Abstract
Background:
Botanical supplements have been proven to provide beneficial health effects. However, they can induce unintended adverse events such as hepatotoxicity. Oroxylum indicum extract (OIE, Sabroxy®) has several health benefits including anti-inflammatory, anti-arthritic, antifungal, antibacterial, and neuroprotective effects. It is currently unknown whether OIE has the potential to induce hepatotoxicity.
Purpose:
In the current study, we sought to determine whether OIE can induce hepatotoxicity in C57BL/6J mouse model.
Methods:
The male mice were fed powdered rodent food (control group) or powdered rodent food mixed with OIE (Sabroxy®, 500mg/kg) daily for 4 weeks. Following the treatment, we assessed liver histology and serum levels of biomarkers commonly associated with liver damage, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP).
Results:
No significant alterations were observed in liver histology, and serum levels of ALT, AST, ALP, bilirubin, albumin, globulin and total protein in the OIE fed mice compared to the control mice.
Conclusion:
Taken together, our results suggest that OIE, when fed at its physiologically relevant dosage, does not induce hepatotoxicity in C57BL/6J mice.
Keywords: Alanine aminotransferase, Alkaline phosphatase, Aspartate aminotransferase, Botanical supplement, Hepatotoxicity, Oroxylum indicum
Introduction
Botanical supplements (BS) are consumed globally as an alternative form of medicine, and are commonly acquired without any medical prescription 1. However, the majority of BS are not evaluated by regulatory bodies due to lack of federal regulations. Therefore, the exact makeup of ingredients in BS may not be described, resulting in uncertainty about the safety and toxicity of the majority of available BS. Although the full scope of BS-induced adverse effects remains to be determined, a number of reports showed an increasing incidence of BS-induced hepatotoxicity 2. For example, Camellia sinensis (green tea extract), Hydroxycut® (a proprietary blend of BS), and Garcinia cambogia have been shown to induce liver injury as exhibited by increases in serum markers of liver function 3. Clinical chemistry and histopathology are the mainstay to detect various types of hepatic injury in pre-clinial animal studies. For example, serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are routinely used to detect hepatocellular injury. Similarly, serum levels of bilirubin and alkaline phosphatases are used to assess the hepatobiliary function 4, 5. In addition, low levels of albumin, an important plasma protein produced by hepatocytes, may also indicate hepatic dysfunction 4, 5.
Oroxylum indicum (OI) is a genus of medium sized tree belonging to the plant family Bignoniaceae. It is native to tropical Asian countries, particularly India, Sri Lanka, Malaysia, China, Thailand, Philippines, and Indonesia 6. OI raw plant parts or OI extract (OIE) are commonly used in the traditional system of medicine for the prevention and treatment of several diseases 7, 8. For example, OI has been shown to exhibit antidiabetic 9, anti-arthritic 10 anti-hyperlipidemic 11, antioxidant 6, anti-inflammatory 12, anticarcinogenic 13, antimicrobial 14, and anti-obesity 15 activities in both in vivo and in vitro studies. OI has also been shown to protect against β-amyloid-induced cell injury 16 and drug-induced renal injury 8, 17.
While OI and OIE have been shown to exhibit many beneficial effects, their potential toxic effects are not fully understood. Here, we set to determine whether the administration of a commercially available OIE Sabroxy®, which contains satandardiazed amounts of bioactive compounds, would induce hepatotoxicity in a rodent model by examining the key serum markers and histopahology indicative of liver injury.
Materials and Methods
Materials:
OI extract (Sabroxy®) was provided by Sabinsa Corporation. (Sabroxy®), the Oroxylum indicum bark extract used in this investigation was prepared using the methods described in US patent number: US10555982B2 18. The identified active compounds in this extract included Oroxylin A, Baicalein, and Chrysin.
Animals and treatment:
8 week old male C57BL/6J mice weighing 25–35 g were housed in a temperature-controlled room with a 12-hour day and night cycle with access to food and water ad libitum. The mice received humane care and in vivo experiments were carried after obtaining appropriate approvals from Auburn University’s Institutional Animal Care and Use Committee (IACUC). Health benefits of OIE were studied using orally administered doses ranging from 100 to 500 mg/kg/day of Sabroxy® in rodents 10, 19–21. To determine the potential hepatotoxic effect of OIE, the treatment group mice (n=6) were fed daily for 4 weeks with 500 mg/kg Sabroxy® mixed in the powdered rodent food. The control group of mice (n=6) were fed the powdered rodent food without Sabroxy® for 4 weeks.
Tissue collection and processing:
After 4 weeks of treatment, the mice from both groups were euthanized through CO2 asphyxiation followed by secondary decapitation. Blood was collected immediately after decapitation and serum was separated for serum biomarker analysis. The mice livers were collected and processed for histological analysis.
Serum biomarker Analysis:
Serum ALT and AST concentrations were measured using a colorimetric enzymatic assay established and validated on the c 311 biochemistry analyzer (Roche, Indianapolis, Indiana) at the Auburn University Clinical Pathology Laboratory. Serum was also used to test for other markers indicative of hepatotoxicity such as total protein, albumin, and globulin. Serum ALP and bilirubin levels were also assessed using the same method used for ALT and AST measurement.
Liver histology analysis:
The liver sections were collected from various liver lobes, fixed in 10% neutral buffered formalin and paraffin embedded. 5 μm thick liver sections were cut and stained with H&E for histological assessment.
Data and statistical analysis:
Data are shown as mean values ± SEM. Analyses were performed using Prism-V software (La Jolla, CA, USA). The significance of the differences between groups was evaluated by Students t-test. P-value of < 0.05 were determined to be statistically significant.
Results
Effect of OIE treatment on liver histology:
OIE treatment did not result in a noticeable change in the liver histology as shown by H&E staining (Figure 1).
Figure 1.
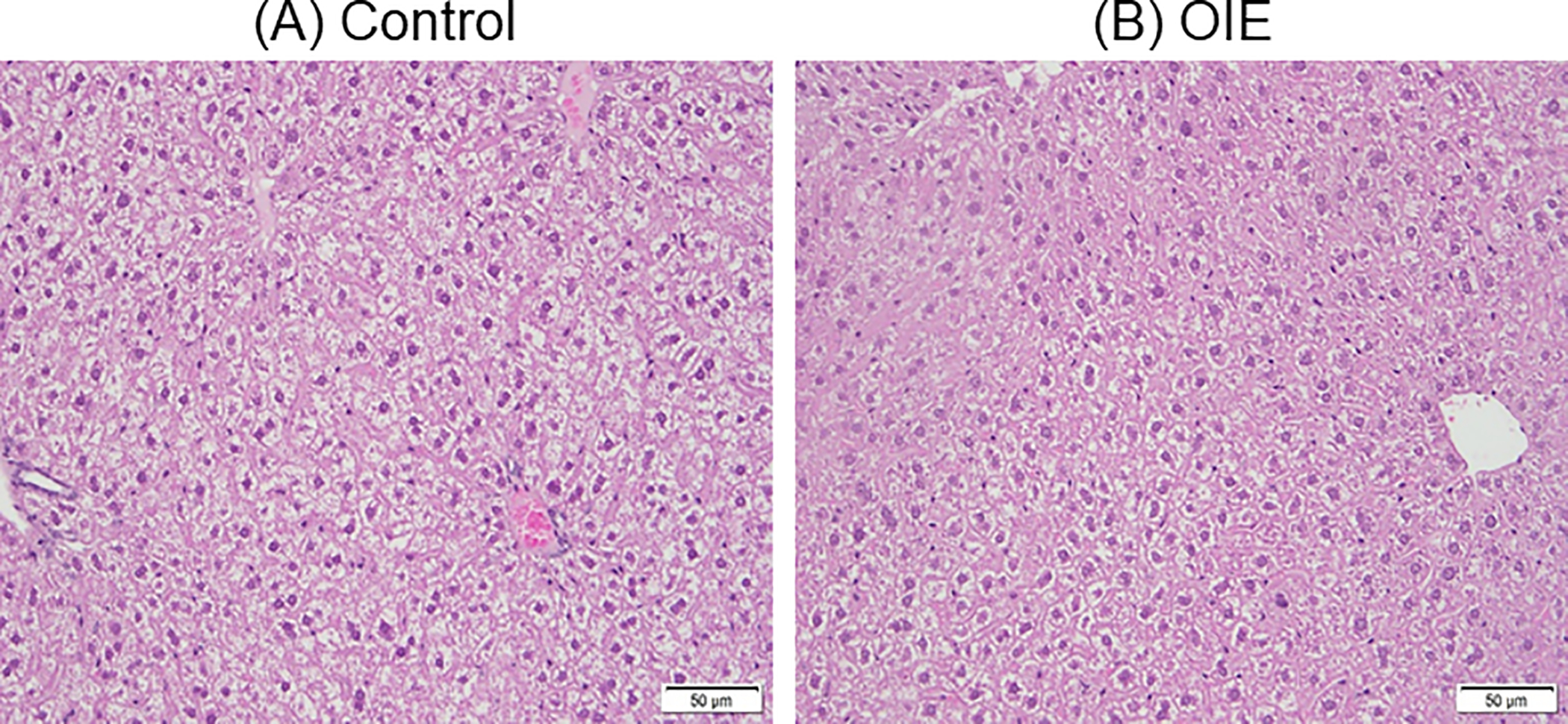
Effect of OIE treatment on liver histology. Liver sections of control (A) and OIE treated (B) mice were stained with H&E for histological assessment.
Effect of OIE treatment on serum biomarkers of liver function:
OIE treatment did not significantly alter the serum levels of ALT, AST, ALP, bilirubin, albumin, globulin, and total protein (Figure 2).
Figure 2:
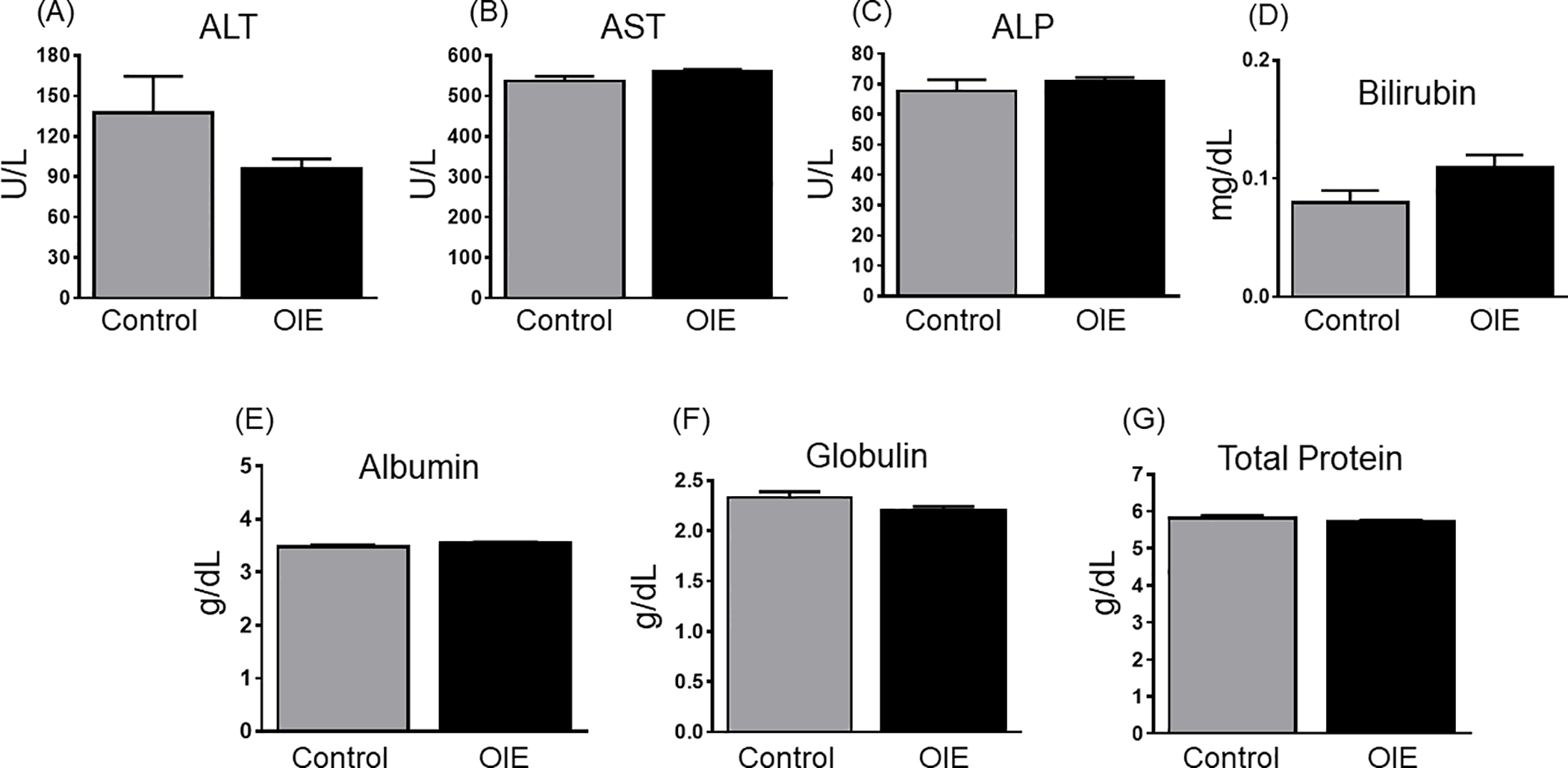
Effect of OIE treatment on serum levels of ALT (A), AST (B), ALP (C), bilirubin (D), albumin (E), globulin (F), and total protein (G). Serum markers were analyzed from control (n =6) and OIE treated mice (n =6). Results are presented as mean values ± SEM. The significance of the differences between groups was evaluated by Students t-test. P < 0.05 values were determined to be statistically significant.
Discussion
In this study, we showed that the administration of Sabroxy® at the dose of 500 mg/kg/day for 4 weeks does not induce hepatotoxicity in C57BL/6J male mice. After treatment, there was no significant change in serum biomarkers including liver transaminases, albumin, bilirubin, and ALP which are commonly used to detect liver injury. Similarly, Sabroxy® supplementation did not lead to histopathological changes in the liver of mice.
Different parts of OI plant, including stem, bark, roots, leaves, and fruits have been shown to exhibit a variety of health benefits 6, 9–15, 19. OIE may display differential biological effects depending on the extraction method 20. One study evaluated the anti-arthritic activity of OI root extracts obtained using different solvents: chloroform, ethyl acetate, and n-butanol. All three extracts were administered into rats through the oral route at a dosage of 300 mg/kg. Results indicated that all three extracts exhibited anti-arthritic activity. However, the relative anti-arthritic activity was found to be highest in ethyl acetate extract (67.69%), followed by chloroform extract (64.61%), and lastly, n-butanol extract (58.46%) 10. The differential effects of OIE could be due to the variation in the amounts of biologiclally active ingredients depending on the plant part and solvent used for extraction method. It is difficult to evaluate the hepatoxocity of OIEs since OIEs can be obtained from various parts of the OI plant using different extraction methods. Therefore, having a standardized extract, such as Sabroxy®, with known amounts of biologically active compunds would be beneficial to understand the potential of OIE to induce hepatotoxicity.
Sabroxy® contains standardized amounts of the Oroxylin-A, Baicalein, and Chrysin. All three compounds show hepatoprotective effects in vivo. Oroxylin A, Baicalein, and Chrysin are flavonoids present in numerous plant extracts including OI, and were shown to suppress inflammation in mice with CCl4-induced acute liver failure 22, 23 and acetaminophen-exposed liver injury in mice 24. Our current study supports the above published studies, as Sabroxy® failed to cause hepatotoxic effects in the mice.
Due to the the fact that the biomarkers were measured at one time point after a 4 week treatment period of OIE, this study is considered as a sub-acute study. Although we did not notice a significant hepatotoxicity at the end of 4 weeks of treatment, it can not be ruled out that the mice may have developed acute hepatic toxicity during the treatment period, and that the mice may have recovered from the early liver injury before our endpoint at 4 weeks when serum biomarkers were studied.
Although the dosage and frequency of administration of OIE used in our study is physiologically relevant, various doses from 100–500 mg/kg are typically used for varying treatment periods. Therefore, future studies in larger cohorts are warranted to determine the dosage and time-dependent hepatic effects of OIE administration to validate the potential of hepatotoxicity OIE. It is also important that future studies examine the levels and activities of markers of oxidative stress and inflammation in liver. Finally, our study included only male mice. It will be interesting to examine whether the effect of OIE administration is sexually dimorphic.
Conclusion:
Our results suggest that Sabroxy®, at its physiologically relevant doasge used for beneficial effects, does not induce hepatotoxicity in the mice after a 4-week treatment.
Acknowledgements
This project was supported by Sabinsa Corporation, Animal Health and Disease Research Grant and Auburn University Research Initiative in Cancer Grant. This work was also supported by NIH R00 HD082686 Grant to Huang C J. The authors thank the Auburn University Clinical Pathology Lab for assistance with serum biomarker analysis.
Abbreviations:
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- OI
Oroxylum indicum
- OIE
Oroxylum indicum extract
Footnotes
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication and all significant financial support for this work has been listed in the acknowledgements. Our institution does not require ethical approval for reporting individual cases or case series. Nagabhushanam K and Majeed M, are involved in designing the experiments and proofreading the manuscript. They did not involve in conducting the experiments and data analyses. All procedures in this study were conducted in accordance with the Auburn University’s Institutional Animal Care and Use Committee-IACUC, (2016-2978) approved protocols. There are no human subjects in this article and informed consent is not applicable.
References
- 1.Tarn DM, Paterniti DA, Good JS, et al. Physician-patient communication about dietary supplements. Patient Educ Couns. June 2013;91(3):287–94. doi: 10.1016/j.pec.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro VJ, Khan I, Bjornsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. January 2017;65(1):363–373. doi: 10.1002/hep.28813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch. toxicol. June 2015;89(6):851–65. doi: 10.1007/s00204-015-1471-3 [DOI] [PubMed] [Google Scholar]
- 4.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. July 2007;74(7):663–71. doi: 10.1007/s12098-007-0118-7 [DOI] [PubMed] [Google Scholar]
- 5.Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. November 22 2009;3:17. [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra SL, Sinhamahapatra PK, Nayak A, Das R, Sannigrahi S. In vitro Antioxidant Potential of Different Parts of Oroxylum indicum: A Comparative Study. Indian J Pharm sci. March 2010;72(2):267–9. doi: 10.4103/0250-474X.65013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinda B, SilSarma I, Dinda M, Rudrapaul P. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: from traditional uses to scientific data for its commercial exploitation. J Ethnopharmacol. February 23 2015;161:255–78. doi: 10.1016/j.jep.2014.12.027 [DOI] [PubMed] [Google Scholar]
- 8.Harminder Singh V, Chaudhary AK. A Review on the Taxonomy, Ethnobotany, Chemistry and Pharmacology of Oroxylum indicum Vent. Indian J Pharm sci. September 2011;73(5):483–90. doi: 10.4103/0250-474X.98981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J, Kakkar P. Modulation of liver function, antioxidant responses, insulin resistance and glucose transport by Oroxylum indicum stem bark in STZ induced diabetic rats. Food Chemical Toxicol. December 2013;62:722–31. doi: 10.1016/j.fct.2013.09.035 [DOI] [PubMed] [Google Scholar]
- 10.Karnati M, Chandra RH, Veeresham C, Kishan B. Anti-arthritic activity of root bark of Oroxylum indicum (L.) vent against adjuvant-induced arthritis. Pharmacognosy Res. April 2013;5(2):121–8. doi: 10.4103/0974-8490.110543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangal P, Khare P, Jagtap S, Bishnoi M, Kondepudi KK, Bhutani KK. Screening of six Ayurvedic medicinal plants for anti-obesity potential: An investigation on bioactive constituents from Oroxylum indicum (L.) Kurz bark. J Ethnopharmacol. Feb 2 2017;197:138–146. doi: 10.1016/j.jep.2016.07.070 [DOI] [PubMed] [Google Scholar]
- 12.Doshi K, Ilanchezhian R, Acharya R, Patel BR, Ravishankar B. Anti-inflammatory activity of root bark and stem bark of Shyonaka. J Ayurveda Integr Med. October 2012;3(4):194–7. doi: 10.4103/0975-9476.104434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naveen Kumar DR, Cijo George V, Suresh PK, Ashok Kumar R. Cytotoxicity, apoptosis induction and anti-metastatic potential of Oroxylum indicum in human breast cancer cells. Asian Pac J Cancer Prev. 2012;13(6):2729–34. doi: 10.7314/apjcp.2012.13.6.2729 [DOI] [PubMed] [Google Scholar]
- 14.Radhika LG, Meena CV, Peter S, Rajesh KS, Rosamma MP. Phytochemical and antimicrobial study of Oroxylum indicum. Anc Sci Life. April 2011;30(4):114–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Hengpratom T, Lowe GM, Thumanu K, Suknasang S, Tiamyom K, Eumkeb G. Oroxylum indicum (L.) Kurz extract inhibits adipogenesis and lipase activity in vitro. BMC Complement Altern Med. June 8 2018;18(1):177. doi: 10.1186/s12906-018-2244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mairuae N, Connor JR, Buranrat B, Lee SY. Oroxylum indicum (L.) extract protects human neuroblastoma SHSY5Y cells against betaamyloidinduced cell injury. Mol Med Rep. August 2019;20(2):1933–1942. doi: 10.3892/mmr.2019.10411 [DOI] [PubMed] [Google Scholar]
- 17.Sultana S, Verma K, Khan R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J Pharm Pharmacol. June 2012;64(6):872–81. doi: 10.1111/j.2042-7158.2012.01470.x [DOI] [PubMed] [Google Scholar]
- 18.Majeed M, Nagabhushanam K, Bhat B, Pande A, inventors; Composition containing oroxylin A and method of extraction thereof. USA: 2020. [Google Scholar]
- 19.Tenpe C, Upaganlawar A, Burle S, Yeole Y. In vitro antioxidant and preliminary hepatoprotective activity of Oroxylum indicum Vent leaf extracts. Pharmacologyonline. 2009;1:35–43. [Google Scholar]
- 20.Begum MM, Islam A, Begum R, et al. Ethnopharmacological Inspections of Organic Extract of Oroxylum indicum in Rat Models: A Promising Natural Gift. Evid Based Complement Alternat Med: eCAM. 2019;2019:1562038. doi: 10.1155/2019/1562038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das BK, Al-Amin MM, Russel SM, Kabir S, Bhattacherjee R, Hannan JM. Phytochemical Screening and Evaluation of Analgesic Activity of Oroxylum indicum. Indian J Pharm sci. Nov-Dec 2014;76(6):571–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu R, Zeng G, Chen Y, et al. Oroxylin A accelerates liver regeneration in CCl(4)-induced acute liver injury mice. PloS one. 2013;8(8):e71612. doi: 10.1371/journal.pone.0071612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermenean A, Mariasiu T, Navarro-Gonzalez I, et al. Hepatoprotective activity of chrysin is mediated through TNF-alpha in chemically-induced acute liver damage: An in vivo study and molecular modeling. Exp Ther Med. May 2017;13(5):1671–1680. doi: 10.3892/etm.2017.4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou HC, Wang H, Shi K, Li JM, Zong Y, Du R. Hepatoprotective Effect of Baicalein Against Acetaminophen-Induced Acute Liver Injury in Mice. Molecules. December 31 2018;24(1)doi: 10.3390/molecules24010131 [DOI] [PMC free article] [PubMed] [Google Scholar]


