Abstract
This commentary presents a scientific basis for managing as one chemical class the thousands of chemicals known as PFAS (per- and polyfluoroalkyl substances). The class includes perfluoroalkyl acids, perfluoroalkylether acids, and their precursors; fluoropolymers and perfluoropolyethers; and other PFAS. The basis for the class approach is presented in relation to their physicochemical, environmental, and toxicological properties. Specifically, the high persistence, accumulation potential, and/or hazards (known and potential) of PFAS studied to date warrant treating all PFAS as a single class. Examples are provided of how some PFAS are being regulated and how some businesses are avoiding all PFAS in their products and purchasing decisions. We conclude with options for how governments and industry can apply the class-based approach, emphasizing the importance of eliminating non-essential uses of PFAS, and further developing safer alternatives and methods to remove existing PFAS from the environment.
Graphical Abstract
INTRODUCTION
When chemicals have similar molecular structures, environmental properties, and/or biological hazards, managing them as a class can be an effective means of reducing adverse effects on human and ecological health.1–4 While a class-based approach to chemical management can pose challenges to the traditional paradigm of individual chemical risk assessment, the extreme persistence and potential for harm from thousands of PFAS (per- and polyfluoroalkyl substances)5,6 demand a more efficient and effective approach. Examples of cases in which substances with common chemical characteristics are currently managed as a class include organophosphate pesticides, organochlorine pesticides, and organohalogen flame retardants.1,7 Thus, a class-based approach not only is feasible but also has already been implemented by regulatory agencies globally.
Here we provide scientific justification for why a class-based approach is appropriate and necessary for all PFAS, defined as chemicals with at least one aliphatic perfluorocarbon moiety (e.g., -CnF2n−).5,6 We discuss the following major subclasses of PFAS in detail: perfluoroalkyl acids and perfluoroalkylether acids (together termed PFAA) and their precursors, fluoropolymers and perfluoropolyethers, and other (primarily less reactive) PFAS (see Figure 1 for examples). PFAA are nonpolymer PFAS with at least one perfluorocarbon moiety (e.g., -CF2-, >CF-) directly linked to an acid functional group. The most well-known are perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). Many other PFAS may transform and yield PFAA in the environment and biota and are thus regarded as precursors to PFAA. Examples are PFAS derived from fluorotelomers and perfluoroalkane-sulfonyl fluorides, including so-called side-chain-fluorinated polymers (i.e., polymers with nonfluorinated backbones and fluorinated side chains). Fluoropolymers and perfluoropolyethers include polymers with backbones being per- or polyfluorinated. Other PFAS in the class include primarily nonpolymeric PFAS with limited chemical reactivity, such as linear and cyclic perfluoroalkanes, perfluoroalkylethers, and perfluoroalkyl amines.
Figure 1.
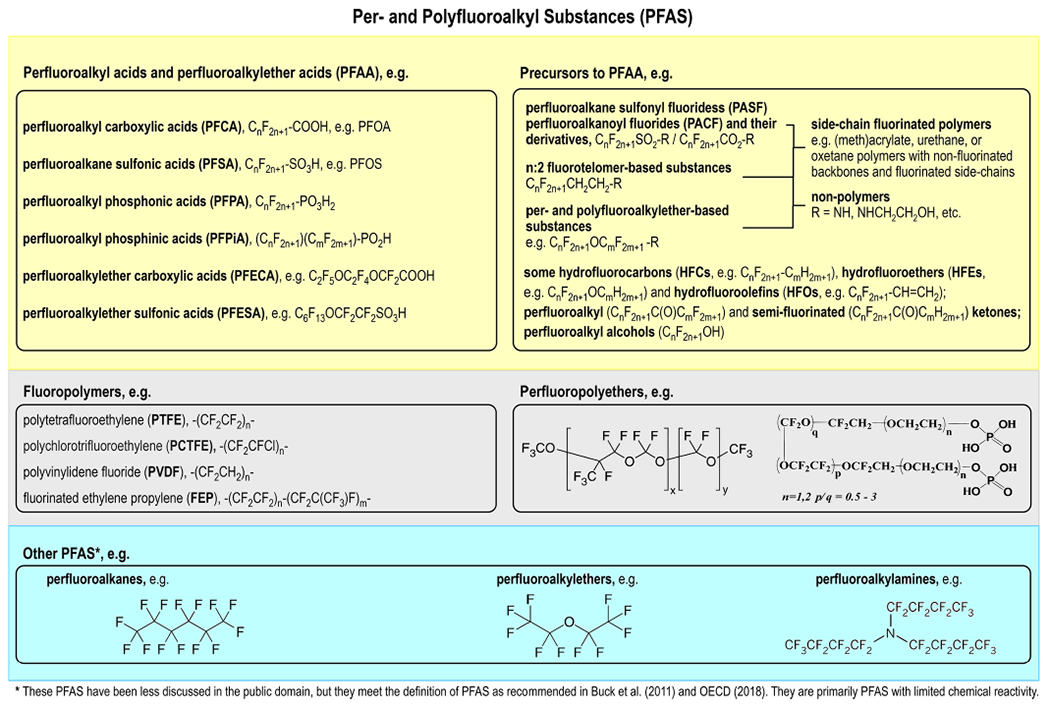
Examples of PFAS chemistries. *These PFAS have been less discussed in the public domain, but they meet the definition of PFAS as recommended in refs 58 and 5. They are primarily PFAS with limited chemical reactivity.
PFAS function in many capacities, including as surfactants, friction reducers, and repellents of water, dirt, and oil. As such, they are used in a wide variety of consumer products to confer nonstick (waterproof, greaseproof, and stainproof) and low-friction properties. Examples of products that contain or are coated with PFAS include carpets, glass, paper, clothing and other textiles, plastic articles, cookware, food packaging, electronics, and personal care products. PFAS are also used directly or as technical aids (dispersants and emulsifiers) in many industrial applications, such as in metal coatings, lubricants for machinery, membranes, and firefighting foams. PFAS are used in the synthesis of or as adjuvants in pesticides, in medical procedures and products, and in many other applications.8
The most consistent feature within the class of PFAS is that their perfluorocarbon moieties do not break down, or do so very slowly under natural conditions, which is why PFAS are often termed “forever chemicals”.9 Because PFAS are persistent, they accumulate or concentrate in the environment, including water, air, sediment, soil, and plants.10 Elevated levels of PFAS and their widespread presence in environmental media and drinking water stem from industrial sites that produce or use PFAS (or have done so in the past), airports, military bases (fire-training and response areas), landfills, wastewater treatment plants, and the spreading of PFAS-contaminated biosolids.11–13 Some PFAS are highly mobile in either air or water, allowing them to travel long distances from their source.
Environmental and human exposure to PFAS can occur throughout the life cycles of these chemicals and products containing them, including during chemical production, product manufacturing, distribution, use, disposal, and recycling. Many PFAS, particularly PFAA, have been detected globally and are in the bodies of nearly all people living in the United States (US), Europe, and other countries worldwide.14,15 Major sources of human exposure to PFAS are from contaminated food, water, air, and other media such as consumer products and house dust.16,17 Limited testing of primarily large public water sources in the US found PFAS in the water supplies serving an estimated 16.5 million people, including 6 million with a combined PFOS and PFOA concentration over the US EPA’s lifetime health advisory of 70 ng/L.11 More recent testing of 25 public water systems in the US identified PFAS in every one, with an average of nearly 10 different PFAS at a combined concentration near 20 ng/L.18 Such testing is lacking in many other parts of the world, including many European countries. PFAS are also found in a variety of foods.19,20 The highest levels are found in fish and shellfish, but meat, eggs, and milk may also contain PFAS if animals have consumed contaminated feed or water. Fruits and vegetables have been shown to contain PFAS taken up from the soil and water used to grow them.21 Food contact materials are another source of exposure,22,23 as are consumer products and house dust.24–28
Exposure to PFAS occurs in complex mixtures of multiple PFAS, yet at present, fewer than 50 individual PFAS (often fewer than 10) are commonly measured in environmental media.29,30 New analytical methods allow for more comprehensive screening such as measuring total fluorine or extractable/adsorbable organofluorine in the environment,31–33 products,22,23,34,35 dust,27 biota,36 and humans.37 These methods reveal evidence that humans and wildlife are exposed to more PFAS than previously estimated. For example, in one study of tap water in five US cities, less than half the total organic fluorine measured in treated drinking water was accounted for by the sum of individually identified PFAS, indicating far more PFAS and other organofluorine compounds were present in the water than were identified with targeted analysis.38
The most well-studied of these substances, PFOA and PFOS, have been linked to a variety of health problems. They are termed “long-chain” PFAS, a designation that includes perfluoroalkylcarboxylic acids (PFCA) with seven or more fluorinated carbons, perfluoroalkanesulfonic acids (PFSA) with six or more fluorinated carbons, and their precursors. When some major manufacturers phased out the production of long-chain PFAS, most industries turned to structurally similar replacements, including homologues with fewer fluorinated carbons (short-chain PFAS) or other less well known PFAS (e.g., per- and polyfluoroalkylether-based substances).39,40 These replacement PFAS were marketed by producers as safer alternatives because of their presumed lower toxicity and lower level of bioaccumulation in human blood.41 However, several lines of evidence suggest that short-chain PFAS are not safer alternatives. Research has demonstrated that short-chain PFAS can be equally environmentally persistent and are even more mobile in the environment and more difficult to remove from drinking water than long-chain PFAS.33,42,43 Bioaccumulation of some short-chain PFAS occurs in humans and animals,44–46 and research in fish suggests they can do so in excess of the long-chain compounds they aimed to replace.47,48 Short-chain PFAS also can be more effectively taken up by plants.49–51 Because short-chain PFAS have, to a large extent, replaced the long-chain PFAS in commerce, the levels of short-chain PFAS, such as perfluorobutanoic acid (PFBA), perfluorobutanesulfonic acid (PFBS), and perfluorohexanoic acid (PFHxA), have increased in environmental media.37,43,52,53 To date, relatively little is known about possible health effects of long-term exposure to short-chain PFAS. However, a growing body of evidence suggests they are associated with similar adverse toxicological effects as long-chain PFAS.54–57 The ongoing accumulation of persistent chemicals that are known or potentially hazardous increases risks to human and environmental health over an indefinite period of time.
Fluoropolymers consist of molecular segments (monomers) that are linked together, with up to hundreds of thousands of linked monomers in high-molecular weight polymers. While they are commonly regarded as PFAS,58 fluorochemical producers now argue that fluoropolymers should be separated from other PFAS for hazard assessment or regulatory purposes.59 However, the production of fluoropolymers and perfluoropolyethers is responsible for extensive environmental PFAS contamination, including releases of both intentionally added PFAA processing aids and unintentional PFAS by products.13,43,60–65 It is estimated that the vast majority (~80%) of PFCA in the environment is from fluoropolymer manufacture and use.60 Below, we discuss reasons why fluoropolymers and perfluoropolyethers should be included in the class approach to managing PFAS.
To date, managing the risk of PFAS has focused primarily on one chemical at a time, or a small group of PFAS. This approach has not been effective at controlling widespread exposure to this large group of chemicals with known and potential hazards. Below, we present scientific justification for managing PFAS as a single chemical class, and we suggest ways in which government and industry can reduce PFAS-related risks. For example, a class-based approach can be implemented to more effectively eliminate non-essential uses of PFAS, develop safer alternatives, and clean up highly contaminated areas. Ultimately, this will reduce and prevent further accumulation of these hazardous chemicals in people and the environment and avoid replacing them with other related and harmful substances.
HEALTH AND ENVIRONMENTAL HAZARDS
With regard to biological activity and the potential for human health impacts, PFAA, particularly PFOA and PFOS, are the most well studied PFAS. Data from toxicokinetic studies of PFAA indicate that they are generally well-absorbed after ingestion.66 After absorption, they distribute from blood to organs and tissues that receive high blood flow, such as the liver, kidney, lung, heart, skin, testis, brain, bone, and spleen.46,66–70 Because PFAA can occupy sites on multiple receptors, proteins, and cell interfaces in the body, they can produce physiological effects across a range of tissues.66 Toxicological (in vitro and in vivo) and epidemiological (in occupational, highly exposed, and general populations) studies have identified a broad range of adverse health outcomes associated with exposure to PFAA in people and animals. In studies of exposed humans, elevated blood levels of PFAA have been associated with kidney and testicular cancer, elevated cholesterol, liver disease, decreased fertility, thyroid problems, changes in hormone functioning, changes in the immune system, and adverse developmental effects.54,71,72 Studies of experimental animals provide biological support for associations seen in human epidemiological studies, and mechanistic studies increase confidence in a causal relationship between PFAA and health effects in humans.72,73 To understand the potential of every PFAS to adversely affect health would require testing across the entire range of different biological end points.
Effects on the immune system are some of the most well studied health effects of PFAA. Multiple lines of evidence support PFAA as immunotoxicants and, more specifically, immunosuppressants at small administered doses in rodents, and measured serum concentrations in humans. Findings of suppressed vaccine response in humans and T cell-dependent antibody response in experimental animals led the US National Toxicology Program (NTP) to classify PFOA and PFOS as presumed immune hazards to humans.72 In a recent draft toxicological profile, the US Agency for Toxic Substances and Disease Registry (ATSDR) extended this finding to PFHxS and perfluorodecanoic acid (PFDeA), identifying all four compounds as suppressants of antibody response in humans.54 These reviews provide strong evidence for immunotoxicity, especially when seen across multiple compounds, species, and studies. Notably, suppressed vaccine response in children indicates the period of early life as an exposure window of specific concern.74,75 As such, developmental toxicity has been used as the basis for managing PFAS in drinking water and food contact materials.76–80 Although the immune system, particularly during development, appears to be sensitive to these chemicals, few PFAS have been studied for such effects.
To date, a majority of human epidemiological studies have focused on long-chain PFAA. In experimental animal models, however, short-chain PFAA have shown effects similar to those of long-chain PFAA. For example, exposure to GenX has been associated with hepatic and renal effects81,82 and suppressed immune function in mice.83 A study of PFBA in rats indicated changes to liver weight, serum cholesterol, and thyroid hormones,84 and a two-generation study of PFBS in rats demonstrated increased liver weight and pathological changes in kidneys.85 Recent reports by NTP found that both PFBS and PFHxA had numerous adverse effects, including decreased thyroid hormones in male and female rats.55,56 Effects on kidneys86 and on reproduction and development87 also have been reported for PFHxA. Notably, effects observed with other PFAA may occur at larger administered doses compared to the long-chain PFAA. However, humans are exposed to multiple PFAS at once, and there is little research to date on the effects of combined exposures. To account for such effects, an additive model for PFAS toxicity is used by the US EPA for two PFAS and in several US states for five to six PFAS. In Europe, an additive model is used by the European Food Safety Authority (EFSA) for four PFAS,88 by the EU drinking water directive listing 20 PFAS, and by individual European countries, including Sweden and Denmark.
Some manufacturers have proposed that fluoropolymers should not be grouped with other PFAS for regulatory purposes, arguing that they are biologically inert because of their high molecular weight.59 However, these chemicals can release low-molecular weight PFAS and other hazardous substances to the environment throughout their life cycle. Thus, we argue for the inclusion of fluoropolymers and perfluoropolyethers in the overall class approach for PFAS, specifically for the following reasons.
(1) During production of fluoropolymers and perfluoropolyethers, low-molecular weight PFAS used as raw materials, processing agents, or additives, or generated as intermediates, can be released into different waste streams (air and water) and current emission filters do not completely capture them, nor is there an effective means of disposing of captured PFAS.13,89–91 For example, it was the production of fluoropolymers and the associated use and release of PFOA that led to the widespread contamination of the US mid-Ohio river valley and its residents.92 In addition, potent greenhouse gases such as HFC-23 [trifluoromethane (CHF3)] can be formed during fluoropolymer production, and emissions to the atmosphere have been reported.93,94
(2) During use, low-molecular weight PFAS may be released, for example, PFCA in personal care products that contain PTFE.95
(3) During disposal, PFAA and other hazardous byproducts may be generated and released, such as when they are incinerated at an insufficiently high temperature for insufficient time.96–100 For example, when PTFE is heated above 350–400 °C, it decomposes and releases various gases that cause the so-called “Teflon fever” in workers.101
Other important considerations are that (1) some perfluoropolyethers (e.g., Krytox 157FS102) are mixtures of PFAA with molecular weights of only several thousand grams per mole and thus potentially biologically active; (2) in the EU and many other countries, substances registered as polymers can consist of fewer than 10 monomers, which are likely to be small and bioavailable molecules;103 (3) fluorine is 19 times heavier than hydrogen, and therefore high-molecular weight PFAS can be relatively small molecules, compared to hydrocarbon molecules of the same weight; and (4) fluoropolymer microplastics contribute to global plastic and microplastics debris,104,105 thus adding to ongoing environmental plastic and PFAS pollution.
Similar to fluoropolymers, other PFAS such as perfluoroalkanes and perfluoroalkylamines are generally inert,106 but they can be very potent greenhouse gases, up to 3 orders of magnitude more potent than CO2.107–113
In sum, scientific evidence supports the possibility that adverse effects of PFAS can occur in several bodily systems, with the developing immune system being particularly sensitive. Health effects have been demonstrated for several PFAS, including long- and short-chain PFAA, and chemicals associated with polymers. However, <1% of all PFAS have been tested for their hazardous effects. Proceeding with the approach of testing one chemical at a time will cause substantial delays in the effort to protect health and the environment from this large class of potentially hazardous chemicals.
ENVIRONMENTAL EXPOSURE: PERSISTENCE, ACCUMULATION, AND MOBILITY
An overarching property of all PFAS is that they have highly stable perfluorocarbon moieties in their molecular structure. Thus, all PFAS either are extremely persistent in the environment and biota or partially transform into extremely persistent PFAS.114–117 Studies have estimated that PFAS such as perfluoroalkanes have lifetimes in the thousands of years.113,118 Thus, PFAS will be present in the environment for centuries or longer, even if environmental releases cease immediately.
The high persistence of PFAS results in long-term accumulation in the environment and living organisms, which increases the risk of harm. A key concern in recent years is that some replacement PFAS, such as PFBA, PFBS, and GenX, have been widely detected in surface water and groundwater.43,65,119 PFAS can concentrate in plants, including food crops, when grown in contaminated soil or irrigated with contaminated water.20,120–122 Bioaccumulation occurs through the food chain, with top predators (e.g., whales, bald eagles, and humans) having the highest levels.123–127 Most concerning is that when PFAS accumulate, they can reach concentrations where hazardous effects are observed in humans and ecosystems, particularly when the effects of combined exposure to multiple PFAS are considered.128,129
The high mobility of many PFAS further exacerbates the concern.130 Many PFAS can travel long distances from their sources. PFAA, particularly short-chain types, are very water-soluble, being distributed readily in groundwater, surface waters, and the oceans.53,131,132 They can be difficult, costly, and sometimes even impossible to remove from water with conventional and even advanced treatment processes.13,43,133,134 Many other PFAS may be highly mobile in air, including the volatile perfluoroalkanes, fluorotelomer alcohols (FTOH), and many other (semi)volatile PFAA precursors.116
The extreme persistence of the fluorocarbon chain, combined with the propensity for accumulation and mobility of many PFAS, has resulted in PFAS being ubiquitous globally, even in remote regions like the Arctic.36,53,135,136 The continued use of PFAS will result in increasing concentrations of PFAS, increasing numbers of exposed organisms,137 and increasing probabilities of harm. Once adverse effects are identified, it will take decades, centuries, or even longer to reverse contamination and reduce the harm to our health and the environment.
MANAGING RISK
Risk management consists of various actions to minimize the chance of harm. Chemical risk management can be carried out by governments and businesses and includes the cessation or restriction of production and use of the chemicals, and efforts to clean up contamination.
Regulatory Approaches.
Many different regulatory frameworks are used for managing the risk of exposure to hazardous chemicals. While traditionally PFAS have been regulated one chemical at a time, subgroups of PFAS have also been regulated, with a focus on PFAA and their precursors.79,138–142 An advantage of targeting chemical subgroups is that the toxicological end points are often assumed to be similar, which allows for extrapolation from well-studied chemicals to those less studied. However, assessing only small subgroups systematically ignores the majority of PFAS and underestimates the overall risk, particularly when many of the chemicals are unknown. For example, the EU drinking water directive, which addresses a relatively large subgroup, covers only 20 PFAS.143
Governments are increasingly using broader management approaches to control PFAS exposure, such as targeting all PFAS within certain use categories. For example, the US states of Maine and Washington banned all PFAS in food contact materials144,145 and Denmark banned PFAS from paper and paperboard food packaging.146,147 South Australia and Washington state (and other US states) enacted bans on PFAS in firefighting foam.148,149 California has proposed to regulate any PFAS used in carpets and rugs.150 In the case of drinking water, a “PFAS - Total” limit was recently adopted by the European Commission.143 Regulatory agencies in Europe and the US are working to advance, validate, and standardize currently available methods to measure total PFAS in certain media.
A more comprehensive risk management approach that has been gaining traction is to limit the uses of hazardous chemicals to only those considered “essential”, while fostering development of safer alternatives. In 1987, the Montreal Protocol defined essentiality (in the case of ozone-depleting chlorofluorocarbons) as being necessary for health or safety, or critical for the functioning of society, and without technically and economically feasible alternatives or substitutes that are acceptable from the standpoint of environment and health.151 In the 2015 “Madrid Statement”, more than 200 scientists advocated using a similar approach for PFAS, i.e., limiting the production and use of the entire class of PFAS, including polymers, to essential uses.152 A more recent publication applied the essentiality concept to specific PFAS use categories and described examples of current PFAS-free alternatives, as well as uses where alternatives still need to be developed.153 In 2019, several European countries committed to phasing out all non-essential uses of PFAS by 2030.154 Limiting PFAS to essential uses would incentivize further development of alternatives that do not require fluorinated chemicals. Focusing on pollution prevention is critical because remediation of PFAS-impacted media, such as polluted groundwater aquifers, is costly, is energy-intensive, and cannot fully reverse the damage.
Managing PFAS as a class has additional benefits. It reduces the likelihood of replacing well-studied hazardous chemicals with poorly studied but structurally similar PFAS that have the potential to be similarly hazardous (i.e., “regrettable substitution”). It can be simpler and less expensive to implement: for example, for premarket regulation of uses for the entire class, for setting procurement standards, for testing for compliance and communicating test results through the supply chain, and for authorities monitoring the extent of PFAS contamination of humans, products, food, water, and the environment. Simpler, cheaper, class-based methods also typically result in more frequent testing, which improves compliance and detection of emerging risks. Methods to screen for fluorine already exist, for example, extractable organic fluorine methods coupled to combustion ion chromatography (EOF-CIC) and particle-induced γ-ray emission (PIGE).23,155,156 Hence, focusing on risk management tools that address PFAS as a class has the potential not only to prevent pollution by known PFAS but also to prevent regrettable substitution, to improve the efficiency and effectiveness of chemical management, and to encourage the selection of treatment approaches that effectively reduce total PFAS exposure when remediating PFAS-contaminated sites.
Marketplace Approaches.
Compared to governments, retailers and manufacturers can make more rapid changes to reduce their use of chemical classes of concern. For instance, home retailer IKEA committed to a complete phase-out of all PFAS in its textile products and reported achieving this goal as of 2016.157 Recently, H&M, Danish COOP, and ChemSec’s corporate initiative called to end the use of PFAS in products and the supply chain.158 Numerous factors are encouraging companies to stop using the entire class of PFAS. Increasing demand for products containing fewer harmful chemicals is one driver. For example, demand from retailers for food contact materials, from textile brands for sportswear, or from large purchasers and green builders for carpets has resulted in safer PFAS-free products on the market. Pressure from environmental groups is another driver. One prominent campaign contributed to the decision of many apparel companies to eliminate PFAS in their textile treatments.159 Companies’ values can also play a role, such as when member-owned retailer COOP Denmark announced a phase-out of all PFAS-containing cosmetics “on the basis of a precautionary principle”.160 Similarly, Kaiser Permanente, Levi Strauss & Co., and Crate and Barrel are phasing out all PFAS based on the companies’ environmental and health values.3
New international, national, state, and local regulations focusing on PFAS in certain products are additional influences,150 as are threats of future litigation and liability.161 While some companies may find it challenging to eliminate all PFAS from their products, others view it as important for mitigating business risks, or as a business opportunity. For example, treating PFAS as a class can help companies avoid multiple cycles of reformulations due to regrettable substitutions. Regulatory action addressing the class of PFAS will encourage further preventive actions from companies and help “level the playing field” by reducing the financial disadvantage of industry front-runners developing safer alternatives while incentivizing even further innovation toward safer alternatives.
OPTIONS MOVING FORWARD
Thousands of PFAS have already been documented across multiple industries and business sectors, and the list is growing.5,6 Managing PFAS one by one is neither feasible nor cost-efficient. More comprehensive solutions are needed, given that traditional approaches have failed to control widespread exposures to PFAS and resulted in inadequate public health protection. For Europe alone, the annual health costs linked to exposure to just a few PFAS are estimated at 52–84 billion Euros, and environmental remediation costs at roughly 17 billion Euros.162 Here we suggest class-based options to more comprehensively and efficiently reduce PFAS exposure.
Government policy makers have already begun limiting PFAS through bans in certain product categories. However, to more effectively manage PFAS, governments can apply the essential uses framework. Examples of essential and non-essential uses of PFAS have already begun to be described.153 To make the criteria fully operational for inclusion in legislation, a more precise set of decision criteria is needed to guide the categorization. Such decisions involve both scientific and ethical considerations and thus require input from a broad set of scientists, civil society, industry, and policymakers.
Limiting the entire class of PFAS, including fluorinated polymers, to essential uses is critical, given that currently, remediating PFAS, once released to the environment, is at best extremely costly and, in some cases, impossible.114,163 Governments can take a class-based approach to cleanup efforts, for example, by prioritizing research and development funding for treatment and disposal/destruction methods that are effective for the entire class of PFAS. Such an approach would ensure that treatment strategies remove all PFAS from all impacted environmental media (water, air, and soil) and that treatment residuals (for example, spent activated carbon and reverse osmosis concentrate) are managed such that the entire PFAS class is destroyed and its degradation products (or minerals) captured, so that unknown fluorinated reaction intermediates and harmful levels of organofluorines and hydrogen fluoride are not reintroduced into the environment. A class approach can also be used in developing cleanup standards, so that responsible industries are held accountable for remediation of all PFAS, not just a few. Additionally, governments can hold responsible parties accountable for exposure and health monitoring in heavily exposed populations, in order to promote effective and lasting solutions.
Regulatory agencies can also adopt class-based strategies to reduce exposure and minimize health risk. For example, they may extrapolate risk from well-understood PFAS when limiting uses of PFAS in commerce or setting protective cleanup levels. They can also assess combined exposures to PFAS (e.g., in drinking water, food, air, consumer products, and waste) as a basis to set regulatory limits and treatment standards. Establishing limits to the class rather than doing so on a chemical-by-chemical basis would result in lower exposure values that better protect vulnerable populations such as pregnant women, children, and workers. In addition, systems that can track historic, current, and future uses of all PFAS, and releases to the environment, could help to guide and prioritize monitoring, for instance, for emerging risk detection and compliance/enforcement testing. The further development, use, and interlaboratory standardization of analytical methods to measure total PFAS would complement this effort, improving the accuracy, speed, and cost of screening for PFAS in the environment, consumer products, and people. Collaboration within and across national and international policy and regulatory bodies to foster class-based strategies would be beneficial. Such concerted efforts could help prevent shifting burdens from one geographical location to another and may evolve into “de facto” industry standards as international actors attempt to minimize costs of complying with multiple different regulations.
Solutions are also available in the marketplace. Chemical manufacturers can move quickly to develop safer non-fluorinated alternatives for PFAS with current essential uses. They can also work with product manufacturers and businesses to rapidly replace all PFAS uses that have technically and economically feasible alternatives that are acceptable from the standpoint of environment and health. Chemical and product manufacturers can be transparent about the use of any PFAS chemistries in the supply chain and monitor and strictly control releases of all PFAS into the environment until their use can be phased out. In addition, PFAS manufacturers can assist in developing better methods to detect, remove, and destroy PFAS, although regulatory incentives or pressures may be needed.
The more we study PFAS, the more we learn about the harm they can do to our health and the environment. However, it is not possible to thoroughly assess every individual PFAS, or combination of PFAS, for their full range of effects in a reasonable time frame. Without effective risk management action around the entire class of PFAS, these chemicals will continue to accumulate and cause harm to human health and ecosystems for generations to come. As demonstrated above, managing PFAS as a class is scientifically sound, will provide business innovation opportunities, and will help protect our health and environment now and in the future.
Box Key Messages.
Per- and polyfluoroalkyl substances (PFAS) make up a class of extremely persistent chemicals, numbering in the thousands, that accumulate in the environment and living organisms and can be highly mobile, leading to global contamination.
The use of PFAS in numerous consumer and industrial applications has led to widespread human and environmental exposure from, for example, drinking water, food, and consumer products.
Toxicological and epidemiological studies have identified a broad range of adverse health outcomes associated with exposure to PFAS in people and animals.
We suggest a class-based approach to managing the human and environmental risks associated with all PFAS, including polymers.
We provide options for how governments and industry can apply the class-based approach, emphasizing the importance of eliminating non-essential uses of PFAS, and further developing safer alternatives and methods to remove all existing PFAS from the environment.
ACKNOWLEDGMENTS
The authors are grateful for the help of Gina Solomon in early discussions of the manuscript topic, Elsie Sunderland and Holly Davies in reviewing the manuscript prior to submission, and Chris Ribbens who contributed administrative support.
Funding
Funding was supplied by charitable contributions to The Endocrine Disruption Exchange (C.F.K. and K.E.P.), the Green Science Policy Institute (T.A.B., A.S., and A.B.), and the Natural Resources Defense Council (A.R.). C.W. was supported by the National Institute for Environmental Health Sciences Superfund Research Program (P42ES027706). D.R.U.K. and J.C.D. were supported by the National Institute for Environmental Health Sciences Superfund Research Program (1 P42 ES031009-01).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.estlett.0c00255
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the European Environment Agency.
The authors declare the following competing financial interest(s): It may be relevant that J.C.D. currently is engaged as a plaintiffs expert witness in several cases involving PFAS.
Contributor Information
Carol F. Kwiatkowski, Department of Biological Sciences, North Carolina State University, Raleigh, North Carolina 27695, United States.
David Q. Andrews, Environmental Working Group, Washington, D.C. 20009, United States
Linda S. Birnbaum, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709, United States.
Thomas A. Bruton, Green Science Policy Institute, Berkeley, California 94709, United States
Jamie C. DeWitt, Department of Pharmacology and Toxicology, Brody School of Medicine, East Carolina University, Greenville, North Carolina 27834, United States
Detlef R. U. Knappe, Department of Civil, Construction, and Environmental Engineering, North Carolina State University, Raleigh, North Carolina 27695, United States.
Maricel V. Maffini, Private Residence, Frederick, Maryland 21701, United States.
Mark F. Miller, National Institute of Environmental Health Sciences and U.S. Public Health Service, Research Triangle Park, North Carolina 27709, United States
Katherine E. Pelch, School of Public Health, University of North Texas Health Science Center, Fort Worth, Texas 76126, United States
Anna Reade, Natural Resources Defense Council, San Francisco, California 94104, United States.
Anna Soehl, Green Science Policy Institute, Berkeley, California 94709, United States.
Xenia Trier, European Environment Agency, DK-1050 Copenhagen, Denmark.
Marta Venier, O’Neill School of Public and Environmental Affairs, Indiana University, Bloomington, Indiana 47401, United States.
Charlotte C. Wagner, Harvard John A. Paulson School of Engineering and Applied Science, Harvard University, Cambridge, Massachusetts 02138, United States
Zhanyun Wang, Chair of Ecological Systems Design, Institute of Environmental Engineering, ETH Zürich, 8093 Zurich, Switzerland.
Arlene Blum, Green Science Policy Institute, Berkeley, California 94709, United States; Department of Chemistry, University of California, Berkeley, California 94720, United States.
REFERENCES
- (1).National Academies of Sciences, Engineering, and Medicine. A Class Approach to Hazard Assessment of Organohalogen Flame Retardants; National Academies Press: Washington DC, 2019. [PubMed] [Google Scholar]
- (2).U.S. Consumer Product Safety Commission. Minutes of commission meeting. Decisional Matter: Petition HP 15—1 Request Rulemaking on Certain Products Containing Organohalgen Flame Retardants. Bethesda, MD, 2017. [Google Scholar]
- (3).Blum A Tackling toxics. Science 2016, 351 (6278), 1117. [DOI] [PubMed] [Google Scholar]
- (4).Cordner A; Richter L; Brown P Can Chemical Class Approaches Replace Chemical-by-Chemical Strategies? Lessons from Recent U.S. FDA Regulatory Action on Per- And Polyfluoroalkyl Substances. Environ. Sci. Technol 2016, 50 (23), 12584–12591. [DOI] [PubMed] [Google Scholar]
- (5).Organisation for Economic Cooperation and Development. Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs): summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs). Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides, and Biotechnology Series on Risk Management, 39th Paris, 2018. [Google Scholar]
- (6).U.S. Environmental Protection Agency. PFAS Master List of PFAS Substances. https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster (accessed 2020-04-26).
- (7).U.S. Environmental Protection Agency Cumulative Assessment of Risk from Pesticides. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/cumulative-assessment-risk-pesticides (accessed 2019-08-14).
- (8).Kissa E Fluorinated surfactants and repellents, 2nd ed.; Marcel Dekker: New York, 2001. [Google Scholar]
- (9).Allen JG These toxic chemicals are everywhere—even in your body. And they wonť ever go away. The Washington Post, 2018. [Google Scholar]
- (10).Ghisi R; Vamerali T; Manzetti S Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res 2019, 169, 326–341. [DOI] [PubMed] [Google Scholar]
- (11).Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA; Higgins CP; Sunderland EM Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett 2016, 3 (10), 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lindstrom AB; Strynar MJ; Delinsky AD; Nakayama SF; McMillan L; Libelo EL; Neill M; Thomas L Application of WWTP biosolids and resulting perfluorinated compound contamination of surface and well water in Decatur, Alabama, USA. Environ. Sci. Technol 2011, 45 (19), 8015–21. [DOI] [PubMed] [Google Scholar]
- (13).Hopkins ZR; Sun M; DeWitt JC; Knappe DRU Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. - Am. Water Works Assoc 2018, 110 (7), 13–28. [Google Scholar]
- (14).U.S. Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables, March 2018. Vol. 1. [Google Scholar]
- (15).Bjerregaard-Olesen C; Bossi R; Liew Z; Long M; Bech BH; Olsen J; Henriksen TB; Berg V; Nost TH; Zhang JJ; Odland JO; Bonefeld-Jorgensen EC Maternal serum concentrations of perfluoroalkyl acids in five international birth cohorts. Int. J. Hyg. Environ. Health 2017, 220, 86–93. [DOI] [PubMed] [Google Scholar]
- (16).Sunderland EM; Hu XC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Exposure Sci. Environ. Epidemiol 2019, 29 (2), 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Land M; de Wit CA; Bignert A; Cousins IT; Herzke D; Johansson JH; Martin JW What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environmental Evidence 2018, 7 (1), n/a. [Google Scholar]
- (18).Boone JS; Vigo C; Boone T; Byrne C; Ferrario J; Benson R; Donohue J; Simmons JE; Kolpin DW; Furlong ET; Glassmeyer ST Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ 2019, 653, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).U.S. Food and Drug Administration. Per and Polyfluoroalkyl Substances (PFAS). https://www.fda.gov/food/chemicals/and-polyfluoroalkyl-substances-pfas (accessed 2019-08-01).
- (20).Universiteit Van Amsterdam. PERFOOD Report Summary; European Union: Amsterdam, 2013. [Google Scholar]
- (21).European Food Safety Authority. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. In EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen HK, Alexander J, Barregard L, Bignami M, Bruschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl-Kraupp B, Hogstrand C, Hoogenboom, Stefano C., Isabelle N, Oswald P, Petersen A., Rose M, Roudot A-C, Vleminckx C, Vollmer G, Wallace H, Bodin L, Cravedi J-P, Halldorsson TI, Haug LS., Johansson N., van Loveren H, Gergelova P, Mackay P, Levorato S, van Manen M, Schwerdtle T, Eds.; John Wiley and Sons Ltd., on behalf of European Food Safety Authority, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Trier X; Taxvig C; Rosenmai AK; Pedersen GA PFAS in paper and board for food contact: Options for risk management of poly- and perfluorinated substances; Nordisk Ministerrad: Copenhagen, 2017. [Google Scholar]
- (23).Schaider LA; Balan SA; Blum A; Andrews DQ; Strynar MJ; Dickinson ME; Lunderberg DM; Lang JR; Peaslee GF Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett 2017, 4 (3), 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Liu W; Chen S; Harada KH; Koizumi A Analysis of perfluoroalkyl carboxylates in vacuum cleaner dust samples in Japan. Chemosphere 2011, 85 (11), 1734–41. [DOI] [PubMed] [Google Scholar]
- (25).Karaskova P; Venier M; Melymuk L; Becanova J; Vojta S; Prokes R; Diamond ML; Klanova J Perfluorinated alkyl substances (PFASs) in household dust in Central Europe and North America. Environ. Int 2016, 94, 315–324. [DOI] [PubMed] [Google Scholar]
- (26).Swedish Chemicals Agency (KEMI). Occurrence and use of highly fluorinated substances and alternatives: Report from a government assignment. Stockholm, 2005. [Google Scholar]
- (27).Norsk Institutt for Luftforskning. PFASs in house dust. Kjeller, Norway, 2015. [Google Scholar]
- (28).Mitro SD; Dodson RE; Singla V; Adamkiewicz G; Elmi AF; Tilly MK; Zota A R Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ. Sci. Technol 2016, 50 (19), 10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wang Z; DeWitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol 2017, 51 (5), 2508–2518. [DOI] [PubMed] [Google Scholar]
- (30).U.S. Environmental Protection Agency. PFAS methods and guidance for sampling and analyzing water and other environmental media (Technical Brief). https://www.epa.gov/water-research/pfas-methods-and-guidance-sampling-and-analyzing-water-and-other-environmental-media (accessed 2019-08-14).
- (31).Houtz EF; Sedlak DL Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ. Sci. Technol 2012, 46 (17), 9342–9349. [DOI] [PubMed] [Google Scholar]
- (32).Koch A; Aro R; Wang T; Yeung LWY Towards a comprehensive analytical workflow for the chemical characterisation of organofluorine in consumer products and environmental samples. TrAC, TrendsAnal. Chem 2020, 123, 115423. [Google Scholar]
- (33).Zhang CH; Hopkins ZR; McCord J; Strynar MJ; Knappe DRU Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett 2019, 6 (11), 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Robel AE; Marshall K; Dickinson M; Lunderberg D; Butt C; Peaslee G; Stapleton HM; Field JA Closing the Mass Balance on Fluorine on Papers and Textiles. Environ. Sci. Technol 2017, 51 (16), 9022–9032. [DOI] [PubMed] [Google Scholar]
- (35).Schultes L; Vestergren R; Volkova K; Westberg E; Jacobson T; Benskin JP Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure. Environmental science. Processes & impacts 2018, 20 (12), 1680–1690. [DOI] [PubMed] [Google Scholar]
- (36).De Silva AO; Spencer C; Ho KC; Al Tarhuni M; Go C; Houde M; de Solla SR; Lavoie RA; King LE; Muir DC.; Fair PA; Wells RS.; Bossart GD Perfluoroalkylphosphinic Acids in Northern Pike (Esox lucius), Double-Crested Cormorants (Phalacrocorax auritus), and Bottlenose Dolphins (Tursiops truncatus) in Relation to Other Perfluoroalkyl Acids. Environ. Sci. Technol 2016, 50 (20), 10903–10913. [DOI] [PubMed] [Google Scholar]
- (37).Yeung LWY; Mabury SA Are humans exposed to increasing amounts of unidentified organofluorine? Environ. Chem 2016, 13 (1), 102–110. [Google Scholar]
- (38).Hu XC; Tokranov AK; Liddie J; Zhang X; Grandjean P; Hart JE; Laden F; Sun Q; Yeung LWY; Sunderland EM Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environ. Health Perspect 2019, 127 (6), 067006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Brendel S; Fetter E; Staude C; Vierke L; Biegel-Engler A Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur 2018, 30 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).European Chemicals Agency. Annex XV Report: Proposal for Identification of a Substance of Very High Concern on the Basis of the Criteria Set Out In REACH Article 57. In Identification of HFPO-DA And Its Salts/Acyl Halides as SVHC; 2019. [Google Scholar]
- (41).Wang Z; Cousins IT; Scheringer M; Hungerbuhler K Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int 2013, 60, 242–8. [DOI] [PubMed] [Google Scholar]
- (42).Cousins IT; Vestergren R; Wang Z; Scheringer M; McLachlan MS The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int 2016, 94, 331–340. [DOI] [PubMed] [Google Scholar]
- (43).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett 2016, 3 (12), 415–419. [Google Scholar]
- (44).Russell MH; Nilsson H; Buck RC Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 2013, 93 (10), 2419–25. [DOI] [PubMed] [Google Scholar]
- (45).Kabadi SV; Fisher J; Aungst J; Rice P Internal exposure-based pharmacokinetic evaluation of potential for biopersistence of 6:2 fluorotelomer alcohol (FTOH) and its metabolites. Food Chem. Toxicol 2018, 112, 375–382. [DOI] [PubMed] [Google Scholar]
- (46).Perez F; Nadal M; Navarro-Ortega A; Fabrega F; Domingo JL; Barcelo D; Farre M Accumulation of perfluoroalkyl substances in human tissues. Environ. Int 2013, 59, 354–62. [DOI] [PubMed] [Google Scholar]
- (47).Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Guo Y; Sun Y; Dai J First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol 2017, 51 (17), 9553–9560. [DOI] [PubMed] [Google Scholar]
- (48).Shi Y; Vestergren R; Zhou Z; Song X; Xu L; Liang Y; Cai Y Tissue Distribution and Whole Body Burden of the Chlorinated Polyfluoroalkyl Ether Sulfonic Acid F-53B in Crucian Carp (Carassius carassius): Evidence for a Highly Bioaccumulative Contaminant of Emerging Concern. Environ. Sci. Technol 2015, 49 (24), 14156–65. [DOI] [PubMed] [Google Scholar]
- (49).Lasee S; Subbiah S; Thompson WA; Karnjanapiboonwong A; Jordan J; Payton P; Anderson TA Plant Uptake of Per- and Polyfluoroalkyl Acids under a Maximum Bioavailability Scenario. Environ. Toxicol. Chem 2019, 38 (11), 2497–2502. [DOI] [PubMed] [Google Scholar]
- (50).Blaine AC; Rich CD; Sedlacko EM; Hyland KC; Stushnoff C; Dickenson ER; Higgins CP Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Environ. Sci. Technol 2014, 48 (24), 14361–8. [DOI] [PubMed] [Google Scholar]
- (51).Blaine AC; Rich CD; Sedlacko EM; Hundal LS; Kumar K; Lau C; Mills MA; Harris KM; Higgins CP Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended soils. Environ. Sci. Technol 2014, 48 (14), 7858–7865. [DOI] [PubMed] [Google Scholar]
- (52).Houtz EF; Sutton R; Park JS; Sedlak M Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res 2016, 95, 142–9. [DOI] [PubMed] [Google Scholar]
- (53).Gomis MI; Wang Z; Scheringer M; Cousins IT A modeling assessment of the physicochemical properties and environmental fate of emerging and novel per- and polyfluoroalkyl substances. Sci. Total Environ 2015, 505, 981–91. [DOI] [PubMed] [Google Scholar]
- (54).Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls (Draft for Public Comment). 2018. [PubMed]
- (55).U.S. National Toxicology Program. Toxicity Report 96: NTP Technical Report on The Toxicity Studies of Perfluoroalkyl Sulfonates (Perfluorobutane Sulfonic Acid, Perfluorohexane Sulfonate Potassium Salt, and Perfluorooctane Sulfonic Acid) Administered by Gavage to Sprague Dawley (Hsd:Sprague Dawley SD) Rats. Research Triangle Park, NC, 2019. [Google Scholar]
- (56).U.S. National Toxicology Program. Toxiciy Report 97: NTP Technical Report on The Toxicity Studies of Perfluoroalkyl Carboxylates (Perfluorohexanoic Acid, Perfluorooctanoic Acid, Perfluorononanoic Acid, and Perfluorodecanoic Acid) Administered by Gavage to Sprague Dawley (Hsd:Sprague Dawley SD) Rat. Research Triangle Park, NC, 2019. [Google Scholar]
- (57).U.S. Environmnetal Protection Agency. GenX and PFBS Draft Toxicity Assessments. https://www.epa.gov/pfas/genx-and-pfbs-draft-toxicity-assessments (accessed 2018-11-19).
- (58).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manage 2011, 7 (4), 513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Henry BJ; Carlin JP; Hammerschmidt JA; Buck RC; Buxton LW; Fiedler H; Seed J; Hernandez O A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers. Integr. Environ. Assess. Manage 2018, 14 (3), 316–334. [DOI] [PubMed] [Google Scholar]
- (60).Prevedouros K; Cousins IT; Buck RC; Korzeniowski SH Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol 2006, 40 (1), 32–44. [DOI] [PubMed] [Google Scholar]
- (61).Hoffman K; Webster TF; Bartell SM; Weisskopf MG; Fletcher T; Vieira VM Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ. Health Perspect 2011, 119 (1), 92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol 2015, 49 (19), 11622–30. [DOI] [PubMed] [Google Scholar]
- (63).McCord J; Strynar M Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol 2019, 53 (9), 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Newton S; McMahen R; Stoeckel JA; Chislock M; Lindstrom A; Strynar M Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environ. Sci. Technol 2017, 51 (3), 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Gebbink WA; van Asseldonk L; van Leeuwen SPJ Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol 2017, 51 (19), 11057–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Kudo N Metabolism and Pharmacokinetics. In Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances; DeWitt JC, Ed.; Humana Press: Cham, Switzerland, 2015; pp 151–175. [Google Scholar]
- (67).Greaves AK; Letcher RJ; Sonne C; Dietz R Brain region distribution and patterns of bioaccumulative perfluoroalkyl carboxylates and sulfonates in east greenland polar bears (Ursus maritimus). Environ. Toxicol. Chem 2013, 32 (3), 713–22. [DOI] [PubMed] [Google Scholar]
- (68).Verreault J; Houde M; Gabrielsen GW; Berger U; Haukas M; Letcher RJ; Muir DC Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian arctic. Environ. Sci. Technol 2005, 39 (19), 7439–45. [DOI] [PubMed] [Google Scholar]
- (69).Van de Vijver KI; Holsbeek L; Das K; Blust R; Joiris C; De Coen W Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea. Environ. Sci. Technol 2007, 41 (1), 315–20. [DOI] [PubMed] [Google Scholar]
- (70).Shi Y; Wang J; Pan Y; Cai Y Tissue distribution of perfluorinated compounds in farmed freshwater fish and human exposure by consumption. Environ. Toxicol. Chem 2012, 31 (4), 71723. [DOI] [PubMed] [Google Scholar]
- (71).C8 Science Panel. Probable Link Reports. http://www.c8sciencepanel.org/prob_link.html (accessed 2019-06-28).
- (72).U.S. National Toxicology Program Office of Health Assessment and Translation. Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS); Research Triangle Park, NC, 2016. [Google Scholar]
- (73).Birnbaum L Testimony before the Senate Committee on Environment and Public Works Hearing on “Examining the Federal response to the risks associated with per- and polyfluoroalkyl substances (PFAS)”. 2019. [Google Scholar]
- (74).Grandjean P; Andersen EW; Budtz-Jorgensen E; Nielsen F; Molbak K; Weihe P; Heilmann C Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA, J. Am. Med. Assoc 2012, 307 (4), 391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Grandjean P; Heilmann C; Weihe P; Nielsen F; Mogensen UB; Timmermann A; Budtz-Jorgensen E Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol 2017, 14 (1), 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).U.S. Environmental Protection Agency Office of Water. Drinking Water Health Advisory for perfluorooctanoic acid (PFOA). Washington, DC, 2016. [Google Scholar]
- (77).U.S. Environmental Protection Agency Office of Water. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). Washington, DC, 2016. [Google Scholar]
- (78).New Hampshire Department of Environmental Services. Technical Background Report for the June 2019 Proposed Maximum Contaminant Levels (MCLs) and Ambient Groundwater Quality Standards (AGQSs) for Perfluorooctane sulfonic Acid (PFOS), Perfluorooctanoic Acid (PFOA), Perfluorononanoic Acid (PFNA), and Perfluorohexane sulfonic Acid (PFHxS) And Letter from Dr. Stephen M. Roberts, Ph.D. dated 6/25/2019. Findings of Peer Review Conducted on Technical Background Report. Concord, NH, 2019. [Google Scholar]
- (79).U.S. Food and Drug Administration. Indirect Food Additives: Paper and Paperboard Components. Federal Register 2016, 81 (1), 5. [Google Scholar]
- (80).Michigan Science Advisory Workgroup. Health-based drinking water value recommendations for PFAS in Michigan. 2019. [Google Scholar]
- (81).Caverly Rae JM; Craig L; Slone TW; Frame SR; Buxton LW; Kennedy GL Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague-Dawley rats. Toxicol Rep 2015, 2, 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Conley JM; Lambright CS; Evans N; Strynar MJ; McCord J; McIntyre BS; Travlos GS; Cardon MC; Medlock-Kakaley E; Hartig PC; Wilson VS; Gray LE Jr. Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats. Environ. Health Perspect 2019, 127 (3), 037008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Rushing BR; Hu Q; Franklin JN; McMahen R; Dagnino S; Higgins CP.; Strynar MJ; DeWitt JC. Evaluation of the immunomodulatory effects of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in C57BL/6 mice. Toxicol. Sci 2017, kfW251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Butenhoff JL; Bjork JA; Chang SC; Ehresman DJ; Parker GA; Das K; Lau C; Lieder PH; van Otterdijk FM; Wallace KB Toxicological evaluation of ammonium perfluorobutyrate in rats: twenty-eight-day and ninety-day oral gavage studies. Reprod. Toxicol 2012, 33 (4), 513–530. [DOI] [PubMed] [Google Scholar]
- (85).Lieder PH; York RG; Hakes DC; Chang SC; Butenhoff JL A two-generation oral gavage reproduction study with potassium perfluorobutanesulfonate (K+PFBS) in Sprague Dawley rats. Toxicology 2009, 259 (1–2), 33–45. [DOI] [PubMed] [Google Scholar]
- (86).Klaunig JE; Shinohara M; Iwai H; Chengelis CP; Kirkpatrick JB; Wang Z; Bruner RH Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague-Dawley rats. Toxicol. Pathol 2015, 43 (2), 209–20. [DOI] [PubMed] [Google Scholar]
- (87).Loveless SE; Slezak B; Serex T; Lewis J; Mukerji P; O’Connor JC; Donner EM; Frame SR; Korzeniowski SH; Buck RC Toxicological evaluation of sodium perfluorohexanoate. Toxicology 2009, 264 (1–2), 32–44. [DOI] [PubMed] [Google Scholar]
- (88).EFSA. PFAS public consultation: draft opinion explained. https://www.efsa.europa.eu/en/news/pfas-public-consultation-draft-opinion-explained (accessed 2020-04-26).
- (89).Davis KL; Aucoin MD; Larsen BS; Kaiser MA; Hartten AS Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 2007, 67 (10), 2011–9. [DOI] [PubMed] [Google Scholar]
- (90).Neltner T Paper mills as a significant source of PFAS contamination, but who’s watching? Environmental Defense Fund, 2018; http://blogs.edf.org/health/2018/05/21/pfas-paper-mills/ (accessed 2020-02-20). [Google Scholar]
- (91).Young CJ; Hurley MD; Wallington TJ; Mabury SA Atmospheric lifetime and global warming potential of a perfluoropolyether. Environ. Sci. Technol 2006, 40 (7), 2242–6. [DOI] [PubMed] [Google Scholar]
- (92).Steenland K; Jin C; MacNeil J; Lally C; Ducatman A; Vieira V; Fletcher T Predictors of PFOA levels in a community surrounding a chemical plant. Environ. Health Perspect 2009, 117 (7), 1083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, and New York, 2013. [Google Scholar]
- (94).Rand S; Yamabe M; Campbell N; Hu J; Lapin P; McCulloch A; Merchant A; Mizuno K; Owens J; Rollet P Nonmedical aerosols, solvents, and HFC 23. In IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System; Fujimoto Y., Pons J, Eds.; Cambridge, U.K., 2005. [Google Scholar]
- (95).Ministry of Environment and Food of Denmark. Risk assessment of fluorinated substances in cosmetic products; The Danish Environmental Protection Agency, 2018. [Google Scholar]
- (96).Ellis DA; Mabury SA; Martin JW; Muir DCG Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001, 412 (6844), 321–324. [DOI] [PubMed] [Google Scholar]
- (97).Ellis DA; Martin JW; Muir DC; Mabury SA The use of 19F NMR and mass spectrometry for the elucidation of novel fluorinated acids and atmospheric fluoroacid precursors evolved in the thermolysis of fluoropolymers. Analyst 2003, 128 (6), 756–64. [DOI] [PubMed] [Google Scholar]
- (98).Feng M; Qu R; Wei Z; Wang L; Sun P; Wang Z Characterization of the thermolysis products of Nafion membrane: A potential source of perfluorinated compounds in the environment. Sci. Rep 2015, 5, 9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Schlummer M; Solch C; Meisel T; Still M; Gruber L; Wolz G Emission of perfluoroalkyl carboxylic acids (PFCA) from heated surfaces made of polytetrafluoroethylene (PTFE) applied in food contact materials and consumer products. Chemosphere 2015, 129, 46–53. [DOI] [PubMed] [Google Scholar]
- (100).Danish Environmental Protection Agency. Belysning af destruktion af visse POP-stoffer pa konventionelle affaldsforbrandingsantag til forbranding af hovedsageligt ikkefarligt og forbraendingsegnet affald. In Lighting of destruction of certain POP drugs on conventional waste incinerator for incineration of mainly non-hazardous and incinerable waste; Danish Environmental Protection Agency, 2019; Environmental Project 2085. [Google Scholar]
- (101).Shusterman DJ Polymer fume fever and other fluorocarbon pyrolysis-related syndromes. Occup. Med 1993, 8 (3), 519–31. [PubMed] [Google Scholar]
- (102).Dupont, DuPont Krytox® 157 FS Fluorinated Oil. 2012. https://samaro.fr/pdf/FT/KRYTOX_FT_157-FS-series_EN_.pdf (accessed 2019-08-13).
- (103).European Chemicals Agency. Guidance for monomers and polymers. Report ECHA-12-G-02-EN; Helsinki, 2012; Vol. 2.0. [Google Scholar]
- (104).Zhao SY; Danley M; Ward JE; Li DJ; Mincer TJ An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Anal. Methods 2017, 9 (9), 1470–1478. [Google Scholar]
- (105).National Toxics Network. Contaminants in marine plastic pollution: ‘the new toxic time-bomb’. 2016. https://ntn.org.au/wp-content/uploads/2016/05/NTN-Contaminants-in-Marine-Plastics-Report-April-2016-1-1.pdf (accessed 2019-10-04).
- (106).Kitazume T Green chemistry development in fluorine science. J. Fluorine Chem 2000, 105 (2), 265–278. [Google Scholar]
- (107).Hong AC; Young CJ; Hurley MD; Wallington TJ; Mabury SA Perfluorotributylamine: A novel long-lived greenhouse gas. Geophys. Res. Lett 2013, 40 (22), 6010–6015. [Google Scholar]
- (108).Bernard F; Papanastasiou DK; Papadimitriou VC; Burkholder JB Infrared absorption spectra of N(CxF2x+1)(3)(,) x = 2–5 perfluoroamines. J. Quant. Spectrosc. Radiat. Transfer 2018, 211, 166–171. [Google Scholar]
- (109).Bera PP; Francisco JS; Lee TJ Identifying the molecular origin of global warming. J. Phys. Chem. A 2009, 113 (45), 12694–9. [DOI] [PubMed] [Google Scholar]
- (110).Bravo I; Aranda A; Hurley MD; Marston G; Nutt DR; Shine KP; Smith K; Wallington TJ Infrared absorption spectra, radiative efficiencies, and global warming potentials of perfluorocarbons: Comparison between experiment and theory. J. Geophys. Res 2010, 115.n/a [Google Scholar]
- (111).Kokkila SI; Bera PP; Francisco JS; Lee TJ A group increment scheme for infrared absorption intensities of greenhouse gases. J. Mol. Struct 2012, 1009, 89–95. [Google Scholar]
- (112).Greenhouse Gas Protocol. Global Warming Potential Values. https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf (accessed 2019-08-13).
- (113).Ivy DJ; Rigby M; Baasandorj M; Burkholder JB; Prinn RG. Global emission estimates and radiative impact of C4F10, C5F12, C6F14, C7F16 and C8F18. Atmos. Chem. Phys 2012, 12 (16), 7635–7645. [Google Scholar]
- (114).Ross I; McDonough J; Miles J; Storch P; Thelakkat Kochunarayanan P; Kalve E; Hurst J; S. Dasgupta S; Burdick J A review of emerging technologies for remediation of PFASs. Remediation 2018, 28 (2), 101–126. [Google Scholar]
- (115).U.S. National Toxicology Program. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm (accessed 2019-01-06).
- (116).Young CJ; Mabury SA Atmospheric perfluorinated acid precursors: chemistry, occurrence, and impacts. In Reviews of Environmental Contamination and Toxicology; Springer: New York, 2010; Vol. 208. [DOI] [PubMed] [Google Scholar]
- (117).Dreyer A; Matthias V; Weinberg I; Ebinghaus R Wet deposition of poly- and perfluorinated compounds in Northern Germany. Environ. Pollut. (Oxford, U. K.) 2010, 158 (5), 1221–7. [DOI] [PubMed] [Google Scholar]
- (118).Velders GJM; Madronich S; Clerbaux C; Derwent R; Grutter M; Hauglustaine D; Incecik S; Ko M; Libre J-M; Nielsen OJ; Stordal F; Zhu T; Blake D; Cunnold D; Daniel J; Forster P; Fraser P; Krummel P; Manning A; Montzka S; Myhre G; O’Doherty S; Oram D; Prather M; Prinn R; Reimann S; Simmonds P; Wallington T; Weiss R Chemical and Radiative Effects of Halocarbons and Their Replacement Compounds. In IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System; Saksen ISA, Jallow BP, Eds.; Cambridge, U.K., 2005; p 48. [Google Scholar]
- (119).Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Sun Y; Guo Y; Dai J Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ. Sci. Technol 2018, 52 (14), 7621–7629. [DOI] [PubMed] [Google Scholar]
- (120).Felizeter S; McLachlan MS; de Voogt P Uptake of Perfluorinated Alkyl Acids by Hydroponically Grown Lettuce (Lactuca sativa). Environ. Sci. Technol 2012, 46 (21), 11735–11743. [DOI] [PubMed] [Google Scholar]
- (121).Lan Z; Zhou M; Yao Y; Sun H Plant uptake and translocation of perfluoroalkyl acids in a wheat-soil system. Environ. Sci. Pollut. Res 2018, 25 (31), 30907–30916. [DOI] [PubMed] [Google Scholar]
- (122).Universiteit Van Amsterdam. https://ibed.fnwi.uva.nl/perfood/publications.html (accessed 2019-08-28).
- (123).Kelly BC; Ikonomou MG; Blair JD; Surridge B; Hoover D; Grace R; Gobas FA Perfluoroalkyl contaminants in an Arctic marine food web: trophic magnification and wildlife exposure. Environ. Sci. Technol 2009, 43 (11), 4037–43. [DOI] [PubMed] [Google Scholar]
- (124).Tomy GT; Budakowski W; Halldorson T; Helm PA; Stern GA; Friesen K; Pepper K; Tittlemier SA; Fisk AT Fluorinated organic compounds in an eastern Arctic marine food web. Environ. Sci. Technol 2004, 38 (24), 6475–81. [DOI] [PubMed] [Google Scholar]
- (125).Haukas M; Berger U; Hop H; Gulliksen B; Gabrielsen GW Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. (Oxford, U. K.) 2007, 148 (1), 360–71. [DOI] [PubMed] [Google Scholar]
- (126).Wu Y; Simon KL; Best DA; Bowerman W; Venier M Novel and legacy per- and polyfluoroalkyl substances in bald eagle eggs from the Great Lakes region. Environ. Pollut. (Oxford, U. K.) 2020, 260, 113811. [DOI] [PubMed] [Google Scholar]
- (127).Dassuncao C; Hu XC; Nielsen F; Weihe P; Grandjean P; Sunderland EM Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environ. Sci. Technol 2018, 52 (6), 3738–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).MacLeod M; Breitholtz M; Cousins IT; de Wit CA; Persson LM; Ruden C; McLachlan MS Identifying chemicals that are planetary boundary threats. Environ. Sci. Technol 2014, 48 (19), 11057–63. [DOI] [PubMed] [Google Scholar]
- (129).Cousins IT; Ng CA; Wang Z; Scheringer M Why is high persistence alone a major cause of concern? Environmental science. Processes & impacts 2019, 21 (5), 781–792. [DOI] [PubMed] [Google Scholar]
- (130).Diamond ML; de Wit CA; Molander S; Scheringer M; Backhaus T; Lohmann R; Arvidsson R; Bergman A; Hauschild M; Holoubek I; Persson L; Suzuki N; Vighi M; Zetzsch C Exploring the planetary boundary for chemical pollution. Environ. Int 2015, 78, 8–15. [DOI] [PubMed] [Google Scholar]
- (131).Ahrens L Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. J. Environ. Monit 2011, 13 (1), 20–31. [DOI] [PubMed] [Google Scholar]
- (132).Yamashita N; Kannan K; Taniyasu S; Horii Y; Petrick G; Gamo T A global survey of perfluorinated acids in oceans. Mar. Pollut. Bull 2005, 51 (8–12), 658–68. [DOI] [PubMed] [Google Scholar]
- (133).Darlington R; Barth E; McKernan J The Challenges of PFAS Remediation. The Military Engineer 2018, 110 (712), 58–60. [PMC free article] [PubMed] [Google Scholar]
- (134).Rahman MF; Peldszus S; Anderson WB Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res. 2014, 50, 318–40. [DOI] [PubMed] [Google Scholar]
- (135).Giesy JP; Kannan K Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol 2001, 35 (7), 1339–42. [DOI] [PubMed] [Google Scholar]
- (136).Yeung LWY; Dassuncao C; Mabury S; Sunderland EM; Zhang X; Lohmann R Vertical Profiles, Sources, and Transport of PFASs in the Arctic Ocean. Environ. Sci. Technol 2017, 51 (12), 6735–6744. [DOI] [PubMed] [Google Scholar]
- (137).Cousins IT; Ng CA; Wang Z; Scheringer M Why is high persistence alone a major cause of concern? Environ. Sci.: Processes Impacts 2019, 21, 781. [DOI] [PubMed] [Google Scholar]
- (138).U.S. Environmental Protection Agency. Perfluoroalkyl Sulfonates; Significant New Use Rule. In 40 CFR 721 ed., 2002; Vol. 67 FR 72854, pp 72854–72867. [Google Scholar]
- (139).U.S. Environmental Protection Agency. Perfluoroalkyl Sulfonates; Significant New Use Rule. In 40 CFR 721 ed., 2007; Vol. 72 FR 57222, pp 57222–57235. [Google Scholar]
- (140).U.S. Environmental Protection Agency. Long-Chain Per- fluoroalkyl Carboxylate and Perfluoroalkyl Sulfonate Chemical Substances; Significant New Use Rule. In 40 CFR 721 ed., 2015; Vol. 80 FR 2885, pp 2885–2898. [Google Scholar]
- (141).United Nations. Report of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants on the work of its ninth meeting; Geneva, 2019. [Google Scholar]
- (142).Eurpoean Chemicals Agency. Annex XVII TO REACH: Conditions of restriction; Council of the European Union: Helsinki. [Google Scholar]
- (143).Council of the European Union. Proposal for a Directive of the European Parliament and of the Council on the quality of water intended for human consumption (recast); General Secretariat of the Council of the European Union, 2020; Vol. 2017/0332(COD). [Google Scholar]
- (144).Department of Ecology, Hazardous Waste and Toxics Reduction Program, Washington State. Focus on: Alternatives to PFAS in Food Packaging; Olympia, WA, 2018. [Google Scholar]
- (145).State of Maine Legislature. An Act To Protect the Environment and Public Health by Further Reducing Toxic Chemicals in Packaging. 2019; Vol. LD, p 1433. [Google Scholar]
- (146).Hunt K Denmark just became the first country to ban PFAS ‘forever chemicals’ from food packaging. CNN, September 4, 2019. [Google Scholar]
- (147).Ministry of the Environment and Food. Minister will ban the use of organic fluorinated substances in cardboard and paper for food. Copenhagen, 2019. [Google Scholar]
- (148).South Australia Environmental Protection Agency. Transitioning to fluorine-free firefighting foam. https://www.epa.sa.gov.au/environmental_info/perfluorinated-compounds (accessed 2019-1106).
- (149).Washington State Legislature. Firefighting agents and equipment - toxic chemical use. 2018; Vol. 70.75A. [Google Scholar]
- (150).Department of Toxic Substances Control. Proposed: Carpets and Rugs with Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). https://dtsc.ca.gov/scp/carpets-and-rugs-with-perfluoroalkyl-and-polyfluoroalkyl-substances-pfass/ (accessed 2019-08-13).
- (151).United Nations. Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer, 13th ed.; Ozone Secretariat: Nairobi, Kenya, 2019; p 942. [Google Scholar]
- (152).Blum A; Balan SA; Scheringer M; Trier X; Goldenman G; Cousins IT; Diamond M; Fletcher T; Higgins C; Lindeman AE; Peaslee G; de Voogt P; Wang Z; Weber R The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs). Environ. Health Perspect 2015, 123 (5), A107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Cousins IT; Goldenman G; Herzke D; Lohmann R; Miller M; Ng CA; Patton S; Scheringer M; Trier X; Vierke L; Wang Z; DeWitt JC The concept of essential use for determining when uses of PFASs can be phased out. Environ. Sci.: Processes Impacts 2019, 21, 1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Environmental Officials from Sweden, the Netherlands, Germany, and Denmark. Elements for an EU-strategy for PFAS. 2019. https://www.documentcloud.org/documents/6586418-EU-Strategy-for-PFASs-FINAL-VERSION-December-19.html (accessed 2019-04-26).
- (155).McDonough CA; Guelfo JL; Higgins CP Measuring total PFASs in water: The tradeoff between selectivity and inclusivity. Current Opinion in Environmental Science & Health 2019, 7, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Kärrman A; Wang T; Roland K; Langseter AM; Grønhovd SM; Ræder EM; Lyche JL; Yeung L; Chen F; Eriksson U; Aro R; Fredriksson F PFASs in the Nordic Environment, Screening of poly- and perfluoroalkyl substances (PFASs) and extractable organic fluorine (EOF) in the Nordic Environment; edited by the Nordic Council of Ministers; TemaNord, 2019. [Google Scholar]
- (157).Ikea. IKEA FAQ: Highly fluorinated chemicals. https://www.ikea.com/ms/en_US/pdf/reports-downloads/product_safety/IKEA_FAQ_highly_fluorinated_chemicals.pdf (accessed 2019-08-13).
- (158).ChemSec H&M, Coop. Coop Denmark join NGO ChemSec in call to end the use of PFAS chemicals. https://chemsec.org/hm-coop-denmark-join-ngo-chemsec-in-call-to-end-the-use-of-pfas-chemicals/ (accessed 2020-03-13).
- (159).Cousins EM; Richter L; Cordner A; Brown P; Diallo S Risky Business? Manufacturer and Retailer Action to Remove Per- and Polyfluorinated Chemicals From Consumer Products. New solutions: a journal of environmental and occupational health policy: NS 2019, 29 (2), 242–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Blume MT The Danish Coop Bans Fluorinated Compounds in All Cosmetics. Business Wire, March 9, 2019. [Google Scholar]
- (161).Dintzer J; Johnson N The New Reality of PFAS Liability in California; Law.com The Recorder, November 20, 2018. [Google Scholar]
- (162).Goldenman G; Fernandes M; Holland M; Tugran T; Nordin A; Schoumacher C; McNeill A The cost of inaction: A socioeconomic analysis of environmental and health impacts linked to exposure to PFAS. Copenhagen, 2019. [Google Scholar]
- (163).Techology Interstate & Council Regulatory. Remediation Technologies and Methods for Per- and Polyfluoroalkyl Substances (PFAS). Washington DC, 2018. [Google Scholar]


