Abstract
Background
Red blood cell (RBC) transfusion remains an essential part of sickle cell disease (SCD) management but it can lead to alloimmunisation, with an increased incidence in this population. Prevention is based on RBC antigen phenotype matching, with complete RH and Kell matching being a standard of care.
Materials and methods
We performed a retrospective, single-centre study analysing alloimmunisation prevalence and risk factors in a cohort of transfused SCD patients.
Results
Eighty-seven patients (96.5% of paediatric age) received 1,781 RBC units (RBCu). Complete RH and Kell matched RBCu represented a median of 100% among total transfusions per patient. Of the 87 patients, 52 (59.8%) underwent chronic transfusion therapy, whereas 35 (40.2%) were only episodically transfused. Seven patients were alloimmunised (8.4%) and eleven antibodies were detected (alloimmunisation rate: 0.62/100 units transfused). 54.6% of these antibodies corresponded to RH-Kell despite the high accomplishment of the RH-Kell matching transfusion protocol. Alloimmunised patients had a median of 90.9% RH-Kell matched transfusions vs 100% in non-alloimmunised patients, but no statistical differences were observed (p=0.127). Number of transfused RBCu (19 vs 7; p=0.023), number of episodic RBCu (8 vs 2; p=0.006), episodic to chronic RBCu ratio (0.57 vs 0.09; p=0.045), number of vaso-occlusive crises (VOC) (4 vs 2; p=0.011), and autoantibody presence (57.1 vs 0%; p<0.001) were all statistically related to alloimmunisation.
Discussion
We report a low alloimmunisation prevalence (8.4%) related to a high grade of RH-Kell matching. However, deviation from 100% translates into alloimmunisation, with >50% of alloantibodies corresponding to RH-Kell. Alloimmunisation risk increases with transfusion burden, particularly during acute complications, and in patients with a higher number of VOC, probably reflecting underlying inflammation and disease severity. Further studies will be needed to elucidate additional risk factors and help prevent alloimmunisation in these patients.
Keywords: sickle cell disease, erythrocyte transfusion, alloimmunisation, immunohaematology
INTRODUCTION
Sickle cell disease (SCD) is an inherited disorder affecting approximately 300,000 newborns annually worldwide. Increasing migratory movements from endemic areas have conditioned distribution changes making SCD a new health challenge in Europe1–3 with an estimated prevalence of 22 per 100,000. In Spain, this has led to the implementation of universal neonatal screening programmes in areas like the region of Madrid where the incidence of SCD for 2003 was 0.16 per 100,000 births4. Posterior data showed a national prevalence of 1.34 per 100,000 inhabitants5, confirming a rising trend in SCD cases.
Red blood cell (RBC) transfusion remains a cornerstone of SCD management, not only for the treatment of acute complications, but also as a preventive tool6,7, effective both for primary and secondary stroke prevention8. However, transfusion can lead to alloimmunisation, resulting not only in a higher risk of delayed haemolytic transfusion reactions (DHTR)9, but also challenging the provision of adequate transfusion support in these patients, which may contribute to premature deaths10,11. The incidence of red blood cell alloimmunisation is higher in SCD compared to the general population with reported rates ranging from 20 to 70%12–15. This higher rate is partially explained by RBC phenotypic disparity conditioned by ethnic differences between donors and patients, especially affecting C, E and K antigens13,16.
Matching for complete RH and Kell antigens reduces alloimmunisation incidence to 5–14%7,17–20 and has become a standard of care21–23. Extended matching (Kidd, Duffy and S) is even more effective17, but incurs additional costs and increases the difficulty in finding suitable RBC units (RBCu)22. Genotyping provides additional information and is especially useful in recently transfused patients and when RH variants are suspected20,24,25. The latest guidelines suggest this approach is to be preferred over serology23. Nevertheless, its widespread application is currently still limited by its higher costs26–28.
In consideration of all these factors, a deeper understanding of alloimmunisation mechanisms and risk factors has become a subject of interest. Highly susceptible patients need to be identified in order to efficiently prevent alloimmunisation15,29.
Besides the established association between transfusion load and alloimmunisation13,25,30,31, inflammation underlying the pathophysiology of SCD itself and the pro-inflammatory state prior to transfusion seem to be involved in alloimmunisation risk13,18. Individual features have also been proposed as risk factors, including sex12,32, age12,30,32, age at first transfusion13,15, or coexistence of an autoantibody12,13,30–32. Most of these studies are based on American populations, whereas information on European countries is scarce31.
Our institution is a national reference centre for erythropathology, mainly hemoglobinopathies, assisting 250 SCD patients with 12 new diagnoses each year. In this setting, we aimed to determine the prevalence of alloimmunisation in our SCD population and to analyse the risk factors involved. Another objective was to evaluate the accomplishment of our SCD transfusion protocol and its contribution to alloimmunisation prevention.
MATERIALS AND METHODS
Study design
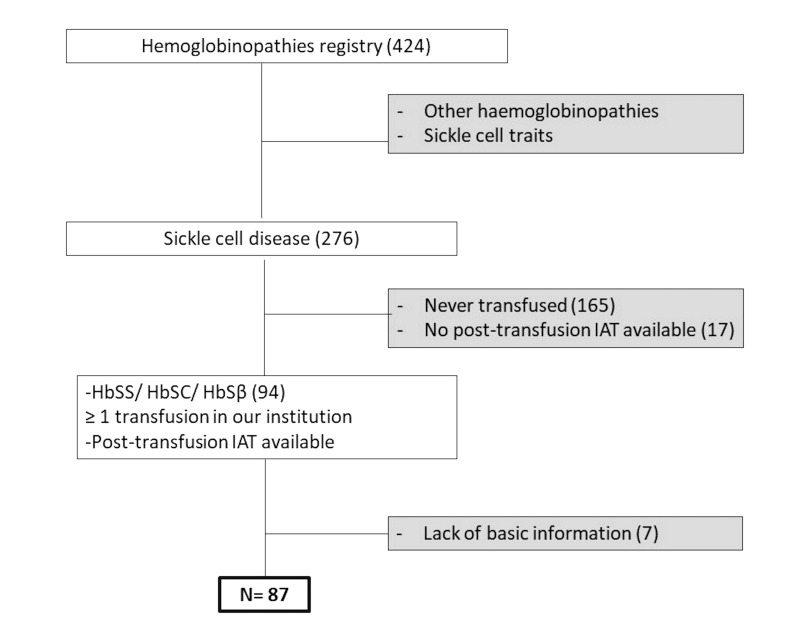
This was a single-centre retrospective study performed in a Spanish tertiary care centre that is a national point of reference for hemoglobinopathies. Inclusion criteria were: SCD diagnosis including SS, SC, and SB phenotypes; transfused at least once in our institution between July 2006 and April 2019, and with an available post-transfusion irregular antibody screening test (IAT). The selection algorithm used is shown in Figure 1. Medical files and transfusion records were systematically reviewed. All information was obtained according to local data protection laws and regulations. The study was approved by the hospital ethics committee.
Figure 1. Patient selection flow chart.
IAT: Irregular antibody screening test.
Collected data included demographic variables such as sex, date of birth, SCD phenotype and geographic origin, as well as clinical data including splenectomy, hydroxyurea treatment, iron chelation, complications during follow-up, and number of admissions due to complications related to the disease. Recorded complications were vaso-occlusive crises (VOC), splenic sequestration (SS), acute chest syndrome (ACS), stroke, aplastic anaemia, dactylitis or febrile episodes.
Transfusion history was registered, including age at first transfusion, number of total RBCu received, and indication of each transfusion. Transfusions were defined as (i) “episodic”: transfused during an acute complication and (ii) “chronic”: transfused within a chronic transfusion therapy programme (CTTP). For each patient, total number of RBCu transfused, number of episodic RBCu, and number of chronic RBCu were registered. The ratio between episodic and chronic RBCu was calculated. Patients were classified into two groups depending on whether they had been included on a CTTP or had only been episodically transfused. For every transfused RBCu, antigen information was compared to the patient’s phenotype. For each patient, we calculated the percentage of complete RH and Kell matched units among total RBCu received. In case of irregular antibody detection, specificity, date of detection and number of previously transfused RBCu were registered.
Transfusion procedures and antibody detection
All transfused RBCu were leukoreduced. Patients undergoing stem cell transplantation (SCT) received irradiated products.
Since 2009, we have been applying a protocol of phenotypically matched RBC transfusion in SCD patients. At diagnosis, RBC serological antigen extended phenotype is performed including ABO; complete RH (C/c, D, E/e), Kell, Duffy (Fya), Kidd (Jka) and MNS (S). Genotyping is only mandatory for patients who have been transfused elsewhere. Genotyping is performed at the Regional Transfusion Center using BLOODchip® (Grifols SA, Barcelona, Spain) molecular genotyping platform including 37 RBC antigens corresponding to Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright and Lutheran systems.
Complete RH and Kell matched RBCu are provided to all SCD patients, and extended matching (Fy, Jk and S) is restricted to alloimmunised patients in which RBCu also need to be negative for the corresponding antigen33.
For IAT, a gel-based test with a 3-cell panel reagent (Ortho Clinical Diagnostics, Raritan, NJ, USA) is performed on an Ortho Autovue® or Vision® automated analyser. Extended 11-cell panels and enzyme-treated panels (Immucor, Barcelona, Spain and Ortho Clinical Diagnostics) are used after positive screening for alloantibody identification.
Autoantibody detection is made by direct antiglobulin test with polyspecific and monospecific reagents for IgG and complement. If positive, elution with DiaCidel® (Bio-Rad, Hercules, CA, USA) is performed to define specificity.
Statistical analysis
For the descriptive analysis, quantitative variables are expressed as median and interquartile range (IQR). Alloimmunisation prevalence was defined as the number of alloimmunised patients within the number of patients included. Alloimmunisation rate was calculated by dividing the number of detected antibodies by the number of received transfusions. Non-parametric tests were performed due to the absence of normal distribution of most of the studied variables.
To assess alloimmunisation risk factors, clinical and transfusion characteristics were statistically compared between alloimmunised and non-alloimmunised patients. Mann Whitney test was used for continuous variables and χ2/Fisher test for categorical variables. Multivariate logistic regression analysis could not be performed due to the low number of registered events. p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistics 25 software.
RESULTS
Eighty-seven patients were analysed (60.9% male). Eighty-four (96.6%) were of paediatric age at the beginning of the study, with a median age of 3 years (1–6 years) at first transfusion in our hospital. Median follow-up was 3 years (1–7 years). Sixty-five (74.7%) were of African origin, 19 (21.8%) Latin-American, 2 (2.3%) Caucasian, and one (1.1%) was Asian. Regarding HbS phenotype, 83 patients (95.4%) were HbSS, 3 (3.4%) HbSB, and one (1.1%) was HbSC. Seventy-four patients (85.1%) had received hydroxyurea, 23 (26.4%) iron chelating agents, and ten (11.8%) had been splenectomised.
During follow-up, 32 patients (36.8%) underwent SCT with a median age of 6 years (2–9 years). Five patients (5.7%) died during follow-up. None of the deaths was related to haemolytic complications.
Patients’ baseline characteristics and RBC antigen frequencies are presented in the Online Supplementary Tables SI and SII.
Transfusion analysis
Eighty-seven patients received a total of 1,781 RBCu. Median of complete RH and Kell matched units per patient was 100% (85–100%). Thirty-nine patients (44.8%) received at least one transfusion outside our centre. In most cases, these transfusions were prior to receiving transfusion support at our centre.
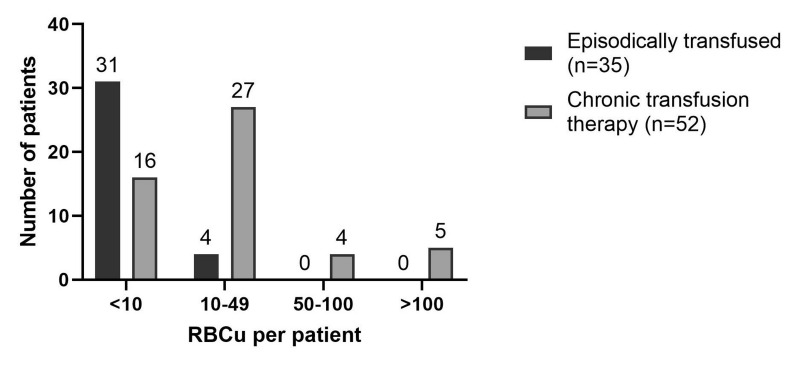
Fifty-two patients (59.8%) were included in CTTP, with a median of 12 (IQR 7–21) RBCu per patient (range 3–225). Another 35 patients (40.2%) were only episodically transfused with a median of 3 (IQR 2–6) RBCu per patient (range 1–22). Transfusion load in both groups is shown in Figure 2.
Figure 2. Transfusion load.
RBCu: red blood cell units; n: number.
Indications for CTTP were: preparation for SCT (53.9%), primary stroke prevention (23.1%), secondary stroke prevention (9.6%), VOC prevention (7.7%), pregnancy (1.9%), pulmonary hypertension (1.9%), and renal impairment (1.9%).
Alloimmunisation
We detected 16 antibodies in 11 patients (12.6%). In 4 patients, all the antibodies were identified before any transfusion in our hospital and were, therefore, excluded from the subsequent analysis. The remaining 7 patients, presumably alloimmunised in our institution, imply an alloimmunisation prevalence of 8.4% with a rate of 0.62 per 100 units transfused.
The alloimmunisation rate was higher in the only episodically transfused group (1.74 per 100 units) than in CTTP patients (0.56 per 100 units), but these differences were not statistically significant (p=0.317).
Median age at first antibody detection was 10 years (4–22). Median number of RBCu received before alloimmunisation was 7 (3–13). Three patients (42.9%) had only one alloantibody while the other 4 (57.1%) had 2 alloantibodies each (Table I).
Table I.
Summary of alloimmunised patients
| Patient | Sex | Demographic origin | Phenotype | CTTP | RH-K matched RBCu1 | Antibody | Age at first antibody detection | Previous RBCu2 | Total RBCu |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | African | C− c+ E− e+ K− Fya− Jka+ S+ | No | 77.8% | Anti-E | 22y | 8 | 9 |
| Anti-C | 9 | ||||||||
| 2 | F | Latin-American | C− c+ E− e+ K− Kpa− Kpb+ Fya− Fyb+ Jka+ Jkb+ S+ s+ | No | 100% | Anti-Fya | 17y | 13 | 22 |
| 3 | M | Latin-American | C+ c+ E− e+ K− Kpa− Fya− Jka+Lea− S− | Yes (VOC) | 98.2% | Anti-Kpa | 4y | 17 | 225 |
| Anti-K | 86 | ||||||||
| 4 | F | Latin-American | C+ c+ E− e+ K− Kpa− Kpb+ Fya+Fyb− Jka+ Jkb+ M+ N+ S+ s+ | Yes (VOC) | 81.8% | Anti-E | 27y | 2 | 11 |
| 5 | F | African | C+ c+ E− K− Kpa− Fya+ Fyb+Jka+ Jkb− Lua+ Lub+ M− N+ S− | Yes (stroke) | 90.9% | Anti-M | 10y | 1 | 11 |
| Anti-S | 7 | ||||||||
| 6 | M | Latin-American | C+ c+ E− e+ K− Fya− Jka+ S+ | No | 95.5% | Anti-Fya | 10m | 3 | 22 |
| 7 | M | Latin-American | C+ c+ E− e+ K− Fya+ Jka+ S+ | Yes (stroke) | 84.2% | Anti-E | 6y | 3 | 19 |
| Anti-K | 4 |
Percentage of total RBCu matched for RH and Kell.
Number of transfusions received before antibody detection.
M: male, F: female, VOC: vaso-occlusive crises; RBCu: red blood cell units; CTTP: chronic transfusion therapy program; y: years; m: months.
Out of the 11 identified antibodies, 6 (54.6%) corresponded to RH or Kell specificities, 3 anti-E (27.3%), 2 anti-K (18.2%), and one anti-C (9%). The remaining corresponded to 2 Fya (18.2%), one Kpa (9.1%), and 2 MNS (18.2%).
We only registered one DHTR (1.19%) in a patient with 2 previous antibodies against E and K antigens. Autoantibodies were detected in 4 patients (4.82%).
Alloimmunisation risk factors
We found a strong association between total number of RBCu transfused per patient and alloimmunisation; 19 (11–22) vs 7 (3–14) (p=0.023) (Table II). Median percentage of RH-Kell matched units was 90.9% (81.8–98.2) in alloimmunised patients vs 100% (86.2–100) in non-alloimmunised patients, but these differences were not statistically significant (p=0.127).
Table II.
Comparison of clinical and transfusion variables between alloimmunised and non-alloimmunised patients (n=83)
| Alloimmunised (n=7) | Non-alloimmunised (n=76) | p | |
|---|---|---|---|
| Female sex | 3/7 (42.8%) | 28/76 (36.8%) | 0.525 |
| HbSS | 7/7 (100%) | 73/76 (96%) | 0.765 |
| Group O | 4/7 (57.1%) | 35/76 (46%) | 0.432 |
| Rh D positive | 7/7 (100%) | 70/76 (92.1%) | 0.579 |
| Age at first transfusion ≥5 y | 5/7 (71.4%) | 26/76 (34.2%) | 0.064 |
| Transfused elsewhere | 4/7 (57.1%) | 34/76 (44.7%) | 0.417 |
| Total RBCu | 19 (11–22) | 7 (3–14) | 0.023 |
| Total episodic RBCu | 8 (4–22) | 2 (1–4) | 0.006 |
| Total chronic RBCu | 8 (0–11) | 5 (0–11) | 0.421 |
| Episodic/chronic RBCu ratio1 | 0.57 (0.13–0.82) | 0.09 (0–0.27) | 0.045 |
| RH-K matched RBCu2 | 90.9% (81.8–98.2) | 100% (86.2–100) | 0.127 |
| Chronic transfusion therapy | 5/7 (71.4%) | 45/76 (59.2%) | 0.420 |
| Erythropheresis | 2/7 (28.6%) | 10/76 (13.2%) | 0.266 |
| N. of severe events (ACS+Stroke+SS) | 2 (1–5) | 1 (1–3) | 0.230 |
| N. of VOC episodes | 6 (1–12) | 1 (0–3) | 0.011 |
| N. of hospital admissions | 4 (1–5) | 1 (1–3) | 0.236 |
| Autoantibody presence | 4/7 (57.1%) | 0/78 (0%) | <0.001 |
Ratio between total number of episodically transfused RBCu and total number of RBCu transfused within chronic transfusion therapy programme.
Percentage of total RBCu matched for RH and Kell.
Four patients were excluded due to alloantibody detection previous to any transfusion in our centre.
Bold indicates statistical significance. y: years; RBCu: red blood cell units; N: number; ACS: acute chest syndrome; SS: splenic sequestration; VOC: vaso-occlusive crises.
Number of episodic RBCu per patient (8 in alloimmunised [4–22]) vs 2 in non-alloimmunised [1–4]) and a higher episodic to chronic transfusion ratio (0.57 [0.13–0.82] vs 0.09 [0–0.27]) were also statistically significant alloimmunisation risk factors (p=0.006 and p=0.045, respectively).
Neither CTTP nor erythrocytapheresis were found to increase alloimmunisation (p>0.05). Regarding clinical features, a higher number of VOC episodes resulted in an increased alloimmunisation risk (6 [1–12] vs 1 [0–3]; p=0.011). Presence of autoantibodies was more frequent in alloimmunised patients (57.1 vs 0%; p<0.001).
DISCUSSION
We describe a relatively low prevalence of alloimmunisation (8.4%)15,25,30,32, probably related to a high fulfilment of complete RH-Kell matched transfusion protocol. Nevertheless, minor deviation from 100% of accomplishment translates into alloimmunisation with >50% of detected alloantibodies corresponding to RH or Kell. Thus, we confirm the importance and need to transfuse RH-Kell matched RBCu to SCD patients.
Alloantibody specificities are consistent with other published series and expected antigenic differences between predominantly Caucasian donors and patients mainly of African descent13,15,18,25,30–32,34. It should be noted that donor-receptor differences become even more significant in our country where virtually all donors are Caucasian.
We verified the relationship between transfusion burden and risk of alloimmunisation (p=0.023)13,25,30–32. Nevertheless, 72.7% of the antibodies were detected before the tenth transfusion proving that highly susceptible patients can present early alloimmunisation15 as a result of risk factors other than transfusion burden.
Alloimmunisation seems to favour the development of subsequent alloantibodies and it is common to find ≥2 alloantibodies per patient. In our study, 57,1% (4 of 7) of alloimmunised patients presented 2 alloantibodies, but none of them accumulated more than 2, unlike reports from other series15,20,30. We believe extended matching may have prevented the development of additional antibodies in these highly susceptible patients.
The incidence of delayed haemolytic transfusion reactions was low (1.19%)35, and there was no alloimmunisation-related mortality. Our analysis supports the hypothesis of a higher alloimmunisation risk when transfusions occur in an acute setting, with alloimmunised patients showing a higher number of episodic transfusions (p=0.006) and a higher episodic to chronic transfusion ratio (p=0.045). Related to this, we found a higher alloimmunisation rate in only episodically transfused patients compared to the CTTP group (1.74 vs 0.56 per 100 units transfused) despite higher transfusion load in the latter. However, these differences were not statistically significant (p=0.317).
As to individual-specific susceptibility factors, we did not find any significant differences regarding sex, unlike other published data25,30,32. As previously described in other paediatric series31, this may be related to the paediatric age of most of our patients since female sex-attributed risk is related to history of pregnancy.
Paediatric age may also have contributed to a globally lower alloimmunisation prevalence, given that age has been reported to be an independent risk factor both in the general population and in SCD12,25,30,32. Age at first transfusion has been related to alloimmunisation by an immune tolerance mechanism affecting transfusions at early ages13,15. We did not observe significant differences between patients firstly transfused in our institution before or after the age of 5. Nevertheless, some patients had been transfused elsewhere before, so this variable did not homogeneously represent age at first transfusion.
Regarding clinical variables, we found a statistically significant relationship between number of VOC and alloimmunisation. Number of VOC could be a surrogate marker of underlying inflammation and disease severity, and thereby reveal a more pro-inflammatory background which may predispose to the development of alloantibodies. This finding supports the role of a pro-inflammatory state, both at baseline and prior to transfusion, in alloimmunisation risk18.
Finally, we have shown the relationship between presence of autoantibody and alloimmunisation (p<0.001), highlighting the immune dysregulation underlying SCD11–13,30–32.
The main detected cause of RH-Kell matching protocol deviation was the inability to identify SCD patients as such at blood-bank services, especially in urgent situations.
In our cohort, 42.9% (3 of 7) of alloimmunised patients became alloimmunised as a result of their first transfusion in our institution, and in all cases, this happened in an acute complication setting. This finding points out how the first admission of these patients in a new institution is a critical moment for transfusion safety and alloimmunisation prevention. SCD patients may present with an acute complication that requires urgent transfusion support, challenging both the possibility of adequate identification and the response capacity from blood bank services. Therefore, the ability to perform extended RBC antigen phenotyping, having adequate previous immunohaematologic records, and the availability of matched RBCu become imperative for hospitals dealing with this pathology21,23. Moreover, SCD patients are frequently assisted in different hospitals. This situation demands the creation of centralised records to ensure the availability of transfusion history and immunohaematologic information to help avoid preventable DHTR22,23.
Despite the proven utility of extended matching17, after assessing our results, and having proved a low alloimmunisation prevalence, we consider it to be reasonable to restrict this procedure to previously alloimmunised patients who are at greater risk of developing subsequent antibodies.
Our study has several limitations, many of which derive from its retrospective nature and the limited number of patients. Several patients were followed in different hospitals. The resulting heterogeneity and the possible loss of information also reflect the complex social reality of many of these patients.
The inclusion of both adult and paediatric patients is another factor compromising the homogeneity of our study. However, we believe that including both types of patients provides a more realistic vision of our SCD population and relevant information regarding alloimmunisation.
Finally, the incidence of alloimmunisation may have been underestimated in our study, as it is in the general population, due to antibody evanescence36 since IAT was not systematically performed after each transfusion.
CONCLUSIONS
We report a low alloimmunisation prevalence related to a high accomplishment of RH-Kell matched transfusion protocol. Nevertheless, >50% of detected alloantibodies corresponded to RH or Kell, proving that a slight deviation from total fulfilment of this transfusion objective clearly translates into alloimmunisation and that RH-Kell matching must be respected in SCD patients.
We confirm a higher risk of alloimmunisation in patients with higher transfusion burden, mainly when transfusions occur during acute complications, and also in patients with a higher number of VOC, probably reflecting an underlying pro-inflammatory state or disease severity.
Red blood cell alloimmunisation is an important complication, and further studies are needed to clarify implicated risk factors in order to identify patients at higher risk, contributing to more effective and efficient prevention.
To our knowledge, this is the first study describing alloimmunisation frequency and risk factors in a Spanish cohort, with only one previously reported series in Europe31. Considering the progressive increase in SCD prevalence in this region, it seems clear that this pathology and the specific transfusional approach adopted in its management deserve our attention.
Supplementary Information
ACKNOWLEDGEMENTS
We would like to thank the technical and nursing teams involved in the study.
Footnotes
AUTHORSHIP CONTRIBUTIONS
IR and AP designed the research study. MG, EC, CB, EB, IP and GP provided clinical data. CP, SM and CF helped with immunohaematologic information. IR performed the statistical analysis. IR, AP and JA wrote the paper. EC helped in the discussion. JA and JLD supervised the project.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Roberts I, de Montalembert M. Sickle cell disease as a paradigm of immigration hematology: new challenges for hematologists in Europe. Haematologica. 2007;92:865–71. doi: 10.3324/haematol.11474. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Tatem AJ, Huang Z, et al. Global migration and the changing distribution of sickle haemoglobin: a quantitative study of temporal trends between 1960 and 2000. Lancet Glob Health. 2014;2:e80–9. doi: 10.1016/S2214-109X(13)70150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobitz S, Telfer P, Cela E, et al. Newborn screening for sickle cell disease in Europe: recommendations from a Pan-European Consensus Conference. Br J Haematol. 2018;183:648–60. doi: 10.1111/bjh.15600. [DOI] [PubMed] [Google Scholar]
- 4.Cela E, Dulin Iniguez E, Guerrero Soler M, et al. [Evaluation of systematic neonatal screening for sickle cell diseases in Madrid three years after its introduction]. An Pediatr (Barc) 2007;66:382–6. doi: 10.1157/13101243. [DOI] [PubMed] [Google Scholar]
- 5.Cela E, Bellon JM, de la Cruz M, et al. National registry of hemoglobinopathies in Spain (REPHem) Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26322. [DOI] [PubMed] [Google Scholar]
- 6.Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med. 2017;376:1561–73. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- 7.Chou ST, Fasano RM. Management of Patients with Sickle Cell Disease Using Transfusion Therapy: Guidelines and Complications. Hematol Oncol Clin North Am. 2016;30:591–608. doi: 10.1016/j.hoc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 9.Coleman S, Westhoff CM, Friedman DF, Chou ST. Alloimmunization in patients with sickle cell disease and underrecognition of accompanying delayed hemolytic transfusion reactions. Transfusion. 2019;59:2282–91. doi: 10.1111/trf.15328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickel RS, Hendrickson JE, Fasano RM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56:107–14. doi: 10.1111/trf.13379. [DOI] [PubMed] [Google Scholar]
- 11.Telen MJ, Afenyi-Annan A, Garrett ME, et al. Alloimmunization in sickle cell disease: changing antibody specificities and association with chronic pain and decreased survival. Transfusion. 2015;55:1378–87. doi: 10.1111/trf.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karafin MS, Westlake M, Hauser RG, et al. Risk factors for red blood cell alloimmunization in the Recipient Epidemiology and Donor Evaluation Study (REDS-III) database. Br J Haematol. 2018;181:672–81. doi: 10.1111/bjh.15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–37. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vichinsky EP, Earles A, Johnson RA, et al. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 15.Sins JW, Biemond BJ, van den Bersselaar SM, et al. Early occurrence of red blood cell alloimmunization in patients with sickle cell disease. Am J Hematol. 2016;91:763–9. doi: 10.1002/ajh.24397. [DOI] [PubMed] [Google Scholar]
- 16.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–7. [PubMed] [Google Scholar]
- 17.Lasalle-Williams M, Nuss R, Le T, et al. Extended red blood cell antigen matching for transfusions in sickle cell disease: a review of a 14-year experience from a single center (CME) Transfusion. 2011;51:1732–9. doi: 10.1111/j.1537-2995.2010.03045.x. [DOI] [PubMed] [Google Scholar]
- 18.Fasano RM, Booth GS, Miles M, et al. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 19.Fasano RM, Meyer EK, Branscomb J, et al. Impact of Red Blood Cell Antigen Matching on Alloimmunization and Transfusion Complications in Patients with Sickle Cell Disease: A Systematic Review. Transfus Med Rev. 2019;33:12–23. doi: 10.1016/j.tmrv.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Yee MEM, Josephson CD, Winkler AM, et al. Red blood cell minor antigen mismatches during chronic transfusion therapy for sickle cell anemia. Transfusion. 2017;57:2738–46. doi: 10.1111/trf.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis BA, Allard S, Qureshi A, et al. British Committee for Standards in H. Guidelines on red cell transfusion in sickle cell disease. Part I: principles and laboratory aspects. Br J Haematol. 2017;176:179–91. doi: 10.1111/bjh.14346. [DOI] [PubMed] [Google Scholar]
- 22.Pirenne F, Yazdanbakhsh K. How I safely transfuse patients with sickle-cell disease and manage delayed hemolytic transfusion reactions. Blood. 2018;131:2773–81. doi: 10.1182/blood-2018-02-785964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4:327–55. doi: 10.1182/bloodadvances.2019001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou ST, Evans P, Vege S, et al. RH genotype matching for transfusion support in sickle cell disease. Blood. 2018;132:1198–207. doi: 10.1182/blood-2018-05-851360. [DOI] [PubMed] [Google Scholar]
- 25.Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–71. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 26.Gehrie EA, Ness PM, Bloch EM, et al. Medical and economic implications of strategies to prevent alloimmunization in sickle cell disease. Transfusion. 2017;57:2267–76. doi: 10.1111/trf.14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kacker S, Ness PM, Savage WJ, et al. Cost-effectiveness of prospective red blood cell antigen matching to prevent alloimmunization among sickle cell patients. Transfusion. 2014;54:86–97. doi: 10.1111/trf.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kacker S, Ness PM, Savage WJ, et al. Economic evaluation of a hypothetical screening assay for alloimmunization risk among transfused patients with sickle cell disease. Transfusion. 2014;54:2034–44. doi: 10.1111/trf.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson KE, Fasano RM, Karafin MS, et al. Mechanisms of alloimmunization in sickle cell disease. Curr Opin Hematol. 2019;26:434–41. doi: 10.1097/MOH.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 30.Nickel RS, Horan JT, Fasano RM, et al. Immunophenotypic parameters and RBC alloimmunization in children with sickle cell disease on chronic transfusion. Am J Hematol. 2015;90:1135–41. doi: 10.1002/ajh.24188. [DOI] [PubMed] [Google Scholar]
- 31.Allali S, Peyrard T, Amiranoff D, et al. Prevalence and risk factors for red blood cell alloimmunization in 175 children with sickle cell disease in a French university hospital reference centre. Br J Haematol. 2017;177:641–7. doi: 10.1111/bjh.14609. [DOI] [PubMed] [Google Scholar]
- 32.Murao M, Viana MB. Risk factors for alloimmunization by patients with sickle cell disease. Braz J Med Biol Res. 2005;38:675–82. doi: 10.1590/s0100-879x2005000500004. [DOI] [PubMed] [Google Scholar]
- 33.Yazer MH, Lozano M, Crighton G, et al. Transfusion service management of sickle-cell disease patients. Vox Sang. 2016;110:288–94. doi: 10.1111/vox.12296. [DOI] [PubMed] [Google Scholar]
- 34.Boateng LA, Ngoma AM, Bates I, Schonewille H. Red Blood Cell Alloimmunization in Transfused Patients With Sickle Cell Disease in Sub-Saharan Africa; a Systematic Review and Meta-Analysis. Transfus Med Rev. 2019;33:162–9. doi: 10.1016/j.tmrv.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Pirenne F. The cause and pathogenesis of hemolytic transfusion reactions in sickle-cell disease. Curr Opin Hematol. 2019;26:488–94. doi: 10.1097/MOH.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 36.Tormey CA, Hendrickson JE. Transfusion-related red blood cell alloantibodies: induction and consequences. Blood. 2019;133:1821–30. doi: 10.1182/blood-2018-08-833962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.