020;148:e199. doi: 10.1017/S0950268820002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. Clin Infect Dis. 2020:ciaa1150. doi: 10.1093/cid/ciaa1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levi JE, Telles PR, Scrivani H, Campana G. Lack of association between ABO blood groups and susceptibility to SARS-CoV-2 infection. Vox Sang. 2021;116:251–2. doi: 10.1111/vox.13015. [DOI] [PubMed] [Google Scholar]
- 38.Ray JG, Schull MJ, Vermeulen MJ, Park AL. Association between ABO and Rh blood groups and SARS-CoV-2 infection or severe COVID-19 illness: a population-based cohort study. Ann Intern Med. 2021;174:308–15. doi: 10.7326/M20-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun. 2020;11:5761. doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May JE, McGwin G, Jr, Gangaraju R, et al. Questioning the association between ABO type and outcomes in patients with COVID-19. Ann Hematol. 2020 doi: 10.1007/s00277-020-04348-0. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñiz-Diaz E, Llopis J, Parra R, Roig I, et al. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021;19:53–63. doi: 10.2450/2020.0256-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnkob MB, Pottegård A, Støvring H, et al. Reduced prevalence of SARS-CoV-2 infection in ABO blood group O. Blood Adv. 2020;4:4990–3. doi: 10.1182/bloodadvances.2020002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pourali F, Afshari M, Alizadeh-Navaei R, et al. Relationship between blood group and risk of infection and death in COVID-19: a live meta-analysis. New Microbes New Infect. 2020;37:100743. doi: 10.1016/j.nmni.2020.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk virus infection. Nature Med. 2003;9:548–53. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 45.Liao Y, Xue L, Gao J, et al. ABO blood group-associated susceptibility to norovirus infection: a systematic review and meta-analysis. Infect Genet Evol. 2020;81:104245. doi: 10.1016/j.meegid.2020.104245. [DOI] [PubMed] [Google Scholar]
- 46.Lin CW, Chang YS, Wu SC, Cheng KS. Helicobacter pylori in gastric biopsies of Taiwanese patients with gastroduodenal diseases. Japan J Med Sci Biol. 1998;51:13–23. doi: 10.7883/yoken1952.51.13. [DOI] [PubMed] [Google Scholar]
- 47.Mohammadali F, Pourfathollah A. Association of ABO and Rh blood groups to blood-borne infections among blood donors in Tehran-Iran. Iran. J Public Health. 2014;43:981–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Reilly J, Meyer N, Shashaty M, et al. ABO blood type A is associated with increased risk of acute respiratory distress syndrome in caucasians following both major trauma and severe sepsis. Chest. 2014;145:753–61. doi: 10.1378/chest.13-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lebiush M, Rannon L, Kark J. The relationship between epidemic influenza A (H 1 N 1) and ABO blood groups. J Hyg (Lond) 1981;87:139–46. doi: 10.1017/s002217240006931x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elnady HG, Abdel SO, Saleh MT, et al. ABO blood grouping in Egyptian children with rotavirus gastroenteritis. Prz Gastroenterol. 2017;12:175–80. doi: 10.5114/pg.2017.70469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murugananthan K, Subramaniyam S, Kumanan T, et al. Blood group AB is associated with severe forms of dengue virus infection. Virusdisease. 2018;29:103–5. doi: 10.1007/s13337-018-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loscertales MP, Owens S, O’Donnell J, et al. ABO blood group phenotypes and Plasmodium falciparum malaria: unlocking a pivotal mechanism. Adv Parasitol. 2007;65:1–50. doi: 10.1016/S0065-308X(07)65001-5. [DOI] [PubMed] [Google Scholar]
- 53.Franchini M, Marano G, Cruciani M, et al. COVID-19-associated coagulopathy. Diagnosis (Berl) 2020;7:357–63. doi: 10.1515/dx-2020-0078. [DOI] [PubMed] [Google Scholar]
- 54.McVey J, Baker D, Parti R, et al. Anti-A and anti-B titers in donor plasma, plasma pools, and immunoglobulin final products. Transfusion. 2015;55(Suppl 2):S98–S104. doi: 10.1111/trf.13114. [DOI] [PubMed] [Google Scholar]
- 55.Liu YJ, Chen W, Wu KW, et al. The development of ABO isohemagglutinins in Taiwanese. Hum Hered. 1996;46:181–4. doi: 10.1159/000154350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.