Abstract
Background
The impact and consequences of the COVID-19 pandemic on people with rheumatic disease are unclear. We developed the COVID-19 Global Rheumatology Alliance Patient Experience Survey to assess the effects of the COVID-19 pandemic on people with rheumatic disease worldwide.
Methods
Survey questions were developed by key stakeholder groups and disseminated worldwide through social media, websites, and patient support organisations. Questions included demographics, rheumatic disease diagnosis, COVID-19 diagnosis, adoption of protective behaviours to mitigate COVID-19 exposure, medication access and changes, health-care access and communication with rheumatologists, and changes in employment or schooling. Adults age 18 years and older with inflammatory or autoimmune rheumatic diseases were eligible for inclusion. We included participants with and without a COVID-19 diagnosis. We excluded participants reporting only non-inflammatory rheumatic diseases such as fibromyalgia or osteoarthritis.
Findings
12 117 responses to the survey were received between April 3 and May 8, 2020, and of these, 10 407 respondents had included appropriate age data. We included complete responses from 9300 adults with rheumatic disease (mean age 46·1 years; 8375 [90·1%] women, 893 [9·6%] men, and 32 [0·3%] participants who identified as non-binary). 6273 (67·5%) of respondents identified as White, 1565 (16·8%) as Latin American, 198 (2·1%) as Black, 190 (2·0%) as Asian, and 42 (0·5%) as Native American or Aboriginal or First Nation. The most common rheumatic disease diagnoses included rheumatoid arthritis (3636 [39·1%] of 9300), systemic lupus erythematosus (2882 [31·0%]), and Sjögren's syndrome (1290 [13·9%]). Most respondents (6921 [82·0%] of 8441) continued their antirheumatic medications as prescribed. Almost all (9266 [99·7%] of 9297) respondents adopted protective behaviours to limit SARS-CoV-2 exposure. A change in employment status occurred in 2524 (27·1%) of 9300) of respondents, with a 13·6% decrease in the number in full-time employment (from 4066 to 3514).
Interpretation
People with rheumatic disease maintained therapy and followed public health advice to mitigate the risks of COVID-19. Substantial employment status changes occurred, with potential implications for health-care access, medication affordability, mental health, and rheumatic disease activity.
Funding
American College of Rheumatology.
Introduction
People with rheumatic disease are at increased risk of infection due to immune dysregulation and the use of immunosuppressive medications.1, 2 Behavioural changes that could mitigate these risks are often discussed as part of the shared decision making that occurs during the management of rheumatic diseases.3 However, at the beginning of the COVID-19 pandemic, little was known to inform discussions about the risks of COVID-19 in people with these rheumatic diseases. As a result, people with rheumatic diseases faced substantial challenges in deciding how to modify their behaviour to reduce their risk of infection with the SARS-CoV-2 virus.
The pandemic also caused substantial disruptions in health-care delivery, including the delay or cancellation of clinic visits, infusions, and procedures4 and impaired access to some antirheumatic medications because these were diverted to prevent or treat COVID-19.5 These challenges also greatly affected employment and education, and consequently, access to health insurance and the ability to obtain health care.6 Understanding the effect of the pandemic on people with rheumatic disease might help rheumatologists better address their patients' needs and inform policies to protect this potentially vulnerable population.
Research in context.
Evidence before this study
We searched PubMed for articles published up to March 1, 2020, regarding the risks of infection in patients with rheumatic disease. We included the Medical Subject Headings “rheumatology” OR “rheumatic diseases” AND “infections” AND “risk.” We did not restrict our search by language or type of publication. We found multiple studies showing that people with rheumatic disease are at increased risk of infection due to immune dysregulation and the use of immunosuppression. We then did a Medical Subject Headings search with “rheumatology” OR “rheumatic diseases AND “COVID-19” AND “behavior” OR “patient reported outcome measures” to our search algorithm and no relevant articles were found. At the beginning of the COVID-19 pandemic, little was known about the risks of COVID-19 in people with rheumatic disease or how people with rheumatic disease changed their behaviours because of the pandemic. Among this population, the impact of the pandemic on health-care access, use of health-care systems, and employment had not been well-characterised.
Added value of this study
Our study is the largest international survey of people with rheumatic disease during the COVID-19 pandemic. We found that people with rheumatic disease adhered to risk-mitigating behaviours such as physical distancing and mask-wearing and avoided potential high-risk exposures; the proportion of participants reporting a diagnosis of COVID-19 during this time period was low. Respondents largely continued their use of antirheumatic and immunosuppressive drugs. More than a quarter of respondents had changes in employment status, with decreases in the number of full-time employees while the number of those unemployed increased. Our study complements and contextualises data gathered from other sources, such as medical records, claims databases, and physician-entered registries.
Implications of all the available evidence
Understanding the behaviours and access to care among people with rheumatic disease during the early phase of the pandemic is essential to inform clinical decision making and structural changes required within health-care systems. Given the substantial changes to employment in people with rheumatic disease, policies that promote remote working might help them to continue working while avoiding potentially high-risk exposures. Future studies should investigate the long-term effects of the COVID-19 pandemic on patients, including COVID-19 vaccination, behavioral modifications, and effect on rheumatic disease activity.
We developed the COVID-19 Global Rheumatology Alliance Patient Experience Survey to assess the effect of the COVID-19 pandemic on patient-reported outcomes and health-related behaviours in people with rheumatic diseases.7 The survey was disseminated through social media, websites, and patient support organisations. It complemented a physician-entered registry of people with rheumatic disease and COVID-19 that focused on clinical outcomes.8 Using real-world data from this survey, we aimed to describe the effect of the COVID-19 pandemic on health-care access, protective health behaviours, employment, and educational opportunities in adults with rheumatic disease.
Methods
Survey development and dissemination and study participants
The COVID-19 Global Rheumatology Alliance Patient Experience Survey was developed by the COVID-19 Global Rheumatology Alliance Steering Committee, patient partners, patient organisation representatives, physicians, and researchers in March 2020.7 The purpose of the survey was to understand the effect of the COVID-19 pandemic on individuals with inflammatory or autoimmune rheumatic diseases globally.
The patient and public involvement in this study prioritised patient-valued questions and allowed perspectives of patient partners and patient organisations to direct survey development, dissemination, and interpretation to improve the quality and relevance of our research.9, 10 Patient partners were involved in the generation of the survey questions, study design, selection and development of measurement instruments, recruitment of participants to the study, contribution to manuscripts, and participation in the COVID-19 Global Rheumatology Alliance Steering Committee.7, 11 Patients and care-partners were viewed as primary stakeholders and therefore most knowledgeable about the essential themes and questions about COVID-19 for those living with rheumatic disease. A full list of all the contributors can be found in the appendix (pp 44–45).
Physicians, patients, researchers, and patient organisation representatives reviewed initial survey items to ensure the inclusion of meaningful questions and use of appropriate language sensitive to diverse cultures and belief systems. This methodology enabled rapid iteration of the survey questions to ensure focus on outcomes most relevant to the patient community and issues of importance to the rheumatologists caring for these patients.
The survey was translated by physician and patient volunteers into nine languages (English, Spanish, Arabic, Chinese (simplified and traditional), French, German, Hebrew, Italian, and Portuguese) and hosted on a Qualtrics server. Patient partners led survey dissemination.11 International patient organisations received a social media kit, including images, text, and survey links designed to explain the survey's purpose and invite participants to the study. Patient organisations disseminated the survey to their members and through social media channels. Additionally, the survey was publicly accessible from the COVID-19 Global Rheumatology Alliance website. A copy of the survey is provided in appendix (pp 1–39).
Adults age 18 years and older with inflammatory or autoimmune rheumatic diseases were eligible to participate. We included all adult respondents who completed the survey between April 3 and May 8, 2020, and provided their age, gender, country of residence, race or ethnicity (or both), rheumatic disease diagnosis, and reported their use of antirheumatic medications. Participants with and without a COVID-19 diagnosis were included. We excluded participants reporting non-inflammatory rheumatic diseases such as fibromyalgia or osteoarthritis.
The project was deemed exempt from requiring ethics review by the Boston Children's Hospital Institutional Review Board. The survey was anonymous and consent was implied by survey completion.
Survey data collection
As part of the survey, participants were required to provide information on demographics and clinical characteristics. Participants self-reported demographics, including age, gender (female, male, non-binary, prefer not to answer), country of residence, and race or ethnicity. Country of residence was grouped by WHO region.12 Race or ethnicity was grouped into mutually exclusive categories: Black, Asian (including East Asian, South Asian, and West Asian), Latin American, White, Native American or Aboriginal or First Nations, Arab, Pacific Islander, and multiple identities (participants with more than one race or ethnicity).
Participants reported tobacco smoking status (current, past, never) and selected from 22 common comorbidities, including those that had been associated with poorer outcomes from COVID-19 (appendix p 40). Individual comorbidity burden was defined by the number of comorbid conditions reported, and categorised as: none, one, two, and three or more comorbidities.
Participants were also required to report their COVID-19 status, and if believed to have the SARS-CoV-2 virus, how it was diagnosed (self-diagnosed on the basis of symptoms, diagnosed by a health-care provider on the basis of symptoms, or via laboratory testing).
The survey additionally included questions on rheumatic disease diagnoses and rheumatic disease activity. Respondents could indicate multiple rheumatic diseases. Rheumatic diseases were categorised as: rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, psoriatic arthritis, antiphospholipid syndrome, spondyloarthritis, vasculitis, other connective tissue disease, autoinflammatory disease, other arthritis, and other rheumatic disease (appendix p 40). Rheumatic disease activity was adapted from a patient global assessment of disease activity on a visual analogue scale.13 Participants were asked “considering all the ways your rheumatic disease affects you, rate how well you are doing today on the following scale,” in which 0 indicated “very well” and 10 indicated “very poor”.
Participants were required to answer questions regarding medication use and availability. Participants identified all antirheumatic medications they took within 3 months of completing the survey from a list of 23 classes of medications, with an option to report medications not listed (appendix p 40). Respondents indicated whether there were any changes to their medication use specifying: “yes, I continue to take this drug”; “no, pharmacy did not have supply”; “no, it was not effective”; or “no, I want to avoid immunosuppression.”
The survey also included questions on adaptive behaviours during the pandemic. Participants were asked whether they contacted their rheumatologist, adopted protective behaviours, and engaged in activities that could increase their risk of COVID-19. Modes of communicating with their rheumatologist included phone call, email or patient portal, telemedicine or video conference, in person visit, unable to communicate, and unnecessary to communicate. The protective behaviours included physical distancing (avoiding crowds and large groups of people), quarantining (staying home and avoiding others as much as possible), using gloves or masks, or both during social interactions, or none. Those reporting quarantining also specified whether it was self-imposed or imposed by their government. We also asked about travel to an area with many COVID-19 cases, close contact with a person with confirmed or probable COVID-19, and presence in a health-care facility where COVID-19 is managed.
Finally, the survey included questions about employment and educational status. Participants indicated their employment or student status as of Jan 1, 2020 (employed full-time, part-time, not employed looking for work, not employed not looking for work, retired, disabled, or full-time student) and whether this had changed at the time of survey completion. Full-time students specified how they were participating in classes at the time of survey completion (in the classroom, virtually on a computer, classes were cancelled, or other).
Statistical analysis
Descriptive statistics, including means and SDs, proportions and 95% CIs, were summarised. Missing data for each question were omitted. A sensitivity analysis was done to determine the effect of including respondents with missing demographics information.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
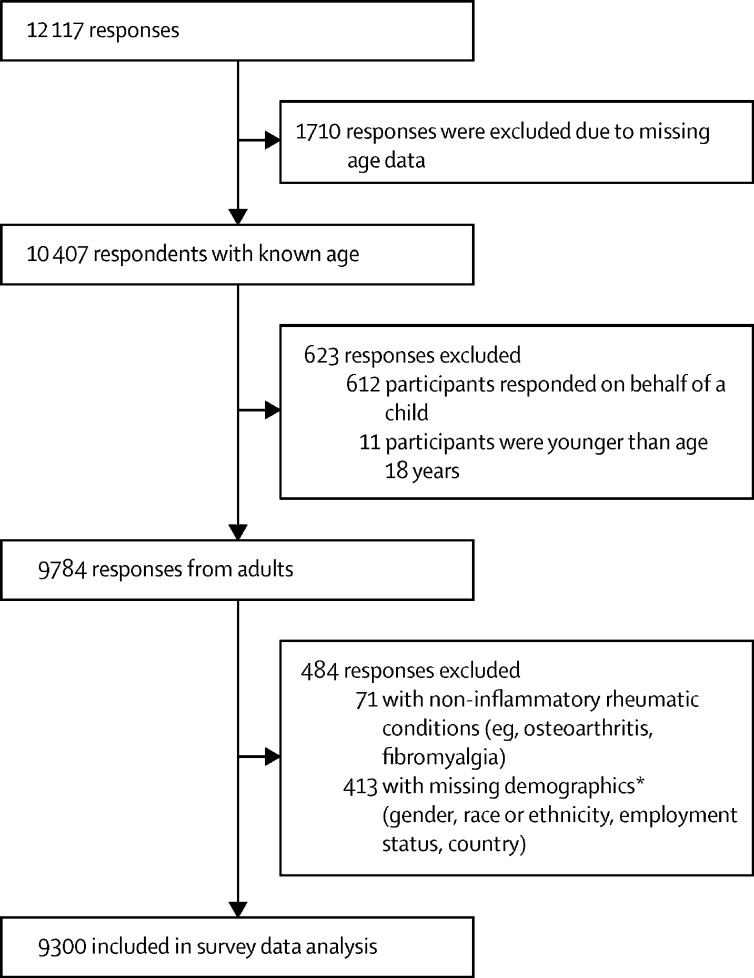
Between April 3 and May 8, 2020, 12 117 responses were received. Of these, 1710 were missing age data, and 10 407 respondents had included adequate age data. 623 responses were excluded on the basis of age, which showed that 9784 responses were received from adults. 484 responses were further excluded and 9300 responses were included in survey data analysis (figure 1 ). A sensitivity analysis showed no effect when including respondents with missing demographics information. Demographics and clinical characteristics of adult participants are outlined in the table . Additional characteristics, stratified by gender, are presented in the appendix (pp 41–42).
Figure 1.
Survey respondent inclusion and exclusion criteria
*We used these criteria as a consequence of limitations to survey design in which participants were not required to enter values for all questions, which led to questions being easily missed; after analysing the missing data pattern and the importance of patient characteristics when describing the sample, we decided to exclude for missing demographics.
Table.
Demographics and clinical characteristics of COVID-19 Global Rheumatology Alliance Patient Experience Survey respondents
| Respondents (n=9300) | |
|---|---|
| Age, years | |
| 18–29 | 966 (10·4%) |
| 30–49 | 4658 (50·1%) |
| 50–69 | 3334 (35·8%) |
| ≥70 | 342 (3·7%) |
| Gender | |
| Female | 8375 (90·1%) |
| Male | 893 (9·6%) |
| Non-binary | 32 (0·3%) |
| Race or ethnicity | |
| Arab | 131 (1·4%) |
| Asian | 190 (2·0%) |
| East Asian | 69/190 (36·3%) |
| South Asian | 113/190 (59·5%) |
| West Asian | 8/190 (4·2%) |
| Black | 198 (2·1%) |
| Latin American | 1565 (16·8%) |
| Multiple identities | 455 (4·9%) |
| Native American, Aboriginal, First Nations | 42 (0·5%) |
| Pacific Islander | 10 (0·1%) |
| White | 6273 (67·5%) |
| Other | 162 (1·7%) |
| Unsure | 154 (1·7%) |
| Prefer not to say | 120 (1·3%) |
| WHO region | |
| Region of the Americas | 6113 (65·7%) |
| European region | 2697 (29·0%) |
| Western Pacific region | 253 (2·7%) |
| Eastern Mediterranean region | 131 (1·4%) |
| African region | 84 (0·9%) |
| South-East Asian region | 22 (0·2%) |
| Rheumatic disease diagnosis* | |
| Rheumatoid arthritis | 3636 (39·1%) |
| Systemic lupus erythematosus | 2882 (31·0%) |
| Sjögren's syndrome | 1290 (13·9%) |
| Other connective tissue disease | 1119 (12·0%) |
| Spondyloarthritis (other than psoriatic arthritis) | 1155 (12·4%) |
| Vasculitis | 706 (7·6%) |
| Psoriatic arthritis | 673 (7·2%) |
| Other inflammatory arthritis | 538 (5·8%) |
| Antiphospholipid syndrome | 497 (5·3%) |
| Autoinflammatory disease | 275 (3·0%) |
| Other rheumatic disease | 444 (4·8%) |
| Antirheumatic medications* | |
| Conventional synthetic DMARDs | 6637 (71·4%) |
| Systemic glucocorticoids | 3248 (34·9%) |
| Biologic DMARDs | 2888 (31·1%) |
| Targeted synthetic DMARDs | 299 (3·2%) |
| Other | 154 (1·7%) |
| None | 615 (6·6%) |
| Patient Global Assessment of Disease Activity† | |
| Mean (SD) | 4·5 (2·5) |
| Comorbidities | |
| None | 3258/8923 (36·5%) |
| 1 comorbidity | 2832/8923 (31·7%) |
| 2 comorbidities | 1519/8923 (17·0%) |
| ≥3 comorbidities | 1314/8923 (14·7%) |
| Tobacco smoking status | |
| Current | 943 (10·1%) |
| Past | 2875 (30·9%) |
| Never | 5449 (58·6%) |
| Missing | 33 (0·4%) |
| COVID-19 status and diagnosis method | |
| Yes | 510 (5·5%) |
| Self-diagnosis | 223/510 (43·7%) |
| Physician diagnosis | 179/510 (35·1%) |
| Laboratory test confirmed | 91/510 (17·8%) |
| Not sure | 11/510 (2·2%) |
| Missing | 6/510 (1·2%) |
| No | 8790 (94·5%) |
Data are n (%) or n/N (%), unless otherwise specified. DMARDs=disease-modifying antirheumatic drugs.
Participants could indicate more than one rheumatic disease and more than one antirheumatic medication. Categorisations and groupings of comorbidities, rheumatic diseases, and medications can be found in the appendix (p 40). An extended table of the clinical characteristics can be found in the appendix (pp 41–42).
n=8962.
Responses were collected from more than 90 countries, with most respondents from the Americas (6113 [65·7%] of 9300) or Europe (2697 [29·0%]). 8375 (90·1%) respondents were women, 893 (9·6%) were men, and 32 (0·3%) identified as non-binary. The mean age of respondents was 46·1 years (SD 12·8). 6273 (67·5%) respondents identified themselves as White, 1565 (16·8%) as Latin American, 198 (2·1%) as Black, 190 (2·0%) as Asian, 42 (0·5%) as Native American or Aboriginal or First Nations. The remaining 1032 (11·1%) respondents indicated that they identified as other races or ethnicities (table). The most common rheumatic disease diagnoses were rheumatoid arthritis (3636 [39·1%]), systemic lupus erythematosus (2882 [31·0%]), and Sjögren's syndrome (1290 [13·9%]). The mean score of the patient global assessment of disease activity was 4·5 (SD 2·5). The most commonly reported antirheumatic medications taken within the previous 3 months were conventional synthetic disease modifying antirheumatic drugs (DMARDs; 6637 [71·4%]), systemic glucocorticoids (3248 [34·9%]), and biologic DMARDs (2888 [31·1%]).
At least one comorbidity was reported by 5665 (63·5%) of 8923 respondents (table). Two or more comorbidities were reported by 2833 (31·7%) respondents. The most common comorbidities were cardiovascular disease (2241 [25·1%]), pain syndromes (1901 [21·3%]), and pulmonary disease (1819 [20·4%]; appendix p 41). Current smoking was reported by 943 (10·1%) of 9300 respondents and past tobacco smoking was reported by 2875 (30·9%) respondents (table). Additionally, 348 (3·8%) of 9266 respondents reported current use of vaping or e-cigarettes, whereas 512 (5·5%) reported past use (appendix p 41).
Of the 9300 participants, 510 (5·5%) reported a COVID-19 diagnosis. Of these, 223 (43·7%) were self-diagnosed on the basis of symptoms, 179 (35·1%) were diagnosed by a health-care provider on the basis of symptoms, and 91 (17·8%) were confirmed by laboratory testing. The remaining 17 (3·3%) respondents indicated that they were unsure of how they were diagnosed or did not complete the question (table).
Communication with a rheumatologist most commonly occurred by telephone (2252 [24·3%] of 9270), followed by email or patient portal (1611 [17·4%]), office visit (ie, in person appointment; 919 [9·9%]), and telemedicine (552 [6·0%]). Other communication methods, including social media and texting, were used by 773 (8·3%) of respondents. More than a third (3291 [35·5%]) of respondents reported that they did not have any reason to contact their rheumatologist, and 1043 (11·3%) could not communicate with their rheumatologist by any method (appendix p 41).
Nearly all respondents (9266 [99·7%] of 9297) adopted at least one protective behaviour (appendix p 41). Protective measures included quarantining (staying home as much as possible; 7952 [85·5%]), physical distancing (7206 [77·5%]), and using gloves or masks, or both (4631 [49·8%]). All the listed protective measures were used by 3620 (38·9%) participants. More than half of those who quarantined were instructed to do so by their local or national governments (4056 [51·1%] of 7935). Most respondents (6921 [82·0%] of 8441) continued their antirheumatic medications as prescribed. The remaining 1520 (18·0%) participants treated with antirheumatic medications discontinued at least one of their medications for reasons including lack of efficacy, concern for immunosuppression, or diminished pharmacy supply (appendix p 42).
About a fifth of respondents (2104 [22·9%] of 9179) engaged in activities that could increase their risk of SARS-CoV-2 exposure (appendix pp 41–42). Of 9179 respondents, 1228 (13·4%) visited a health-care facility where COVID-19 had been managed, 394 (4·3%) had close contact with a confirmed or probable case of COVID-19, and 365 (4·0%) travelled to an area with a high prevalence of COVID-19. Other potential exposures were reported by 477 (5·2%) participants, including close interactions in the workplace, shopping, taking public transport, and secondary transmission from their children attending school or relatives and friends travelling. Of 2104 respondents who engaged in activities that might have increased SARS-CoV-2 exposure, 1781 (84·6%) reported one activity, while 290 (13·8%) reported two activities, and 33 (1·6%) reported three or more activities that could increase their risk of exposure.
As of Jan 1, 2020, almost half of the respondents reported that they were employed full-time (4066 [43·7%] of 9300), 1434 (15·4%) were employed part-time, while 1058 (11·4%) were not employed (including those not looking and those looking for work). 1321 (14·2%) were disabled and unable to work, 301 (3·2%) were full-time students, and 1120 (12·0%) were retired (appendix p 43).
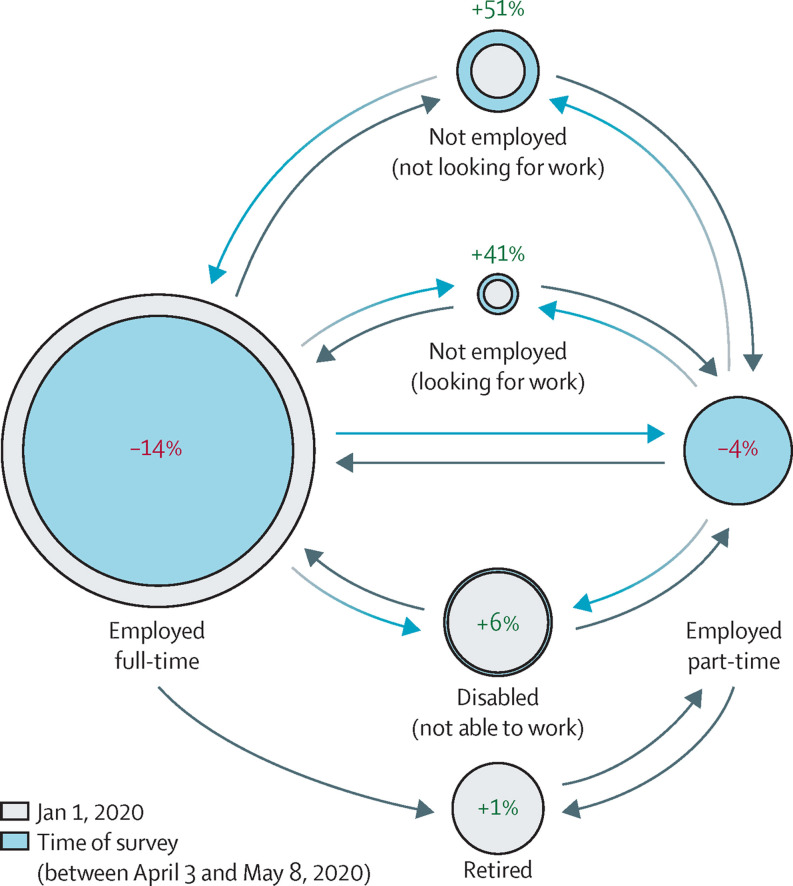
A change in employment status was reported by 27·1% (2524 of 9300) of respondents, and these transitions are depicted in figure 2 . The most common transition was from full-time employment to another category, experienced by 21·9% (552 of 2524) of respondents reporting a change in employment. The proportion of individuals who classified under the employed full-time status decreased by 13·6% (from 4066 to 3514) at the time of the survey.
Figure 2.
Employment changes reported by patients with rheumatic disease in the early stages of the COVID-19 pandemic
The spheres indicate the percentage of change noted in each employment status between Jan 1, 2020, (grey spheres) and at the time of the survey response (blue spheres). Arrow directions depict migration between the different categories. Detailed data are provided in the appendix (p 43).
Full-time students also experienced changes, with 207 (68·8%) of 301 transitioning to virtual classes, 52 (17·3%) having classes cancelled, 5 (1·7%) continuing to attend classes in person, and 35 (11·6%) reporting other changes such as having finished classes at the time of the survey (appendix p 42).
Discussion
Our study shows that almost all respondents with rheumatic disease adopted protective behaviours during the early phase of the pandemic, with most practicing physical distancing or self-isolation strategies, using masks and gloves, and avoiding activities that could increase their risk of SARS-CoV-2 exposure. Most respondents also continued their antirheumatic medications. Moreover, the pandemic had severe effects on participants' employment and education, with an increase in unemployment and most students transitioning to virtual classes.
The COVID-19 Global Rheumatology Alliance Patient Experience Survey was unique due to the international involvement of multiple stakeholders in developing questions to ensure patient-important outcomes were collected. This survey fills a gap and complements results from physician-reported registries, medical records, and claims databases by collecting data regarding patient perceptions and behavioural adaptations. Dissemination of the survey through social media and patient organisations enabled participation from people who did not access health care and would not have been captured in medical records-based studies.
In our study, most participants continued their antirheumatic medications, including immunosuppressants. This practice conforms to current recommendations for people with rheumatic conditions during the pandemic,14, 15, 16 which were not yet available at the time of the survey. The presence of rheumatic disease and the decision to continue immunosuppression might have created an increased perceived risk of infection, which perhaps explains the frequent use of protective behaviours and avoidance of potential exposures to COVID-19 in this population. Other studies have found that those with rheumatic disease are more likely to isolate than are matched friends or family controls,17 and that people taking biologic drugs are more likely to practice shielding (ie, to quarantine) than are those not taking biologics.18 Whether or not people with rheumatic disease are at increased risk of infection or complications than the general population remains unclear.19, 20 People with rheumatic diseases might be more likely to be tested for COVID-19 because of their underlying diagnoses, thus creating biases in the outcomes of many studies.21 Additionally, those with rheumatic disease might have increased prevalence of comorbidities such as chronic lung disease, a known risk factor for poor COVID-19 outcomes, which has been associated with increased risk of death among this patient population.22
Health-care systems adapted quickly to the COVID-19 pandemic, even during the early stages, with participants engaging in telemedicine as early as April, 2020. Concerningly, a portion of respondents indicated that they could not communicate with their rheumatologist, perhaps because of the closures of many clinics and delays in adapting to new types of health-care delivery. How this could magnify existing disparities in rheumatology care related to access to technology and the ability to use telemedicine remains to be elucidated.
We found a shift in employment in nearly a third of respondents, with a rise in unemployment and a decrease in full-time employment. Increases in unemployment and underemployment occurred in the USA and UK during the early months of the pandemic.23 The impact of the COVID-19 pandemic on employment in people with rheumatic disease is further complicated by work limitations and lower workforce participation already present in this population, including for younger people.24 The loss of employer-sponsored health insurance could be particularly catastrophic for those living in countries without universal health care, who might not be able to afford out-of-pocket medical costs.6 Policies that promote remote working might help people with rheumatic diseases continue working while avoiding potentially high-risk exposures.
Strengths of our study include the strong engagement of stakeholders through all phases of the research, international scope and reach, and responses from more than 9000 people with rheumatic disease. Several limitations must be acknowledged. Given the online nature of our survey, there could be limited generalisability to the general rheumatic disease patient population, although our study probably gives voice to groups that would not be included in more traditional medical studies. Individuals who had severe symptoms from COVID-19 are probably underrepresented because they were not able to take the survey. Few patients with reports of COVID-19 had confirmatory laboratory tests, and the accuracy of self-reported COVID-19 is unknown. Although these details might have increased the risk of misclassification in this study, they might also reflect the limited availability of testing early in the pandemic. There was limited male and racial and ethnic diversity within the cohort, which has been shown to affect the risk and severity of COVID-19,25, 26 although challenges to minority recruitment for research are not limited to this study. Barriers to enrollment of non-white participants in research studies include structural racism and distrust of research given the history of mistreatment of vulnerable individuals.27 The female predominance in this study probably reflects the increased prevalence of rheumatic diseases in women, as well as the increased participation of women in online studies.28 Relying on self-reported data, we cannot rule out misclassification of diagnosis or other relevant clinical or demographic data. Finally, we lacked a control population of people without rheumatic disease, so some of the findings of this study might not necessarily be attributable to the presence of rheumatic disease, but rather might reflect changes that occurred in the general population.
In summary, we describe adaptations employed by people with rheumatic disease early in the pandemic, including those aimed to reduce their perceived risk of COVID-19, as well as the disruptions in health care that occurred. The results of this international survey complement and provide a context for data gathered from other sources, such as medical records, claims databases, and physician-entered registries. The engagement of patients, physicians, and researchers to develop, disseminate, and analyse the results of this survey provides a model of collaboration among the rheumatology community. Understanding the early behaviours of people with inflammatory and autoimmune conditions is necessary to assess the effects of the pandemic on this population, and not only those who became infected with SARS-CoV-2. A far-reaching consequence of the pandemic at the time of data collection was the abrupt change to employment, and many people with rheumatic disease were faced with delayed or reduced income. Unique within the field of rheumatology, our study illustrates the direction and magnitude of employment change from Jan 1, 2020, to May 8, 2020. Further work should address the consequences of employment status changes for health-care access, medication affordability, mental health, and rheumatic disease activity. With an improved understanding of COVID-19 and the existence of patient recommendations from professional organisations, future studies should address changes in behaviours, perceptions, and concerns in this population, including COVID-19 vaccination, COVID-19 sequelae, and the long-term effect of the pandemic on patient outcomes.
Data sharing
Researchers interested in performing additional analyses from survey data are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at rheum-covid.org. For approved projects, we will be able to provide summary tables and data analyses as requested. We do not currently have IRB approval to make the raw data available to other researchers.
Declaration of interests
JSH reports grants from Childhood Arthritis and Rheumatology Research Alliance and Rheumatology Research Alliance; and personal fees from Novartis, Pfizer, and Biogen, outside of the submitted work. JWL reports grants from Pfizer, outside of the submitted work. JAS reports grants and personal fees from Bristol-Myers Squibb; and personal fees from Gilead, Inova Diagnostics, Optum, and Pfizer, outside of the submitted work. CH reports personal fees from AstraZeneca and Aurinia Pharmaceuticals, outside of the submitted work. MJL reports grants from American College of Rheumatology during the conduct of the study and consulting fees from AbbVie, Amgen, Actelion, Boehringer Ingelheim, BMS, Celgene, Gilead, Johnson & Johnson, Mallinckrodt, Novartis, Pfizer, Roche, Sandoz, Sanofi, Sobi, and UCB, outside of the submitted work. SES is supported by the Vasculitis Clinical Research Consortium and Vasculitis Foundation outside of the submitted work. KLD reports grants from Novartis, Sobi, National Institutes of Health, and Horizon Bio, outside of the submitted work. EFM reports that the Liga Portuguesa Contra as Doenças Reumaticas received support for specific activities: grants from Abbvie, Novartis, Janssen-Cilag, Lilly Portugal, Sanofi, Grünenthal SA, MSD, Celgene, Medac, Pharmakern, GAfPA, AMGEN, A Menarini Portugal; grants and non-financial support from Pfizer; and non-financial support from Grünenthal GmbH and Tilray, outside of the submitted work. DPR is the volunteer Vice President of the Canadian Arthritis Patient Alliance, which is primarily supported by independent grants from pharmaceutical companies. DPR reports consulting fees from NovoNordisk Canada and speaking fees and an honoraria from Eli Lilly Canada, outside of the submitted work. DPR also lives with rheumatoid arthritis. SB reports personal fees from Novartis, AbbVie, Pfizer, and Horizon Pharma, outside of the submitted work. RG reports personal fees from AbbVie New Zealand, Cornerstones, Janssen New Zealand; and personal fees and non-financial support from Pfizer New Zealand, (all <$10 000) outside of the submitted work. PMM reports personal fees from Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and grants and personal fees from Orphazyme, outside of the submitted work. PCR reports personal fees from Abbvie, Gilead, Lilly, and Roche; grants and personal fees from Novartis, UCB Pharma, Janssen, and Pfizer; and non-financial support from BMS, outside of the submitted work. PS reports honoraria from being a social media editor for @ACR_Journals, outside of the submitted work. ZSW reports grants from National Institutes of Health, BMS, and Sanofi; and personal fees from Viela Bio and MedPace, outside of the submitted work. JY reports personal fees from Pfizer and Eli Lilly, and grants and personal fees from Astra Zeneca, outside of the submitted work. ES is a Board Member of the Canadian Arthritis Patient Alliance, which is a patient-run, volunteer-based organisation whose activities are primarily supported by independent grants from pharmaceutical companies. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We would like to thank all the clinicians, health-care providers, and patient organisations who helped to develop and disseminate this survey. A full list of all the contributors can be found in the appendix (pp 44–45). Preliminary results were presented at the American College of Rheumatology 2020 conference. The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology, the European League Against Rheumatism, the UK National Health Service, the National Institute for Health Research, the UK Department of Health, or any other organisation.
Contributors
JSH, KK, JFS, JWL, JAS, TTM, CH, MJL, ML, SES, TS, GF, SS, and LT contributed to data collection, data quality control, data analysis, and interpretation. They drafted and revised the manuscript critically for important intellectual content and gave final approval of the version to be published. RPB, KLD, EFM, SM, MN, CAP, and DPR contributed to planning and data collection, reviewed the manuscript, and provided important intellectual content. DFLL and CLH critically revised the manuscript and provided important intellectual content. SB, WC, RG, PMM, PCR, PS, ZSW, and JY contributed to the acquisition, analysis, and interpretation of the data. They drafted and revised the manuscript critically for important intellectual content and gave final approval of the version to be published. ES directed the work, designed the data collection methods, and contributed to the analysis and interpretation of the data. ES drafted and revised the manuscript critically for important intellectual content and gave final approval of the version to be published. JSH, ES, and KK had full access to the study data and verify the credibility of the underlying data. All authors have read, revised, and approved this manuscript and had final responsibility for the decision to submit for publication.
Contributor Information
COVID-19 Global Rheumatology Alliance:
Philip C. Robinson, Suleman Bhana, Jean W. Liew, Paul H. Sufka, Namrata Singh, Richard A. Howard, Alfred H.J. Kim, Tiffany Westrich-Robertson, Emily Sirotich, Edmund Tsui, Ali Duarte-Garcia, Jeffrey A. Sparks, Herman Tam, Arundathi Jayatilleke, Maximilian F. Konig, Elizabeth R. Graef, Michael S. Putman, Reema H. Syed, Peter Korsten, Elsa Mateus, Sebastian E. Sattui, Zachary S. Wallace, Upton A. Laura, Kilian Adam, Yu Pei Eugenia Chock, Douglas W. White, Geraldine T. Zamora, Lisa S. Traboco, Aarat M. Patel, Rebecca Grainger, Manuel F. Ugarte-Gil, Milena A. Gianfrancesco, Isabelle Amigues, Catalina Sanchez-Alvarez, Laura Trupin, Lindsay R. Jacobsohn, Richard P. Beesley, Bimba F. Hoyer, Pedro M. Machado, Kavita Makan, Laure Gossec, Chaudhary Priyank, Jan Leipe, Beth Wallace, Sheila T. Angeles-Han, Ibrahim A. Almaghlouth, Wysham D. Katherine, Anthony S. Padula, Francis Berenbaum, Erin M. Treemarcki, Rashmi Sinha, Laura B. Lewandowski, Kate Webb, Kristen J. Young, Inita Bulina, Sebastian Herrera Uribe, Tamar B. Rubinstein, Marc W. Nolan, Elizabeth Y. Ang, Swamy R. Venuturupalli, Jonathan S. Hausmann, Maureen Dubreuil, Cecilia N. Pisoni, Micaela A. Cosatti, Jose Campos, Julia F. Simard, Richard Conway, Tiffany M. Peterson, Carly O. Harrison, Christele Felix, Dawn P. Richards, Laurie Proulx, Akpabio A. Akpabio, Angus B. Worthing, Lynn R. Laidlaw, Pankti Reid, Candace A. Palmerlee, Maria I. Danila, Lotfi-Emran Sahar, Ngo Q. Linh, Arnav Agarwal, Paul Studenic, David F.L. Liew, Maggie J. Larche, Serena A.M. Mingolla, Erick A. Zamora, Saskya S. Angevare, Rashmi R. Sinha, Karen L.W. Durrant, Andrea Peirce, Emily C. Somers, Laura C. Cappelli, Brittany A. Frankel, Bharat Kumar, Sonia D. Silinsky Krupnikova, Jorge A. Rosario Vega, Jourdan Frankovich, Ruth Fernandez-Ruiz, Marcela Posada Velásquez, Su-Ann Yeoh, Maria Marino, Michal Nudel, Chrisiaan Scott, Cecilia Rodríguez, Ana I. Martín Mancheño, Philip Seo, Rocío V. Gamboa-Cárdenas, Victor R. Pimentel-Quiroz, Cristina Reátegui-Sokolova, Mari Kihara, Chung M.A. Lin, Dheera Kattula, Girgis Laila, Loreto Carmona, John Wallace, Jinoos Yazdany, Wendy Costello, Monique C. Gore-massy, Laura-Ann Tomasella, and Moré A. Kodek
Supplementary Material
References
- 1.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 2.Barber MRW, Clarke AE. Systemic lupus erythematosus and risk of infection. Expert Rev Clin Immunol. 2020;16:527–538. doi: 10.1080/1744666X.2020.1763793. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 4.Dejaco C, Alunno A, Bijlsma JWJ, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218697. published online Nov 6. [DOI] [PubMed] [Google Scholar]
- 5.Mendel A, Bernatsky S, Thorne JC, Lacaille D, Johnson SR, Vinet É. Hydroxychloroquine shortages during the COVID-19 pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217835. published online May 20. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal D, Fowler EJ, Abrams M, Collins SR. Covid-19—implications for the health care system. N Engl J Med. 2020;383:1483–1488. doi: 10.1056/NEJMsb2021088. [DOI] [PubMed] [Google Scholar]
- 7.Sirotich E, Dillingham S, Grainger R, et al. capturing patient-reported outcomes during the covid-19 pandemic: development of the COVID-19 Global Rheumatology Alliance Patient Experience Survey. Arthritis Care Res. 2020;72:871–873. doi: 10.1002/acr.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianfrancesco MA, Hyrich KL, Gossec L, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton CB, Leese JC, Hoens AM, Li LC. Framework for advancing the reporting of patient engagement in rheumatology research projects. Curr Rheumatol Rep. 2017;19:38. doi: 10.1007/s11926-017-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358 doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirotich E. Putting patients at the centre of COVID-19 research. Nature. 2020 doi: 10.1038/d41586-020-02226-3. published online July 24. [DOI] [PubMed] [Google Scholar]
- 12.WHO Definition of regional groupings. http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/
- 13.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Cl. Arthritis Care Res. 2011;63:S14–S36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- 14.Landewé RBM, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 15.Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 2. Arthritis Rheumatol. 2020;72:e1–12. doi: 10.1002/art.41437. [DOI] [PubMed] [Google Scholar]
- 16.Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704–1716. doi: 10.1016/j.jaad.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooijberg F, Boekel L, Vogelzang EH, et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2:e583–e585. doi: 10.1016/S2665-9913(20)30286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahil SK, Yates M, Langan SM, et al. Risk-mitigating behaviours in people with inflammatory skin and joint disease during the COVID-19 pandemic differ by treatment type: a cross-sectional patient survey. Br J Dermatol. 2021;185:80–90. doi: 10.1111/bjd.19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79:1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. published online Oct 13. [DOI] [PubMed] [Google Scholar]
- 21.Ferri C, Giuggioli D, Raimondo V, et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin Rheumatol. 2020;39:3195–3204. doi: 10.1007/s10067-020-05334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell DNF, Blanchflower DG. US and UK labour markets before and during the COVID-19 crash. Natl Inst Econ Rev. 2020;252:R52–R69. [Google Scholar]
- 24.Berkovic D, Briggs AM, Ayton D, Parker C, Ackerman I. Arthritis-related work outcomes experienced by younger to middle-aged adults: a systematic review. Occup Environ Med. 2021;78:225–236. doi: 10.1136/oemed-2020-106640. [DOI] [PubMed] [Google Scholar]
- 25.Abrams EM, Szefler SJ. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020;8:659–661. doi: 10.1016/S2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianfrancesco MA, Leykina LA, Izadi Z, et al. Association of race and ethnicity with COVID-19 outcomes in rheumatic disease: data from the COVID-19 Global Rheumatology Alliance Physician Registry. Arthritis Rheumatol. 2021;73:374–380. doi: 10.1002/art.41567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirotich E, Hausmann JS. Removing barriers and disparities in health: lessons from the COVID-19 pandemic. Nat Rev Rheumatol. 2021;17:125–126. doi: 10.1038/s41584-020-00524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton L, Batterham PJ, Fassnacht DB, Kay-Lambkin F, Calear AL, Hunt S. Recruiting for health, medical or psychosocial research using Facebook: Systematic review. Internet Interv. 2016;4:72–81. doi: 10.1016/j.invent.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers interested in performing additional analyses from survey data are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at rheum-covid.org. For approved projects, we will be able to provide summary tables and data analyses as requested. We do not currently have IRB approval to make the raw data available to other researchers.