Abstract
In the context of the coronavirus disease 2019 (COVID-19) pandemic, the global healthcare community has raced to find effective therapeutic agents against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To date, dexamethasone is the first and an important therapeutic to significantly reduce the risk of death in COVID-19 patients with severe disease. Due to powerful anti-inflammatory and immunosuppressive effects, dexamethasone could attenuate SARS-CoV-2-induced uncontrolled cytokine storm, severe acute respiratory distress syndrome and lung injury. Nevertheless, dexamethasone treatment is a double-edged sword, as numerous studies have revealed that it has significant adverse impacts later in life. In this article, we reviewed the literature regarding the adverse effects of dexamethasone administration on different organ systems as well as related disease pathogenesis in an attempt to clarify the potential harms that may arise in COVID-19 patients receiving dexamethasone treatment. Overall, taking the threat of COVID19 pandemic into account, we think it is necessary to apply dexamethasone as a pharmaceutical therapy in critical patients. However, its adverse side effects cannot be ignored. Our review will help medical professionals in the prognosis and follow-up of patients treated with dexamethasone. In addition, given that a considerable amount of uncertainty, confusion and even controversy still exist, further studies and more clinical trials are urgently needed to improve our understanding of the parameters and the effects of dexamethasone on patients with SARS-CoV-2 infection.
Keywords: Dexamethasone, COVID-19, Patients, SARS-CoV-2, Necrosis of the femoral head, Depression, Diabetes
Key Summary Points
| It is necessary to apply dexamethasone as a therapy in critical COVID-19 patients, but its adverse side effects cannot be ignored. |
| Through a variety of molecular pathways, dexamethasone can interfere with normal organ functions and cause numerous clinical manifestations, intensifying the risk and severity of sequelae of COVID-19 disease, such as osteonecrosis of the femoral head, hypertension and diabetes. |
| Regular follow-up and evaluation of physical conditions in accordance with the time line are crucial for COVID-19 patients who have received dexamethasone treatment. |
Introduction
After emerging in December 2019, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread across the world and led to high morbidity and mortality. Globally, as of 18 April 2021, there have been 140 million confirmed cases, including 3 million deaths, reported to the World Health Organization (WHO) [1]. The clinical spectrum of COVID-19 appears to be wide, ranging from asymptomatic infection to critical illness. The common symptoms of infection are fever (94%), cough (79%), fatigue (23%), loose stools (16%), anosmia and dysgeusia (70–84%) [2, 3]. Sepsis (59%) is the most frequently observed complication, followed by respiratory failure (54%), acute cardiac injury (23%) and septic shock (20%) [4]. Cytokine storm, clots, disseminated intravascular coagulation and thrombocytopenia have also been reported [5, 6]. Notably, advanced age and several comorbidities including impaired renal function and thrombosis are reportedly associated with a worse disease course and increased mortality rate [7–9]. Hence, there is an urgent need to develop effective therapies to prevent the progression of disease. An array of drugs and therapeutic agents used to be considered as having potential efficacy against SARS-CoV-2, encompassing antiviral agents (remdesivir, chloroquine or hydroxychloroquine, azithromycin and lopinavir), blood-derived products (convalescent plasma and immunoglobulin products), anticoagulants (heparin and low-molecular-weight heparin) and immunomodulators (corticosteroids, interferons, interleukin-1 [IL-1] inhibitors, IL-6 inhibitors and kinase inhibitors) [10–14]. Disappointingly, on 15 October 2020, the interim results from the Solidarity trial coordinated by WHO indicated that remdesivir, hydroxychloroquine, lopinavir and interferon regimens appeared to have little or no effect on hospitalized COVID-19 patients [15], while dexamethasone, declared as the world’s first treatment to significantly reduce the risk of death [16], is still the only therapeutic agent shown to be effective for patients with severe disease [17].
As a broad-spectrum immunosuppressor, the potency and duration of action of dexamethasone are greater and longer than those of cortisone. Its immunosuppressive effect is exerted through a variety of ways. In B cells, glucocorticoids impair upstream B cell receptor and Toll-like receptor 7 signaling while promoting significant upregulation of the genes encoding the immunomodulatory cytokine IL-10 [18]. Similarly, dexamethasone exposure causes defects in cell division for both CD4 and CD8 T cells and dampens T cell receptor signaling and cytokine expression [19, 20]. Moreover, in dendritic cells, dexamethasone could inhibit antigen presentation by increasing expression and activity of Na+/Ca2+ exchanger [21]. In parallel, the anti-inflammatory actions of glucocorticoids occur by decreasing the gene transcription of pro-inflammatory cytokines (IL-1, TNF and IL-6), chemokines and adhesion molecules [22]. Studies have demonstrated that SARS-CoV-2-induced uncontrolled cytokine storm [23], severe acute respiratory distress syndrome (ARDS) and multiorgan failure, which are the main causes of COVID-19-related mortality, can be attenuated by the use of dexamethasone [24].
At the beginning of the pandemic, the use of corticosteroids was considered controversial, although Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial provided evidence that dexamethasone 6 mg once daily for 10 days reduced mortality in COVID-19 patients receiving oxygen therapy [16]. Some investigators noted that early corticosteroid use might lead to increased virus replication and thus higher viral loads [25]. Meanwhile, there is an analysis suggesting that the initiation of treatment in the second week of symptom onset, when the immunopathological phenomenon dominates, may be beneficial [16]. However, many researchers pointed out that, in practice, the symptom onset is usually impossible to ascertain, and the signs of severity often appear late. In a study that assessed the association between the dexamethasone initiation time and mortality benefits, Sulaiman et al. (2021) found survival benefits with the early initiation in critically ill COVID-19 patients [26]. The WHO panel concluded that it is preferable that critical COVID-19 patients receive corticosteroids (even if within 7 days of symptom onset) but non-critical patients do not (even if after 7 days of symptoms onset) [27]. Although described as ‘low-dose dexamethasone therapy,’ the dose of 6 mg per day is five to six times higher than that for therapeutic glucocorticoid replacement [28–31]. At this dose, patients suffer side effects such as skin thinning, weight gain, osteoporosis, hypertension and diabetes [20]. Hyperglycemia, gastrointestinal hemorrhage and psychosis were also considered related to dexamethasone treatment in the RECOVERY trial [16]. Recently, Weng and colleagues claimed that patients with gastrointestinal sequelae at 90 days were treated more often with corticosteroids and proton pump inhibitors than were patients without such sequelae [32]. Hence, many researchers are cautious about its extensive use for critically ill patients. Based on results of previous studies in SARS patients, avascular necrosis and osteoporosis were more likely to occur among patients with higher-dose steroid therapy. For example, in a retrospective study of 539 patients with steroid treatment following SARS, the incidence of steroid-induced osteonecrosis of the femoral head (ONFH) was 24% [33]. Notably, all the meta-analyses found patients on corticosteroid treatment were more likely to have secondary infections such as bacterial infection, invasive fungal infection or exacerbation of pre-existing conditions [34]. Due to the immunosuppressive effect, a strong association exists between corticosteroid use and respiratory infectious disease (e.g., active tuberculosis and pulmonary aspergillosis) [35, 36]. Similarly, Strongyloides hyperinfection/dissemination in low- and middle-income countries induced by dexamethasone treatment cannot be ignored [37]. Moreover, short-term or protracted dexamethasone therapy might contribute to other potential side effects, such as neuromuscular weakness, psychiatric symptoms and even iatrogenic Cushing syndrome [38–40].
Although dexamethasone is recommended in critical patients infected by COVID-19, it is essential for us to develop deeper insights into the potential damage of dexamethasone in view of the large number of patients. Therefore, we searched PubMed, Scopus, the Web of Science and Google Scholar up to April 2021 for papers on the effects of dexamethasone therapy on different organs and summarized current understanding of the mechanisms of complications and sequelae induced by dexamethasone in this review. This will assist medical professionals in managing COVID-19 patients treated with dexamethasone and directing post-acute and long-term follow-up. Additionally, we hope this will provide a valuable basis for future studies. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Musculoskeletal System
Long-term or excessive use of glucocorticoids is the most common non-traumatic cause of ONFH [41, 42] and secondary osteoporosis [43, 44] in patients (Fig. 1). Owing to the use of glucocorticoids during the SARS epidemic in 2003, some patients had varying degrees of ONFH [33], and the risk of ONFH showed an aggravating trend with the increase of cumulative doses and treatment durations of steroids in SARS patients [45]. In 2014, Guo et al. found that more male SARS patients (51/129, 40%) were diagnosed with ONFH compared to female patients (79/410, 19%) [33]. Recently, a large-scale global statistical analysis revealed that while males and females are at equivalent risk of SARS-CoV-2 infection, male sex is associated with a higher risk for the development of severe disease as measured by intensive therapy unit admission [46], which indicated that male patients are more likely to receive dexamethasone treatment and suffer from ONFH. Therefore, to further explore the risk of ONFH in COVID-19 patients, we sum up the mechanisms of dexamethasone-induced ONFH. Its pathogenesis is mainly related to the differentiation of mesenchymal stem cells (MSCs) and osteoblast apoptosis. MSCs have the potential to differentiate into cells of mesodermal lineage, such as adipocytes, osteocytes and chondrocytes [47]. Yin et al. proved that dexamethasone can directly induce MSCs to differentiate into adipocytes [48]. On the one hand, adipogenesis of MSCs leads to excessive accumulation of marrow fat and increased intraosseous pressure, thus inducing venous stasis, arterial obstruction and eventually ischemic osteonecrosis [48, 49]. On the other hand, dexamethasone inhibits osteogenesis of stem cells, reducing the ability to reshape bone and repair necrotic bone and accounting for the onset of osteonecrosis [48, 50]. In addition, previous studies have demonstrated that dexamethasone could induce apoptosis of MSCs in a time- and concentration-dependent manner, which is probably the mechanism of pathogenesis of steroid-induced ONFH [51, 52]. Excessive use of dexamethasone can promote the apoptosis of osteoblasts through multiple signaling mechanisms. First, forkhead box transcription factor O1 (FOXO1) targets genes involved in apoptosis, autophagy and cell cycle arrest [53]. Dexamethasone could upregulate FOXO1 expression, inhibit the viability of osteoblasts and promote apoptosis [54]. Second, the phosphatidylinositol 3-kinase/protein kinase 3 (PI3K/AKT) signaling pathway controls many cellular functions by participating in the signal transduction pertaining to proliferation, survival and motility [55]. Dexamethasone can inhibit the activation of the PI3K/AKT pathway in osteoblasts by suppressing the expression of p-PI3K and p-AKT, thereby inducing osteoblast apoptosis [56]. Third, after dexamethasone treatment, the expression of glycogen synthase kinase 3β (GSK3β) in osteoblasts is significantly upregulated, which can induce mitochondrial apoptotic and lead to ONFH [56, 57]. Zhu et al. claimed that miR-124 accumulation following circHIPK3 downregulation appears to be the primary mechanism of dexamethasone-induced cytotoxicity and programmed necrosis in human osteoblasts [58]. Therefore, to prevent dexamethasone-induced ONFH, corticosteroids should be applied only to patients in critical cases at low-to-moderate doses and short courses. In the 1st year after prescription of corticosteroids, patients with suspected ONFH should receive periodic magnetic resonance imaging examination during follow-up to diagnose and treat the disease in the early stage [59].
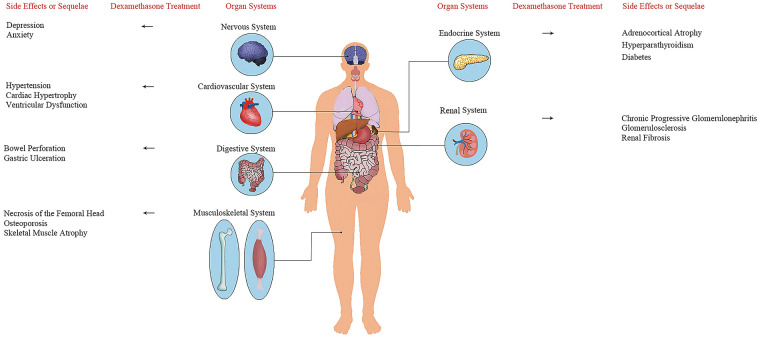
Fig. 1.
Organ systems affected by dexamethasone and the side effects or sequelae
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture [60]. As mentioned before, glucocorticoids cause apoptosis of osteoblasts and a depletion of the osteoblastic cell population, accounting for the reduction in bone formation and trabecular width [61]. On the flip side, following dexamethasone treatment, the decline in levels of the antiresorptive molecule osteoprotegerin and the increase in levels of the osteoclastogenesis-inducing molecule, the receptor activator of nuclear factor-κB ligand, promote bone resorption by osteoclasts [62]. Taken together, these changes lead to glucocorticoid-induced osteoporosis, mainly via reduced bone formation. More recently, there have been several discoveries of key proteins that have further increased our understanding of the molecular mechanism. Dexamethasone-induced osteoporosis can be caused by suppression of the canonical Wnt signal. Secreted frizzled-related proteins (SFRPs) could compete with membrane-bound frizzled proteins for Wnt binding [63], and dickkopf-1 (Dkk-1) also mediates the inhibition of Wnt signal [64]. Then, several studies have reported dexamethasone increases the expression of Dkk-1 and SFRP1 and represses Wnt/β-catenin signaling in human osteoblasts to reduce mineral density and trabecular bone volume [64–67]. Additionally, dexamethasone can downregulate the expression of matrix marker biglycan [68], and the lack of biglycan contributes to the reduction in trabecular bone volume, mineral deposition rate and bone formation rate [69].
Skeletal muscle atrophy occurs also as a side effect of dexamethasone treatment [70, 71], leading to severe muscle weakness, inactivity and reduced quality of life for the patients [72]. In general, muscle atrophy results from the imbalance between protein synthesis and degradation. Qin et al. observed that the upregulation of myostatin gene expression caused by dexamethasone was associated with the myostatin gene promoter and glucocorticoid responsive element along the promoter [73], and the increased myostatin led to the changes in ultrastructure of skeletal muscle, including the inhibition of myoblast proliferation and induction of muscle atrophy [74, 75]. Moreover, dexamethasone induces depletion of myosin heavy chain protein and the upregulation of muscle RING-finger protein-1 [70, 76], which affects muscle integrity by increasing protein breakdown of an important component of the sarcomere and results in muscle atrophy [67]. Concomitantly, it has been reported that dexamethasone could induce mitochondrial dysfunction, bringing about ATP deprivation and subsequently AMP-activated protein kinase activation, which further activates the FOXO3/Atrogenes and ultimately leads to protein degradation as well as muscle atrophy [77].
Cardiovascular System
As a common cardiovascular disease, hypertension is also the most frequent comorbidity (51%) in COVID-19 patients, followed by diabetes (19%) and atrial fibrillation (11%) [78]. At the same time, patients with severe COVID-19 infection commonly have a history of hypertension. Wu et al. (2020) reported that compared with COVID-19 patients without ARDS, patients who developed ARDS had a higher proportion of comorbidities such as hypertension (14% and 27%, respectively) and diabetes (5% and 19%, respectively) [79]. In Italy, almost 75% of patients who have died from COVID-19 had hypertension [80]. It is worth noting that dexamethasone, the first drug shown to reduce deaths from the coronavirus disease [81], can induce hypertension in clinical studies (Table 1) and animal experiments (Table 2) through a variety of mechanisms [82, 83]. On the one hand, dexamethasone enhances vasoconstriction. A recent study has found that dexamethasone increased synthesis of catecholamines by inducing the transcription of the rate-limiting enzyme tyrosine hydroxylase, and excessive catecholamine levels induce direct vasoconstriction [84]. Increasing calcium influx in vascular smooth muscle is also a way in which dexamethasone causes hypertension [85]. On the other hand, dexamethasone-induced hypertension is associated with reduction of vasodilating mediators such as prostacyclin, nitric oxide (NO) and hydrogen sulfide (H2S). There have been reports indicating that dexamethasone can inhibit the biosynthesis of prostaglandins via the inhibition of phospholipase A2 activity [86–88]. Schafer et al. claimed dexamethasone could reduce NO production by means of several mechanisms including induction of oxidative stress as well as downregulation of cationic amino acid transporter-1 and endothelial NO synthase [89]. H2S has been proposed as a candidate for endothelium-derived hyperpolarizing factor, involved in inducing vasodilation. H2S biosynthesis could be inhibited by dexamethasone via the impairment of cystathionine-β-synthase and cystathionine-γ-lyase expression [90]. The vascular endothelial glucocorticoid receptor (GR) plays a critical role in mediating blood pressure response to steroids [91]. Although there is no report confirming the use of dexamethasone in the treatment of COVID-19 leads to hypertension, we still suggest follow-up, evaluation and close monitoring of blood pressure after recovery from SARS-CoV-2 infection, especially for the hypertensive patients who received dexamethasone treatment.
Table 1.
Organ systems of human affected by dexamethasone treatment: symptoms and sequelae
| Organ system affected | Symptom/sequelae | Subjects’ characteristic | Dosage | Disease incidence | Onset time | Precaution/therapy | Monitoring indicator or method | References |
|---|---|---|---|---|---|---|---|---|
| Musculoskeletal system | Necrosis of the femoral head | Multiple myeloma patients | 16.7 mg/kg × 56 days (median) | 7% | 11.1 months (median) | Bisphosphonates, vitamin E, anticoagulants and vasodilators | MRI | [42, 59] |
| Skeletal muscle atrophy | Hemiplegic patients | 24 mg/day × 4 days | - | Within 10 days |
1. Physical exercises 2. Vitamin D and calcium supplementation |
1. Urine creatine levels 2. Muscle biopsy 3. Ultrasonography |
[71, 198, 199] | |
| Cardiovascular system | Hypertension | Infants with a birth weight of 714–1920 g (median 1087 g) and CLD | 0.6 mg/kg/day × 3 days + 0.3 mg/kg/day × 3 days + taper the dose over a 3-week period | 41% | Within 2 weeks | Exercise training |
1. Blood pressure 2. Electrocardiogram |
[82, 83, 200, 201] |
| Pediatric ALL patients | 6 mg/m2/day × 5 days | 6% | 4 days | |||||
| Ventricular hypertrophy | PI with a birth weight of 500–2054 g (median 815 g) | 0.4–0.6 mg/kg/day × 3 weeks | 94% | 2–3 days |
1. Curcumin 2. Statins |
1. Echocardiogram 2. MiR‐30a, MiR‐181b, VEGF‐B |
[92, 100, 101, 202–204] | |
| PI with CLD | 0.5 mg/kg/day (taper in a standardized fashion over a total 42-day course) | 57% | Within 6 weeks | |||||
| PI with BPD | 0.5 mg/kg/day (taper at successive 3-day intervals during the next 5 to 6 weeks) | - | Within 1 week | |||||
| Digestive system | Bowel perforation | PI with a birth weight of 501–1000 g | 0.15 mg/kg/day × 3 days + 0.10 mg/kg/day × 3 days + 0.05 mg/kg/day × 2 days + 0.02 mg/kg/day × 2 days | 13% | Within 2 weeks |
1. Oral PGE2 analogs 2. Breast feeding |
CT | [124, 205, 206] |
| Renal system | Renal calcification | PI with a birth weight of 440–990 g and CLD | 3.8 mg/kg | 83% | 26 days | - | Sonography | [130, 131] |
| Nervous system | Persistent nerve injury | Patients undergoing surgical procedures distal to the tibial meta-diaphyseal junction | 3–4 mg | 65% | 2 weeks | - | Sensory neurological examination | [149] |
| Endocrine system | Adrenal insufficiency | Early B-cell lineage acute lymphoblastic leukemia patients | 0.2 mg/kg/day × 28 days | 100% | 29 days | Taper doses of glucocorticoids | Adrenal stimulation testing | [156] |
| Diabetes | Subjects are relatives of non-insulin-dependent diabetic patients | 4 mg/day × 5 days | 20% | 4–5 days |
1. Exercise, diet therapy and oral hypoglycemic drugs (blood glucose level < 200 mg/dl) 2. Insulin therapy (blood glucose level ≥ 200 mg/dl) |
1. Plasma glucose and insulin concentrations 2. OGTT 3. IVGTT 4. Hemoglobin A1c tests |
[171, 172, 207] | |
| Non-diabetic patients with newly diagnosed gastrointestinal cancer | 10–12 mg/day × 1 day + 7–8 mg/day × 2 days (+ 7-8 mg/day × 1 day) (administer every 2–4 weeks in at least three cycles) | 22% | 3 months |
MRI magnetic resonance imaging, CLD chronic lung disease, ALL acute lymphoblastic leukemia, PI preterm infants, BPD bronchopulmonary dysplasia, MiR microRNA, VEGF vascular endothelial growth factor, PGE2 prostaglandin E2, CT computed tomography, OGTT oral glucose tolerance test, IVGTT intravenous glucose tolerance test
Table 2.
Organ systems of animal models affected by dexamethasone treatment: symptoms and sequelae
| Organ system affected | Symptom/sequela | Species | Dosage | Onset time | Pathogenic mechanism | References |
|---|---|---|---|---|---|---|
| Musculoskeletal system | Osteoporosis | Female mice (BALB/c) | 1 mg/kg/day × 3 weeks | 3 weeks | Osteocalcin decrease and leptin increase | [62] |
| Female rats (Sprague-Dawley) | 0.6 mg/kg/3 days × 12 weeks | 12 weeks | Biglycan, LRP5, OPG and RUNX2 downregulation; Col1α1 upregulation | [68] | ||
| Skeletal muscle atrophy | Female mice (Kun-Ming) | 20 mg/kg/day × 8 days | 8 days | Myostatin promoter activity upregulation | [73] | |
| Male mice (C57BL/J) | 5 mg/kg/day × 18 days | 6 days | Activation of AMPK/FOXO3/agtrogenes signaling | [77] | ||
| Cardiovascular system | Hypertension | Male rats (Fisher 344) | 0.03, 0.3 or 3 mg/kg/day × 10 days | 8, 6 or 5 days | Increased transcription of TH | [84] |
| Male rabbits (New Zealand) | 1000 mg/kg | Within 6 weeks | Increased transmembrane Ca2+ in VSM | [85] | ||
| Neonatal rats (Wistar) | 0.5 mg/kg + 0.3 mg/kg + 0.1 mg/kg | 3 months | - | [127] | ||
| Male rats (Wistar) | 1.5 mg/kg/day × 8 days | 2 days | Impaired expression of CBS and CSE | [90] | ||
| Cardiac hypertrophy | Female neonatal rats (Wistar) | 0.5 mg/kg + 0.3 mg/kg + 0.1 mg/kg | 45 weeks | Increased promoter methylation in the CcnD2 gene | [94, 96] | |
| Neonatal rats (Wistar) | 0.5 mg/kg + 0.3 mg/kg + 0.1 mg/kg | Within 15 months | - | [127] | ||
| Arrhythmias | Male rats (Wistar) | 2 mg/kg/day × 7 days | 1 week | Increased ROS generation | [103] | |
| Diastolic dysfunction | Male rats (Wistar) | 35 mg/kg/day × 15 days | 15 days | Impaired calcium handling and calcineurin signaling pathway activation | [102] | |
| Ocular system | Glaucoma | Mice (C57BL/6) | 0.1% × (~ 20 μl) × 3 times/day × 20 weeks | 20 weeks | Myocilin, actin and ECM proteins increase | [110] |
| Digestive system | Gastric ulceration | Male rats (Wister) | 1 mg/kg | 26 h | Inhibit prostaglandin synthetase and peroxidase | [121] |
| Renal system | Chronic progressive glomerulonephritis | Neonatal rats (Wistar) | 0.5 mg/kg + 0.3 mg/kg + 0.1 mg/kg | Within 15 months | - | [127] |
| Glomerulosclerosis | 32 weeks | Accumulation of inflammatory factors | [128] | |||
| Renal fibrosis | ||||||
| Reduction of nephron | 8 and 24 weeks | |||||
| 4 weeks | Suppression of mitotic activity | [129] | ||||
| Nervous system | Anxiety | Male albino mice | 10 mg/kg | 0.5 h | Dysregulation of the HPA axis | [136] |
| Male rats (Sprague-Dawley) | 5 mg/kg/day × 7 days | 1 week | Excessive CREB phosphorylation and BDNF upregulation | [138] | ||
| Depressive behavior | Male mice (C57BL/6 J) | 4 mg/kg/day × 21 days | 20 days | GR mRNA decrease | [137] | |
| Male mice (ICR) | 60 mg/kg/day × 21 days | 22 days | GR protein expression reduction | [143] | ||
| Cerebral edema | Male rats in SE (Sprague-Dawley) | 2 mg/kg | 2 days | - | [150] | |
| Rats with acidosis (Sprague-Dawley) | 3 mg/kg | 4 h | AQP-1–mediated pathways | [151] | ||
| Endocrine system | Adrenocortical atrophy | Female rats (Wistar) | 0.15 mg/kg/day × 7 days | Within 1 week | Inhibition of ACTH synthesis and secretion | [160] |
| Hyperglycemia | Mice (C57BL/6 J) | 1 mg/kg/2 days × 2 months | Within 2 months | GR/KLF9/PGC1α signaling pathway | [181] | |
| Diabetes | Rats (Wistar) | 5 mg/kg/day × 24 days | Within 5 days | Insulin resistance | [182] | |
| Female rats Zucker (fa/fa) | 0.2–0.4 mg/kg × 24 days | Promptly |
LRP-5 low-density lipoprotein5, OPG osteoprotegerin, RUNX2 runt-related transcription factor 2, AMPK AMP-activated protein kinase, FOXO3 forkhead box O 3, TH tyrosine hydroxylase, VSM vascular smooth muscle, CBS cystathionine-β-synthase, CSE cystathionine-γ-lyase, CcnD2 gene cyclinD2 gene, ROS reactive oxygen species, ECM extracellular matrix, HPA hypothalamic-pituitary-adrenal, CREB anti-cAMP responsive element binding protein, BDNF brain-derived neurotrophic factor, GR glucocorticoid receptor, SE status epilepticus, AQP-1 aquaporin-1, ACTH adreno-cortico-tropic-hormone, KLF9 Krüppel-like factor 9, PGC1α peroxisome proliferator-activated receptor γ coactivator 1 α
A clinical trial suggested retardation of heart growth among dexamethasone-treated infants compared with control infants [92]. Cardiomyocytes can only divide within a limited time after birth, and once the proliferation of cardiomyocytes is inhibited during this period, it will have a negative repercussion on the total number of cardiomyocytes later in life [93]. The use of dexamethasone in newborn rats increased promoter methylation in the cardiomyocytes CcnD2 gene, which causes the decrease of D2 protein and inhibition of cardiomyocyte proliferation [94, 95]. Eventually, neonatal dexamethasone treatment in rat pups leads to a permanent decrease in heart weight, as well as reduced number, hypertrophy and early degeneration of cardiomyocytes during adult life [93, 96]. Since most COVID-19-positive newborns are mildly affected with cases of severe disease being very rare [97] and dexamethasone is mainly shown to be effective against the novel coronavirus for severe patients, we believe that dexamethasone is not suitable for the overwhelming majority of neonates. During the treatment of 66 babies with SARS-CoV-2 infection in a UK survey, only 2 (3%) were treated with corticosteroids [97]. Meanwhile, we recently indicated that SARS-CoV-2 infection poses a great threat to pregnant women and fetuses [98], and according to the above survey, of total 66 neonates, 16 (25%) were premature babies [97]. Bensley et al. claimed that cardiomyocyte proliferation can be inhibited by premature birth, which may adversely affect heart growth, cardiac function, functional reserve and repair ability throughout postnatal life [99]. Therefore, dexamethasone treatment may bring about more serious damage to the heart in preterm infants [100, 101].
Recent evidence suggests that dexamethasone treatment in adults could result in hypertension, pathologic cardiac remodeling, cardiac hypertrophy associated with maladaptive remodeling and ultimate ventricular dysfunction [102, 103]. Cardiac hypertrophy, considered an adaptive response, allows heart to withstand glucocorticoid-induced hypertension [102]. Roy et al. (2009) and De et al. (2011) reported that excess of dexamethasone treatment could induce cardiac hypertrophy, myocardial fibrosis, hypoxia and ventricular dysfunction via angiotensin II signaling pathway [104, 105]. Pathologic heart remodeling occurs at the same time as the enhanced collagen synthesis, which leads to interstitial fibrosis [104]. Fibrosis is directly responsible for reduced blood flow to the heart and increase in cell apoptosis [102]. Additionally, myocardial fibrosis increases the stiffness of the myocytes, causing systolic or diastolic disorders [106]. However, Macedo et al. demonstrated that a short-term therapeutic regimen of dexamethasone will not decrease ventricular contractility [103]. In terms of the effect of dexamethasone on heart rate, cardiac fibrosis impairs the electrical conduction and subsequent generation of reentry circuits [107, 108]. By increasing sympathetic modulation, but reducing the parasympathetic one, dexamethasone induces autonomic imbalance, which may make the dexamethasone-treated animals more susceptible to develop harmful forms of ventricular arrhythmias through increasing reactive oxygen species generation [103].
Ocular System
Several ophthalmic complications, such as glaucoma and cataracts, have been demonstrated to be probably linked with the use of corticosteroids. Most patients (88%) with steroid-induced glaucoma experienced an increase in intraocular pressure (IOP), a major associated risk factor leading to glaucoma [109]. Similarly, the murine model of dexamethasone-induced glaucoma exhibited an elevation of IOP, structural and functional loss of retinal ganglion cells and axonal degeneration [110] (Table 2). This is mainly because dexamethasone treatment can increase the deposition of myocilin, actin and extracellular matrix proteins, which are relevant to the induction of endoplasmic reticulum stress. It may cause dysfunction of the trabecular meshwork, resulting in the elevated resistance to aqueous humor outflow and subsequent IOP [111–113].
The impact of corticosteroids on the incidence of cataracts remains a source of much controversy and considerable debate [114]. Posterior subcapsular cataract (PSC) has been reported in a few adults and children treated with beclomethasone dipropionate or dexamethasone aerosol, but the risk seems to be much lower than when taking these corticosteroids systemically [115]. Moreover, patients taking inhaled corticosteroids were observed to have a risk that appeared negligible, even if high doses were used [116]. Contrarily, another review claimed that exposure to inhaled corticosteroids increased the prevalence of PSC about twofold [117]. There is even a third view, which argues that no firm link exists between them [118]. Further research is necessary to help elucidate the association between dexamethasone treatment and the incidence of PSC.
Digestive System
Compared with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2-induced adverse effects on the gastrointestinal tract are of particular concern [119]. Patients with COVID-19 were prone to develop gastrointestinal symptoms such as nausea and diarrhea, which still bothered 44% of COVID-19 survivors at 90 days after discharge from hospital [32], even lasting ≥ 6 months [120].
Even worse, it has been reported that dexamethasone makes the gastric mucosa susceptible to ulceration, but the mechanism of the ulcerogenic action remains controversial. Specifically, an earlier study indicated that dexamethasone diminishes gastroprotection and damages the mucosa by inhibiting the activity of prostaglandin synthetase and peroxidase, respectively [121]. However, Filaretova et al. held that long-lasting maintenance of blood glucose level accompanied by the signs of catabolic effects may be the reason for pathologic ulcerogenic caused by dexamethasone treatment [122].
By thinning the circumferential smooth muscle and making the bowel wall more vulnerable, dexamethasone could increase the risk of bowel perforation [123]. Stark et al. claimed that early treatment with dexamethasone has no effect on mortality or chronic lung disease but is associated with spontaneous gastrointestinal perforation and decreased growth in preterm infants [124]. In addition, dexamethasone inhibits small intestinal growth via both increased degradation and decreased synthesis of protein [125]. When dexamethasone was given to growing rats, there was a significant decrease in the weights of the stomach, small intestine and colon [126].
Renal System
Neonatal dexamethasone treatment might increase the risk of renal damage in adulthood. The number of glomeruli and kidney weight were lower in neonatal rats with dexamethasone administration, and the kidneys showed signs of chronic progressive glomerulonephritis with glomerulosclerosis and extensive renal fibrosis, presumably because of an early inflammatory trigger that elicits a persistent pro-fibrotic process [127–129]. On the other hand, in clinical studies, dexamethasone could contribute to renal calcification formation by increasing urinary calcium excretion [130], with nephrocalcinosis occurring in 15 (83%) of 18 infants [131] (Table 1).
Nervous System
A survey of 402 adults surviving COVID-19 revealed that 42% of them suffered from anxiety, 40% from insomnia, 31% from depression, 28% from post-traumatic stress disorder and 20% from obsessive-compulsive symptoms, while females and younger patients suffered from higher levels of depression and sleep disturbances [132]. These psychiatric consequences can result from one or several combined factors. Due to the neurotropic properties with neuroinvasive activity [133], SARS-CoV-2 could induce neuronal injuries via directly infecting the central nervous system. Besides, cytokine storm involved in the immune response to coronaviruses may cause neuroinflammation, which indirectly leads to psychopathologic sequelae [134]. Simultaneously, the psychological impact of patients’ fear of severe illness with a very high risk of death, uncertainty about future, stigma, traumatic memories of severe illness and social isolation in an intensive care unit setting cannot be ignored [132, 135].
Similarly, dexamethasone treatment can be associated with neuropsychiatric diseases and neurotoxicity. Peripheral administration of dexamethasone induces biphasic effects on anxiety-related behaviors: anxiolytic effects at low and anxiogenic effects at high doses [136]. According to a report from the US Food and Drug Administration, about 4% of 50,000 dexamethasone users had developed severe anxiety as an adverse effect of therapy [137]. A finding has indicated that hyperphosphorylation of cAMP-responsive element-binding protein and reduced expression of brain-derived neurotrophic factor in the cerebral cortex might be involved in high levels of anxiety-like behavior in dexamethasone-treated rats [138]. Besides, dexamethasone-treated mice demonstrated a host of depression-like behaviors, such as increased time of immobility in the forced swim test and a reduced preference for saccharin consumption. The following mechanisms are considered: first, dexamethasone treatment can cause insufficient cell energy supply through glucose inhibition [139], thereby affecting the regulation of glutamate release and reuptake. Calcium-dependent proteases, triggered by increased glutamate-mediated transmission, could cause degeneration of cytoskeletal proteins and lipases, possibly generating free radicals [140], which leads to neuronal damage and depression [141]. The second possible explanation is the hyperfunction of the hypothalamic-pituitary-adrenal axis (HPAA) caused by dexamethasone treatment, which is mainly attributed to the decreased GR mRNA [137] and protein [142, 143] expressions and consequent impairment of GR-mediated negative feedback [144]. The raised level of cortisol in the blood will exacerbate depression by impairing brain functions, such as neuronal survival, neuronal excitability, neurogenesis and memory acquisition [144]. Notably, Skupio et al. have found that the co-chaperone FK506 binding protein 51 and serum-and glucocorticoid-inducible-kinase-1 proteins increased in the prefrontal cortex, hippocampus and striatum of mice treated with dexamethasone [137], which could regulate GR sensitivity [145], mediate glucocorticoid effects on neuronal function and contribute to major depressive disorder [146].
It is worth mentioning that peripheral nerve block is often used for postoperative analgesia; however, the pain relief lasts only a few hours [147]. As dexamethasone could prolong the analgesic duration by inducing vasoconstriction, it has been used in peripheral nerve block as an adjuvant [148]. By contrast, a recent study reported a twofold increased risk of persistent neurologic symptoms when perineural dexamethasone was applied after foot and ankle surgery [149] (Table 1). Besides, dexamethasone is widely used in clinics for alleviating cerebral edema. However, Duffy et al. (2014) found that after status epilepticus induced by lithium-pilocarpine, regional administration of dexamethasone (2 mg/kg) led to increased transverse magnetization relaxation time at 2 days and reduced hippocampal volumes at 3 weeks, representing aggravated cerebral edema and brain injury, respectively [150]. Another study observed that under acidotic conditions, dexamethasone also worsened the cerebral edema, which could be attenuated by selective blockage of aquaporin-1 channels with HgCl2 [151]. Therefore, although recent studies demonstrated dexamethasone could slow Huntington’s disease progression [152] and showed protective effects against Parkinson [153] and Alzheimer’s disease-related cognitive impairments in mice [154], there is an urgent need to continue to monitor the potential influence of dexamethasone administration on mental state and damage to nerves when it is used in COVID-19 patients.
Endocrine System
A range of mechanisms that contributes to endocrine disorders has been reported in association with the use of dexamethasone, one of the most unpredictable of which is the inhibition of the HPAA. Dexamethasone mainly binds to GRs in the pituitary, where it inhibits the expression of proopiomelanocortin as well as secretion of adreno-cortico-tropic-hormone (ACTH) and subsequent adrenocortical cortisol [155, 156]. Simultaneously, compared with other preparations with shorter half-lives, dexamethasone, as the most effective ACTH suppressant with a longer half-life, can lead to more serious HPAA inhibition [157, 158]. For example, in cancer patients receiving chemotherapy, adrenal response suppression and adrenal insufficiency have been reported after dexamethasone use [156, 159] (Table 1). Additionally, low-dose dexamethasone administered chronically could give rise to partial adrenocortical atrophy in rats [160]. There is evidence that the suppressive effects of dexamethasone at the hypothalamic-pituitary level are not only confined to ACTH, but the serum levels of thyroid-stimulating hormone (TSH) and prolactin (PRL) could be reduced by directly restraining the anterior pituitary [161]. Dexamethasone also attenuates the stimulation of the release of TSH and PRL by thyroid-releasing hormone [161]. Concurrently, there is a decrease in serum-3, 3′, 5-triiodothyronine (T3) and thyroxine (T4) serum levels with dexamethasone treatment, which is probably the consequence of the inhibitory effect of dexamethasone on TSH secretion by the pituitary, and a direct inhibitory effect on thyroid release of T3 and T4 cannot be neglected [161]. Therefore, more detailed studies are needed to better determine the mechanism involved in these effects.
Changes in growth hormone (GH), insulin-like growth factor (IGF), melatonin and parathyroid hormone levels are associated with dexamethasone-induced endocrine disorders. Jux et al. demonstrated that GH- or IGF-1-stimulated growth plate chondrocyte growth is dose-dependently blunted by dexamethasone [162]. Melatonin is considered an output signal mediated by the circadian system. Dexamethasone could diminish melatonin synthesis by reducing the expression of the key enzymes such as tryptophan hydroxylase, arylalkylamine N-acetyltransferase and hydroxyindole-O-methyltransferase [163]. In parallel, previous studies have found that dexamethasone can increase parathyroid hormone synthesis, which may be an important pathogenic role in persisting hyperparathyroidism [164, 165].
It is well known that in skeletal muscle and adipocytes, insulin stimulates the translocation of glucose transporter (GLUT) 4 from intracellular vesicles to the cell membrane for glucose uptake [166]. As soon as insulin binds to its receptor, the receptor undergoes tyrosine phosphorylation and recruits insulin receptor substrates (IRSs) for tyrosine phosphorylation. Once phosphorylated, IRSs bind to and activate PI3K, acting as a molecular switch to phosphorylate downstream protein kinase B (PKB) [167]. Akt substrate of 160 kDa (also called AS160 or TBC1D4), which is phosphorylated by activated PKB, plays a crucial role in regulating GLUT4 transport [168]. In addition, PKB could inhibit glycogen synthase (GS) activity by mediating glycogen synthase kinase 3 (GSK-3) phosphorylation [169, 170].
Dexamethasone, as an exogenous glucocorticoid, can significantly influence the glucose metabolism in the human body [171, 172]. (1) In adipocytes, dexamethasone treatment affects the normal absorption of glucose by reducing the expression level of GLUT1 protein. Meanwhile, dexamethasone therapy decreases PKB expression and insulin-stimulated phosphorylation and downregulates GS expression in adipocytes [166]. In muscle, treatment with dexamethasone can not only reduce insulin-mediated PI3K and PKB activation but also increase the phosphorylation sites of GS [173, 174]. Besides, under dexamethasone treatment, the insulin-stimulated GLUT4 translocation to the cell surface decreases without altering the GLUT4 protein in total lysates in muscle and adipose tissue [175, 176]. All of these may lead to a decrease in insulin-stimulated glucose uptake, which causes insulin resistance (IR). (2) Moreover, it has been reported that elevated plasma free fatty acids (FFAs) could induce IR [177], and dexamethasone increases FFA content by interfering with fatty acid metabolism [166]. Studies have found that long-term incubation of soleus muscle strips with FFAs impaired insulin-stimulated PKB and reduced glucose uptake and glycogen synthesis [178]. Another possible mechanism involves peroxisome proliferator-activated receptor (PPAR) [179], a transcription factor activated by FFA, and Bernal-Mizrachi et al. have proven that human hepatocytes treated with dexamethasone induced PPARA gene expression and identified hepatic activation of PPAR-α as a mechanism underlying dexamethasone-induced IR [180]. Therefore, the increase in circulating FFA caused by dexamethasone use may make an important contribution to muscle IR [166]. (3) Furthermore, our previous work suggests that dexamethasone induces the expression of Krüppel-like factor 9 (KLF9) in the liver, which plays a critical role in the regulation of hepatic glucose metabolism. KLF9 may regulate IR via KLF9/PGC1α/TRB-3 signaling pathway and promote hepatic gluconeogenesis and hyperglycemia. Conversely, the lack of KLF9 alleviated hyperglycemia induced by dexamethasone treatment [181]. Then, due to the impaired function of insulin-stimulated glucose uptake in peripheral tissues and/or the weakened effect of insulin to suppress the liver from producing endogenous glucose, dexamethasone-induced IR can result in hyperglycemia and diabetes [182], the current common side effects in acute care settings such as emergency rooms and urgent care centers [180, 183]. (4) In addition, Guo et al. found that dexamethasone could induce apoptosis of pancreatic β cells through activation of GSK-3β [184].
Diabetes has seriously affected the prognosis of patients with COVID-19, and according to data from Wuhan, compared with non-diabetic patients, diabetic patients have more complications and shorter overall survival time [185]. Possible explanations for this phenomenon are as follows: (1) generally, infectious diseases are more common and/or severe in diabetic patients since the hyperglycemic environment can lead to immune dysfunction, vascular disease, neuropathy and decrease in antibacterial activity of the digestive tract [186]. (2) Acute hyperglycemia has been shown to upregulate the expression of angiotensin-converting enzyme (ACE) 2 while chronic hyperglycemia reduces ACE2 expression [187]. Recently, Wijnant et al. demonstrated increased expression of ACE2 protein in the bronchi and alveoli of diabetic patients may affect the infectivity and clinical outcome of COVID-19 [188]. (3) In the case of uncontrolled hyperglycemia, the abnormally increased glycosylation of glycosylated ACE2 and the viral spike protein may promote the virus binding and inflammation [189]. (4) The fourth underlying mechanism that may explain the link between COVID-19 and diabetes involves dipeptidyl peptidase-4 (DPP-4/CD26) [190], acting as a potential receptor for SARS-CoV-2 [191]. Compared with nondiabetic subjects, DPP4 expression is enhanced on blood T lymphocytes from type 2 diabetic patients [192]. Interestingly, in 2019, Kulcsar et al. found the diabetic DPP4H/M mice, which could express human DPP4 in the nonciliated epithelial cells and alveolar type 2 cells, exhibited more severe clinical symptoms characterized by a prolonged period of weight loss and clinical disease with a delay in the initiation of inflammation in the lung and slower inflammatory resolution after infection with MERS-CoV [193]. In addition, researchers discovered that COVID-19 may also cause hyperglycemia. The potential pathways are as follows: (1) ACE2 is expressed at high levels in pancreatic islet cells [194], and in 2003 SARS produced a transient impairment of pancreatic islet cell function. Similarly, SARS-CoV-2 may also impair β-cell insulin secretion, causing hyperglycemia, or exacerbate pre-existing diabetes [195]. (2) COVID-19 infection is accompanied by increases of many cytokines, which can induce or exacerbate IR [196].
In summary, dexamethasone has a remarkable effect on glucose homeostasis in the body, accounting for hyperglycemia and diabetes, which are risk factors for COVID-19 and adversely affect prognosis. Simultaneously, COVID-19 infection can contribute to hyperglycemia. Based on the above evidence, we make the following recommendations. (1) Blood sugar changes of COVID-19 patients treated with dexamethasone should be strictly monitored. After stopping dexamethasone therapy, IR will fall. Therefore, it is necessary to adjust insulin dose to avoid hypoglycemia. (2) More and more type 2 diabetes patients may have an increased risk for pronounced inflammatory responses, including cytokine storms, so screening for excessive inflammation is essential to improve the prognosis. (3) Regular follow-up is crucial for preventing new-onset diabetes, which may be caused by the virus and dexamethasone, and hemoglobin A1c (HbA1c), a glycosylated hemoglobin formed by the specific binding of glucose to the N-terminal valine of the hemoglobin β chain, is recommended as an annual assessment indicator for this process [197].
Conclusion
In conclusion, the impacts of COVID-19 are not just confined to the lungs, but lead to the involvement of almost all the organs of the body, including heart, brain, kidney and intestines. While dexamethasone can reduce the mortality in treating critically ill patients with COVID-19, consequences for various organ systems have also been reported. Through a variety of molecular pathways, dexamethasone can interfere with normal organ functions and cause numerous clinical manifestations, which may further intensify the risk and severity of sequelae of COVID-19 infection, such as ONFH, hypertension and diabetes. Hence, we suggest close monitoring of blood pressure, HbA1c and other necessary parameters when managing COVID-19 patients treated with dexamethasone and taking timely measures. Furthermore, regular follow-up and evaluation of physical conditions according to the monitoring indicators provided in this article are crucial for patients after recovery from SARS-CoV-2 infection.
Acknowledgements
Funding
This work was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2020QC100) and Innovation and Entrepreneurship Training Program for College Students of Jining Medical University (grant no. cx2019033). The Rapid Service Fee was funded by the Natural Science Foundation of Shandong Province (grant no. ZR2020QC100) and Innovation and Entrepreneurship Training Program for College Students of Jining Medical University (grant no. cx2019033).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Chen F conceived the idea, analyzed the data, and drafted the manuscript; Hao L, Zhu S and Yang X contributed towards the conception, wrote part of the article; Shi W, Zheng K, Wang T and Chen H proofread the manuscript and contributed to editing; all authors provided critical review and approved the final manuscript before submission.
Disclosures
Fei Chen, Lanting Hao, Shiheng Zhu, Xinyuan Yang, Wenhao Shi, Kai Zheng, Tenger Wang and Huiran Chen have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.World Health Organization. WHO Coronavirus Disease (COVID19) Dashboard 2021. https://covid19.who.int/. Accessed 20 Apr 2021.
- 2.Ianiro G, Porcari S, Settanni CR, et al. Letter: prevalence and patterns of gastrointestinal symptoms in a large Western cohort of patients with COVID-19. Aliment Pharmacol Ther. 2020;52:902–903. doi: 10.1111/apt.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou JJ, Movassaghi M, Gordy D, et al. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol. 2021;2:2. doi: 10.17879/freeneuropathology-2021-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ombrello MJ, Schulert GS. COVID-19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome? Transl Res. 2021 doi: 10.1016/j.trsl.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahu KK, Cerny J. A review on how to do hematology consults during COVID-19 pandemic. Blood Rev. 2021;47:100777. doi: 10.1016/j.blre.2020.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Gennaro F, Vittozzi P, Gualano G, et al. Active pulmonary tuberculosis in elderly patients: A 2016–2019 retrospective analysis from an Italian referral hospital. Antibiotics. 2020;9:489. doi: 10.3390/antibiotics9080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Castelnuovo A, Bonaccio M, Costanzo S, et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30:1899–1913. doi: 10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Gennaro F, Marotta C, Storto M, et al. SARS-CoV-2 transmission and outcome in neuro-rehabilitation patients hospitalized at neuroscience hospital in Italy. Mediterr J Hematol Infect Dis. 2020;12:e2020063. doi: 10.4084/MJHID.2020.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Castelnuovo A, Costanzo S, Antinori A, et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: the multicenter Italian CORIST Study. Thromb Haemost. 2021 doi: 10.1055/a-1347-6070. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alghamdi AN, Abdel-Moneim AS. Convalescent plasma: a potential life-saving therapy for Coronavirus Disease 2019 (COVID-19) Front Public Health. 2020;8:437. doi: 10.3389/fpubh.2020.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingraham NE, Lotfi-Emran S, Thielen BK, et al. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8:544–546. doi: 10.1016/S2213-2600(20)30226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consortium WHOST, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid-19—Interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. [DOI] [PMC free article] [PubMed]
- 16.Group RC. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xinhua. Trial finds therapeutics including remdesivir have 'little or no' effect on COVID-19 patients: WHO. 2020. http://www.xinhuanet.com/english/2020-10/17/c_139446018.htm. Accessed 20 Jan 2021.
- 18.Franco LM, Gadkari M, Howe KN, et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J Exp Med. 2019;216:384–406. doi: 10.1084/jem.20180595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cain DW, Cidlowski JA. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Immunol. 2020;20:587–588. doi: 10.1038/s41577-020-00421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heise N, Shumilina E, Nurbaeva MK, et al. Effect of dexamethasone on Na+/Ca2+ exchanger in dendritic cells. Am J Physiol Cell Physiol. 2011;300:C1306–C1313. doi: 10.1152/ajpcell.00396.2010. [DOI] [PubMed] [Google Scholar]
- 22.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdin SM, Elgendy SM, Alyammahi SK, Alhamad DW, Omar HA. Tackling the cytokine storm in COVID-19, challenges and hopes. Life Sci. 2020;257:118054. doi: 10.1016/j.lfs.2020.118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentice RE, Al-Ani A, Christensen B. Managing COVID-19 in patients with inflammatory bowel disease: navigating unprecedented challenges. Intern Med J. 2021;51:284–287. doi: 10.1111/imj.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulaiman KA, Alhubaishi A, Juhani OA, et al. Early versus late use of dexamethasone in critically ill patients with covid-19: a multicenter, prospective cohort study. Research Square. 2021. 10.21203/rs.3.rs-349677/v1.
- 27.World Health Organization. Therapeutics and COVID-19: living guideline, 31 March 2021. No. WHO/2019-nCoV/therapeutics/2021.1. 2021. https://apps.who.int/iris/bitstream/handle/10665/340374/WHO-2019-nCoV-therapeutics-2021.1-eng.pdf?sequence=1. Accessed 30 Apr 2021.
- 28.Tang C, Wang Y, Lv H, Guan Z, Gu J. Caution against corticosteroid-based COVID-19 treatment. Lancet. 2020;395:1759–1760. doi: 10.1016/S0140-6736(20)30749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayman G, Lumb AN, Kennon B, et al. Dexamethasone therapy in COVID-19 patients: implications and guidance for the management of blood glucose in people with and without diabetes. Diabetic Med. 2021;38:e14378. doi: 10.1111/dme.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6:344–346. doi: 10.1016/S2468-1253(21)00076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo KJ, Zhao FC, Guo Y, Li FL, Zhu L, Zheng W. The influence of age, gender and treatment with steroids on the incidence of osteonecrosis of the femoral head during the management of severe acute respiratory syndrome: a retrospective study. Bone Jt J. 2014;96-B:259–262. doi: 10.1302/0301-620X.96B2.31935. [DOI] [PubMed] [Google Scholar]
- 34.Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixit D, Kuete NT, Bene P, Khan I, Oprea-Ilies G, Flenaugh E. invasive pulmonary aspergillosis with hydropneumothorax in a patient taking high-dose glucocorticoids. Am J Case Rep. 2020;21:e928499. doi: 10.12659/AJCR.928499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopalaswamy R, Subbian S. Corticosteroids for COVID-19 therapy: potential implications on tuberculosis. Int J Mol Sci. 2021;22:3773. doi: 10.3390/ijms22073773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivera MJ. Dexamethasone and COVID-19: strategies in low- and middle-income countries to Tackle steroid-related strongyloides hyperinfection. Am J Trop Med Hyg. 2021 doi: 10.4269/ajtmh.20-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra GP, Mulani J. Corticosteroids for COVID-19: the search for an optimum duration of therapy. Lancet Respir Med. 2021;9:e8. doi: 10.1016/S2213-2600(20)30530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutta D, Shivaprasad KS, Ghosh S, Mukhopadhyay S, Chowdhury S. Iatrogenic Cushing's syndrome following short-term intranasal steroid use. J Clin Res Pediatr Endocrinol. 2012;4:157–159. doi: 10.4274/Jcrpe.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes JM, Hichens M, Booze GW, Thorner MO. Cushing's syndrome from the therapeutic use of intramuscular dexamethasone acetate. Arch Intern Med. 1986;146:1848–1849. [PubMed] [Google Scholar]
- 41.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X, Geng C, Sun W, Tan M. Incidence and risk factors of osteonecrosis of femoral head in multiple myeloma patients undergoing dexamethasone-based regimens. Biomed Res Int. 2020;2020:7126982. doi: 10.1155/2020/7126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Uyl D, Bultink IE, Lems WF. Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep. 2011;13:233–240. doi: 10.1007/s11926-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 45.Zhao R, Wang H, Wang X, Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos Int. 2017;28:1027–1034. doi: 10.1007/s00198-016-3824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 48.Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J. 2006;119:581–588. [PubMed] [Google Scholar]
- 49.Atsumi T, Kuroki Y. Role of impairment of blood supply of the femoral head in the pathogenesis of idiopathic osteonecrosis. Clin Orthop Relat Res. 1992;277:22–30. [PubMed] [Google Scholar]
- 50.Ma L, Feng X, Wang K, Song Y, Luo R, Yang C. Dexamethasone promotes mesenchymal stem cell apoptosis and inhibits osteogenesis by disrupting mitochondrial dynamics. FEBS Open Bio. 2019;10:211–220. doi: 10.1002/2211-5463.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oshina H, Sotome S, Yoshii T, et al. Effects of continuous dexamethasone treatment on differentiation capabilities of bone marrow-derived mesenchymal cells. Bone. 2007;41:575–583. doi: 10.1016/j.bone.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 52.Fan Q, Zhan X, Li X, Zhao J, Chen Y. Vanadate inhibits dexamethasone-induced apoptosis of rat bone marrow-derived mesenchymal stem cells. Ann Clin Lab Sci. 2015;45:173–180. [PubMed] [Google Scholar]
- 53.Wang S, Xia P, Huang G, et al. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat Commun. 2016;7:11023. doi: 10.1038/ncomms11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing L, Zhang X, Feng H, et al. Silencing FOXO1 attenuates dexamethasone-induced apoptosis in osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 2019;513:1019–1026. doi: 10.1016/j.bbrc.2019.04.112. [DOI] [PubMed] [Google Scholar]
- 55.Guntur AR, Rosen CJ. The skeleton: a multi-functional complex organ: new insights into osteoblasts and their role in bone formation: the central role of PI3Kinase. J Endocrinol. 2011;211:123–130. doi: 10.1530/JOE-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng S, Dai G, Chen S, et al. Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3beta signaling pathway. Biomed Pharmacother. 2019;110:602–608. doi: 10.1016/j.biopha.2018.11.103. [DOI] [PubMed] [Google Scholar]
- 57.Nie Z, Chen S, Peng H. Glucocorticoid induces osteonecrosis of the femoral head in rats through GSK3beta-mediated osteoblast apoptosis. Biochem Biophys Res Commun. 2019;511:693–699. doi: 10.1016/j.bbrc.2019.02.118. [DOI] [PubMed] [Google Scholar]
- 58.Zhu CY, Yao C, Zhu LQ, She C, Zhou XZ. Dexamethasone-induced cytotoxicity in human osteoblasts is associated with circular RNA HIPK3 downregulation. Biochem Biophys Res Commun. 2019;516:645–652. doi: 10.1016/j.bbrc.2019.06.073. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B, Zhang S. Corticosteroid-induced osteonecrosis in COVID-19: a call for caution. J Bone Miner Res. 2020;35:1828–1829. doi: 10.1002/jbmr.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peck WA, Burkhardt P, Christiansen C, et al. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 61.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Investig. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLaughlin F, Mackintosh J, Hayes BP, et al. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone. 2002;30:924–930. doi: 10.1016/s8756-3282(02)00737-8. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- 64.Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 2005;329:177–181. doi: 10.1016/j.bbrc.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 65.Wang FS, Lin CL, Chen YJ, et al. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146:2415–2423. doi: 10.1210/en.2004-1050. [DOI] [PubMed] [Google Scholar]
- 66.Ohnaka K, Taniguchi H, Kawate H, Nawata H, Takayanagi R. Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: novel mechanism of glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun. 2004;318:259–264. doi: 10.1016/j.bbrc.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 67.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Investig. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren H, Liang D, Jiang X, et al. Variance of spinal osteoporosis induced by dexamethasone and methylprednisolone and its associated mechanism. Steroids. 2015;102:65–75. doi: 10.1016/j.steroids.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Xu T, Bianco P, Fisher LW, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 70.Clarke BA, Drujan D, Willis MS, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Moukas M, Vassiliou MP, Amygdalou A, Mandragos C, Takis F, Behrakis PK. Muscular mass assessed by ultrasonography after administration of low-dose corticosteroids and muscle relaxants in critically ill hemiplegic patients. Clin Nutr. 2002;21:297–302. doi: 10.1054/clnu.2001.0532. [DOI] [PubMed] [Google Scholar]
- 72.Aguilar-Agon KW, Capel AJ, Fleming JW, Player DJ, Martin NRW, Lewis MP. Mechanical loading of tissue engineered skeletal muscle prevents dexamethasone induced myotube atrophy. J Muscle Res Cell Motil. 2020 doi: 10.1007/s10974-020-09589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin J, Du R, Yang YQ, et al. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res Vet Sci. 2013;94:84–89. doi: 10.1016/j.rvsc.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 74.Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 75.Zimmers TA, Davies MV, Koniaris LG, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 76.Chromiak JA, Vandenburgh HH. Glucocorticoid-induced skeletal muscle atrophy in vitro is attenuated by mechanical stimulation. Am J Physiol. 1992;262:C1471–C1477. doi: 10.1152/ajpcell.1992.262.6.C1471. [DOI] [PubMed] [Google Scholar]
- 77.Liu J, Peng Y, Wang X, et al. Mitochondrial dysfunction launches dexamethasone-induced skeletal muscle atrophy via AMPK/FOXO3 signaling. Mol Pharm. 2016;13:73–84. doi: 10.1021/acs.molpharmaceut.5b00516. [DOI] [PubMed] [Google Scholar]
- 78.Rodilla E, Saura A, Jimenez I, et al. Association of hypertension with all-cause mortality among hospitalized patients with COVID-19. J Clin Med. 2020;9:3136. doi: 10.3390/jcm9103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020;116:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ledford H. Steroid is first drug shown to prevent deaths from Covid-19. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 82.Warris LT, van den Akker EL, Bierings MB, et al. Acute activation of metabolic syndrome components in pediatric acute lymphoblastic leukemia patients treated with dexamethasone. PLoS ONE. 2016;11:e0158225. doi: 10.1371/journal.pone.0158225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smets K, Vanhaesebrouck P. Dexamethasone associated systemic hypertension in low birth weight babies with chronic lung disease. Eur J Pediatr. 1996;155:573–575. doi: 10.1007/BF01957907. [DOI] [PubMed] [Google Scholar]
- 84.Soto-Pina AE, Franklin C, Rani CS, Gottlieb H, Hinojosa-Laborde C, Strong R. A novel model of dexamethasone-induced hypertension: use in investigating the role of tyrosine hydroxylase. J Pharmacol Exp Ther. 2016;358:528–536. doi: 10.1124/jpet.116.234005. [DOI] [PubMed] [Google Scholar]
- 85.Kornel L, Prancan AV, Kanamarlapudi N, Hynes J, Kuzianik E. Study on the mechanisms of glucocorticoid-induced hypertension: glucocorticoids increase transmembrane Ca2+ influx in vascular smooth muscle in vivo. Endocr Res. 1995;21:203–210. doi: 10.3109/07435809509030436. [DOI] [PubMed] [Google Scholar]
- 86.Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278:456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- 87.Hirata F, Schiffmann E, Venkatasubramanian K, Salomon D, Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci USA. 1980;77:2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russo-Marie F, Duval D. Dexamethasone-induced inhibition of prostaglandin production dose not result from a direct action on phospholipase activities but is mediated through a steroid-inducible factor. Biochem Biophys Acta. 1982;712:177–185. doi: 10.1016/0005-2760(82)90100-x. [DOI] [PubMed] [Google Scholar]
- 89.Schafer SC, Wallerath T, Closs EI, et al. Dexamethasone suppresses eNOS and CAT-1 and induces oxidative stress in mouse resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H436–H444. doi: 10.1152/ajpheart.00587.2004. [DOI] [PubMed] [Google Scholar]
- 90.d'Emmanuele di Villa Bianca R, Mitidieri E, Donnarumma E, et al. Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide Biol Chem. 2015;46:80–6. [DOI] [PubMed]
- 91.Goodwin JE, Zhang J, Gonzalez D, Albinsson S, Geller DS. Knockout of the vascular endothelial glucocorticoid receptor abrogates dexamethasone-induced hypertension. J Hypertens. 2011;29:1347–1356. doi: 10.1097/HJH.0b013e328347da54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Werner JC, Sicard RE, Hansen TW, Solomon E, Cowett RM, Oh W. Hypertrophic cardiomyopathy associated with dexamethasone therapy for bronchopulmonary dysplasia. J Pediatr. 1992;120:286–291. doi: 10.1016/s0022-3476(05)80446-9. [DOI] [PubMed] [Google Scholar]
- 93.de Vries WB, Bal MP, Homoet-van der Kraak P, et al. Suppression of physiological cardiomyocyte proliferation in the rat pup after neonatal glucocorticosteroid treatment. Basic Res Cardiol. 2006;101:36–42. [DOI] [PubMed]
- 94.Gay MS, Li Y, Xiong F, Lin T, Zhang L. Dexamethasone treatment of newborn rats decreases cardiomyocyte endowment in the developing heart through epigenetic modifications. PLoS ONE. 2015;10:e0125033. doi: 10.1371/journal.pone.0125033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gay MS, Dasgupta C, Li Y, Kanna A, Zhang L. Dexamethasone induces cardiomyocyte terminal differentiation via epigenetic repression of Cyclin D2 Gene. J Pharmacol Exp Ther. 2016;358:190–198. doi: 10.1124/jpet.116.234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Vries WB, van der Leij FR, Bakker JM, et al. Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr Res. 2002;52:900–906. doi: 10.1203/00006450-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 97.Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021;5:113–121. doi: 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jing Y, Run-Qian L, Hao-Ran W, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bensley JG, Moore L, De Matteo R, Harding R, Black MJ. Impact of preterm birth on the developing myocardium of the neonate. Pediatr Res. 2018;83:880–888. doi: 10.1038/pr.2017.324. [DOI] [PubMed] [Google Scholar]
- 100.Skelton R, Gill AB, Parsons JM. Cardiac effects of short course dexamethasone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F133–F137. doi: 10.1136/fn.78.2.f133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Israel BA, Sherman FS, Guthrie RD. Hypertrophic cardiomyopathy associated with dexamethasone therapy for chronic lung disease in preterm infants. Am J Perinatol. 1993;10:307–310. doi: 10.1055/s-2007-994747. [DOI] [PubMed] [Google Scholar]
- 102.de Salvi GF, de Moraes WM, Bozi LH, et al. Dexamethasone-induced cardiac deterioration is associated with both calcium handling abnormalities and calcineurin signaling pathway activation. Mol Cell Biochem. 2017;424:87–98. doi: 10.1007/s11010-016-2846-3. [DOI] [PubMed] [Google Scholar]
- 103.Macedo FN, Souza DS, Araujo J, et al. NOX-dependent reactive oxygen species production underlies arrhythmias susceptibility in dexamethasone-treated rats. Free Radical Biol Med. 2020;152:1–7. doi: 10.1016/j.freeradbiomed.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 104.Roy SG, De P, Mukherjee D, et al. Excess of glucocorticoid induces cardiac dysfunction via activating angiotensin II pathway. Cell Physiol Biochem. 2009;24:1–10. doi: 10.1159/000227803. [DOI] [PubMed] [Google Scholar]
- 105.De P, Roy SG, Kar D, Bandyopadhyay A. Excess of glucocorticoid induces myocardial remodeling and alteration of calcium signaling in cardiomyocytes. J Endocrinol. 2011;209:105–114. doi: 10.1530/JOE-10-0431. [DOI] [PubMed] [Google Scholar]
- 106.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 107.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 108.Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rahayu NK, Emily A. Clinical profile of steroid-induced glaucoma in Bali Mandara Eye Hospital year 2019. Intisari Sains Med. 2021;12:6–8. [Google Scholar]
- 110.Zode GS, Sharma AB, Lin X, et al. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Investig. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 112.Johnson D, Gottanka J, Flugel C, Hoffmann F, Futa R, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch Ophthalmol. 1997;115:375–383. doi: 10.1001/archopht.1997.01100150377011. [DOI] [PubMed] [Google Scholar]
- 113.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 114.Langarizadeh MA, Ranjbar Tavakoli M, Abiri A, Ghasempour A, Rezaei M, Ameri A. A review on function and side effects of systemic corticosteroids used in high-grade COVID-19 to prevent cytokine storms. EXCLI J. 2021;20:339–365. doi: 10.17179/excli2020-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993;148:S1–26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. [DOI] [PubMed] [Google Scholar]
- 116.Hanania NA, Chapman KR, Kesten S. Adverse effects of inhaled corticosteroids. Am J Med. 1995;98:196–208. doi: 10.1016/S0002-9343(99)80404-5. [DOI] [PubMed] [Google Scholar]
- 117.Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337:8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 118.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–1317. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 119.Renu K, Prasanna PL, Valsala GA. Coronaviruses pathogenesis, comorbidities and multi-organ damage—a review. Life Sci. 2020;255:117839. doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bandyopadhyay U, Biswas K, Bandyopadhyay D, Ganguly CK, Banerjee RK. Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidase–two important gastroprotective enzymes. Mol Cell Biochem. 1999;202:31–36. doi: 10.1023/a:1007018212822. [DOI] [PubMed] [Google Scholar]
- 122.Filaretova L, Podvigina T, Bagaeva T, Morozova O. Dual action of glucocorticoid hormones on the gastric mucosa: how the gastroprotective action can be transformed to the ulcerogenic one. Inflammopharmacology. 2009;17:15–22. doi: 10.1007/s10787-008-8046-3. [DOI] [PubMed] [Google Scholar]
- 123.Gordon PV, Price WA, Stiles AD. Dexamethasone administration to newborn mice alters mucosal and muscular morphology in the ileum and modulates IGF-I localization. Pediatr Res. 2001;49:93–100. doi: 10.1203/00006450-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 124.Stark AR, Carlo WA, Tyson JE, et al. Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 125.Burrin DG, Wester TJ, Davis TA, Fiorotto ML, Chang X. Dexamethasone inhibits small intestinal growth via increased protein catabolism in neonatal pigs. Am J Physiol. 1999;276:E269–E277. doi: 10.1152/ajpendo.1999.276.2.E269. [DOI] [PubMed] [Google Scholar]
- 126.Read LC, Tomas FM, Howarth GS, et al. Insulin-like growth factor-I and its N-terminal modified analogues induce marked gut growth in dexamethasone-treated rats. J Endocrinol. 1992;133:421–431. doi: 10.1677/joe.0.1330421. [DOI] [PubMed] [Google Scholar]
- 127.Kamphuis PJ, de Vries WB, Bakker JM, et al. Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr Res. 2007;61:72–76. doi: 10.1203/01.pdr.0000249980.95264.dd. [DOI] [PubMed] [Google Scholar]
- 128.Liu Y, van Goor H, Havinga R, et al. Neonatal dexamethasone administration causes progressive renal damage due to induction of an early inflammatory response. Am J Physiol Renal Physiol. 2008;294:F768–F776. doi: 10.1152/ajprenal.00163.2007. [DOI] [PubMed] [Google Scholar]
- 129.de Vries WB, van den Borne P, Goldschmeding R, et al. Neonatal dexamethasone treatment in the rat leads to kidney damage in adulthood. Pediatr Res. 2010;67:72–76. doi: 10.1203/PDR.0b013e3181bf570d. [DOI] [PubMed] [Google Scholar]
- 130.Kamitsuka MD, Williams MA, Nyberg DA, Fox KA, Lee DL, Hickok D. Renal calcification: a complication of dexamethasone therapy in preterm infants with bronchopulmonary dysplasia. J Perinatol. 1995;15:359–363. [PubMed] [Google Scholar]
- 131.Cranefield DJ, Odd DE, Harding JE, Teele RL. High incidence of nephrocalcinosis in extremely preterm infants treated with dexamethasone. Pediatr Radiol. 2004;34:138–142. doi: 10.1007/s00247-003-1090-7. [DOI] [PubMed] [Google Scholar]
- 132.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Flores G. SARS-COV-2 (COVID-19) has neurotropic and neuroinvasive properties. Int J Clin Pract. 2021;75:e13708. [DOI] [PubMed]
- 134.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vafaei AA, Rashidy-Pour A, Taherian AA. Peripheral injection of dexamethasone modulates anxiety related behaviors in mice: an interaction with opioidergic neurons. Pak J Pharm Sci. 2008;21:285–289. [PubMed] [Google Scholar]
- 137.Skupio U, Tertil M, Sikora M, Golda S, Wawrzczak-Bargiela A, Przewlocki R. Behavioral and molecular alterations in mice resulting from chronic treatment with dexamethasone: relevance to depression. Neuroscience. 2015;286:141–150. doi: 10.1016/j.neuroscience.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 138.Park DI, Kim HG, Jung WR, Shin MK, Kim KL. Mecamylamine attenuates dexamethasone-induced anxiety-like behavior in association with brain derived neurotrophic factor upregulation in rat brains. Neuropharmacology. 2011;61:276–282. doi: 10.1016/j.neuropharm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 139.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiat. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 140.McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–206. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- 141.Haynes LE, Barber D, Mitchell IJ. Chronic antidepressant medication attenuates dexamethasone-induced neuronal death and sublethal neuronal damage in the hippocampus and striatum. Brain Res. 2004;1026:157–167. doi: 10.1016/j.brainres.2004.05.117. [DOI] [PubMed] [Google Scholar]
- 142.Unemura K, Kume T, Kondo M, Maeda Y, Izumi Y, Akaike A. Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J Pharmacol Sci. 2012;119:30–39. doi: 10.1254/jphs.12047fp. [DOI] [PubMed] [Google Scholar]
- 143.Ruksee N, Tongjaroenbuangam W, Mahanam T, Govitrapong P. Melatonin pretreatment prevented the effect of dexamethasone negative alterations on behavior and hippocampal neurogenesis in the mouse brain. J Steroid Biochem Mol Biol. 2014;143:72–80. doi: 10.1016/j.jsbmb.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 144.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 146.Dattilo V, Amato R, Perrotti N, Gennarelli M. The emerging role of SGK1 (serum- and glucocorticoid-regulated kinase 1) in major depressive disorder: hypothesis and mechanisms. Front Genet. 2020;11:826. doi: 10.3389/fgene.2020.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112:427–439. doi: 10.1093/bja/aet417. [DOI] [PubMed] [Google Scholar]
- 148.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27:285–288. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 149.Gagne OJ, Cheema A, Abuhantash M, et al. Effect of dexamethasone in peripheral nerve blocks on recovery of nerve function. Foot Ankle Int. 2021;42:23–30. doi: 10.1177/1071100720952075. [DOI] [PubMed] [Google Scholar]
- 150.Duffy BA, Chun KP, Ma D, Lythgoe MF, Scott RC. Dexamethasone exacerbates cerebral edema and brain injury following lithium-pilocarpine induced status epilepticus. Neurobiol Dis. 2014;63:229–236. doi: 10.1016/j.nbd.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tran ND, Kim S, Vincent HK, et al. Aquaporin-1-mediated cerebral edema following traumatic brain injury: effects of acidosis and corticosteroid administration. J Neurosurg. 2010;112:1095–1104. doi: 10.3171/2009.8.JNS081704. [DOI] [PubMed] [Google Scholar]
- 152.Maheshwari M, Bhutani S, Das A, et al. Dexamethasone induces heat shock response and slows down disease progression in mouse and fly models of Huntington's disease. Hum Mol Genet. 2014;23:2737–2751. doi: 10.1093/hmg/ddt667. [DOI] [PubMed] [Google Scholar]
- 153.Joshi N, Singh S. Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res. 2018;96:379–390. doi: 10.1002/jnr.24185. [DOI] [PubMed] [Google Scholar]
- 154.Hui Z, Zhijun Y, Yushan Y, et al. The combination of acyclovir and dexamethasone protects against Alzheimer's disease-related cognitive impairments in mice. Psychopharmacology. 2020;237:1851–1860. doi: 10.1007/s00213-020-05503-1. [DOI] [PubMed] [Google Scholar]
- 155.Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. A prospective study. J Psychiatric Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 156.Felner EI, Thompson MT, Ratliff AF, White PC, Dickson BA. Time course of recovery of adrenal function in children treated for leukemia. J Pediatr. 2000;137:21–24. doi: 10.1067/mpd.2000.107385. [DOI] [PubMed] [Google Scholar]
- 157.Paragliola RM, Papi G, Pontecorvi A, Corsello SM. Treatment with synthetic glucocorticoids and the hypothalamus-pituitary-adrenal axis. Int J Mol Sci. 2017;18:2201. doi: 10.3390/ijms18102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Helfer EL, Rose LI. Corticosteroids and adrenal suppression. Characterising and avoiding the problem. Drugs. 1989;38:838–845. doi: 10.2165/00003495-198938050-00008. [DOI] [PubMed] [Google Scholar]
- 159.Han HS, Shim YK, Kim JE, et al. A pilot study of adrenal suppression after dexamethasone therapy as an antiemetic in cancer patients. Support Care Cancer. 2012;20:1565–1572. doi: 10.1007/s00520-011-1248-z. [DOI] [PubMed] [Google Scholar]
- 160.Lesniewska B, Nowak KW, Malendowicz LK. Dexamethasone-induced adrenal cortex atrophy and recovery of the gland from partial, steroid-induced atrophy. Exp Clin Endocrinol. 1992;100:133–139. doi: 10.1055/s-0029-1211193. [DOI] [PubMed] [Google Scholar]
- 161.Sowers JR, Carlson HE, Brautbar N, Hershman JM. Effect of dexamethasone on prolactin and TSH responses to TRH and metoclopramide in man. J Clin Endocrinol Metab. 1977;44:237–241. doi: 10.1210/jcem-44-2-237. [DOI] [PubMed] [Google Scholar]
- 162.Jux C, Leiber K, Hugel U, et al. Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology. 1998;139:3296–3305. doi: 10.1210/endo.139.7.6099. [DOI] [PubMed] [Google Scholar]
- 163.Meneses-Santos D, Buonfiglio DDC, Peliciari-Garcia RA, et al. Chronic treatment with dexamethasone alters clock gene expression and melatonin synthesis in rat pineal gland at night. Nat Sci Sleep. 2018;10:203–215. doi: 10.2147/NSS.S158602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sugimoto T, Brown AJ, Ritter C, Morrissey J, Slatopolsky E, Martin KJ. Combined effects of dexamethasone and 1,25-dihydroxyvitamin D3 on parathyroid hormone secretion in cultured bovine parathyroid cells. Endocrinology. 1989;125:638–641. doi: 10.1210/endo-125-2-638. [DOI] [PubMed] [Google Scholar]
- 165.Peraldi MN, Rondeau E, Jousset V, et al. Dexamethasone increases preproparathyroid hormone messenger RNA in human hyperplastic parathyroid cells in vitro. Eur J Clin Invest. 1990;20:392–397. doi: 10.1111/j.1365-2362.1990.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 166.Buren J, Lai YC, Lundgren M, Eriksson JW, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys. 2008;474:91–101. doi: 10.1016/j.abb.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 167.Shepherd PR. Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol Scand. 2005;183:3–12. doi: 10.1111/j.1365-201X.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 168.Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem. 2006;281:29174–29180. doi: 10.1074/jbc.M603274200. [DOI] [PubMed] [Google Scholar]
- 169.McManus EJ, Sakamoto K, Armit LJ, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cohen P. Dissection of the protein phosphorylation cascades involved in insulin and growth factor action. Biochem Soc Trans. 1993;21(Pt 3):555–567. doi: 10.1042/bst0210555. [DOI] [PubMed] [Google Scholar]
- 171.Henriksen JE, Alford F, Ward GM, Beck-Nielsen H. Risk and mechanism of dexamethasone-induced deterioration of glucose tolerance in non-diabetic first-degree relatives of NIDDM patients. Diabetologia. 1997;40:1439–1448. doi: 10.1007/s001250050847. [DOI] [PubMed] [Google Scholar]
- 172.Jeong Y, Han HS, Lee HD, et al. A pilot study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Treat. 2016;48:1429–1437. doi: 10.4143/crt.2015.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Investig. 1993;92:2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48:2119–2130. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 175.Sakoda H, Ogihara T, Anai M, et al. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000;49:1700–1708. doi: 10.2337/diabetes.49.10.1700. [DOI] [PubMed] [Google Scholar]
- 176.Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metab Clin Exp. 1998;47:3–6. [DOI] [PubMed]
- 177.Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Long-term high-fat feeding leads to severe insulin resistance but not diabetes in Wistar rats. Am J Physiol Endocrinol Metab. 2002;282:E1231–E1238. doi: 10.1152/ajpendo.00173.2001. [DOI] [PubMed] [Google Scholar]
- 178.Thompson AL, Lim-Fraser MY, Kraegen EW, Cooney GJ. Effects of individual fatty acids on glucose uptake and glycogen synthesis in soleus muscle in vitro. Am J Physiol Endocrinol Metab. 2000;279:E577–E584. doi: 10.1152/ajpendo.2000.279.3.E577. [DOI] [PubMed] [Google Scholar]
- 179.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 180.Bernal-Mizrachi C, Weng S, Feng C, et al. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003;9:1069–1075. doi: 10.1038/nm898. [DOI] [PubMed] [Google Scholar]
- 181.Cui A, Fan H, Zhang Y, et al. Dexamethasone-induced Kruppel-like factor 9 expression promotes hepatic gluconeogenesis and hyperglycemia. J Clin Investig. 2019;129:2266–2278. doi: 10.1172/JCI66062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ogawa A, Johnson JH, Ohneda M, et al. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Investig. 1992;90:497–504. doi: 10.1172/JCI115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 184.Guo B, Zhang W, Xu S, Lou J, Wang S, Men X. GSK-3beta mediates dexamethasone-induced pancreatic beta cell apoptosis. Life Sci. 2016;144:1–7. doi: 10.1016/j.lfs.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shang J, Wang Q, Zhang H, et al. The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort Study in Wuhan, China. Am J Med. 2021;134:e6–e14. doi: 10.1016/j.amjmed.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wijnant SRA, Jacobs M, Van Eeckhoutte HP, et al. Expression of ACE2, the SARS-CoV-2 receptor, in lung tissue of patients with Type 2 diabetes. Diabetes. 2020;69:2691–2699. doi: 10.2337/db20-0669. [DOI] [PubMed] [Google Scholar]
- 189.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J Med Virol. 2020;92:770–775. doi: 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diab Res Clin Pract. 2020;162:108125. doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee SA, Kim YR, Yang EJ, et al. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:2553–2561. doi: 10.1210/jc.2012-4288. [DOI] [PubMed] [Google Scholar]
- 193.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4:1774. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diab Metab Res Rev. 2020;36:e33213321. doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ceriello A, De Nigris V, Prattichizzo F. Why is hyperglycaemia worsening COVID-19 and its prognosis? Diabetes Obes Metab. 2020;22:1951–1952. doi: 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3:446–451. doi: 10.1177/193229680900300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cielen N, Maes K, Gayan-Ramirez G. Musculoskeletal disorders in chronic obstructive pulmonary disease. BioMed Res Int. 2014;2014:965764. doi: 10.1155/2014/965764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dekhuijzen PN, Decramer M. Steroid-induced myopathy and its significance to respiratory disease: a known disease rediscovered. Eur Respir J. 1992;5:997–1003. [PubMed] [Google Scholar]
- 200.Herrera NA, Jesus I, Shinohara AL, Dionisio TJ, Santos CF, Amaral SL. Exercise training attenuates dexamethasone-induced hypertension by improving autonomic balance to the heart, sympathetic vascular modulation and skeletal muscle microcirculation. J Hypertens. 2016;34:1967–1976. doi: 10.1097/HJH.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 201.Bird K, Chan G, Lu H, et al. Assessment of hypertension using clinical electrocardiogram features: a first-ever review. Front Med. 2020;7:583331. doi: 10.3389/fmed.2020.583331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Habibi E, Esmaeeli H. A review of the effects of curcumin on histone acetyltransferase activity in the prevention of cardiac hypertrophy. J Babol Univ Med Sci. 2017;19:27–35. [Google Scholar]
- 203.Liao JK. Statin therapy for cardiac hypertrophy and heart failure. J Investig Med. 2004;52:248–253. doi: 10.1136/jim-52-04-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu L, Li C, Liu Q, Xu W, Zhou X. Molecular biomarkers in cardiac hypertrophy. J Cell Mol Med. 2019;23:1671–1677. doi: 10.1111/jcmm.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ng PC, Brownlee KG, Dear PR. Gastroduodenal perforation in preterm babies treated with dexamethasone for bronchopulmonary dysplasia. Arch Dis Child. 1991;66:1164–1166. doi: 10.1136/adc.66.10_spec_no.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee NK, Kim S, Hong SB, et al. CT diagnosis of non-traumatic gastrointestinal perforation: an emphasis on the causes. Jpn J Radiol. 2020;38:101–111. doi: 10.1007/s11604-019-00910-7. [DOI] [PubMed] [Google Scholar]
- 207.Tamez-Perez HE, Quintanilla-Flores DL, Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Tamez-Pena AL. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6:1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.