Abstract
Background
For many people with HIV (PWH), taking antiretroviral therapy (ARV) every day is difficult.
Methods
Average adherence (Av-Adh) and log-transformed treatment interruption (TI) to ARV were prospectively measured over 6 months using electronic drug monitoring (EDM) in several cohorts of PWH. Multivariate linear regression models including baseline confounders explored the influence of EDM-defined adherence (R2) on 6-month log10 HIV-RNA. Multivariate logistic regression models were used to compare the risk of HIV-RNA detection (VR) within subgroups stratified by lower (≤95%) and higher (>95%) Av-Adh.
Results
Three hundred ninety-nine PWH were analyzed with different ARVs: dolutegravir (n = 102), raltegravir (n = 90), boosted PI (bPI; n = 107), and NNRTI (n = 100). In the dolutegravir group, the influence of adherence pattern measures on R2 for HIV-RNA levels was marginal (+2%). Av-Adh, TI, and Av-Adh × TI increased the R2 for HIV-RNA levels by 54% and 40% in the raltegravir and bPI treatment groups, respectively. TI increased the R2 for HIV-RNA levels by 36% in the NNRTI treatment group. Compared with the dolutegravir-based regimen, the risk of VR was significantly increased for raltegravir (adjusted odds ratio [aOR], 45.6; 95% CI, 4.5–462.1; P = .001), NNRTIs (aOR, 24.8; 95% CI, 2.7–228.4; P = .005), and bPIs (aOR, 28.3; 95% CI, 3.4–239.4; P = .002) in PWH with Av-Adh ≤95%. Among PWH with >95% Av-Adh, there were no significant differences in the risk of VR among the different ARVs.
Conclusions
These findings support the concept that dolutegravir in combination with 2 other active ARVs achieves greater virological suppression than older ARVs, including raltegravir, NNRTI, and bPI, among PWH with lower adherence.
Keywords: adherence, dolutegravir, PWH, missed doses
Ninety-five percent adherence rule revisited: compared to older antiretroviral combinations, dolutegravir-based triple therapy had a unique forgiveness profile to low-to-moderate electronic drug monitoring adherence levels in terms of HIV RNA replication and emerging resistance mutations amongst PWH.
Suboptimal adherence to antiretroviral therapy can result in insufficient viral suppression [1,2] and promotes the emergence of drug-resistant viral strains [3]. A landmark study with unboosted protease inhibitor antiretrovirals (ARVs) proposed that >95% adherence was required to achieve and maintain virological suppression, which led to the concept that an undetectable HIV viral load (VL) was equivalent to full adherence [1]. Modern antiretroviral therapies with once-daily dosing and low pill burden improved the level of adherence compared with more complex regimens [4,5]. Simpler regimens have improved adherence [4,6], and potent regimens with more favorable pharmacokinetic profiles have allowed more forgiveness with regard to missed doses. Studies investigating non-nucleoside reverse transcriptase inhibitors (NNRTI), boosted protease inhibitors (bPIs), and integrase strand-transfer inhibitors (INSTI) as a part of ARV drug combinations demonstrated that the lowest level of ARV adherence required to sustain virological suppression may be ~80% [7,8]. However, the methods used to measure adherence, such self-report and pharmacy refills, did not capture treatment interruptions, another independent driver of virological failure [9] and resistance [10]. In addition, these studies did not specifically investigate second-generation INSTI-based ARV combinations, despite their being widely recommended.
Real-world studies of the “forgiveness” to missed doses of ARV regimen are important, as they may help to predict regimen durability and risk of resistance in a context where suboptimal adherence is probably common [11]. We hypothesized that the pharmacokinetic profile and genetic barrier provided by second-generation INSTIs, namely dolutegravir-based ARV combinations, would allow a high rate of virological suppression at low to moderate adherence levels. Therefore, we aimed to investigate the patterns of adherence to dolutegravir associated with virological replication in comparison with older third agents.
METHODS
Study Design and Participants
We conducted an international multicenter prospective cohort study of people with HIV (PWH) treated with a dolutegravir-based regimen. The DOLUTECAPS study took place in France and Switzerland between May 2015 and December 2018. The details of the inclusion criteria are described in the clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT02878642. Briefly, adults with HIV starting once- or twice-a-day dolutegravir-based regimens were included at the physician’s discretion. Because we were interested in covering a large range of different pill-taking behaviors, the participation of PWH perceived by their treating physician to be at risk of suboptimal adherence was encouraged. Subjects had a genotypic sensitivity score of ≥3, including dolutegravir. The genotypic sensitivity score represents the total number of ARV drugs in the regimen to which a patient’s HIV is susceptible (score 1), possibly susceptible (score 0.5), or resistant (score 0), according to version 30 of the ANRS AC-43 resistance group algorithm (http://www.hivfrenchresistance.org/2019/tab6.html). We did not include people using pillbox organizers and those who were not responsible for taking their antiretroviral pills. Three groups of participants were defined: antiretroviral-naïve individuals who initiated dolutegravir (STARTING group), antiretroviral-experienced individuals who switched to dolutegravir for virological failure (FAILING group), and antiretroviral-experienced individuals who switched to dolutegravir while HIV-RNA was supressed (SWITCHING group). Dolutegravir combined with abacavir/lamivudine as a single-tablet regimen and multitablet regimens containing dolutegravir plus at least 2 other active ARVs were allowed.
Several centers from our group have incorporated the use of EDM devices in routine practice. In addition, we previously investigated adherence–virological outcome relationships for older ARVs such as NNRTIs [12], bPIs [13,14], and raltegravir [15]. We contrasted the DOLUTECAPS cohort findings with other antiretroviral therapies from our EDM database (Supplementary Figure 1). All participants were followed prospectively with electronic adherence monitoring and an HIV-RNA determination at 6 months as the primary outcome.
Data Collection and Adherence Pattern Measures
Baseline characteristics including sociodemographic factors and clinical characteristics were collected at baseline for the 4 groups: dolutegravir, raltegravir, bPIs, and NNRTIs. Patients were asked to use electronic drug monitoring (EDM; Aardex, Switzerland) devices to prospectively characterize their pattern of adherence to the third agent for 6 months. The same monitoring strategy and devices were used for the 4 groups. Other ARV pills (eg, backbone nucleos/tide reverse transcriptase inhibitors), if any, were not monitored. Two measures were extracted from electronic dosing history for each participant: (1) the average percent dose adherence corresponding to the number of observed electronic pill cap opening events divided by the expected events; (2) the log10-transformed duration of the longest treatment interruption (in hours). EDM records were read and reviewed at all study visits and allowed participants to add any doses taken when they knew they did not use the device. Seventeen participants with no EDM events during the 2 weeks prior to month 6 were excluded. This is because nonpersistence to any short-acting antiretroviral drug is known to be associated with virological replication in most situations.
Patient Consent Statement
The Institutional Review Board of the University of Caen, France (which covers all French sites), and the Committee on Human Subjects Research of the University of Lausanne, Switzerland, approved all study procedures, and the participants provided written informed consent.
Outcomes
The primary outcome was HIV-1 RNA in plasma, measured using the test available in each center with a limit of detection ≤50 HIV-1 RNA copies/mL. We defined virological replication as failure to suppress or sustain HIV-RNA to <50 copies/mL at 6 months. A value of ≤50 was imputed to PWH who had a lower limit of detection >50 copies/mL for 34 participants in the NNRTI group and 56 participants in the bPI treatment group. Emergence of resistance to dolutegravir and to raltegravir was investigated by genotyping the integrase coding sequence of the virus after the development of virological replication.
Statistical Analysis
Continuous variables were summarized as mean values, median values, SDs, and interquartile ranges (IQRs), depending on their distributions. Dichotomous data were summarized as numbers and proportions. Regarding baseline and follow-up characteristics, quantitative variables were compared between ARV classes using an analysis of variance or Kruskal-Wallis test, as appropriate, and qualitative variables were compared using the Fisher exact test. In order to characterize the adherence pattern associated with HIV-RNA replication, we displayed a 3-dimensional scatter plot reporting the level of log HIV-RNA (vertical z-axis) according to the average adherence (horizontal x-axis) and log longest treatment interruption (horizontal y-axis). Because the original data did not contain enough combinations of x, y, and z values to generate an empirically derived surface plot with <64% average adherence, we censored these observations for data visualization. In addition, we used a smoothing spline interpolation method with λ = 0.1 as a trade-off between closeness to the original data and smoothness. For each antiretroviral group, we computed 3 different linear regression models—(i) with average adherence (Model 1); (ii) with treatment interruption (Model 2); (iii) with average adherence, treatment interruption, and the product (interaction) of average adherence × treatment interruption (Model 3)—as independent covariables with the log HIV-RNA at 6 months as the dependent variable. These models were also adjusted for potential confounders (age, sex, baseline treatment scenarios [ie, naïve, switch, or treatment failure], baseline HIV-RNA, and CD4 cell count). The influence of the EDM-defined adherence pattern on 6-month HIV-RNA was assessed in 2 ways: (i) by testing the slope of each adherence pattern parameter coefficient to 0; (ii) by assessing the incremental R2 value (or variance explained) for each model, compared with a model without EDM adherence measurement (ie, including only baseline factors).
The effect size of factors associated with the probability of virological detection (HIV-RNA >50 copies/mL) was estimated by calculating odds ratios (ORs) and adjusted ORs using univariate and multivariate logistic regression models, respectively. This analysis was performed in the overall cohort and in subgroups with higher (>95%) and lower (≤95%) average adherence [1]. Analyses were performed using PowerView, version 2.3.3 (Aardex Group, Sion, Switzerland), and SAS, version 9.4 (SAS Institute, Cary, NC, USA). All reported P values are 2-sided, with a P value of ≤.05 denoting statistical significance.
RESULTS
Baseline Characteristics
The baseline characteristics of the participants are shown in Table 1. Seventy-two percent of the participants treated with a dolutegravir-based regimen were men, and the mean age was 47.7 years. Approximately one-quarter of the participants treated with a dolutegravir-based regimen were treatment-naïve at baseline. Fourty-seven (46%) PWH had a plasma HIV-RNA <50 copies/mL at study entry. The median baseline CD4 cell count (IQR) was 494 (290–705), and the median baseline plasma HIV-RNA level (IQR) was 2.1 (1.6–4.1) log10. The baseline characteristics from PWH treated with other third agents are presented in Table 1. In the NNRTI-based group, the third agent was nevirapine for 70 PWH, efavirenz for 12 PWH, and rilpivirine for 18 PWH. In the boosted PI group, the third agent was lopinavir for 54 PWH, atazanavir for 48 PWH, and other boosted PI for 3 PWH.
Table 1.
Baseline and Follow-up Characteristics by Antiretroviral Class
| Variables | Dolutegravir-Based (n = 102) | Raltegravir-Based (n = 90) | NNRTI-Based (n = 100) | bPI-Based (n = 107) | P Value |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, mean (SD), y | 47.7 (13.2) | 46.2 (11.2) | 46.8 (10.6) | 41.3 (7.6) | <.001 |
| Male, sex, No. (%) | 73 (72) | 65 (72) | 86 (86) | 88 (82) | .028 |
| CD4+ cells, median (IQR) | 494 (290–705) | 490 (309–709) | 510 (383–723) | 311 (229–450) | <.001 |
| Treatment groups, no. (%)/log HIV-1 RNA, median [IQR] | |||||
| Switched treatment | 47 (46)/1.7 | 69 (77)/1.7 | 100 (100)/1.7 | 31 (29)/1.7 | <.001 |
| Treatment-naïve | 26 (26)/4.6 [4.0–5.0] | 10 (11)/5.4 [3.6–5.5] | 0 (0)/- | 43 (40)/4.4 [3.8–5.1] | |
| Failed treatment | 29 (28)/3.2 [2.6–4.1] | 11 (12)/4.6 [4.0–5.0] | 0 (0)/- | 33 (31)/2.8 [2.3–3.6] | |
| Backbone | |||||
| TDF/FTC or TAF/FTC | 22 (22) | 49 (54) | 22 (22) | 56 (52) | <.001 |
| ABC/3TC | 47 (46) | 6 (7) | 38 (38) | 10 (9) | |
| Other NRTI combination | 8 (8) | 12 (13) | 40 (40) | 13 (12) | |
| Other class combination | 25 (25) | 23 (26) | 0 (0) | 28 (26) | |
| Adherence follow-up | |||||
| Average adherence, median (IQR) | 96.0 (87.0–99.0) | 97.0 (91.0–100.0) | 96.0 (84.5–99.0) | 95.3 (82.0–100.0) | .32 |
| Longest TI, median (IQR), d | 2.1 (1.3–2.8) | 1.5 (1.0–2.7) | 1.3 (1.1–1.9) | 2.0 (1.0–7.0) | <.001 |
Abbreviations: 3TC, lamivudine; ABC, abacavir; bPI, boosted protease inhibitor; FTC, emtricitabine; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; TAF, tenofovir alafenamine; TDF, tenofovir; TI, treatment interruption.
HIV-RNA at Month 6
In the dolutegravir treatment group (Table 2), 8 PWH had low levels of HIV-RNA replication: 5/24 (17%) in the failing group (median [range] HIV-RNA, 132 [88–168] cp/mL), 2/26 (8%) in the starting group (HIV-RNA, 80 and 161 cp/mL), and 1/46 (2%) in the switching group (HIV-RNA, 73 cp/mL). Among these, 3/8 were amplified, and none demonstrated a resistance mutation to the INSTI class. In the raltegravir treatment group (Table 2), 18 PWH had HIV-RNA replication (median [range] HIV-RNA, 362 [57–51 300] copies/mL) and 14/18 were subjected to nucleic acid amplification and sequencing: 4 samples harbored INSTI conferring resistance to raltegravir (Q148H, N155H, Q148R, and Y143A). In the NNRTI treatment group (Table 2), 12 PWH had HIV-RNA replication (median [range] HIV-RNA, 854 [66-15 000] copies/mL). In the bPI group (Table 2), 26 PWH had HIV-RNA replication (median [range] HIV-RNA, 11 000 [59–801 400] copies/mL). No data were available for resistance testing in the bPI and NNRTI groups.
Table 2.
Factors Associated With Virological Replication (>50 Copies/mL) at Month 6 in the Overall Cohort (n = 399)
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Variables | No VR (n = 335) | VR (n = 64) | P Value | aOR [95% CI] | P Value |
| Age, mean (SD), y | 44.5 (11.8) | 41.6 (11.3) | .07 | 0.88 [0.63–1.22] | .44 |
| Male | 259 (77.3) | 53 (82.8) | .41 | 1.36 [0.6–3.2] | .48 |
| CD4 cells, mean (SD) | 494 (256) | 402 (250) | .009 | 0.97 [0.86–1.14] | .92 |
| Log HIV-RNA, mean (SD), cp/mL | 2.44 (1.23) | 2.90 (1.37) | .008 | 1.61 [0.97–2.68] | .07 |
| Third antiretroviral agent | .005 | ||||
| Dolutegravir-based | 94 (28.1) | 8 (12.5) | Ref. | ||
| Raltegravir-based | 72 (21.5) | 18 (28.1) | 7.7 [2.4–25.2] | .0007 | |
| bPI-based | 81 (24.2) | 26 (40.6) | 1.9 [0.6–6.0] | .29 | |
| NNRTI-based | 88 (26.3) | 12 (18.8) | 3.4 [0.9–12.7] | .07 | |
| Treatment group | <.0001 | ||||
| Switched treatment | 221 (66.0) | 26 (40.6) | Ref. | ||
| Treatment-naïve | 70 (20.9) | 9 (14.1) | 0.6 [0.1–4.2] | .63 | |
| Failed treatment | 44 (13.1) | 29 (41.3) | 4.4 [1.4–14.0] | .012 | |
| Adherence class | <.0001 | ||||
| >95% | 211 (63.0) | 20 (31.2) | Ref. | ||
| 90%–95% | 39 (11.6) | 3 (4.7) | 0.5 [0.1–2.1] | .35 | |
| 80%–90% | 47 (14.0) | 5 (7.8) | 0.8 [0.2–2.6] | .69 | |
| 60%–80% | 29 (8.7) | 13 (20.3) | 3.2 [1.0–10.0] | .043 | |
| <60% | 9 (2.7) | 23 (35.9) | 5.9 [1.5–23.7] | .012 | |
| Longest treatment interruption, log mean (SD), h | 1.63 (0.28) | 2.06 (0.48) | <.0001 | 4.6 [1.3–16.9] | .02 |
Abbreviations: aOR, adjusted odds ratio; bPI, boosted protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; VR, virological replication with HIV-RNA >50 cp/mL.
Adherence Pattern and HIV-RNA Relationships
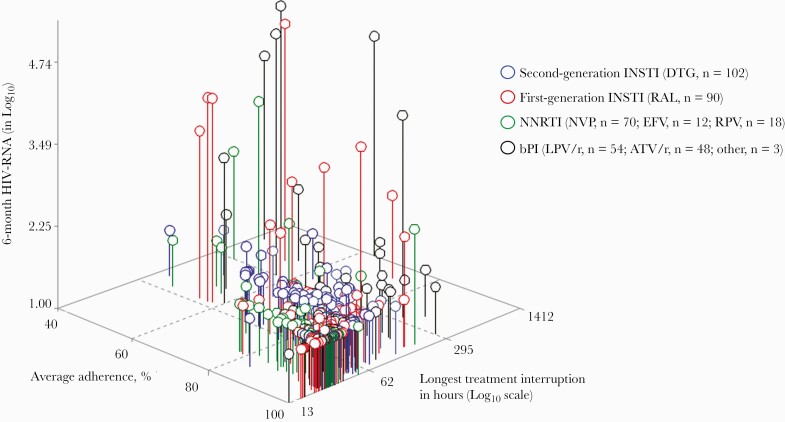
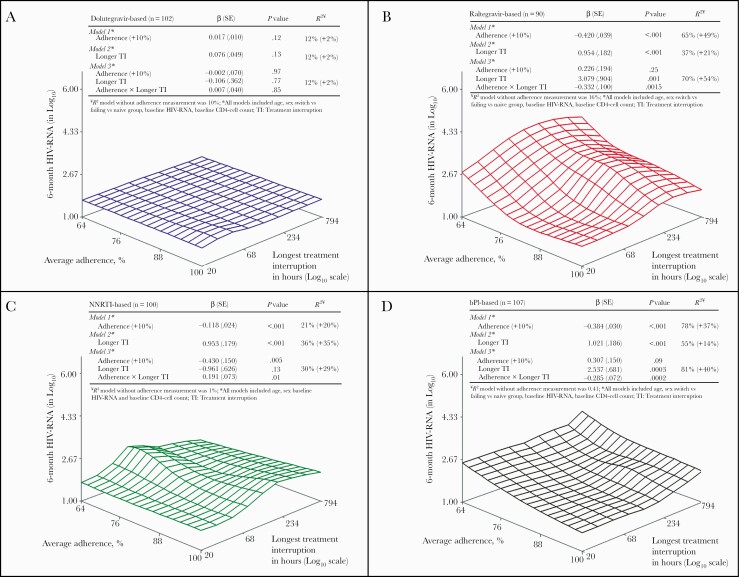
Figure 1 displays the 6-month log10 HIV-RNA according to the EDM-defined adherence pattern by antiretroviral regimen among 399 PWH. As shown in Figure 2A, the surface plot for dolutegravir-based triple therapy is flat, indicating a low level of HIV-RNA replication regardless of the adherence pattern. None of the models including adherence pattern parameters was significantly associated with 6-month HIV-RNA level (Figure 2A), and the incremental HIV-RNA variance explained by the inclusion of adherence pattern variables was minimal (2%). In contrast, the adherence patterns were significantly associated with the level of virological replication above the detection threshold for all older ARVs, with an incremental variance explained (R2) ranging from 14% (Figure 2D, model 2) to 54% (Figure 2B, model 3). Model 3 had the highest HIV-RNA level variance explained for the raltegravir group (Figure 2B) and the bPI group (Figure 2D), suggesting that the influence of 1 adherence measure depends on the value of the other. Regarding the NNRTI group (Figure 2C), the longer treatment interruption (model 2) had the highest variance explained to predict HIV-RNA level.
Figure 1.
Virological replication levels (log HIV-RNA) at 6 months by EDM adherence pattern according to antiretroviral regimen class (n = 399). Each circle symbol represents a PWH connected to the plane by a vertical needle. The length of the needle represents the HIV-RNA level of replication at 6 months in log cp/mL (z-axis). The horizontal plane coordinates correspond to the EDM-defined adherence pattern during the 6-month period, with average adherence on the x-axis and the longest treatment interruption in log10 hours on the y-axis. PWH with higher adherence are those in the bottom corner. Abbreviations: ATV/r, atazanavir/ritonavir; bPI, boosted protease inhibitor; DTG, dolutegravir; EDM, electronic drug monitoring; EFV, efavirenz; LPV/r, lopinavir/ritonavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PWH, people with HIV; RPV, rilpivirine.
Figure 2.
Surface forgiveness plot and linear regression models of adherence patterns explaining HIV-RNA by antiretroviral regimen.
Predictors of HIV-RNA >50 Copies/mL
In the overall cohort, factors associated with virological detection (ie, probability of 6-month HIV-RNA >50 copies/mL) in the univariate and multivariate analyses are shown in Table 2.
In the subgroup analyses based on average adherence, the risk of virological detection was similar between all ARVs among PWH with >95% adherence levels (Table 3) in the multivariate analysis. Among PWH with ≤95% adherence levels (Table 3) and compared with those receiving a dolutegravir-based regimen, the risk of virological detection was significantly and independently increased for the raltegravir-based regimen, NNRTIs, and bPIs in the multivariate analysis.
Table 3.
Predictors of Virological Replication by Average Adherence Subgroups in Multivariate Analysisa
| Higher Adherence (>95%) | Lower Adherence (≤95%) | |||
|---|---|---|---|---|
| n = 211 | n = 188 | |||
| Third Antiretroviral Agent | aOR [95% CI] | P Value | aOR [95% CI] | P Value |
| Dolutegravir-based | Ref. | Ref. | ||
| Raltegravir-based | 3.7 [0.9–16.1] | .08 | 45.6 [4.5–462.1] | .001 |
| bPI-based | 0.6 [0.1–3.1] | .50 | 28.3 [3.4–239.4] | .002 |
| NNRTI-based | 3.4 [0.3–36.2] | .31 | 24.8 [2.7–228.4] | .005 |
Abbreviations: aOR, adjusted odds ratio; bPI, boosted protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
aAdjusting for age, sex, baseline CD4 cell count, baseline HIV-RNA, and treatment group (failing, switching, or starting).
DISCUSSION
In this cohort of PWH followed by EDM, the adherence pattern to dolutegravir-based triple therapy was not a predictor of suppressed HIV-RNA. This picture contrasts with the strong association between adherence pattern and HIV-RNA level found for older regimens, including raltegravir-, NNRTI-, and bPI-based ARV therapies. In addition, dolutegravir therapy outperformed all other ARV strategies in terms of virological suppression below the limit of detection among PWH in the lower adherence subgroup in the multivariate analysis, adjusting for age, sex, CD4 cell count, group (starting, switching, and failing), and baseline HIV-RNA. No emerging mutation conferring resistance to INSTI was detected in the dolutegravir group with detectable HIV-RNA, in contrast with the raltegravir group. Taken together, these results suggest that dolutegravir-based ARV therapies are more forgiving to missed doses (either by average adherence or treatment interruptions) than the other investigated ARVs regarding the risk of HIV-RNA replication. In addition, dolutegravir-based triple therapy is more forgiving to missed doses than raltegravir-based triple therapy regarding risk resistance.
Most of the previous studies in this area of research have attempted to identify an adherence threshold required for virological suppression with the aim to challenge the >95% historical threshold. For example, Byrd et al. [8] reported that 75% average adherence defined by pharmacy refill to INSTI-based ARV therapy was required to suppress 90% of the treated patients. In this large cohort, first- and second-generation INSTIs were pooled, although we found that the level of forgiveness between dolutegravir and raltegravir strongly differed (as suggested in Figure 2A and B, respectively). Overall, the multivariate analysis of the risk of virological replication (n = 399) (Table 1) is consistent with the >80% level of average adherence found in other studies [2,7,8] but reaffirms the importance of treatment interruption length (adjusted odds ratio [aOR], 4.6; 95% CI, 1.3–16.9; P = .02) as an independent risk factor [9,10,16].
Importantly, the use of dolutegravir was significantly and independently associated with a lower risk of virological replication compared with other ARVs in the subgroup with lower adherence. Although the 95% confidence intervals were large due to the smaller sample size in this subgroup, this superiority is consistent with a network meta-analysis of 20 randomized trials in which a significantly higher proportion of naïve PWH starting dolutegravir achieved virological suppression at week 96 compared with protease inhibitors, efavirenz, and cobicistat-boosted elvitegravir [17]. The pharmacokinetic forgiveness of dolutegravir-based regimens is supported by the 14-hour terminal elimination half-life of dolutegravir and its duration of inhibitory effect (>2-fold higher than the IC90 for 72 hours after the last dose) [18]. In contrast, raltegravir, boosted atazanavir, and boosted lopinavir have shorter terminal elimination half-lives: 10–12 hours [19], 8.3 hours, and 2.4 hours [20], respectively. NNRTIs do have a long plasma half-life but a relatively low genetic barrier to HIV-1 resistance. While prolonged plasma exposure improves pharmacokinetic forgiveness [21], a period of functional monotherapy following NNRTI-based treatment interruption may select for low-frequency resistant strains. Despite a high genetic barrier, dolutegravir monotherapy does promote INSTI-resistant strains [22], but only after a long period of exposure. This scenario is unlikely to occur when combined with other nucleosides in a fixed-dose combination or when combined with nucleotides dosed once daily whose anabolites have long intracellular half-lives.
There are several important limitations to this study. Adherence was monitored by EDM, so we cannot prove that the ARVs were ingested. The duration of the study, 6 months, was short, and any virologic breakthrough before 6 months remained unnoticed. We compared nonrandomized groups of PWH with different baseline characteristics. In particular, the distribution of PWH who started treatment as a switch, treatment failure, or first therapy was different among treatment groups (Table 1). The use of multivariate analysis adjusting for important predictors of virological replication may have contributed to attenuating this risk of bias, although residual confounding may remain. The cohorts comparing different treatment groups were not contemporaneously studied. We investigated dolutegravir-based triple therapy. Therefore, our results regarding dolutegravir should not be generalized to dual therapies (with either rilpivirine or lamivudine) or to bictegravir-based triple therapy. Further reseach in this area is warranted.
This study has also strengths. Our large sample size of 399 PWH representing 73 017 EDM events with various ARVs including dolutegravir makes this cohort unique. The diversity of ARV and adherence pattern behaviors allowed us to identify significant interactions between average adherence and treatment interruption. While prior work has demonstrated that short-term treatment interruptions can be reliably predicted by average adherence [23], our results suggest that (i) they are not interchangeable measures [24] and (ii) their influence differs by ARV [21]. In addition, the use of historical controls allowed us to contrast virological outcomes with dolutegravir with older regimens at similar adherence patterns (Figure 2A–D) or different adherence strata (Table 3).
Consistently high adherence should remain the goal of treatment, despite the high rates of suppression across a wide range of adherence levels, and patterns suggest a high degree of short-term forgiveness on dolutegravir-based regimens. Low levels of tenofovir by dried blood spots predicted future virological replication among PWH receiving INSTI-based regimens (aOR, 1.9; 95% CI, 1.0–3.4; P = .036) [25]. Moreover, suboptimal adherence [26] to ARVs and ARV treatment interruptions [27] were both associated with higher levels of inflammation among people with full suppression and were associated with clinically significant morbidity in treated PWH [28].
As the World Health Organization recommends, the transition from NNRTI- to dolutegravir-based HIV treatment regimens in resource-limited settings should limit the risk or resistance following unstructured drug interruption due to toxicities, poor retention in care, and drugs being out of stock locally. Our results are in line with the current International Antiviral Society–USA Panel guideline [29] in which second-generation INSTIs are the preferred ARV treatment for most PWH. However, our results do not support the European AIDS Clinical Society 2020 guideline [30]. Starting a raltegravir-based regimen as preferred initial antiretroviral therapy may expose PWH with suboptimal adherence to the risks of incomplete viral replication and potential emergence of resistance to INSTIs.
CONCLUSIONS
Our findings suggest that PWH treated with dolutegravir-based combination therapy may be at lower risk of detectable virological replication than those treated with older regimens at similar low to moderate adherence levels. While many factors not evaluated in this work should influence the choice of ARV therapy, including tolerance, pill burden, PWH preference, the risk of drug–drug interaction, and costs, we recommend using dolutegravir-based regimens for PWH who struggle to achieve high levels of adherence or are at risk of treatment interruptions.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors wish to thank all participants and the staff from all participating centers, in particular Pascale Goubin, Arnaud de la Blanchardière, Sylvie Dargère, Aurélie Baldolli, Jocelyn Michon, and Anne Martin.
Financial support. This work was funded by ViiV Healthcare.
Disclaimer. All operational aspects of the study, including monitoring, data collection, and statistical analyses, were managed by Caen University Hospital. The funder had no role in the study design, data collection, data analysis, data interpretation, or manuscript writing. All authors had final responsibility for the decision to submit for publication.
Potential conflicts of interest. J.-J.P. reports personal fees and nonfinancial support from Gilead Sciences, MSD, and ViiV Healthcare outside the submitted work. L.C. reports personal fees and nonfinancial support from AbbVie, Janssen Cilag, Gilead Sciences, MSD, and ViiV Healthcare outside the submitted work. L.H. reports personal fees from AbbVie, Gilead, Janssen, Merck, and ViiV Healthcare outside the submitted work. All other authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J.-J.P., L.C., M.C., D.R.B., and L.H. contributed to the study concept and design. All authors were involved in acquisition of data. J.-J.P., A.L.F., J.-J.D., E.M.L., and F.C. analyzed data. J.-J.P., M.C., and L.H. contributed to clinical oversight of the study. J.-J.P. provided statistical expertise. J.-J.P., A.L.F., L.C., M.-P.S., D.R.B., M.C., R.V., and L.H. participated in data interpretation and drafted the report. All authors provided input to the report and approved the final version.
Prior presentation. This work has been previously presented in part at the 12th International Conference on HIV Treatment and Prevention Adherence, held in Miami, Florida, June 4–6, 2017 (Abstract 389).
References
- 1. Paterson DL, Swindells S, Mohr J, et al. . Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 2. Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure. Medicine 2016; 95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gardner EM, Burman WJ, Steiner JF, et al. . Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS 2009; 23:1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nachega JB, Parienti JJ, Uthman OA, et al. . Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58:1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemmige V, Flash CA, Carter J, et al. . Single tablet HIV regimens facilitate virologic suppression and retention in care among treatment naïve patients. AIDS Care 2018; 30:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis 2009; 48:484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viswanathan S, Detels R, Mehta SH, et al. . Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015; 19:601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrd KK, Hou JG, Hazen R, et al. ; Patient-Centered HIV Care Model Team . Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr 2019; 82:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Genberg BL, Wilson IB, Bangsberg DR, et al. ; MACH14 Investigators . Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. AIDS 2012; 26:1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parienti JJ, Massari V, Descamps D, et al. . Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis 2004; 38:1311–6. [DOI] [PubMed] [Google Scholar]
- 11. Mills EJ, Nachega JB, Buchan I, et al. . Adherence to antiretroviral therapy in Sub-Saharan Africa and North America: a meta-analysis. JAMA 2006; 296:679–90. [DOI] [PubMed] [Google Scholar]
- 12. Parienti JJ, Das-Douglas M, Massari V, et al. . Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 2008; 3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parienti JJ, Ragland K, Lucht F, et al. ; ESPOIR and REACH study groups . Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis 2010; 50:1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parienti JJ, Barrail-Tran A, Duval X, et al. . Adherence profiles and therapeutic responses of treatment-naive HIV-infected patients starting boosted atazanavir-based therapy in the ANRS 134-COPHAR 3 trial. Antimicrob Agents Chemother 2013; 57:2265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gras G, Schneider MP, Cavassini M, et al. . Patterns of adherence to raltegravir-based regimens and the risk of virological failure among HIV-infected patients: the RALTECAPS cohort study. J Acquir Immune Defic Syndr 2012; 61:265–9. [DOI] [PubMed] [Google Scholar]
- 16. Meresse M, March L, Kouanfack C, et al. ; Stratall ANRS 12110/ESTHER Study Group . Patterns of adherence to antiretroviral therapy and HIV drug resistance over time in the Stratall ANRS 12110/ESTHER trial in Cameroon. HIV Med 2014; 15:478–87. [DOI] [PubMed] [Google Scholar]
- 17. Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis 2021; 21:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elliot E, Amara A, Jackson A, et al. . Dolutegravir and elvitegravir plasma concentrations following cessation of drug intake. J Antimicrob Chemother 2016; 71:1031–6. [DOI] [PubMed] [Google Scholar]
- 19. Iwamoto M, Wenning LA, Petry AS, et al. . Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther 2008; 83:293–9. [DOI] [PubMed] [Google Scholar]
- 20. Boffito M, Else L, Back D, et al. . Pharmacokinetics of atazanavir/ritonavir once daily and lopinavir/ritonavir twice and once daily over 72 h following drug cessation. Antivir Ther 2008; 13:901–7. [PubMed] [Google Scholar]
- 21. Morrison A, Stauffer ME, Kaufman AS. Relationship between adherence rate threshold and drug ‘forgiveness’. Clin Pharmacokinet 2017; 56:1435–40. [DOI] [PubMed] [Google Scholar]
- 22. Hocqueloux L, Raffi F, Prazuck T, et al. ; MONCAY study group . Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic human immunodeficiency virus infection: the randomized noninferiority monotherapy of tivicay trial. Clin Infect Dis 2019; 69:1498–505. [DOI] [PubMed] [Google Scholar]
- 23. Harris RA, Haberer JE, Musinguzi N, et al. . Predicting short-term interruptions of antiretroviral therapy from summary adherence data: development and test of a probability model. PLoS One 2018; 13:e0194713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parienti JJ, Paterson DL. Number of missed doses: why 1 × 7 does not make 7 × 1? AIDS 2012; 26:1437–40. [DOI] [PubMed] [Google Scholar]
- 25. Morrow M, MaWhinney S, Coyle RP, et al. . Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2019; 220:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. . Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musinguzi N, Castillo-Mancilla J, Morrow M, et al. . Antiretroviral therapy adherence interruptions are associated with systemic inflammation among ugandans who achieved viral suppression. J Acquir Immune Defic Syndr 2019; 82:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lederman MM, Funderburg NT, Sekaly RP, et al. . Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saag MS, Gandhi RT, Hoy JF, et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European AIDS Clinical Society. Initial regimens: ART-naïve adult. Available at: https://eacs.sanfordguide.com/art/initial-regimens-arv-naive-adults. Accessed 30 November 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.