Severe forms of intraventricular hemorrhage (IVH) continue to occur in up to 15% of extremely premature infants,1 and more than half of these infants develop post-hemorrhagic ventricular dilatation (PHVD).2 PHVD is a term that represents the progressive ventricular dilatation caused by IVH and encompasses other terms, such as post-hemorrhagic hydrocephalus. PHVD is associated with a high risk for subsequent adverse motor and cognitive neurodevelopmental outcomes. Despite many decades of investigations, there is no consensus among neonatologists, pediatric neurologists, and pediatric neurosurgeons as to the best management of PHVD. The lack of agreement relates in part to difficulty in assessing the relative contributions to adverse outcomes of: (1) the primary brain injury associated with severe IVH; (2) the presence of blood products mediating brain injury; and (3) PHVD with distension causing injury that could be interrupted with cerebrospinal fluid (CSF) drainage. Over the last 5–10 years, substantial new evidence acquired from both experimental models and human clinical investigations, including randomized trials, has coalesced to inform the neonatologist, pediatric neurologist, pediatric neurosurgeon and pediatrician caring for these patients.
Our goal is to outline the current evidence supporting the premise that PHVD contributes to the brain injury which underlies adverse neurodevelopmental outcomes and that this injury may be mitigated or prevented by targeted approaches to management. We will emphasize that the utilization of ventricular size, quantified by sequential cranial ultrasonography, is a critical measurement for the consideration of intervention and is the approach associated with the lowest risk for adverse neurodevelopmental outcomes.
Pathophysiology and Neuropathology of PHVD
The relation of PHVD to brain injury and dysmaturation in the human premature infant has been supported by a variety of studies of experimental models and of human infants. These studies should be placed in the context of the rapid and complex developmental events occurring in the premature brain in the third trimester. The immature cerebral white matter, particularly the actively differentiating preo-ligodendrocyte (pre-OL), is particularly vulnerable to injury. Combined with a propensity of the immature microglia and astrocytes to pro-inflammatory activation, this developmental period is unique in the neuropathological consequences of PHVD.
Brain Maturation during the Premature Period.
Multiple active developmental events occur in the human brain from 24–40 weeks’ gestation.3–5 The rapidity and complexity of these cellular events underlie their vulnerability to perturbation. The principal components involved include the pre-oligodendrocyte (pre-OL), cerebral white matter axons, subplate neurons, cerebral cortex (including hippocampus), thalamus, basal ganglia, and cerebellum. The principal cellular targets in PHVD appear to be the pre-OL, axon, cerebral cortex (especially hippocampus), thalamus, and cerebellum. (see later)
Microglia and astrocytes are important glial elements in the development of the white and gray matter structures just noted. They are involved in axonal development, pre-OL differentiation – myelination, vascularization, synaptogenesis and neural circuit formation.6, 7 However, these cells, when “activated”, can have multiple deleterious effects4. Notably, a large population of potentially activatable microglia are present in normal developing white matter during the premature period.8 These “activated” cells can impair pre-OL maturation and other maturational events by release of reactive oxygen or nitrogen species or cytokines that act on pre-OLs, and likely on other cellular elements.9 Moreover, pro-inflammatory microglia have been shown to induce formation of neurotoxic reactive astrocytes,10 also important in failure of maturation of pre-OLs and likely other cellular elements.11 The shift in microglial and astroglial phenotypes to pro-inflammatory phenotypes diverts the roles of these glia from the normal developmental events just noted. As noted later, abundant activated microglia and reactive astrocytes are present in cerebral white matter of neonatal IVH, (non-hemorrhagic) ventriculomegaly, and PHVD.
Effects of Intraventricular Hemorrhage without Hydrocephalus.
The vast majority of progressive ventricular dilation in premature infants occurs as a consequence of severe germinal matrix-intraventricular hemorrhage (GMH-IVH). A series of experimental studies, primarily performed in neonatal rats, demonstrated deleterious effects of intraventricular blood and blood components on the developing brain.12–17 The injury scenario begins with hemoglobin release into the ventricular system. The ependymal injury related to the IVH likely facilitates penetration of hemoglobin and heme into brain parenchyma. Heme released from hemoglobin is taken up by microglial cells. Iron facilitates the formation of injurious reactive oxygen species, which affect both pre-OLs and axons. Heme can also be taken up by contiguous neurons which are then injured. Such neurons could include those adjacent to blood in the ventricles (eg, hippocampus, thalamus) or in the subarachnoid space (e.g., external granule cells of cerebellum). The role of free iron in the scenario leading to injury is supported by the beneficial effects of systemically administered deferoxamine in an animal model of IVH.15 Additional blood products, especially thrombin and fibrin, are important in contributing to parenchymal injury after experimental IVH,16 especially by promoting microglial and astroglial activation. These neuroinflammatory responses would be expected to injure vulnerable pre-OLs, axons and contiguous neurons. The importance of the neuroinflammatory response is supported by experimental studies showing prevention of brain injury and PHVD by early systemic administration of minocycline, erythropoietin, melatonin, or mesenchymal stem cells.18–22
The few studies of IVH in the human premature brain postmortem and in vivo appear consistent with the experimental data. The principal findings include: (1) disruption of the ventricular zone with reactive astrogliosis in areas of ependymal loss; (2) impairment of proliferation of oligodendroglial precursor cells and neuronal precursor cells in the subventricular zone of the ganglionic eminence, and in the cerebral white matter, (3) impairment of pre-OL maturation, (4) axonal injury, and (5) microglial activation.23–25 Concerning pathophysiology, large amounts of non-protein-bound iron are present in CSF of infants after large GMH-IVH (with PHVD),26 and quantitative susceptibility map analysis by magnetic resonance imaging (MRI) with severe GMH-IVH shows changes consistent with accumulation of hemosiderin/ferritin iron throughout cerebral white matter.27 The latter observations suggest that intraparenchymal diffusion of extracellular hemoglobin, and ultimately iron, is widespread after severe IVH. The mechanistic implications are clear and likely involve the free radical-mediated disturbances identified in experimental models, as described earlier.
Effects of Neonatal/Infantile Hydrocephalus without Intraventricular Hemorrhage.
To assess the impact of neonatal PHVD on the developing brain, we must consider the effects of hydrocephalus per se, without prior IVH. Experimental studies based largely on the study of kaolin-induced hydrocephalus suggest that the principal initiating events leading to parenchymal injury are periventricular and cerebral white matter vascular impairment resulting initially in tissue hypoxia-ischemia.28–31 A well-documented prominent inflammatory response with activated microglia and reactive astrocytes in cerebral white matter is likely involved in the elevation of free radicals and associated white matter injury.30, 32, 33 The morphological consequences involve, specifically, oligodendroglial precursors (presumably pre-OLs) and axons. In a well-characterized animal model, shunting at 1 week, but not at 4 weeks when axonal loss had occurred, allowed recovery of myelination.34, 35 Moreover, studies in rat models of PHVD also support particular value for early intervention.22 The experimental data thus suggest that earlier rather than later intervention can prevent the pre-OL and axonal dysmaturational disturbances.
Effects of Neonatal Post-hemorrhagic Ventricular Dilatation.
The previous sections concerning the effects on brain structure and pathophysiology of (a) IVH alone, and (b) hydrocephalus alone, set the stage for addressing the central issue of the effects of PHVD in the preterm infant. Multiple experimental studies in developing animals have documented the occurrence of hydrocephalus after intraventricular injection of blood, hemoglobin or iron into CSF.18, 20–22, 36 Intraventricular injection of hemoglobin or iron in neonatal rats led acutely to hydrocephalus, pronounced gliosis with increased inflammatory cytokines in cerebral white matter, impaired myelination, and “cell death” in the hippocampus and “cortex”. The importance of iron in the deleterious effects was shown by the lack of effect of intraventricular injection of iron-deficient protoporphyrin, the correlation of injury to hippocampus and cortex with the enhanced levels of heme oxygenase in those structures (heme oxygenase releases iron from heme), and the amelioration of hydrocephalus, inflammation and tissue injury by the iron chelator deferoxamine.36
Studies of the deleterious effects of PHVD in premature infants suggest disturbances in cerebral hemodynamics and neurophysiological function. These disturbances and their amelioration by CSF removal have been reviewed elsewhere.31 Existing (although limited) studies of the morphological consequences of PHVD in human infants at the cellular level indicate (1) ependymal disruption, (2) axonal injury, (3) microglial infiltration, and (4) impaired myelination.23, 28, 37–39 Overall, the findings suggest a disturbance in development of cerebral white matter, deep nuclear structures (especially the thalamus), cerebral cortex (especially the hippocampus), and cerebellum, i.e., structures contiguous to intraventricular and subarachnoid blood.
In summary, the mechanisms of the deleterious effects on the brain of the human infant with PHVD are likely multifactorial. In addition to cerebral ischemia, mechanical distortion and neuroinflammation, factors related to the presence of blood (leading ultimately to iron release and free radical formation) are pathogenic. Because the hydrocephalic state would be expected to enhance the intraparenchymal movement of blood products, the early institution of interventions that involve CSF drainage seems important. Optimal timing clearly needs further clarification.
Timing of Interventions – Relationship of ventricular size to outcome
Very preterm infants who develop a severe IVH (a grade III hemorrhage filling the lateral ventricle >50%, and resulting acutely in ventricular dilatation), or a periventricular hemorrhagic infarction (PVHI, a hemorrhagic lesion in the brain parenchyma associated with an ipsilateral grade II, or grade III hemorrhage) are prone to develop PHVD. Due to an imbalance in CSF production and reabsorption, the ventricles may dilate around 7–14 days following the onset of the IVH. PHVD can progress slowly or rapidly. In infants with slow progression, stabilization or regression occurs in 65%. In 30–35% of infants, there is rapid progression in ventricular size over days to weeks.2
There is as yet no agreement when best to intervene, with some centers starting intervention based on sequential cranial ultrasound (cUS) measurements whereas others base decisions on clinical symptoms (apneas, vomiting, full fontanel, sunsetting, splaying sutures, rapid increase in head circumference).40, 41 In the preterm infant there are two factors that reduce the sensitivity of fontanel pressure or head circumference changes for evaluating the degree of significant ventricular dilatation. The first factor is that compliance is higher in the preterm infant and lower pressures are sufficient to cause progressive ventricular distension resulting in compression of the cortical mantle before an effect is apparent on the sutures or head circumference. The second factor relates to the larger extracerebral space in very preterm infants requiring considerable ventricular dilatation before changes in fontanel pressure and a rapid increase in head circumference occur. Consistent with this notion, a poor correlation was found between the Evans ratio (the ratio of bifrontal horn diameter to the biparietal bone diameter) and the head circumference.42 Therefore, sequential cUS in preterm infants with IVH are required to diagnose and follow the progression of PHVD.
Cranial Ultrasound Measurements.
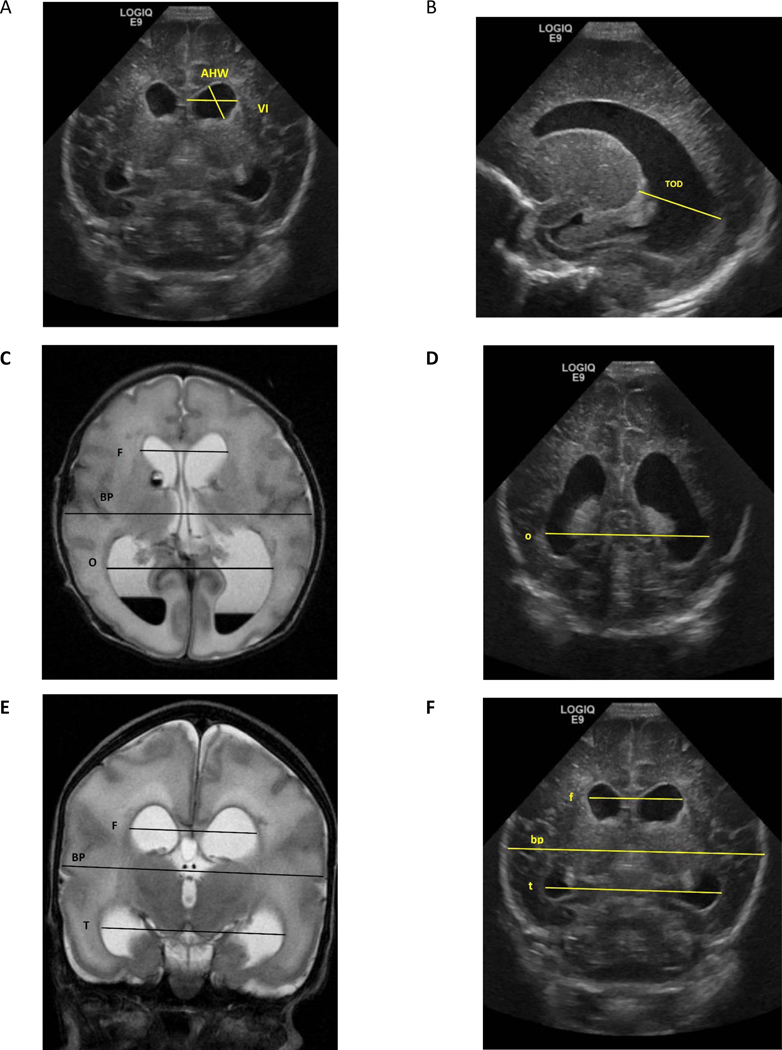
The “ventricular index” (VI), defined by Levene and Starte43, is the most commonly used measurement and is assessed in the coronal view. The VI is the distance between the midline (falx) and the lateral border of the lateral ventricle at the level of foramen of Monro. (Figure 1, A) Another commonly used measurement is the “anterior horn width” (AHW), also measured in the coronal view just anterior to the thalamic notch.44 (Figure 1, A) For both measurements, normal values and cut-off values for PHVD have been reported.43–45 The “thalamo-occipital distance” (TOD) measures the occipital horn in the parasagittal plane and is of additional value because there can be a discrepancy in size between the anterior and occipital horns of the lateral ventricle. (Figure 1, B) The AHW and TOD measures can indicate the ‘ballooning’ shape of the lateral ventricles, and these measures correlate best with 3 dimensional volumetric ultrasound measurements.46 The morphology of the ventricles can assist in the differentiation between dilatation due to PHVD or ventricular dilatation due to cerebral atrophy, because ventricular dilatation is typically associated with ballooning of the ventricles. In addition, assessing the extracellular space will assist in differentiating the two causes of dilatation. The absence of any extra-axial space supports the conclusion that the dilatation relates to CSF accumulation, whereas the presence of extra-axial space suggests cerebral atrophy.47
Figure 1: Demonstration of different ventricular measures and ratios.
Images from cUS and brain MRI of one-week old boy born at 30 weeks of gestation. A and F= Coronal cUS at level of Foramen of Monro, B= Parasagittal cUS, C= Axial View of T2 MRI. D= Coronal cUS at level of occipital horn E= Coronal View of T2 MRI, VI= Ventricular Index, AHW= Anterior Horn Width, TOD= Thalamo-Occipital Diameter MRI dimensions are in Capital letters= F= bifrontal horn, BP= biparietal, T= bitemporal horn, O =bioccipital horn
cUS dimensions are in small letters= f= bifrontal horn, bp= biparietal, t= bitemporal horn, o =bioccipital horn
Evans Ratio= F/BP by MRI or f/bp by US
Frontal and Temporal Horn Ratio= (F+T/2)/ BP by MRI or (f+t/2)/bp by cUS
Frontal and Occipital Horn Ratio (FOHR)= (F+O/2)/BP by MRI or (f+o/2)/bp by cUS
Other measures are derived mainly from axial MRI and computed tomography scans and include the Evans ratio (described above) (F/BP, Figure 1, C) and the Frontal and Occipital Horn Ratio (FOHR), which is the average of the frontal and occipital horn dimensions divided by the biparietal diameter [(F+O)/2)/BP] (Figure 1, C) The FOHR has shown high inter-observer reliability (especially when compared with the Evans ratio only reflecting the anterior horns) and correlation with ventricular volumes.48, 49 cUS measurements of the FOHR, as well as the Frontal and Temporal Horn Ratio, were significantly correlated with the same measures derived from MRI.50 (Figure 1, C–F).
Other Measurements.
Use of the Doppler US resistive index, the difference between peak systolic flow velocity and lowest diastolic flow velocity divided by the peak systolic flow velocity, provides information about cerebral hemodynamics, especially cerebrovascular resistance. With progressive PHVD, initially a rise in systolic flow is seen, followed by a decrease, absent or even inverted diastolic flow.51 Significant changes in the resistive index following application of pressure to the anterior fontanel (a measure of cerebral compliance) have been correlated with intracranial pressure and future need for shunt placement.52 In addition, near infrared spectroscopy (NIRS) studies have shown that regional cerebral oxygen saturation is progressively impaired with increasing dilatation, and gradually improves following CSF drainage.53, 54 As these changes occur when PHVD is severe, intervention is best started before the presence of these changes.
Intervention Studies.
Intervention typically starts with temporizing lumbar punctures (LPs) to decompress the ventricle on an urgent basis while neurosurgical evaluation and surgical planning occur. Lumbar punctures also may prevent the need for further neurosurgical intervention, as was demonstrated in the ELVIS study, in which 2–3 LPs reduced the need for surgery in up to one quarter of all cases.55 Thus, the use of LPs in the immediate period after identification of significant ventricular dilatation can be helpful in temporizing or avoiding surgical intervention.
Earlier data related to the role of LPs in the management of PHVD are summarized in a Cochrane review that reported no evidence that LPs alone were effective in avoiding shunt surgery or improving neurological development.56 This review included 4 trials performed from 1980–1990 with significant variation in indication, timing, number and effectiveness of LPs. The majority of these studies included infants with severely dilated ventricles, with LPs starting relatively late, and high variation in number of LPs (1–40) and volume drained. (Table I; available at www.jpeds.com). Of note, in the largest study in this Cochrane review, >50% of the early intervention group eventually received invasive ventricular taps. 57 We hypothesize that the late, variable and invasive approaches used in these studies explain the higher rates of VP-shunt placement and worse neurodevelopmental outcomes when compared with more recent studies which focused on early effective drainage of CSF. (Table II; available at www.jpeds.com)
Table 1:
Comparisons of Lumber Punctures in Studies Included in the Cochrane Review1
| Anwar et al.2 (n=24) | Mantovani et al.3 (n=19) | Dykes et al.4 (n=22) | Ventriculomegaly Group5, 6 (n= 79) |
||
|---|---|---|---|---|---|
| Indications | Infants with Grade III-IV IVH | Infants with Grade II-III IVH | Asymptomatic severe progressive posthemorrhagic hydrocephalus | VI > 97th percentile + 4 mm | |
| Time to start LPs | 11 Days (SD=5) | 19 days (SD=11) | |||
| Number of LPs | 14 (SD= 10) | Median (10–90th %) 7 (1–37) | |||
| Aimed volume and end point | • Daily LP till flow stops • LP were done every other day, or less if CSF removed at tap was <3 ml. • LPs stopped if the ventricular size decreased or remained unchanged for 2 consecutive weeks |
• Daily LP until the CSF was clear, colorless, and had a protein concentration less than 180 | • Daily LP for a minimum of 1 week and a maximum of 3 weeks. | • LP for max 2% of body weight • Repeat as many aspossible if increase VI > 2mm above measurement before first tap • If LP < 2 ml then shift for Ventricular tap |
|
| Volume (ml) | Total: 67 (SD=10) | Median (10–90th%) 116 (14–678) | |||
| Volume per tap (ml) | Mean= 3 (SD=2) | 3–5 ml | 2–21 ml | ||
| Duration | 20 days (SD= 16) | 18 days (range 7–34) | 1–3 weeks | ||
| Special Description | Screening done to all infants using LP at 24 hours and 72 hours of life. If concerning, CT was done. | Spinal tap only (42%) Ventricular tap (9%) Spinal and Ventricular tap or reservoir (42%) |
|||
| Outcomes | |||||
| VPS | NA | 4/19 (21%) | 9/22 (41%) | 41/79 (52%) | |
| Death or Disability | 14/21 (67%) | 61/73 (84%) | |||
Whitelaw A, Lee-Kelland R. Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage. The Cochrane database of systematic reviews. 2017;4:Cd000216.
Anwar M, Kadam S, Hiatt IM, Hegyi T. Serial lumbar punctures in prevention of post-hemorrhagic hydrocephalus in preterm infants. J Pediatr. 1985;107:446–50.
Mantovani JF, Pasternak JF, Mathew OP, Allan WC, Mills MT, Casper J, et al. Failure of daily lumbar punctures to prevent the development of hydrocephalus following intraventricular hemorrhage. J Pediatr. 1980;97:278–81.
Dykes FD, Dunbar B, Lazarra A, Ahmann PA. Posthemorrhagic hydrocephalus in high-risk preterm infants: natural history, management, and long-term outcome. J Pediatr. 1989;114:611–8.
Group VT. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Ventriculomegaly Trial Group. Arch Dis Child. 1990;65:3–10.
Group VT. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation: results at 30 months. Ventriculomegaly Trial Group. Arch Dis Child Fetal Neonatal Ed. 1994;70:F129–36.
Table 2:
Comparisons between the criteria, interventions, numbers and outcomes reported in major studies of management of PHVD
| Study | Ventriculomegaly trial Group- Early 1 | Ventriculomegaly trial Group- Late1 | Acetazolamide Trial (Standard Arm)2 | DRIFT – Cases3 | DRIFT-Control3 | HCRN 4 | ELVIS Early5 | ELVIS-Late5 | Two Dutch Units6 | North America6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Criteria | VI > 97th percentile + 4 mm | VI > 97th percentile + 4 mm | (a) VI 4 mm over the 97th centile OR (b) all of the following: - AHW > 4 mm - TOD > 26 mm - third ventricle width > 3 mm OR (c) Measurements above a or b on 1 side combined with obvious midline shift indicating a pressure effect. |
FOHR >0.55 + 2 of 3 of: - Bradycardia - Split suture - Bulging Fontanel |
VI > 97th percentile +AHW >6 mm and/or the TOD >25 mm | VI > 97th percentile + 4mm +AHW >10 mm | Based on ventricular measurements VI exceeding the 97th centile and increasing towards the p97+4mm line and an AHW >6mm | Based on signs of increased intracranial pressure | ||
| Intervention | Immediate CSF Drainage • LP for max 2% of body weight • Repeat as many as possible if increase VI > 2mm above measurement before first tap • If LP < 2 ml then shift for Ventricular tap • Shunt if tapping continued for 4 weeks and head enlargement continues and other factors |
Only CSF Drainage if: • Increase in head circumference of twice the normal velocity for gestational age after entry to the trial Or • Symptomatic raised intracranial pressure with a measured cerebrospinal fluid pressure greater than 12 mm Hg) |
The study advised for removal of CSF to be delayed until either head growth exceeded twice the normal rate for 2 weeks or the infant showed clinical symptoms or signs of raised intracranial pressure |
DRIFT Drainage Irrigation and Fibrinolytic Therapy (DRIFT) till fluid clears (usually 72 h) |
Standard Intervention only if 1- Signs of raised ICP (irritability, apnea, reduced consciousness, bulging fontanelle, or loss of diastolic velocities on cerebral arteries) or excessive Or 2- head enlargement over time (> 2 mm/d) Intervention: LP x 2 or failed LP then reservoir |
• Temporize till 1800–2000 gm | • LPs (max 3), and followed by insertion and taps from a VR, aiming for VI<p97 over the next 7–10 days. • Ten mL/kg were removed one or two taps a day, the volume adjusted according to cUS. • When taps from a VR were still needed 28days after insertion to keep the VI well below the p97+4 mm, one or two ‘challenges’ were performed with discontinuation of taps. Reservoir taps were resumed in case of expanding ventricles, clinical symptoms and/or excessive head growth. |
LPs or reservoir followed by VP shunt in the absence of stabilization | LP, reservoir or VP shunt (mostly immediate VP shunt placement) | |
| Number | 79 | 78 | 89 | 39 | 38 | 102 Temporize | 64 | 62 | 78 (48 received intervention) | 49 (24 received intervention) |
| Parenchyma l Lesions | 46 (58%) | 55 (71%) | 40 (45%) | 20 (51%) | 18 (47%) | 54 (53%) | 24 (38%) | 19 (30.6%) | ||
| VP shunt | 41 (52%) | 42 (54%) | 40 (45%) | 16 (41%) | 15 (39%) | 66 (65%) | 12 (19%) | 14 (23%) | 10 (13%) | 22 (45%) |
| Death | 14 (18%) | 18 (23%) | 10 (11%) | 3 (8%) | 5 (13%) | 13 (13%) | 8 (12.5%) | 9 (15%) | 11 (14%) | 20 (41%) |
| Numbers at FU | 59 | 53 | 79 | 39 | 38 | 50 | 45 | 62 | 27 | |
| FU age (CA) | 30 m | 30 m | 12 m | 24m | 24m | 24 m | 24 m | 18–24 m | 18–24 m | |
| Impairment |
Overall Griffiths Scale < 70 29/59 (49%) |
Overall Griffiths Scale < 70 25/53 (47%) |
Impaired or Disabled 52/79 (66%) |
Severe Disability 18/39 (46%) |
Severe Disability 22/38 (58%) | ? |
Combined Cognitive and Motor Scores < 70= 6 (12%) |
Combined Cognitive and Motor Scores < 70 11 (24%) |
Combined Cognitive and Motor Scores < 70= 3 (4.8%) | Combined Cognitive and Motor Scores < 70= 14 (52%) |
Group VT. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation: results at 30 months. Ventriculomegaly Trial Group. Arch Dis Child Fetal Neonatal Ed. 1994;70:F129–36.
Kennedy CR, Ayers S, Campbell MJ, Elbourne D, Hope P, Johnson A. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: follow-up at 1 year. Pediatrics. 2001;108:597–607.
Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 2010;125:e852–8.
Wellons JC, 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni AV, Limbrick DD, Jr., et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. Journal of neurosurgery Pediatrics. 2017;20:19–29.
Cizmeci MN, Groenendaal F, Liem KD, van Haastert IC, Benavente-Fernandez I, van Straaten HLM, et al. Randomized Controlled Early versus Late Ventricular Intervention Study (ELVIS) in Posthemorrhagic Ventricular Dilatation: Outcome at 2 Years. Submitted. 2020.
Leijser LM, Miller SP, van Wezel-Meijler G, Brouwer AJ, Traubici J, van Haastert IC, et al. Posthemorrhagic ventricular dilatation in preterm infants: When best to intervene? Neurology. 2018;90:e698-e706.
In more recent studies, when stabilization or regression of ventricular size did not occur spontaneously, or 2–3 LPs were not successful, the insertion of a ventricular reservoir or ventricular subgaleal shunt has been used as a temporizing measure. Many centers defer such intervention until the VI has crossed the 97th percentile +4mm line or clinical symptoms have developed. Two retrospective, observational studies showed a reduction in shunt requirement when LPs and reservoir insertion were performed before the 97th percentile +4 mm line was crossed.58, 59 In another retrospective study of 32 preterm infants, external ventricular drainage (using bedside insertion of an intravenous catheter connected to a closed drainage plastic container) performed early (defined as ≤25 days after birth) was associated with better scores for adaptive, personal social, communication, and cognitive functions at 6 years of age compared with those with later intervention.60 A more recent observational study has shown results favoring earlier therapeutic intervention for PHVD using cUS measures.40 In the study by Leijser et al, neurodevelopmental outcomes at 18–24 months were compared between preterm infants with and without intervention within cohorts undergoing either an early cUS based approach in two Dutch centers or late intervention following onset of clinical signs in a Canadian center. Infants undergoing early cUS-based intervention had 2-year outcomes indistinguishable from infants not requiring CSF drainage (cognitive test score of 100 vs 95; P = .6; motor score of 97 vs 97; p=0.6), even when they eventually needed insertion of a VP-shunt (20%). In contrast, intervention initiated once clinical signs had occurred was associated with an increased risk of unfavorable outcome (cognitive score of 90 vs 68; p=0.002; motor score of 90 vs 60; p=0.03) and intervention-related complications, with the need for a VP-shunt in 92%, compared with infants not needing intervention. There were important confounders in this study, however, including a differing referral pattern between the Dutch and Canadian centers.
The most recent randomized controlled trial was the ELVIS trial (Early versus Late Ventricular Intervention Study) which enrolled 126 preterm infants born at ≤ 34 weeks of gestation with progressive PHVD following a severe IVH. Treatment was either started at a low threshold (LT) (VI > 97th percentile and AHW > 6 mm and/or TOD > 25 mm), or at a higher threshold (HT) (VI > 97th percentile +4 mm and AHW > 10 mm). There was no significant difference between the two groups for the primary outcome of death and or VP-shunt placement (30% LT and 37% for HT infants). A VP-shunt was inserted in 12/64 (19%) infants in the LT and in 14/62 (23%) infants in the HT group, the lowest rates reported thus far in the literature. More neurosurgical interventions were required in the LT group.55 In a nested sub study, brain injury was assessed by the Kidokoro score on MRI performed at term equivalent age.61 The total Kidokoro score was lower in the infants in the LT group (n = 44) than in the HT group (n = 44; median, 8 [IQR, 5–12] vs median 12 [IQR, 9–17], respectively; P < .001). Ventricular volumes could be calculated in 47 infants and were smaller in the LT group (P = .03). At 2 years of age the risk of developmental impairment was low among both study groups, with 75% of all infants tested achieving a cognitive composite score of ≥ 85. There was no significant difference for adverse outcome (composite outcome: death/cerebral palsy/developmental test score <70) between the two groups.62 However, in the multivariable analysis, children in the LT group had a decreased risk of composite adverse outcome, after correcting for gestational age and hemorrhage severity (adjusted odds ratio [aOR]: 0.24, 95% confidence interval [CI], 0.07 to 0.87, p=0.03). Furthermore, a significant relationship was established between the maximal FOHR and cognitive and motor outcome for all infants combined. These findings support the conclusion that timely intervention with reduction of the extent of ventricular dilatation may mitigate brain injury associated with ventricular dilatation and lead to improved neurodevelopmental outcomes.
Relation of Maximal Ventricular Size to Neurodevelopmental Outcome.
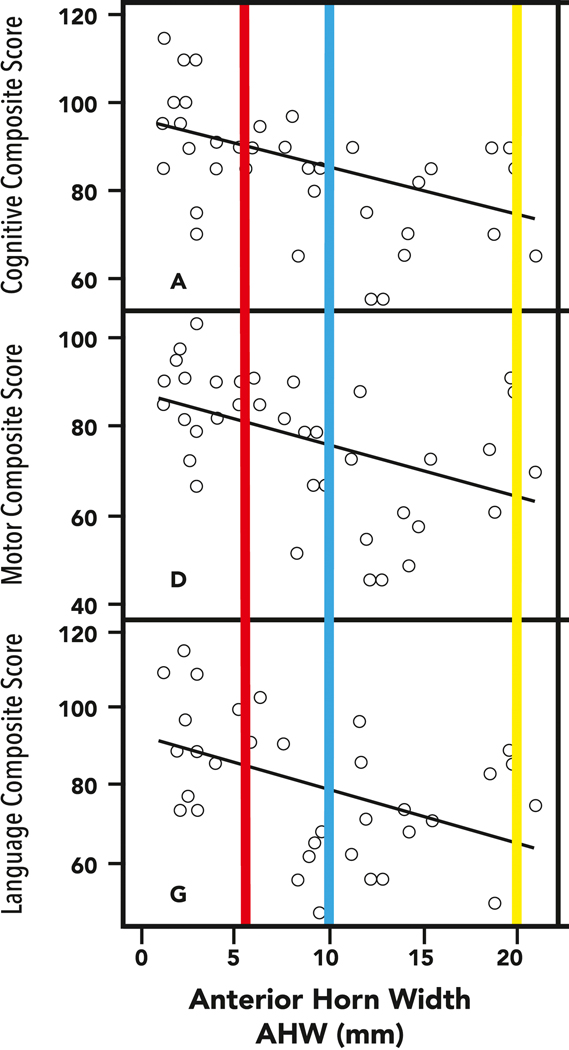
To justify the need for therapeutic reduction in ventricular size, an association with improved outcomes should be demonstrable. In contrast to data derived from postnatal post-inflammatory hydrocephalus in term born infants and children,63 the association between the maximal size of ventricular measurements in PHVD in preterm infants and neurodevelopmental outcomes has been supported by more than one study.40, 62, 64 This association is additionally demonstrated in Figure 2 where the relationship of maximal anterior horn width (AHW) during the neonatal intensive care unit period in preterm infants with PHVD is shown to be related to neurodevelopmental outcomes at 18–24 months on assessment using the Bayley Scales of Infant Development (2nd edition) assessment.64 The bars overlaid on this graph represent the data reported from a single center observational study of a North American population (yellow bar), with concordant mean cognitive scores of 68 and motor scores of 60.40 These values contrast with those from the earlier intervention study (the ELVIS trial) with early (red) and late (blue) interventions both having smaller ventricular measures than the North American cohort. The outcomes in the ELVIS study of early intervention (mean cognitive score 95) and later intervention (mean cognitive 91)62 are consistent with the relatively small differences between study arms in the measurements used as threshold to treat. However, when the ventricular size is much larger, as is typical in the North American cohort, the associated developmental outcomes are much worse, supporting the need to avoid such large degrees of ventricular dilatation, if possible.
Figure 2:
Anterior Horn Width in relation to neurodevelopmental outcomes at 18–24 months in 39 preterm infants with PHVD. The maximal size of the AHW in the North American group of the study by Leijser et al, (yellow bar) and the corresponding neurodevelopmental impairments with performance at 60–70 points, contrasts with the results in the ELVIS study with early (red) and late (blue) demonstrating less significant differences in outcomes. It is noteworthy that timing of the “late” intervention arm in ELVIS is much earlier than in the North American sites.
Alternative Intervention (DRIFT)
Drainage, Irrigation and Fibrinolytic Therapy (DRIFT) was conceived due to evidence that a) blood and its breakdown products persist for months and contribute to free radical injury and inflammation and b) earlier reduction in ventricular enlargement may improve outcome. The intervention is intended to wash out the ventricles and reduce ventricular size. The DRIFT randomized trial recruited 77 infants with PHVD and minimum VI 97th percentile +4 mm, but there was no upper limit for ventricular size at entry. Trial recruitment was stopped early because of excess secondary hemorrhage (35%). Most of these hemorrhages were small, and drainage and irrigation continued until clearance of CSF.65 Of the infants randomized to DRIFT, 21/39 (54%) died or were severely disabled versus 27/38 (71%) in the standard treatment group (adjusted OR 0.25 [95% CI: 0.08–0.82]). Among the survivors, 11/35 (31%) in the DRIFT group had severe cognitive impairments (Bayley cognitive scores <55) versus 19/32 (59%) in the standard treatment group (adjusted OR: 0.17 [95% CI: 0.05–0.57]).66 At 10 years of age, 52 children (28 from the DRIFT group and 24 with standard treatment) were assessed again.67 The mean cognitive quotient (CQ) score was 15.7 points higher (95% confidence interval (CI) - 2.9 to 34.2 points; p = 0.096) in the DRIFT group (69.3 + 30.1 SD) compared with the standard treatment group (53.7 + 35.7 SD). Following adjustment for sex , birth weight and grade of IVH, significantly more children in the DRIFT group were alive without severe cognitive disability, with a difference of 23.5 points. Similarly, another preliminary clinical study, a combination of ventricular lavage therapy using External Ventricular Drainage and intermittent urokinase slow intraventricular injections was associated with less VP-shunt placements and better functional outcomes at 36 weeks of age. 68
Although the DRIFT trial provides support for the hypothesis that washing out harmful substances from the ventricles reduces overall brain injury after PHVD, DRIFT is a complicated procedure requiring highly skilled clinical monitoring and intervention over 72 hours. A simpler intervention which is easier to teach and disseminate is needed.
Considerations for neurosurgical interventions
Renewed interest in optimizing the neurosurgical treatment of infants with PVHD stems from: 1) recognition that PHVD is the most common etiology of hydrocephalus in Europe and North America69; 2) increasing trends in the incidence and prevalence of PHVD70; 3) the severe neurological morbidity and lifelong, complex neurosurgical care required; 4) data suggesting potential benefit to standardized intervention in PHVD; and 5) basic and translational science providing a scientific rationale for earlier intervention.71, 72 Despite this interest, however, the data concerning neurosurgical interventions for PHVD remain limited. Recent evidence-based guidelines under the auspices of the Joint American Association of Neurological Surgeons and Congress of Neurological Surgeons Pediatric Section failed to identify sufficient data to support strong recommendations in the neurosurgical treatment of PHVD.73
Because of the lack of strong evidence, there is great variation in practices between centers with respect to neurosurgical interventions for PHVD. This variation relates to two main treatment decision considerations: the timing of intervention and the type of intervention. Concerning the former, the HCRN (Hydrocephalus Clinical Research Network), a multi-institutional North American research group, identified the single most significant factor driving the timing of neurosurgical intervention of infants with PHVD to be the center at which they were treated.41, 73 As a result, the HCRN standardized the clinical assessment of these infants and developed standard definitions for reliable assessment of a bulging fontanel or split cranial sutures74. However, the presence of adequate inter-rater reliability did not address whether this assessment correlates well with increased ICP, in contrast to previous reports which did not show such correlation75, or if such “late” signs should be the target for intervention.
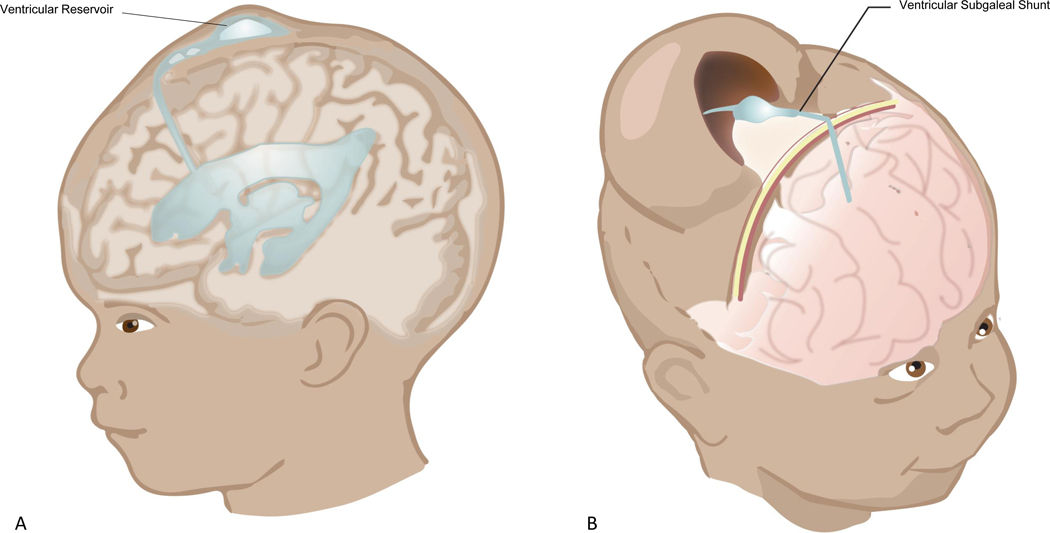
The HCRN also showed that the assessment and timing of intervention in these infants can be standardized with high compliance across multiple centers, and developed standardized operative protocols to decrease VP-shunt infection in these high-risk infants.76, 77 The HCRN study titled “Shunting Outcomes in Post-hemorrhagic Hydrocephalus (SOPHH)”, compared the impact of ventricular reservoir versus ventricular subgaleal shunt (Figure 3; available at www.jpeds.com) as temporizing measure on death or requirement for shunt in a prospective, multicenter observational study.78 In SOPHH, FOHR was used with an intervention threshold ≥0.55, which would exceed the HT group of the ELVIS trial or the threshold for the DRIFT trial. This threshold was based on previously established practice patterns and consensus agreement among the participating centers. Of note, despite agreement to intervene at FOHR ≥0.55, at the time of intervention the FOHR exceeded the intervention threshold (0.68±0.07 for the ventricular subgaleal shunt group and 0.67±0.06 for the ventricular reservoir group). Also notable is that the treatment threshold required a combination of a progressive increase in head circumference, full fontanel or splaying of the sagittal suture ≥2 mm in the mid-parietal region. SOPHH enrollment occurred at 6 sites over 39 months and included placement of 66 ventricular reservoirs and 36 ventricular subgaleal shunts. Overall 65% of infants with temporizing measures were converted to permanent shunts. Developmental follow-up in this cohort with Bayley-III cognitive, language, and motor outcomes is forthcoming.
Figure 3;
online: An illustration of the commonly used temporizing measures for PHVD: A) Ventricular Reservoir (VR), and B) Ventricular Subgaleal Shunt (VSGS)
Complications reported with temporizing measures include obstruction, infection, need for revision and other rare complications including CSF leak, porencephalic cysts and intracranial hemorrhage 79–81 (Table 3; available at www.jpeds.com). In the SOPHH study, there was no difference between ventricular reservoir and ventricular subgaleal shunt in rates of infection, CSF leak, death or VP-shunt rate. Although the ventricular reservoir entails more frequent skin breaks than ventricular subgaleal shunt, minimizing complications from reservoir drainage can be obtained by systematic care, including strict sterile precautions, using a trained provider for removal of the CSF, removing the fluid slowly at not >1ml/minute and carefully considering skin care over the site of the reservoir.82
Table 3:
Complications Associated with Ventricular R eservoir vs. Subgaleal Shunt
| Ventricular Reservoir | Ventricular Subgaleal Shunt | |
|---|---|---|
| Common 1 | ||
| Obstruction | 7.3% (95% CI 5.0–10.4) | 9.6% (95% CI 5.6–16.0) |
| Infection | 9.5% (95% CI 7.0–12.8) | 9.2% (95% CI 6.3–13.3 |
| Revision | 10.8% (95% CI 7.4–15.5) | 12.2% (95% CI 8.8–16.5) |
| Others | ||
| CSF Leak2−4 | 6% | 4.7–6.5% |
| Porencephalic Cysts2 | 8.7% | |
| Intracranial Hemorrhage3 | 1.1% | |
Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, Kulkarni AV. Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. Journal of neurosurgery Pediatrics. 2015;16(5):545–555.
Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. Journal of neurosurgery Pediatrics. 2014;14(5):447–454.
Tubbs RS, Banks JT, Soleau S, et al. Complications of ventriculosubgaleal shunts in infants and children. Childs Nerv Syst. 2005;21(1):48–51.
Wellons JC, 3rd, Shannon CN, Holubkov R, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. Journal of neurosurgery Pediatrics. 2017;20(1):19–29.
In the absence of strong evidence about timing and types of neurosurgical interventions, neurosurgical groups have focused on standardizing the assessment and management protocols. Although helpful, such strategies have tended to result in late interventions and with an unclear benefit on neurodevelopmental outcomes.
Future Opportunities
On the horizon are potentially promising new diagnostic and therapeutic approaches that might be of further benefit to infants with PHVD. Although none are yet in clinical use, quantitative neuroimaging and analysis of CSF and blood-based analytes72, 83–86 show promise as potential diagnostic biomarkers of PHVD that could guide treatment and prediction of outcome. In addition, animal studies have demonstrated the potential protective effect of mesenchymal stem cells by intraventricular20 as well as intravenous21 infusion following severe IVH. Mesenchymal stem cells attenuated the degree of PHVD as well as degree of brain injury in a severe IVH rat model, especially when given at 2 days rather than 7 days after induction of IVH.22 The same group also published the results of a phase I trial using intraventricular transplantation of mesenchymal stem cells in 9 premature infants.87 Other studies suggest benefit from erythropoietin/melatonin19, minocycline18, potentially via anti-inflammatory mechanisms, and deferoxamine, via iron chelation36. Additionally, several centers now apply the principle of “washing out PHVD” by using endoscopic ventricular lavage in the operating room. Schultz et al reported on the feasibility and safety of this technique in 19 infants.88 In addition, d’Arcangues et al reported 56 infants with PHVD treated with endoscopic ventricular lavage and concluded that the procedure may reduce the need for shunt surgery and subsequent shunt revisions.89 Another approach, which could potentially reduce the long-term shunt-related morbidity in these infants, is endoscopic third ventriculostomy without or with choroid plexus cauterization. This intervention could serve as an alternative to CSF shunting in PHVD, though reported success rates are variable, at least early in infancy in this population.83, 90, 91
Management Approach
Combining the evidence from preclinical data, supporting the ability of early interventions to reverse brain injury, and from clinical data, indicating that the best neurodevelopmental outcomes have been reported in centers and trials using ventricular measurements as criteria for interventions, (Table 2) it appears prudent to adopt this evidence into clinical practice. The authors of this manuscript agree on the following approach to be considered for overall management of PHVD.
The recognition of increased intracranial pressure using clinical signs as rapid head growth, separated sutures or bulging fontanelles is important. However, waiting for late signs can lead to late interventions and the potential for worsening brain injury. Nonetheless, the development of such signs should be concerning enough to initiate interventions even with modest increases in ventricular measurements. Doppler US and NIRS may aid with decision given the alterations described previously.
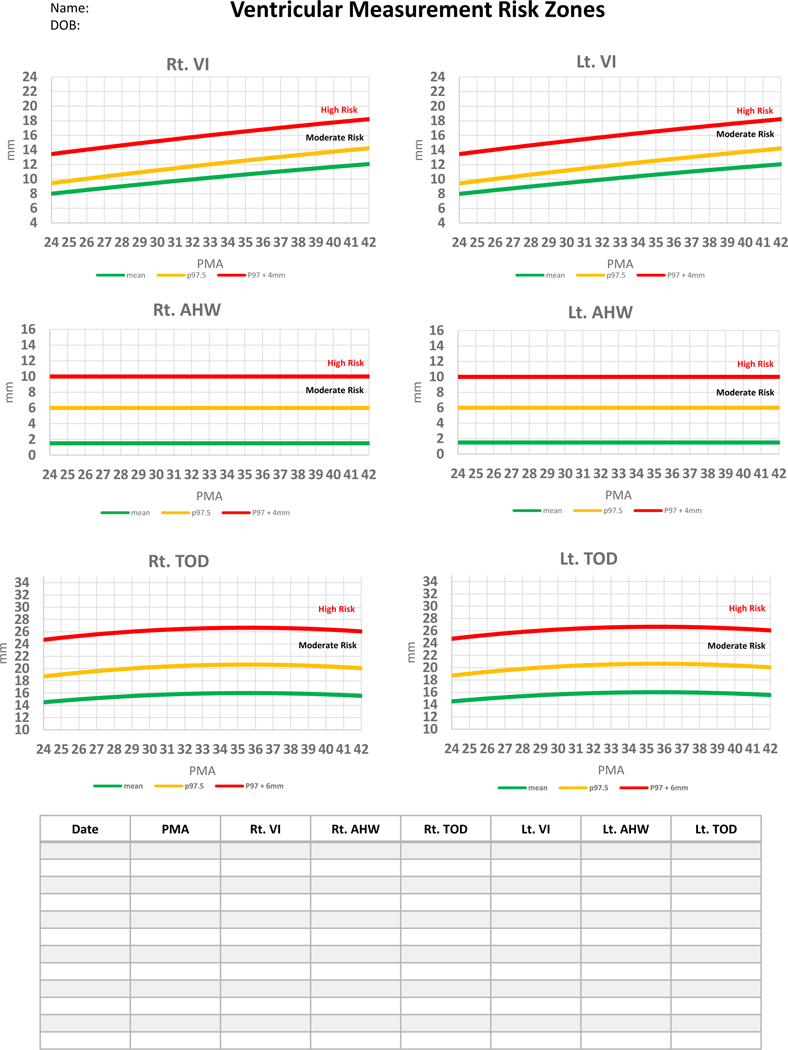
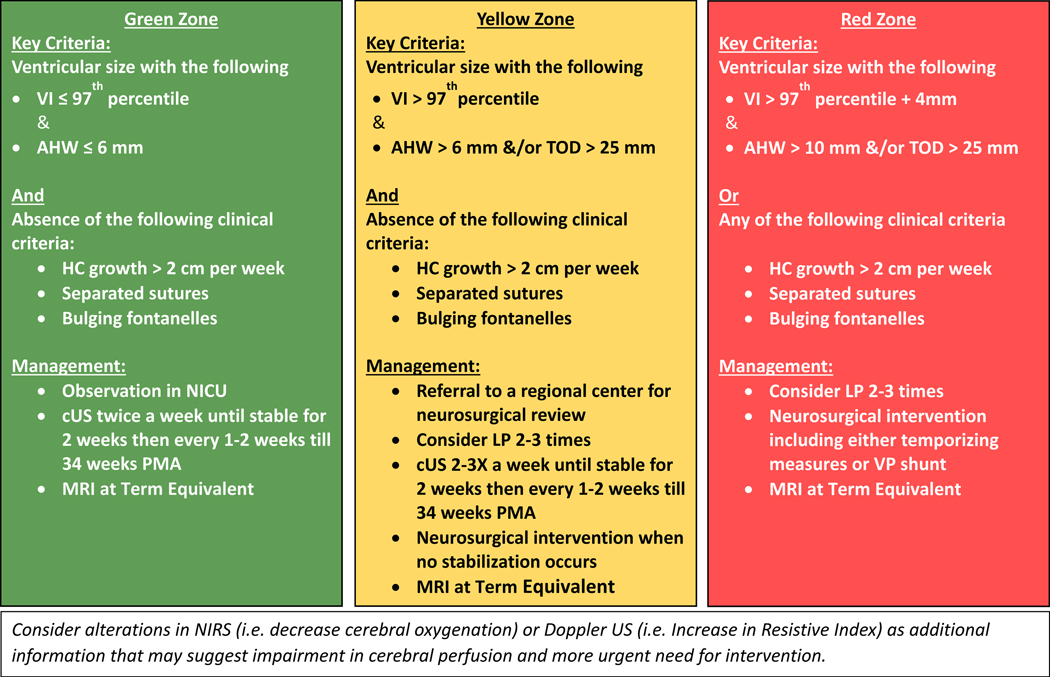
Based on these principles, we are proposing that a framework which relies on standardized ventricular measurements, clinical signs and supplementary tests can be used to assign risk categories for infants with PHVD. Based on this risk assessment, providers can determine the frequency and types of assessments, the timing of temporizing measures, neurosurgical referral and neurosurgical interventions. Recognition of the risk category is facilitated by the use of standardized ventricular measurements (namely VI, AHW and TOD) in every cUS done in infants with PHVD. A simple tool was developed from data from the study by Brouwer et al to help the clinician plot ventricular measurements over time to assess risk progress (Figure 4).45 An electronic spreadsheet is available to be downloaded, stored in secure servers and used for individual patients in two formats: one is for postmenstrual age 24–42 weeks (https://tinyurl.com/PHVD-Measures-1) and another for postmenstrual age 24–29 weeks (https://tinyurl.com/PHVD-Measures-2). This spreadsheet categorizes infants with PHVD into 3 risk groups, i.e., low, moderate or high risk.
Figure 4: A practical clinical tool to monitor commonly used ventricular measures.
Individual measures can be plotted in mm in the table as well as on the corresponding postmenstrual age in the graph to identify risk zone. VI= Ventricular Index, AHW= Anterior Horn Width, TOD= Thalamo-Occipital Diameter
In our opinion, the low-risk group (Green zone) should be monitored closely and evaluated by cUS at least twice weekly until the ventricles are stable for 2 weeks and then every 1–2 weeks until 34 weeks of postmenstrual age. A term equivalent MRI can be useful to delineate the degree of brain injury as well as any alterations in brain maturation.
Infants in the moderate-risk group (yellow zone) may benefit from neurosurgical consultation and 2–3 LPs, aiming for removal of 10 ml/kg of CSF. LPs can be started as soon as infants fulfill criteria for measurements of ventricular size, without any restriction on gestation or birth weight. cUS should be performed before and preferably also following the LP to assess the effect, and the LP should not be performed more often than once a day. cUS should be performed at least 2–3 times per week until the ventricles are stable for 2 weeks, and then every 1–2 weeks. A term equivalent MRI is also recommended.
Infants who reach the high-risk group (Red zone) also may benefit from 2–3 LPs done acutely, while planning neurosurgical interventions. Neurosurgical interventions could include ventricular reservoir or ventricular subgaleal shunt (likely after the infant’s weight is ≥700 grams) based on center preference. Interventions might also include a VP shunt, based on such factors as center preferences, age, weight, clinical condition, and CSF protein and red cell count. Success rates for endoscopic third ventriculostomy without or with choroid plexus cauterization, performed following temporizing measures, are enhanced by selecting infants who are close to or preferably well beyond term equivalent age and who have no obstruction of the prepontine cistern on preoperative MRI.92
Conclusion
PHVD in the preterm infant continues to be a significant problem associated with adverse neurodevelopmental outcomes. The mechanisms involved in brain injury are multifactorial and include cerebral ischemia, mechanical distortion and neuroinflammation secondary to brain exposure to blood products, especially iron. There is growing evidence that early interventions for CSF removal based on ventricular measurements can reduce brain injury and improve neurodevelopmental outcomes. Although advances are expected in this field, current evidence supports the stepwise approach described here to prevent and or attenuate the progression of injury and neurodevelopmental impairment.
Figure 5: Proposed risk stratification and management of infants with PHVD.
Abbreviations
- PHVD
Post-hemorrhagic ventricular dilatation
- IVH
Intraventricular hemorrhage
- cUS
cranial ultrasound
- NIRS
Near-infrared spectroscopy
- LP
Lumbar puncture
- CSF
cerebrospinal fluid
- pre-OL
pre-oligodendrocyte
- VI
ventricular index
- AHW
anterior horn width
- GMH
germinal matrix hemorrhage
- TOD
thalamo-occipital distance
- FOHR
Frontal and Occipital Horn Ratio
- MRI
magnetic resonance imaging
- VP
ventriculo-peritoneal
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, et al. Posthaemorrhagic ventricular dilatation in the premature infant: Natural history and predictors of outcome. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2002;87:F37–F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kinney HC, Volpe JJ. Organizational events In: Volpe JJ, Inder TE, Darras BT, deVries LS, duPlessis AJ, Neil JJ, et al. , editors. Volpe’s Neurology of the Newborn. Chapter 7, 6th ed. Philadelphia, PA: Elsevier; 2018. p. 145–75. [Google Scholar]
- [4].Volpe JJ. Dysmaturation of premature brain: Importance, cellular mechanisms and potential interventions. Pediatr Neurol. 2019;95:42–66. [DOI] [PubMed] [Google Scholar]
- [5].Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 2009;8:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reemst K, Noctor SC, Lucassen PJ, Hol EM. The indispensable roles of microglia and astrocytes during brain development. Front Hum Neurosci. 2016;10:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hammond TR, Robinton D, Stevens B. Microglia and the Brain: Complementary Partners in Development and Disease. Annu Rev Cell Dev Biol. 2018;34:523–44. [DOI] [PubMed] [Google Scholar]
- [8].Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. [DOI] [PubMed] [Google Scholar]
- [9].Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Devl Neurosci. 2011;29:423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134:331–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23:997–1003. [DOI] [PubMed] [Google Scholar]
- [13].Juliet PA, Frost EE, Balasubramaniam J, Del Bigio MR. Toxic effect of blood components on perinatal rat subventricular zone cells and oligodendrocyte precursor cell proliferation, differentiation and migration in culture. J Neurochem. 2009;109:1285–99. [DOI] [PubMed] [Google Scholar]
- [14].Vinukonda G, Dohare P, Arshad A, Zia MT, Panda S, Korumilli R, et al. Hyaluronidase and Hyaluronan Oligosaccharides Promote Neurological Recovery after Intraventricular Hemorrhage. J Neurosci. 2016;36:872–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garton TP, He Y, Garton HJ, Keep RF, Xi G, Strahle JM. Hemoglobin-induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage. Brain Res. 2016;1635:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert opinion on therapeutic targets. 2017;21:1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karimy JK, Reeves BC, Damisah E, Duy PQ, Antwi P, David W, et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nature reviews Neurology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guo J, Chen Q, Tang J, Zhang J, Tao Y, Li L, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res. 2015;1594:115–24. [DOI] [PubMed] [Google Scholar]
- [19].Robinson S, Conteh FS, Oppong AY, Yellowhair TR, Newville JC, Demerdash NE, et al. Extended Combined Neonatal Treatment With Erythropoietin Plus Melatonin Prevents Posthemorrhagic Hydrocephalus of Prematurity in Rats. Front Cell Neurosci. 2018;12:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497–504. [DOI] [PubMed] [Google Scholar]
- [21].Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Im GH, et al. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. PLoS One. 2015;10:e0132919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Park WS, Sung SI, Ahn SY, Sung DK, Im GH, Yoo HS, et al. Optimal Timing of Mesenchymal Stem Cell Therapy for Neonatal Intraventricular Hemorrhage. Cell Transplant. 2016;25:1131–44. [DOI] [PubMed] [Google Scholar]
- [23].McAllister JP, Guerra MM, Ruiz LC, Jimenez AJ, Dominguez-Pinos D, Sival D, et al. Ventricular Zone Disruption in Human Neonates With Intraventricular Hemorrhage. J Neuropathol Exp Neurol. 2017;76:358–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain. 2011;134:1344–61. [DOI] [PubMed] [Google Scholar]
- [25].Supramaniam V, Vontell R, Srinivasan L, Wyatt-Ashmead J, Hagberg H, Rutherford M. Microglia activation in the extremely preterm human brain. Pediatr Res. 2013;73:301–9. [DOI] [PubMed] [Google Scholar]
- [26].Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilation. Pediatr Res. 2001;49:208–12. [DOI] [PubMed] [Google Scholar]
- [27].Tortora D, Severino M, Sedlacik J, Toselli B, Malova M, Parodi A, et al. Quantitative susceptibility map analysis in preterm neonates with germinal matrix-intraventricular hemorrhage. J Magn Reson Imaging. 2018;48:1199–207. [DOI] [PubMed] [Google Scholar]
- [28].Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Del Bigio MR, Khan OH, da Silva Lopes L, Juliet PA. Cerebral white matter oxidation and nitrosylation in young rodents with kaolin-induced hydrocephalus. J Neuropathol Exp Neurol. 2012;71:274–88. [DOI] [PubMed] [Google Scholar]
- [30].Del Bigio MR, Di Curzio DL. Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS. 2016;13:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Inder TE, Perlman JM, Volpe JJ. Preterm intraventricular hemorrhage/posthemorrhagic hydrocephalus. In: Inder TE, Volpe JJ, Darras BT, de Vries LS, du Plessis A, Neil JJ, et al. , editors. Volpe’s Neurology of the Newborn. Chapter 24, 6th ed. Philadelphia, PA: Elsevier; 2018. p. 637–98. [Google Scholar]
- [32].Deren KE, Packer M, Forsyth J, Milash B, Abdullah OM, Hsu EW, et al. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;226:110–9. [DOI] [PubMed] [Google Scholar]
- [33].Xu H, Zhang SL, Tan GW, Zhu HW, Huang CQ, Zhang FF, et al. Reactive gliosis and neuroinflammation in rats with communicating hydrocephalus. Neuroscience. 2012;218:317–25. [DOI] [PubMed] [Google Scholar]
- [34].Del Bigio MR, Kanfer JN, Zhang YW. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. J Neuropathol Exp Neurol. 1997;56:1053–66. [DOI] [PubMed] [Google Scholar]
- [35].Del Bigio MR, Wilson MJ, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol. 2003;53:337–46. [DOI] [PubMed] [Google Scholar]
- [36].Strahle JM, Garton T, Bazzi AA, Kilaru H, Garton HJ, Maher CO, et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery. 2014;75:696–705; discussion 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weller RO, Wisniewski H, Ishii N, Shulman K, Terry RD. Brain tissue damage in hydrocephalus. Dev Med Child Neurol Suppl. 1969;20:1–7. [DOI] [PubMed] [Google Scholar]
- [38].Weller RO, Shulman K. Infantile hydrocephalus: clinical, histological, and ultrastructural study of brain damage. J Neurosurg. 1972;36:255–65. [DOI] [PubMed] [Google Scholar]
- [39].Ulfig N, Bohl J, Neudorfer F, Rezaie P. Brain macrophages and microglia in human fetal hydrocephalus. Brain Dev. 2004;26:307–15. [DOI] [PubMed] [Google Scholar]
- [40].Leijser LM, Miller SP, van Wezel-Meijler G, Brouwer AJ, Traubici J, van Haastert IC, et al. Posthemorrhagic ventricular dilatation in preterm infants: When best to intervene? Neurology. 2018;90:e698–e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Riva-Cambrin J, Shannon CN, Holubkov R, Whitehead WE, Kulkarni AV, Drake J, et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. Journal of neurosurgery Pediatrics. 2012;9:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ingram MC, Huguenard AL, Miller BA, Chern JJ. Poor correlation between head circumference and cranial ultrasound findings in premature infants with intraventricular hemorrhage. Journal of neurosurgery Pediatrics. 2014;14:184–9. [DOI] [PubMed] [Google Scholar]
- [43].Levene MI, Starte DR. A longitudinal study of post-haemorrhagic ventricular dilatation in the newborn. Arch Dis Child. 1981;56:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Davies MW, Swaminathan M, Chuang SL, Betheras FR. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2000;82:F218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brouwer MJ, De Vries LS, Groenendaal F, Koopman C, Pistorius LR, Mulder EJH, et al. New reference values for the neonatal cerebral ventricles. Radiology. 2012;262:224–33. [DOI] [PubMed] [Google Scholar]
- [46].Benavente-Fernandez I, Lubian-Gutierrez M, Jimenez-Gomez G, Lechuga-Sancho AM, Lubian-Lopez SP, Neonatal Neurology F. Ultrasound lineal measurements predict ventricular volume in posthaemorrhagic ventricular dilatation in preterm infants. Acta Paediatr. 2017;106:211–7. [DOI] [PubMed] [Google Scholar]
- [47].De Vries LS, van Haastert IC, Benders MJNL, Groenendaal F. Myth: Cerebral palsy cannot be predicted by neonatal brain imaging. Seminars in Fetal and Neonatal Medicine. 2011;16:279–87. [DOI] [PubMed] [Google Scholar]
- [48].Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Measurement of ventricular size: reliability of the frontal and occipital horn ratio compared to subjective assessment. Pediatr Neurosurg. 1999;31:65–70. [DOI] [PubMed] [Google Scholar]
- [49].O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: A linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–9. [DOI] [PubMed] [Google Scholar]
- [50].Radhakrishnan R, Brown BP, Kralik SF, Bain D, Persohn S, Territo PR, et al. Frontal Occipital and Frontal Temporal Horn Ratios: Comparison and Validation of Head Ultrasound-Derived Indexes With MRI and Ventricular Volumes in Infantile Ventriculomegaly. AJR Am J Roentgenol. 2019;213:925–31. [DOI] [PubMed] [Google Scholar]
- [51].van Alfen-van der Velden AA, Hopman JC, Klaessens JH, Feuth T, Sengers RC, Liem KD. Cerebral hemodynamics and oxygenation after serial CSF drainage in infants with PHVD. Brain Dev. 2007;29:623–9. [DOI] [PubMed] [Google Scholar]
- [52].Taylor GA, Madsen JR. Neonatal hydrocephalus: hemodynamic response to fontanelle compression-- correlation with intracranial pressure and need for shunt placement. Radiology. 1996;201:685–9. [DOI] [PubMed] [Google Scholar]
- [53].Norooz F, Urlesberger B, Giordano V, Klebermasz-Schrehof K, Weninger M, Berger A, et al. Decompressing posthaemorrhagic ventricular dilatation significantly improves regional cerebral oxygen saturation in preterm infants. Acta Paediatr. 2015;104:663–9. [DOI] [PubMed] [Google Scholar]
- [54].Kochan M, McPadden J, Bass WT, Shah T, Brown WT, Tye GW, et al. Changes in Cerebral Oxygenation in Preterm Infants With Progressive Posthemorrhagic Ventricular Dilatation. Pediatr Neurol. 2017;73:57–63. [DOI] [PubMed] [Google Scholar]
- [55].de Vries LS, Groenendaal F, Liem KD, Heep A, Brouwer AJ, van ‘t Verlaat E, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104:F70–f5. [DOI] [PubMed] [Google Scholar]
- [56].Whitelaw A, Lee-Kelland R. Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage. The Cochrane database of systematic reviews. 2017;4:Cd000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Group VT. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Ventriculomegaly Trial Group. Arch Dis Child. 1990;65:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].de Vries LS, Liem KD, van Dijk K, Smit BJ, Sie L, Rademaker KJ, et al. Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in The Netherlands. Acta Paediatr. 2002;91:212–7. [DOI] [PubMed] [Google Scholar]
- [59].Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648–54. [DOI] [PubMed] [Google Scholar]
- [60].Bassan H, Eshel R, Golan I, Kohelet D, Ben Sira L, Mandel D, et al. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. Eur J Paediatr Neurol. 2012;16:662–70. [DOI] [PubMed] [Google Scholar]
- [61].Cizmeci MN, Khalili N, Claessens NHP, Groenendaal F, Liem KD, Heep A, et al. Assessment of Brain Injury and Brain Volumes after Posthemorrhagic Ventricular Dilatation: A Nested Substudy of the Randomized Controlled ELVIS Trial. J Pediatr. 2019;208:191–7 e2. [DOI] [PubMed] [Google Scholar]
- [62].Cizmeci MN, Groenendaal F, Liem KD, van Haastert IC, Benavente-Fernandez I, van Straaten HLM, et al. Randomized Controlled Early versus Late Ventricular Intervention Study (ELVIS) in Posthemorrhagic Ventricular Dilatation: Outcome at 2 Years. Submitted. 2020. [DOI] [PubMed] [Google Scholar]
- [63].Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E, Mugamba J, Ssenyonga P, Donnelly R, et al. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. N Engl J Med. 2017;377:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Srinivasakumar P, Limbrick D, Munro R, Mercer D, Rao R, Inder T, et al. Posthemorrhagic ventricular dilatation-impact on early neurodevelopmental outcome. Am J Perinatol. 2013;30:207–14. [DOI] [PubMed] [Google Scholar]
- [65].Whitelaw A, Evans D, Carter M, Thoresen M, Wroblewska J, Mandera M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119:e1071–8. [DOI] [PubMed] [Google Scholar]
- [66].Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 2010;125:e852–8. [DOI] [PubMed] [Google Scholar]
- [67].Luyt K, Jary SL, Lea CL, Young GJ, Odd DE, Miller HE, et al. Drainage, irrigation and fibrinolytic therapy (DRIFT) for posthaemorrhagic ventricular dilatation: 10-year follow-up of a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Park YS, Kotani Y, Kim TK, Yokota H, Sugimoto T, Nakagawa I, et al. Efficacy and safety of intraventricular fibrinolytic therapy for post-intraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: a preliminary clinical study. Childs Nerv Syst. 2020. [DOI] [PubMed] [Google Scholar]
- [69].Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE, et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg. 2018:1–15. [DOI] [PubMed] [Google Scholar]
- [70].Christian EA, Jin DL, Attenello F, Wen T, Cen S, Mack WJ, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. Journal of neurosurgery Pediatrics. 2016;17:260–9. [DOI] [PubMed] [Google Scholar]
- [71].Eskandari R, Packer M, Burdett EC, McAllister JP 2nd. Effect of delayed intermittent ventricular drainage on ventriculomegaly and neurological deficits in experimental neonatal hydrocephalus. Childs Nerv Syst. 2012;28:1849–61. [DOI] [PubMed] [Google Scholar]
- [72].Isaacs AM, Smyser CD, Lean RE, Alexopoulos D, Han RH, Neil JJ, et al. MR diffusion changes in the perimeter of the lateral ventricles demonstrate periventricular injury in post-hemorrhagic hydrocephalus of prematurity. NeuroImage Clinical. 2019;24:102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr., Rogido M, Mitchell L, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. Journal of neurosurgery Pediatrics. 2014;14 Suppl 1:8–23. [DOI] [PubMed] [Google Scholar]
- [74].Wellons JC 3rd, Holubkov R, Browd SR, Riva-Cambrin J, Whitehead W, Kestle J, et al. The assessment of bulging fontanel and splitting of sutures in premature infants: an interrater reliability study by the Hydrocephalus Clinical Research Network. Journal of neurosurgery Pediatrics. 2013;11:12–4. [DOI] [PubMed] [Google Scholar]
- [75].Kaiser AM, Whitelaw AG. Intracranial pressure estimation by palpation of the anterior fontanelle. Arch Dis Child. 1987;62:516–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kestle JR, Riva-Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. Journal of neurosurgery Pediatrics. 2011;8:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kestle JR, Holubkov R, Douglas Cochrane D, Kulkarni AV, Limbrick DD Jr., Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. Journal of neurosurgery Pediatrics. 2016;17:391–6. [DOI] [PubMed] [Google Scholar]
- [78].Wellons JC 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni AV, Limbrick DD Jr., et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. Journal of neurosurgery Pediatrics. 2017;20:19–29. [DOI] [PubMed] [Google Scholar]
- [79].Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, Kulkarni AV. Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. Journal of neurosurgery Pediatrics. 2015;16:545–55. [DOI] [PubMed] [Google Scholar]
- [80].Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. Journal of neurosurgery Pediatrics. 2014;14:447–54. [DOI] [PubMed] [Google Scholar]
- [81].Tubbs RS, Banks JT, Soleau S, Smyth MD, Wellons JC 3rd, Blount JP, et al. Complications of ventriculosubgaleal shunts in infants and children. Childs Nerv Syst. 2005;21:48–51. [DOI] [PubMed] [Google Scholar]
- [82].Brouwer AJ, Groenendaal F, Han KS, de Vries LS. Treatment of neonatal progressive ventricular dilatation: a single-centre experience. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28 Suppl 1:2273–9. [DOI] [PubMed] [Google Scholar]
- [83].Stone SS, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. Journal of neurosurgery Pediatrics. 2014;14:439–46. [DOI] [PubMed] [Google Scholar]
- [84].Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: Predictors and prognosis. Pediatrics. 2014;134:e444–e53. [DOI] [PubMed] [Google Scholar]
- [85].Dorner RA, Allen MC, Robinson S, Soares BP, Perin J, Ramos E, et al. Early neurodevelopmental outcome in preterm posthemorrhagic ventricular dilatation and hydrocephalus: Neonatal ICU Network Neurobehavioral Scale and imaging predict 3–6-month motor quotients and Capute Scales. Journal of neurosurgery Pediatrics. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Limbrick DD Jr., Castaneyra-Ruiz L, Han RH, Berger D, McAllister JP, Morales DM. Cerebrospinal Fluid Biomarkers of Pediatric Hydrocephalus. Pediatr Neurosurg. 2017;52:426–35. [DOI] [PubMed] [Google Scholar]
- [87].Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal Stem Cells for Severe Intraventricular Hemorrhage in Preterm Infants: Phase I Dose-Escalation Clinical Trial. Stem cells translational medicine. 2018;7:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Schulz M, Buhrer C, Pohl-Schickinger A, Haberl H, Thomale UW. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. Journal of neurosurgery Pediatrics. 2014;13:626–35. [DOI] [PubMed] [Google Scholar]
- [89].d’Arcangues C, Schulz M, Buhrer C, Thome U, Krause M, Thomale UW. Extended Experience with Neuroendoscopic Lavage for Posthemorrhagic Hydrocephalus in Neonates. World neurosurgery. 2018;116:e217–e24. [DOI] [PubMed] [Google Scholar]
- [90].Kulkarni AV, Riva-Cambrin J, Rozzelle CJ, Naftel RP, Alvey JS, Reeder RW, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: a prospective study by the Hydrocephalus Clinical Research Network. Journal of neurosurgery Pediatrics. 2018;21:214–23. [DOI] [PubMed] [Google Scholar]
- [91].Riva-Cambrin J, Kestle JRW, Rozzelle CJ, Naftel RP, Alvey JS, Reeder RW, et al. Predictors of success for combined endoscopic third ventriculostomy and choroid plexus cauterization in a North American setting: a Hydrocephalus Clinical Research Network study. Journal of neurosurgery Pediatrics. 2019:1–11. [DOI] [PubMed] [Google Scholar]
- [92].Warf BC, Campbell JW, Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst. 2011;27:1063–71. [DOI] [PubMed] [Google Scholar]