Abstract
Background
Carbapenem-nonsusceptible and multidrug-resistant (MDR) P. aeruginosa, which are more common in patients with lower respiratory tract infections (LRTIs) and in patients in intensive care units (ICUs), pose difficult treatment challenges and may require new therapeutic options. Two β-lactam/β-lactamase inhibitor combinations, ceftolozane/tazobactam (C/T) and imipenem/relebactam (IMI/REL), are approved for treatment of hospital-acquired/ventilator-associated bacterial pneumonia.
Methods
The Clinical and Laboratory Standards Institute–defined broth microdilution methodology was used to determine minimum inhibitory concentrations (MICs) against P. aeruginosa isolates collected from patients with LRTIs in ICUs (n = 720) and non-ICU wards (n = 914) at 26 US hospitals in 2017–2019 as part of the Study for Monitoring Antimicrobial Resistance Trends (SMART) surveillance program.
Results
Susceptibility to commonly used β-lactams including carbapenems was 5–9 percentage points lower and MDR rates 7 percentage points higher among isolates from patients in ICUs than those in non-ICU wards (P < .05). C/T and IMI/REL maintained activity against 94.0% and 90.8% of ICU isolates, respectively, while susceptibility to all comparators except amikacin (96.0%) was 63%–76%. C/T and IMI/REL inhibited 83.1% and 68.1% of meropenem-nonsusceptible (n = 207) and 71.4% and 65.7% of MDR ICU isolates (n = 140), respectively. Among all ICU isolates, only 2.5% were nonsusceptible to both C/T and IMI/REL, while 6.7% were susceptible to C/T but not to IMI/REL and 3.5% were susceptible to IMI/REL but not to C/T.
Conclusions
These data suggest that susceptibility to both C/T and IMI/REL should be considered for testing at hospitals, as both agents could provide important new options for treating patients with LRTIs, especially in ICUs where collected isolates show substantially reduced susceptibility to commonly used β-lactams.
Keywords: ceftolozane/tazobactam, ICU, imipenem/relebactam, Pseudomonas aeruginosa, respiratory tract infection
Carbapenem-resistant Pseudomonas aeruginosa is listed as a “Priority 1: Critical” pathogen on the Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics, which was compiled by the World Health Organization in 2017 [1]. Isolates with this phenotype and other resistant subsets of P. aeruginosa, including multidrug-resistant isolates, have been found more commonly among patients in intensive care units (ICUs) than other wards [2–7]. Resistant phenotypes of P. aeruginosa have also been found more commonly among respiratory tract isolates compared with intraabdominal, urinary tract, and skin/wound isolates [3, 7–12]. These problematic pathogens require new treatment options, especially for patients in ICUs and those with respiratory tract infections.
The cephalosporin ceftolozane was developed specifically to have enhanced antibacterial activity against P. aeruginosa. Ceftolozane is less susceptible to hydrolysis by AmpC β-lactamases (PDC), is a weak substrate for efflux pumps, and is not affected by OprD loss [13, 14]. The β-lactamase inhibitor tazobactam inhibits most class A and some class C β-lactamases (eg, DHA) and was combined with ceftolozane to broaden the gram-negative spectrum of coverage to many ESBL-producing Enterobacterales, yet it does not contribute to the antipseudomonal activity of ceftolozane. Ceftolozane/tazobactam (C/T) retains activity against the large majority of isolates resistant to carbapenems and other commonly used antipseudomonal β-lactams [15]. However, when isolates become resistant to C/T, treatment options are extremely limited. Imipenem/relebactam (IMI/REL) is a carbapenem (IMI) combined with cilastatin and a novel β-lactamase inhibitor (REL) that is active against class A and C β-lactamases and has been shown to restore imipenem susceptibility among Enterobacterales and P. aeruginosa [16]. Both agents are approved for the treatment of hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP) [17, 18].
Resistance in P. aeruginosa isolates can be complex and is often mediated by multiple chromosomally encoded enzymatic and nonenzymatic mechanisms as well as acquired enzymes. Both C/T and IMI/REL are active against the majority of P. aeruginosa isolates with derepressed chromosomally encoded AmpC (PDC) and porin defects or upregulated efflux transport [13, 14, 16, 19, 20]. However, C/T and IMI/REL are affected by mechanisms of resistance in various ways that can permit P. aeruginosa isolates to be nonsusceptible to one agent while still remaining susceptible to other agents. For example, C/T is not active against P. aeruginosa carrying metallo-β-lactamases (MBLs), KPCs, most isolates carrying ESBLs (eg, PER, VEB), PDC subtypes with mutations that increase hydrolysis of ceftolozane and ceftazidime, or isolates producing PDC at very high levels [13, 14, 16, 19–24]. IMI/REL is not active against P. aeruginosa carrying MBLs or some GES subtypes, or isolates with porin defects that also hyperproduce AmpC at very high levels [16, 19, 20]. Although published data are lacking, IMI/REL is not expected to show activity against P. aeruginosa carrying OXA-type β-lactamases, some of which may be susceptible to C/T [25]; however, it should be noted that several OXA-type enzymes with extended spectrum activity (eg, OXA-14) have been reported to confer resistance to C/T [22, 26].
Because of differences in the susceptibility profiles of the 2 agents, both may have a role in the treatment of resistant P. aeruginosa isolates. We compared the activity of C/T and IMI/REL against recent clinical isolates of P. aeruginosa collected in the United States as part of the global Study for Monitoring Antimicrobial Resistance Trends (SMART) surveillance program. Because of the high prevalence and increased resistance of P. aeruginosa among isolates from lower respiratory tract infections, we focused on this infection source, and because of higher resistance among isolates from ICU patients, we compared isolates from patients in ICUs and non-ICU wards.
METHODS
Bacterial Isolates
Twenty-six clinical laboratories in 18 states (California, Colorado, Florida, Georgia, Illinois, Indiana, Kentucky, Michigan, Minnesota, Nebraska, New York, North Carolina, Ohio, Pennsylvania, Texas, Utah, Washington, and Wisconsin), covering 8 of the 9 United States Census Bureau Divisions (all except New England), each collected up to 100 consecutive clinically relevant isolates of aerobic or facultative gram-negative bacilli from patients with lower respiratory tract infections per year. Only 1 isolate per species per patient per year was accepted into the study. All isolates were transported to a central laboratory (IHMA, Schaumburg, IL, USA), where they were re-identified using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA).
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed following the Clinical and Laboratory Standards Institute (CLSI) reference broth microdilution method [27, 28], using custom-made dehydrated broth microdilution panels manufactured by TREK Diagnostic Systems in 2017 (Thermo Fisher Scientific, Waltham, MA, USA) and frozen broth microdilution panels prepared at IHMA in 2018 and 2019. Relebactam and tazobactam were tested at a fixed concentration of 4 µg/mL, in combination with doubling dilutions of imipenem and ceftolozane, respectively. Avibactam was obtained from BioChemPartner (www.biocompartner.com) and tested at a fixed concentration of 4 µg/mL combined with ceftazidime, starting in 2018.
Minimum inhibitory concentrations (MICs) were interpreted as susceptible, intermediate, or resistant using 2021 CLSI breakpoints [28]. MDR isolates were defined phenotypically as those isolates resistant to ≥3 of the following 7 sentinel antimicrobial agents: amikacin, aztreonam, cefepime, levofloxacin, colistin, imipenem, and piperacillin/tazobactam. Pan-β-lactam-nonsusceptible isolates were defined as nonsusceptible (with intermediate or resistant MICs) to the following tested β-lactams (cefepime, ceftazidime, aztreonam, piperacillin/tazobactam, imipenem, meropenem). Difficult-to-treat resistance (DTR) was defined as isolates testing as nonsusceptible to all tested β-lactams (excluding C/T, IMI/REL, and ceftazidime/avibactam) and fluoroquinolones (ciprofloxacin [only tested in 2017–2018] and levofloxacin) [29].
Statistical Analysis
Differences in proportions of susceptible and nonsusceptible phenotypes between isolates collected from patients in ICUs and non-ICU wards were assessed for statistical significance with the Fisher exact test using XLSTAT, version 2020.1.3. A P value <.05 was considered statistically significant.
RESULTS
A total of 2578 and 2456 gram-negative pathogens were collected from patients with LRTI in ICUs and non-ICU wards, respectively. P. aeruginosa was the most common species, with 720 (27.9%) and 914 (37.2%) collected isolates, respectively, followed by Klebsiella pneumoniae (338 [13.1%] and 241 [9.8%], respectively) and Escherichia coli (291 [11.3%] and 225 [9.2%], respectively).
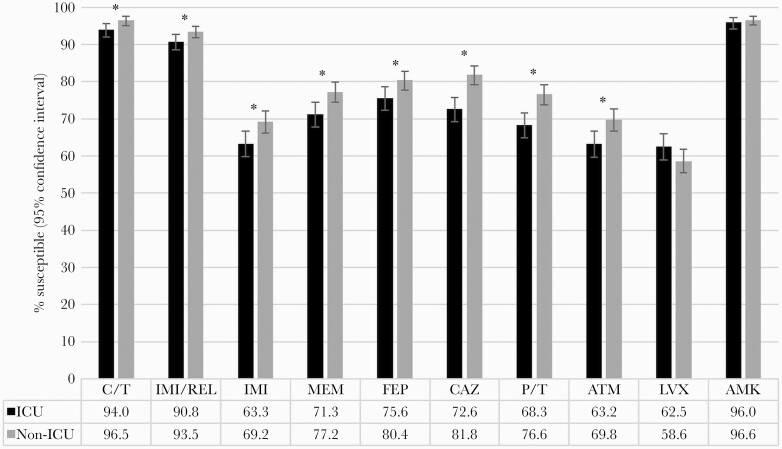
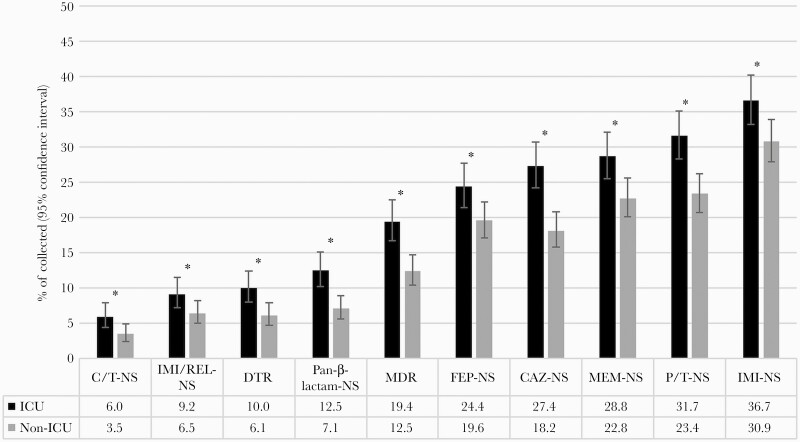
Antimicrobial susceptibility was generally lower among P. aeruginosa isolates from patients in ICUs compared with non-ICU wards, with susceptibility to commonly used β-lactams being 5–9 percentage points lower in ICUs than non-ICU wards (P < .05) (Figure 1). The differences were smaller (≤3 percentage points) for C/T (P = .02), IMI/REL (P = .049), and amikacin (P = .43). These 3 agents maintained susceptibility rates of >90% among isolates from patients in both ICUs and non-ICU wards. Susceptibility to all other comparators was 63%–76% in ICUs. The proportion of nonsusceptible phenotypes among P. aeruginosa isolates collected in ICUs and non-ICU wards is shown ranked by prevalence in Figure 2. C/T-nonsusceptible and IMI/REL-nonsusceptible isolates were less common than MDR, pan-β-lactam-nonsusceptible, and DTR isolates in both ICUs and non-ICU wards. The latter 3 subsets were significantly more prevalent among ICU isolates (10.0%–19.4%) than non-ICU isolates (6.1%–12.5%; P < .01).
Figure 1.
Antimicrobial susceptibility to C/T, IMI/REL, and comparators among all collected P. aeruginosa isolates from patients in ICUs (n = 720) and non-ICU wards (n = 914). *Statistically significant difference between isolates from ICUs and non-ICU wards (P < .05). Abbreviations: AMK, amikacin; ATM, aztreonam; C/T, ceftolozane/tazobactam; CAZ, ceftazidime; FEP, cefepime; ICU, intensive care unit; IMI, imipenem; IMI/REL, imipenem/relebactam; LVX, levofloxacin; MEM, meropenem; P/T, piperacillin/tazobactam.
Figure 2.
Proportion of isolates with resistant phenotypes among all P. aeruginosa collected from patients in ICUs (n = 720) and non-ICU wards (n = 914). *Statistically significant difference between isolates from ICUs and non-ICU wards (P < .05). Abbreviations: CAZ, ceftazidime; C/T, ceftolozane/tazobactam; DTR, difficult-to-treat resistance; FEP, cefepime; ICU, intensive care unit; IMI, imipenem; IMI/REL, imipenem/relebactam; MEM, meropenem; MDR, multidrug-resistant; NS, nonsusceptible (intermediate or resistant MICs); P/T, piperacillin/tazobactam.
Table 1 shows the activity of C/T, IMI/REL, and comparators against isolates with the studied resistance phenotypes. Susceptibility in general was lower in ICUs than non-ICU wards; however, statistically significant differences were not common and were found mostly for ceftazidime and aztreonam. C/T remained active against 77%–87% of ICU isolates that were nonsusceptible to at least 1 commonly used β-lactam and against 61%–71% of MDR, pan-β-lactam-nonsusceptible, and DTR isolates from ICU patients. The activity of IMI/REL was 1 to 15 percentage points lower than C/T among the studied nonsusceptible subsets collected from ICU patients; however, it maintained activity against 58.1% and 59.4% of C/T-nonsusceptible ICU and non-ICU isolates, respectively, while the tested carbapenems were active against 19%–31% of C/T-NS isolates. Similarly, C/T maintained activity against 72.7% and 78.0% of IMI/REL-nonsusceptible ICU and non-ICU isolates, respectively, and the tested cephalosporins and piperacillin/tazobactam were active against 21%–54% of isolates.
Table 1.
Antimicrobial Susceptibility to C/T, IMI/REL, and Comparators Among P. aeruginosa With Nonsusceptible Phenotypes
| % Susceptible | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype/Ward Type, No. | Ceftolozane/ Tazobactam | Imipenem/Relebactam | Imipenem | Meropenem | Cefepime | Ceftazidime | Piperacillin/Tazobactam | Aztreonam | Levofloxacin | Amikacin |
| Meropenem-NS | ||||||||||
| ICU (207) | 83.1 | 68.1 | 4.4 | 0.0 | 45.9 | 47.8a | 36.2 | 26.1 | 27.1 | 90.8 |
| Non-ICU (208) | 88.9 | 72.6 | 9.6 | 0.0 | 48.6 | 59.1a | 44.2 | 31.3 | 29.3 | 92.3 |
| Piperacillin/tazobactam-NS | ||||||||||
| ICU (228) | 82.5 | 77.2 | 39.9 | 42.1 | 26.8 | 20.2a | 0.0 | 7.9a | 34.2 | 91.7 |
| Non-ICU (214) | 87.4 | 79.4 | 47.7 | 45.8 | 34.6 | 31.3a | 0.0 | 15.9a | 32.7 | 92.5 |
| Cefepime-NS | ||||||||||
| ICU (176) | 76.7 | 72.2 | 34.7 | 36.4 | 0.0 | 11.9a | 5.1 a | 4.6a | 30.7 | 89.2 |
| Non-ICU (179) | 84.4 | 77.1 | 38.6 | 40.2 | 0.0 | 30.2a | 21.8 a | 18.4a | 27.4 | 87.2 |
| Ceftazidime-NS | ||||||||||
| ICU (197) | 78.2 | 79.2 | 41.1 | 45.2 | 21.3 | 0.0 | 7.6 | 9.6 | 38.1 | 90.4 |
| Non-ICU (166) | 81.3 | 83.7 | 45.8 | 48.8 | 24.7 | 0.0 | 11.5 | 12.1 | 33.1 | 90.4 |
| Imipenem-NS | ||||||||||
| ICU (264) | 87.1a | 75.0 | 0.0 | 25.0a | 56.4 | 56.1a | 48.1a | 40.2a | 36.0 | 92.1 |
| Non-ICU (282) | 92.2a | 79.4 | 0.0 | 33.3a | 61.0 | 68.1a | 60.3a | 50.4a | 40.8 | 92.2 |
| MDR | ||||||||||
| ICU (140) | 71.4 | 65.7 | 15.7 | 18.6 | 11.4 | 15.7 | 5.7 | 2.9 | 17.9 | 84.3 |
| Non-ICU (114) | 77.2 | 67.5 | 17.5 | 18.4 | 12.3 | 19.3 | 10.5 | 4.4 | 14.0 | 83.3 |
| Pan-β-lactam-NS | ||||||||||
| ICU (90) | 65.6 | 56.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15.6 | 86.7 |
| Non-ICU (65) | 72.3 | 63.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.8 | 84.6 |
| DTR | ||||||||||
| ICU (72) | 61.1 | 54.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 83.3 |
| Non-ICU (56) | 69.6 | 57.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 82.1 |
| IMI/REL-NS | ||||||||||
| ICU (66) | 72.7 | 0.0 | 0.0 | 0.0 | 25.8 | 37.9 | 21.2 | 13.6 | 15.2 | 80.3 |
| Non-ICU (59) | 78.0 | 0.0 | 1.7b | 3.4 | 30.5 | 54.2 | 25.4 | 17.0 | 11.9 | 81.4 |
| C/T-NS | ||||||||||
| ICU (43) | 0.0 | 58.1 | 20.9 | 18.6 | 4.7 | 0.0 | 7.0 | 4.7 | 18.6 | 67.4 |
| Non-ICU (32) | 0.0 | 59.4 | 31.3 | 28.1 | 12.5 | 3.1 | 15.6 | 6.3 | 9.4 | 71.9 |
Abbreviations: C/T, ceftolozane/tazobactam; DTR, difficult-to-treat resistance; ICU, intensive care unit; IMI/REL, imipenem/relebactam; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; NS, nonsusceptible (intermediate or resistant MICs).
aStatistically significant difference between isolates from ICUs and non-ICU wards (P < .05).
bOne isolate tested with an IMI/REL MIC of 4 µg/mL (intermediate) and an IMI MIC of 2 µg/mL (susceptible).
More detailed cross-susceptibility analyses showed that among the 720 isolates collected from patients in ICUs, only 2.5% were nonsusceptible to both C/T and IMI/REL, while a larger proportion (10.1%) was susceptible to either C/T or IMI/REL but not to the other agent (C/T-susceptible IMI/REL-nonsusceptible, 6.7%; IMI/REL-susceptible C/T-nonsusceptible, 3.5%) (Table 2A). Among non-ICU isolates, the percentages of isolates that were nonsusceptible to both agents (1.4%) or to one agent but not the other (7.1%) were slightly smaller (Table 2B). When limiting this analysis to only MDR isolates ("Table 3A and B), the proportions of isolates that were susceptible to C/T or IMI/REL but not to the other agent were more pronounced, especially among ICU isolates (37.1%); 87.1% of MDR ICU isolates were susceptible to either C/T or IMI/REL. Similar results were found when limiting this analysis to pan-β-lactam-nonsusceptible isolates (Supplementary Table 1a and b) and DTR isolates (Supplementary Table 2a and b): The proportion of isolates that were susceptible to C/T or IMI/REL but not to the other agent was 37.8% and 40.3% among pan-β-lactam-nonsusceptible and DTR ICU isolates, respectively; 80.0% of pan-β-lactam-nonsusceptible and 77.8% of DTR ICU isolates were susceptible to either C/T or IMI/REL.
Table 2.
Activity of C/T and IMI/REL Against all P. aeruginosa Collected From Patients in (A) ICUs and (B) Non-ICU Wards
| A, ICU | ||||
|---|---|---|---|---|
| IMI/REL, No. (%) | No. of Isolates | |||
| Susceptible | Nonsusceptible | |||
| C/T | Susceptible | 629 (87.4) | 48 (6.7) | 677 |
| Nonsusceptible | 25 (3.5) | 18 (2.5) | 43 | |
| No. of isolates | 654 | 66 | 720 | |
| B, Non-ICU | ||||
| IMI/REL, No. (%) | No. of Isolates | |||
| Susceptible | Nonsusceptible | |||
| C/T | Susceptible | 836 (91.5) | 46 (5.0) | 882 |
| Nonsusceptible | 19 (2.1) | 13 (1.4) | 32 | |
| No. of isolates | 855 | 59 | 914 |
Abbreviations: C/T, ceftolozane/tazobactam; ICU, intensive care unit; IMI/REL, imipenem/relebactam.
Table 3.
Activity of C/T and IMI/REL Against MDR P. aeruginosa Collected From Patients in (A) ICUs and (B) Non-ICU Wards
| A, ICU | ||||
|---|---|---|---|---|
| IMI/REL, No. (%) | No. of Isolates | |||
| Susceptible | Nonsusceptible | |||
| C/T | Susceptible | 70 (50.0) | 30 (21.4) | 100 |
| Nonsusceptible | 22 (15.7) | 18 (12.9) | 40 | |
| No. of isolates | 92 | 48 | 140 | |
| B, Non-ICU | ||||
| IMI/REL, No. (%) | No. of Isolates | |||
| Susceptible | Nonsusceptible | |||
| C/T | Susceptible | 64 (56.1) | 24 (21.1) | 88 |
| Nonsusceptible | 13 (11.4) | 13 (11.4) | 26 | |
| No. of isolates | 77 | 37 | 114 |
Abbreviations: C/T, ceftolozane/tazobactam; ICU, intensive care unit; IMI/REL, imipenem/relebactam; MDR, multidrug-resistant.
Because susceptibility data for ceftazidime/avibactam (CZA) were only available for isolates collected in 2018 and 2019, analyses comparing the activity of CZA with the other tested agents were restricted to these years and are shown in Supplementary Table 3. Among the 3 newer β-lactam/β-lactamase inhibitor agents, C/T generally showed the highest activity against P. aeruginosa collected in the ICU setting among all isolates and the nonsusceptible phenotypes tested. CZA also showed appreciable activity among isolates with the nonsusceptible phenotypes listed and was generally comparable to IMI/REL; however, both agents demonstrated activity that was 5–19 percentage points lower than observed for C/T among ICU isolates. The differences in activity were more prominent among isolates with more resistant phenotypes such as MDR, pan-β-lactam-nonsusceptible, and DTR subsets. In general, percentages of susceptibility to C/T and CZA were similar among isolates from non-ICU settings. CZA retained activity against C/T-nonsusceptible isolates from both the ICU (35.0%) and non-ICU (42.1%) settings, but susceptibility was ~25 percentage points lower than susceptibility to IMI/REL in this scenario. Similarly, CZA retained activity against 66.7% and 79.4% of ICU and non-ICU isolates that were IMI/REL-nonsusceptible, but these values were 14 percentage points lower than observed for C/T among ICU isolates. Both C/T and IMI/REL maintained 42%–63% activity against CZA-resistant isolates. It should be noted that many of these comparisons should be interpreted with caution due to small sample sizes, especially for C/T-nonsusceptible isolates.
DISCUSSION
Prior studies, including reports from both the SMART and SENTRY programs, have reported higher resistance among isolates from patients in ICUs than non-ICU wards [4, 5, 7], as well as among LRTI isolates [7–12]. In the current study, we were able to expand these findings as we focused on LRTI isolates from US patients and compared the 2 ward types. In contrast to the study by McCann et al., which did not see a higher rate of carbapenem-nonsusceptible P. aeruginosa isolates among respiratory isolates from patients in ICUs compared with non-ICU wards [7], we found that the ICU/non-ICU pattern persisted among LRTI isolates, with antimicrobial susceptibility among LRTI isolates significantly reduced for ICU isolates compared with those from non-ICU wards. For example, susceptibility to ceftazidime was 72.6% and 81.8%, respectively, and susceptibility to meropenem was 71.3% and 77.2%, respectively (P < .05). Susceptibility among ICU isolates was generally lower than that reported by Sader et al. among P. aeruginosa isolates collected from patients with pneumonia hospitalized in US ICUs (81.3% and 73.9% susceptible to ceftazidime and meropenem, respectively) [29]. These differences may reflect participation in the 2 studies of different hospitals in different regions of the United States as well as the fact that Sader et al. analyzed isolates collected from 2015 to 2017, while the current SMART study describes isolates collected from 2017 to 2019. A study by Asempa et al. of isolates collected in 2017–2018 showed susceptibility rates among respiratory isolates from patients in US ICUs that were more similar to those found in the current study (74.6% and 69.1% susceptible to ceftazidime and meropenem, respectively) [30]. In the current study, C/T and IMI/REL maintained activity against 94% and 91% of P. aeruginosa isolates from patients in ICUs, with the latter value being very similar to the finding in the study by Asempa et al. (90.1% susceptible to IMI/REL) [30].
Among the nonsusceptible subsets, the differences in antimicrobial susceptibility between isolates from ICUs and non-ICU wards were smaller and often did not reach statistical significance. For example, susceptibility to ceftazidime was 47.8% and 59.1% among meropenem-nonsusceptible isolates from ICUs and non-ICU wards, respectively (P < .05), while among MDR isolates susceptibility to ceftazidime was 15.7% and 19.3% (P > .05). C/T and IMI/REL maintained activity against 71% and 66% of MDR P. aeruginosa from ICUs, respectively. Even among DTR isolates, a novel category focusing on treatment-limiting resistance to all first-line agents, C/T and IMI/REL maintained activity against 61% and 54% of isolates, respectively. These in vitro data are promising, especially for infections with pan-β-lactam-nonsusceptible and DTR isolates, although clinical outcome data will be critical in determining the ultimate role of these agents in patient treatment. CZA, for which data were only available in 2018–2019 in the current study, was active against 51% of DTR ICU isolates, 16 and 5 percentage points lower than observed for C/T and IMI/REL, respectively, using the limited data set. Only amikacin consistently exceeded the activity of C/T and IMI/REL among nonsusceptible subsets, but it is associated with significant morbidity, including nephrotoxicity, and is typically used only in combination with another agent. Furthermore, colistin was until recently considered a last-resort option for treatment of infections caused by resistant isolates; however, the CLSI guidelines consider all P. aeruginosa isolates nonsusceptible to colistin, as clinical and PK/PD data have demonstrated limited clinical efficacy [28].
The activity of IMI/REL was slightly lower than that of C/T by 3 percentage points, but IMI/REL maintained activity against 58%–59% of C/T-nonsusceptible isolates, 28–58 percentage points higher than all comparator agents except amikacin. Similar observations were seen in a recent study of a large collection of P. aeruginosa isolates from Spain [20]. Fraile-Ribot et al. found susceptibility to IMI/REL among C/T- and ceftazidime/avibactam-resistant isolates that showed resistance mechanisms such as ESBL production (eg, PER and GES) or AmpC (PDC) mutations [20]. Conversely, C/T maintained activity against 73%–78% of IMI/REL-nonsusceptible isolates. Similarly, when eliminating the more susceptible isolates by limiting the analysis to MDR isolates from ICU patients, a more pronounced proportion of the remaining isolates were susceptible to 1 of the 2 agents but not to the other (52 of 140 MDR isolates collected from ICU patients, 37.1%). Given these findings as well as the limited data available for ceftazidime/avibactam in the current study, which also showed cross-susceptibility with C/T and IMI/REL, it appears prudent to include these newer agents in the susceptibility testing protocol, as this increases the chance of identifying an effective agent for infections caused by nonsusceptible P. aeruginosa phenotypes that can be very challenging to treat. The combined susceptibility testing of C/T and IMI/REL would identify a potentially effective antibiotic for 98%–99% of all P. aeruginosa isolates collected for the current study and may be useful in offering expeditious susceptibility data for clinical use. In fact, given the overarching goal to improve antimicrobial stewardship and timely appropriate therapy, it seems crucial to have susceptibility testing results to all newer agents available as soon as possible, including cefiderocol, which was not included in the SMART testing protocol but has been shown to be a potential treatment option for drug-resistant P. aeruginosa [31, 32]. However, given the practical limitations of testing all isolates against these agents, especially the testing of cefiderocol (which requires a special medium), it may be reasonable to restrict testing to isolates collected from patients at risk for resistance or with a history of resistance, or to settings with <90% susceptibility of P. aeruginosa to traditional antipseudomonal agents. This would be in line with the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) HAP/VAP guidelines, which recommend 2 antipseudomonal antibiotics from different classes for the empiric treatment of suspected VAP in these situations [33]. Early testing of newer agents could decrease considerably the use of combination therapy and result in earlier initiation of adequate therapy against drug-resistant pathogens, potentially leading to better clinical outcomes, shorter hospital stays, and reduced health care costs [6, 34–36].
CONCLUSIONS
Resistance to C/T or IMI/REL was uncommon among recent LRTI isolates of P. aeruginosa collected in the United States. There are differences in the mechanisms that result in resistance to C/T and IMI/REL, as evidenced by a considerable proportion of isolates testing as nonsusceptible to one agent and susceptible to the other, especially among isolates from patients in ICUs. These data suggest that susceptibility testing for both agents should be considered at hospitals, as both IMI/REL and C/T could provide important new options for treating patients with infections caused by nonsusceptible P. aeruginosa isolates, especially considering the substantially reduced susceptibilities to commonly used β-lactams.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank all SMART participants for their contributions to the program.
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Potential conflicts of interest. S.H.L., K.M.K., and D.F.S. work for IHMA, which receives funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, for the SMART global surveillance program. D.D.D., C.A.D., K.Y., and M.R.M. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and own stock and options in Merck & Co., Inc., Kenilworth, NJ, USA. The IHMA authors do not have personal financial interests in the sponsor of this paper (Merck Sharp & Dohme Corp.). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Not applicable; the study does not include factors necessitating patient consent.
References
- 1. World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: World Health Organization; 2017. [Google Scholar]
- 2. Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care 2011; 1:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lob SH, Hoban DJ, Young K, et al. . Activity of imipenem/relebactam against gram-negative bacilli from global ICU and non-ICU wards: SMART 2015-2016. J Glob Antimicrob Resist 2018; 15:12–9. [DOI] [PubMed] [Google Scholar]
- 4. Sader HS, Castanheira M, Flamm RK, et al. . Ceftazidime/avibactam tested against gram-negative bacteria from intensive care unit (ICU) and non-ICU patients, including those with ventilator-associated pneumonia. Int J Antimicrob Agents 2015; 46:53–9. [DOI] [PubMed] [Google Scholar]
- 5. Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis 2014; 78:443–8. [DOI] [PubMed] [Google Scholar]
- 6. Trinh TD, Zasowski EJ, Claeys KC, et al. . Multidrug-resistant Pseudomonas aeruginosa lower respiratory tract infections in the intensive care unit: prevalence and risk factors. Diagn Microbiol Infect Dis 2017; 89:61–6. [DOI] [PubMed] [Google Scholar]
- 7. McCann E, Srinivasan A, DeRyke CA, et al. . Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sader HS, Castanheira M, Duncan LR, Flamm RK. Antimicrobial susceptibility of Enterobacteriaceae and Pseudomonas aeruginosa isolates from United States medical centers stratified by infection type: results from the International Network For Optimal Resistance Monitoring (INFORM) surveillance program, 2015-2016. Diagn Microbiol Infect Dis 2018; 92:69–74. [DOI] [PubMed] [Google Scholar]
- 9. Lob SH, Hoban DJ, Young K, et al. . Activity of ceftolozane-tazobactam and comparators against Pseudomonas aeruginosa from patients in different risk strata - SMART United States 2016-2017. J Glob Antimicrob Resist 2020; 20:209–13. [DOI] [PubMed] [Google Scholar]
- 10. Karlowsky JA, Lob SH, Young K, et al. . Activity of imipenem-relebactam against multidrug-resistant Pseudomonas aeruginosa from the United States - SMART 2015-2017. Diagn Microbiol Infect Dis 2019; 95:212–5. [DOI] [PubMed] [Google Scholar]
- 11. Lob SH, Karlowsky JA, Young K, et al. . Activity of imipenem/relebactam against MDR Pseudomonas aeruginosa in Europe: SMART 2015-17. J Antimicrob Chemother 2019; 74:2284–8. [DOI] [PubMed] [Google Scholar]
- 12. Flamm RK, Farrell DJ, Sader HS, Jones RN. Ceftazidime/avibactam activity tested against gram-negative bacteria isolated from bloodstream, pneumonia, intra-abdominal and urinary tract infections in US medical centres (2012). J Antimicrob Chemother 2014; 69:1589–98. [DOI] [PubMed] [Google Scholar]
- 13. Zhanel GG, Chung P, Adam H, et al. . Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014; 74:31–51. [DOI] [PubMed] [Google Scholar]
- 14. Livermore DM, Mushtaq S, Meunier D, et al. ; BSAC Resistance Surveillance Standing Committee . Activity of ceftolozane/tazobactam against surveillance and ‘problem’ Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 2017; 72:2278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moise PA, Gonzalez M, Alekseeva I, et al. . Collective assessment of antimicrobial susceptibility among the most common gram-negative respiratory pathogens driving therapy in the ICU. JAC-Antimicrobial Resist. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young K, Painter RE, Raghoobar SL, et al. . In vitro studies evaluating the activity of imipenem in combination with relebactam against Pseudomonas aeruginosa. BMC Microbiol 2019; 19:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. United States Food and Drug Administration. Zerbaxa highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206829s008lbl.pdf. 2019. Accessed 10 December 2020.
- 18. United States Food and Drug Administration. Recarbrio highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212819s002lbl.pdf. 2020. Accessed 10 December 2020.
- 19. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68:2286–90. [DOI] [PubMed] [Google Scholar]
- 20. Fraile-Ribot PA, Zamorano L, Orellana R, et al. . Activity of imipenem-relebactam against a large collection of Pseudomonas aeruginosa clinical isolates and isogenic β-lactam-resistant mutants. Antimicrob Agents Chemother 2020; 64:e02165-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berrazeg M, Jeannot K, Ntsogo Enguéné VY, et al. . Mutations in β-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother 2015; 59:6248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fraile-Ribot PA, Cabot G, Mulet X, et al. . Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother 2018; 73:658–63. [DOI] [PubMed] [Google Scholar]
- 23. Cabot G, Bruchmann S, Mulet X, et al. . Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother 2014; 58:3091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz de la Rosa JM, Nordmann P, Poirel L. ESBLs and resistance to ceftazidime/avibactam and ceftolozane/tazobactam combinations in Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother 2019; 74:1934–9. [DOI] [PubMed] [Google Scholar]
- 25. Mushtaq S, Meunier D, Vickers A, et al. . Activity of imipenem/relebactam against Pseudomonas aeruginosa producing ESBLs and carbapenemases. J Antimicrob Chemother 2020; 76:434–42. [DOI] [PubMed] [Google Scholar]
- 26. Fournier D, Carrière R, Bour M, et al. . Mechanisms of resistance to ceftolozane/tazobactam in Pseudomonas aeruginosa: results of the GERPA multicenter study. Antimicrob Agents Chemother 2021; 65:e01117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. CLSI Standard M07. 11th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 28. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. 31st ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sader HS, Castanheira M, Mendes RE, Flamm RK. Frequency and antimicrobial susceptibility of gram-negative bacteria isolated from patients with pneumonia hospitalized in ICUs of US medical centres (2015-17). J Antimicrob Chemother 2018; 73:3053–9. [DOI] [PubMed] [Google Scholar]
- 30. Asempa TE, Nicolau DP, Kuti JL. Carbapenem-nonsusceptible Pseudomonas aeruginosa isolates from intensive care units in the United States: a potential role for new β-lactam combination agents. J Clin Microbiol 2019; 57:e00535-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kazmierczak KM, Tsuji M, Wise MG, et al. . In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53:177–84. [DOI] [PubMed] [Google Scholar]
- 32. Parsels KA, Mastro KA, Steele JM, et al. . Cefiderocol: a novel siderophore cephalosporin for multidrug-resistant gram-negative bacterial infections. J Antimicrob Chemother 2021; 76:1379–91. [DOI] [PubMed] [Google Scholar]
- 33. Kalil AC, Metersky ML, Klompas M, et al. . Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lodise TP Jr, Patel N, Kwa A, et al. . Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 2007; 51:3510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Micek ST, Lloyd AE, Ritchie DJ, et al. . Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 2005; 49:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.