Abstract
Background The long-acting somatostatin analog lanreotide autogel is effective in the treatment of patients with neuroendocrine tumors.
Objective To evaluate the long-term treatment response in patients with neuroendocrine tumors receiving lanreotide autogel in routine clinical practice.
Methods Non-interventional, 24-month study in patients with neuroendocrine tumors treated with lanreotide autogel (NCT01840449).
Results Patients (n=80) from 26 centers in Germany and Austria were enrolled. Neuroendocrine tumors were mainly grade 1/2, metastasized, intestinal, and associated with carcinoid syndrome; 88.9% had received previous neuroendocrine tumor treatment. Of those, 84.4% had previous surgery, 18.7% had received octreotide. The primary endpoint, defined by a <50% chromogranin A increase at month 12 compared with the lowest value between baseline and month 3 was achieved by 89.5% patients. Stable disease according to Response Evaluation Criteria in Solid Tumors 1.1 was observed in 76.9 and 75.0% patients at months 12 and 24 of lanreotide treatment, respectively. Mean change of chromogranin A levels from baseline to month 24 was −0.12 × upper limit of normal (95% CI, −0.22; −0.45). In a post hoc analysis, 38.5% of the subgroup of patients with carcinoid syndrome had daily diarrhea at baseline vs. 21.4% at month 24. At baseline, 27.8% of patients received lanreotide 120 mg every 4 weeks vs. 56.7% at month 24. Quality of life data were heterogeneous. No new safety issues arose and/or required further investigation.
Conclusions Our study reflects routine lanreotide autogel use in patients with advanced/metastatic neuroendocrine tumors. This analysis shows effectiveness with stabilization of disease-related symptoms and good tolerability of lanreotide autogel in clinical practice.
Key words: chromogranin A, carcinoid syndrome, diarrhea, flushing, quality of life
Introduction
Neuroendocrine tumors (NET) are rare tumors usually localized in the gastrointestinal tract, lung, and pancreas 1 . They have a low, but increasingly reported, incidence of up to 7/100 000 1 . Most patients present with advanced, unresectable disease due to local extension or metastasis. Treatment options for gastroenteropancreatic (GEP) NET include curative resection of the primary tumor and metastases, and systemic therapies to control tumor growth and alleviate hormonal syndromes. In addition, treatment aims to maintain or improve patients’ quality of life 2 . Prognosis depends on primary tumor location, presence of metastasis in the liver and extrahepatic tissues, proliferative activity (tumor grade) and functional activity 1 .
Advanced unresectable low-grade NETs are usually treated by antiproliferative drugs such as somatostatin analogs. Upon progression, peptide receptor radionuclide therapy (PRRT) or targeted therapies as well as loco-regional treatments represent further therapy options; in pancreatic NET systemic chemotherapy (Streptozocin or temozolomide based) is another established therapy 3 . In high-grade neuroendocrine carcinomas (NECs) cytotoxic chemotherapy with cisplatin and etoposide is first line therapy 3 .
Approximately 30–40% of patients with well-differentiated NET present with carcinoid syndrome due to excessive production of serotonin and other bioactive compounds by the tumor 4 . Hallmarks of disease are diarrhea and flushing. In these patients, somatostatin analogs are a choice for antisecretory treatment 3 . A number of randomized, controlled and observational studies have shown that the long-acting somatostatin analog lanreotide autogel (LAN) effectively controls functional activity and symptoms, particular in serotonin-secreting NETs 5 6 7 8 9 .
In somatostatin receptor (SSTR) positive non-functional NET grade 1 (G1) with low tumor burden both, somatostatin analogs or ‘watch and wait’ are considered as clinical options. Furthermore, somatostatin analogs are indicated for first-line treatment to control tumor growth of patients with SSTR positive, low to intermediate proliferative (Ki-67≤10%) NET G2 with or without high tumor burden or with progressive disease or symptoms 3 . However, some experts consider a lower Ki-67 cut-off (e. g., 5%) a more appropriate threshold to choose more aggressive treatment options 3 . Anti-tumor effects for LAN were confirmed in the randomized controlled 96-week CLARINET trial 10 11 . LAN prolonged progression-free survival over placebo in patients with metastatic G1 or G2 (proliferation index, Ki-67 ≤10%) SSTR positive NET of pancreatic, intestinal or of unknown primary origins. Most patients had prior stable disease (RECIST 1.0), and efficacy of LAN was observed irrespective of hepatic tumor volume and grade 10 11 . LAN is currently licensed in Europe for the treatment of grade 1 (G1) and a subset of grade 2 (Ki-67 index ≤10%) NETs of midgut, pancreatic or unknown origin, in adult patients with unresectable locally advanced or metastatic disease 12 , and in the US for the treatment of patients with unresectable, well-or moderately-differentiated, locally advanced or metastatic GEP-NETs to improve progression-free survival 13 .
This non-interventional study was designed to evaluate the long-term response to LAN treatment in patients with NET in routine clinical practice, and to investigate factors that might correlate with treatment success. The secondary objective was to better understand the patient population receiving treatment with LAN for NET in Germany and Austria.
Methods
Patients
Adult patients aged 18 years and older diagnosed with functional or non-functional NET to be treated de novo with LAN or who had previously been on treatment with LAN for less than 6 months (maximum one-third of the enrolled patients allowed) could be included. In patients previously treated with LAN, baseline data before starting LAN treatment and all other available data since baseline were assessed retrospectively. Prospective documentation followed from the time of inclusion. Patients were recruited from medical practices or clinics in Germany and Austria with special expertise in the treatment of patients with NET. Target enrolment was 76 patients. Written informed consent was obtained from all patients prior to inclusion. The efficacy population comprised all enrolled patients for whom core data (age, gender, date of baseline, date of LAN administration at baseline, dose of LAN administration at baseline and diagnosis of NET) were collected in the electronic case report form; who did not start LAN treatment more than 30 days prior to baseline; and who had a chromogranin A (CgA) value from at least two time points including baseline and/or month 3, and months 3, 6, 12, 18 and/or month 24.
Study design
This was a multicenter, non-interventional, observational study in Germany and Austria. Both prospective and retrospective (maximum one-third of the enrolled patients) documentation was allowed. The patients received treatment as prescribed by the investigator and in accordance with routine practice. All diagnostic and therapeutic decisions were in the hands of the treating physician and completely independent of the decision to include the patient in this study. Routine visits were recorded at baseline and at approximately 1, 3, 6, 12, 18 and 24 months.
This study was conducted in compliance with independent ethics committees/institutional review boards, informed consent regulations, the Declaration of Helsinki (Version 2013) and International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) Guidelines.
Endpoints
The primary endpoint was the long-term response, i. e. the control rate after 1 year, defined by CgA level < 50% increase compared with lowest CgA level between baseline and month 3. Secondary endpoints included changes of symptoms, global evaluation of effectiveness by the physician, global evaluation of tolerability by the patients, and quality of life (QoL).
Assessments
Effectiveness parameters (all assessed as per routine practice) included basal CgA serum levels, 24-hour urinary excretion of 5-hydroxyindoleacetic acid (5-HIAA) in patients with carcinoid syndrome and clinical parameters such as presence and frequency of diarrhea and flushing. Additional laboratory assessments included fasting blood glucose, and glycosylated hemoglobin [HbA 1c ]). All laboratory parameters were tested by local laboratories. Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 was used to assess tumor response, the Eastern Co-operative Oncology Group (ECOG) scale was used to evaluate the performance status. Global evaluation of overall effectiveness was subjectively rated by the investigator by categories (very poor, poor, moderate, good, and very good). Quality of life (QoL) was assessed by the German validated version of the European Organization for Research and Treatment of Cancer (EORTC) Study Group's 30-item Quality of Life Questionnaire (QLQ-C30) (subscores: global health status; physical, role, emotional, cognitive and social functional scales and symptom scales) and, in addition, by the disease-specific 21-item EORTC QLQ-GINET21 questionnaire (subscores: treatment-related symptoms, weight gain, information/communication function, sexual function, endocrine symptoms, gastrointestinal symptoms, social function, disease-related worries, muscle/bone pain symptoms and body image). For health-related quality of life scores a change of 10-points is frequently considered a minimal clinically important difference 14 15 . If routinely performed, quality of life was assessed in the patients at baseline and at approximately 1, 6, 12, 18 and 24 months.
If available, tumor tissue from previous surgery could be submitted optionally for analysis of SSTR status (subtypes 2, 3, and 5). SSTR analyses were all performed by immunohistochemistry (IHC) at the Department of Neuropathology, University Hospital Eppendorf, Hamburg, Germany. For immunohistochemistry polyclonal rabbit anti-SSTR2A, rabbit anti-SSTR5 (both Zytomed Systems, Bargteheide, Germany) and rabbit anti-SSTR3 (Thermo Fisher, Rockford, USA) antibodies were used.
Adverse event (AE) reporting followed regulations related to non-interventional post-authorization studies. Any non-serious or serious AEs related to the product as well as any serious AE, independently of the relationship to the product, were reported to the clinical research organization and pharmacovigilance. Unrelated non-serious AEs were not collected routinely, as they were not considered likely to add relevant new information on the safety of the product. The presence of gallbladder stones was assessed by ultrasound as per clinical practice. Global evaluation of tolerability was assessed by the patient by categories (very poor, poor, moderate, good, and very good).
Statistical analysis
All analyses are descriptive. Data are presented as mean and 95% confidence interval (CI) or as median and range. The primary analysis was performed on the efficacy population (see above). In addition, post hoc analyses were performed for biochemical response, symptoms and SSTR status in the subpopulation of patients with carcinoid syndrome. SSTR expression based on scintigraphy results were presented according to location and functional activity of the primary tumor.
Results
Patient disposition
Patients with NET (n=80) were enrolled from 26 medical practices and clinics, of whom 25 patients were documented retrospectively. Each site contributed between one and eleven patients. 48 patients continued the study until month 12, 31 patients continued until month 24. Reasons for discontinuation until month 24 (n=48) were decreasing efficacy of treatment (n=13), loss to follow-up (n=12), AEs (n=6), withdrawal of consent (n=2), death (n=9), and other reasons (n=6). The efficacy population consisted of 42 patients. Of these, 20 patients discontinued prematurely before month 24 due to decreasing efficacy of treatment (n=8), loss to follow-up (n=4), death (n=4), and other reasons (n=4). 22 patients of the efficacy population completed the study.
Patient characteristics
Table 1 shows the baseline characteristics of the enrolled and efficacy populations. Mean age of enrolled patients was 65.7 years (95% CI, 62.9–68.5), 54.4% were male. Median disease duration was 1.5 years (95% CI, 0.5–2.6) after symptom onset. Of the enrolled patients, 50 (63.3%) NETs were intestinal. Of these, 35 tumors were located in the ileum, four in the stomach, three were located in each, duodenum and jejunum, five in the caecum, and one in each appendix, colon and rectum. 24.1% of tumors were located in the pancreas and 22.8% were located in other sites. In 69.6% of patients, NETs were classified as functioning, in particular as carcinoid syndrome in 64.6% of enrolled patients. Two patients had an insulinoma, and one patient a gastrinoma or somatostatinoma each. Non-functioning NETs were present in 30.0% of patients. 88.6% of patients had metastatic disease, mainly localized in the liver (72.2%) and in the lymph nodes (62.0%). Of the tumors, 53.8% were G1, 41.0% G2, 5.1% G3. Of the patients, 88.9% had received previous treatment for NET. Of these pre-treated patients, 84.4% had previous surgery and 18.7% had been medically treated with octreotide LAR. Few patients had been treated with other options such as PRRT, chemotherapy, targeted therapy, local ablative procedures, or interferon. Most frequent comorbidities at baseline were diabetes mellitus (16.5%) and heart failure (8.9%).
Table 1 Baseline characteristics of the patients.
| Variable | Enrolled population* | Efficacy population | ||
|---|---|---|---|---|
| N | N | |||
| Age, mean (95% CI) | 79 | 65.7 (62.9–68.5) | 42 | 65.6 (61.8–69.4) |
| Female/male, n (%) | 79 | 36 (45.6) / 43 (54.4) | 42 | 19 (45.2) / 23 (54.8) |
| BMI, kg/m 2 , mean (95% CI) | 68 | 25.2 (24.1–26.2) | 37 | 25.3 (23.7–26.8) |
| Duration of NET, median yrs (95% CI) | 79 | 1.5 (0.5–2.6) | 42 | 1.7 (0.5–2.6) |
| Duration of symptoms, median yrs (95% CI) | 79 | 1.8 (0.8–2.6) | 42 | 1.8 (0.7–4.2) |
| NET localizations, n (%) * | 79 | 42 | ||
| Intestinal | 50 (63.3) | 29 (69.0) | ||
| Stomach | 4 (5.1) | 2 (4.8) | ||
| Duodenum | 3 (3.8) | 1 (2.4) | ||
| Jejunum | 3 (3.8) | 1 (2.4) | ||
| Ileum | 35 (44.3) | 22 (52.4) | ||
| Appendix | 1 (1.3) | 1 (2.4) | ||
| Caecum | 5 (6.3) | 2 (4.8) | ||
| Colon | 1 (1.3) | 1 (2.4) | ||
| Rectum | 1 (1.3) | 0 (0.0) | ||
| Pancreas | 19 (24.1) | 11 (26.2) | ||
| Other | 18 (22.8) | 6 (14.3) | ||
| Classification, n (%) | 79 | 42 | ||
| Carcinoid syndrome | 51 (64.6) | 26 (61.9) | ||
| Insulinoma | 2 (2.5) | 2 (4.8) | ||
| Gastrinoma | 1 (1.2) | 0 (0.0) | ||
| Somatostatinoma | 1 (1.2) | 0(0.0) | ||
| Other (non-functioning) | 24 (30.0) | 14 (33.3) | ||
| Metastases, n (%) | 79 | 42 | ||
| Any | 70 (88.6) | 38 (90.5) | ||
| Liver | 57 (72.2) | 29 (69.0) | ||
| Lymph nodes | 49 (62.0) | 28 (66.7) | ||
| Lung | 6 (7.6) | 2 (4.8) | ||
| Other | 26 (32.9) | 12 (28.6) | ||
| Grading, n (%) | 78 | 42 | ||
| G1 | 42 (53.8) | 23 (54.8) | ||
| G2 | 32 (41.0) | 16 (38.1) | ||
| G3 | 4 (5.1) | 3 (7.1) | ||
| Positive SSTR scintigraphy, n (%) By type | ||||
| Carcinoid syndrome | 35 | 28 (80.0) | 21 | 17 (81.0) |
| Other | 19 | 15 (78.9) | 12 | 9 (75.0) |
| By localization | ||||
| Intestinal | 32 | 25 (78.1) | 21 | 16 (76.2) |
| Pancreas | 11 | 9 (81.8) | 8 | 6 (75.0) |
| Other | 7 | 5 (71.4) | 2 | 2 (100.0) |
| By grade | ||||
| G1 | 32 | 25 (78.1) | 19 | 14 (73.7) |
| G2 | 18 | 14 (77.8) | 11 | 9 (81.8) |
| G3 | 4 | 4 (100.0) | 3 | 3 (100.0) |
| Previous treatments, n/N (%) * | ||||
| Any | 72 | 64 (88.9) | 37 | 34 (91.9) |
| Surgery | 64 | 54 (84.4) | 34 | 32 (94.1) |
| Octreotide LAR | 64 | 12 (18.7) | 34 | 8 (23.5) |
| PRRT | 64 | 7 (10.9) | 34 | 4 (11.8) |
| Chemotherapy | 64 | 6 (9.4) | 34 | 2 (5.9) |
| Targeted therapy | 64 | 6 (9.4) | 34 | 3 (8.8) |
| Local ablative procedures | 64 | 4 (6.2) | 34 | 2 (5.9) |
| Interferon | 64 | 1 (1.6) | 34 | 1 (2.9) |
| Concomitant diseases, n/N (%) * | 79 | 42 | ||
| Any | 20/79 (25.3) | 9 (21.4) | ||
| Diabetes | 13/79 (16.5) | 4 (9.5) | ||
| Heart failure | 7/79 (8.9) | 4 (9.5) | ||
| Impaired glucose tolerance | 1/79 (1.3) | 1 (2.4) | ||
| ECOG status, n (%) | 78 | 42 | ||
| 0 (fully active) | 39 (50.0) | 20 (47.6) | ||
| 1 (restricted activity) | 31 (39.7) | 18 (42.9) | ||
| 2 (no work activities) | 6 (7.7) | 4 (9.5) | ||
| 3 (only limited self-care) | 2 (2.6) | 0 (0.0) | ||
| 4 (completely disabled) | 0 (0.0) | 0 (0.0) | ||
* multiple entries possible. BMI: body mass index, CI: confidence interval, G: grade, LAR: long-acting release, PRRT: peptide receptor radionuclide therapy, SD: standard deviation, SSTR: somatostatin receptor, ULN: upper limit of normal, yrs: years. *There are 80 patients in the enrolled population, but missing data on some of the baseline characteristics, yielding N’s of 79 in the analyses.
NET tissue samples to study SSTR expression by IHC were collected for 17 patients. Of these, 94.1% expressed SSTR2/2a, 52.9% SSTR5 and 17.6% SSTR3. SSTR2/2a-positive patients mainly showed high (+++, 64.7%) levels of receptor expression ( Fig. 1 ). In the patients with carcinoid syndrome and available SSTR status (n=13), expression of SSTR2/2a was present in 92.3% patients, SSTR5 in 53.8%, and SSTR3 in 23.1% patients.
Fig. 1.
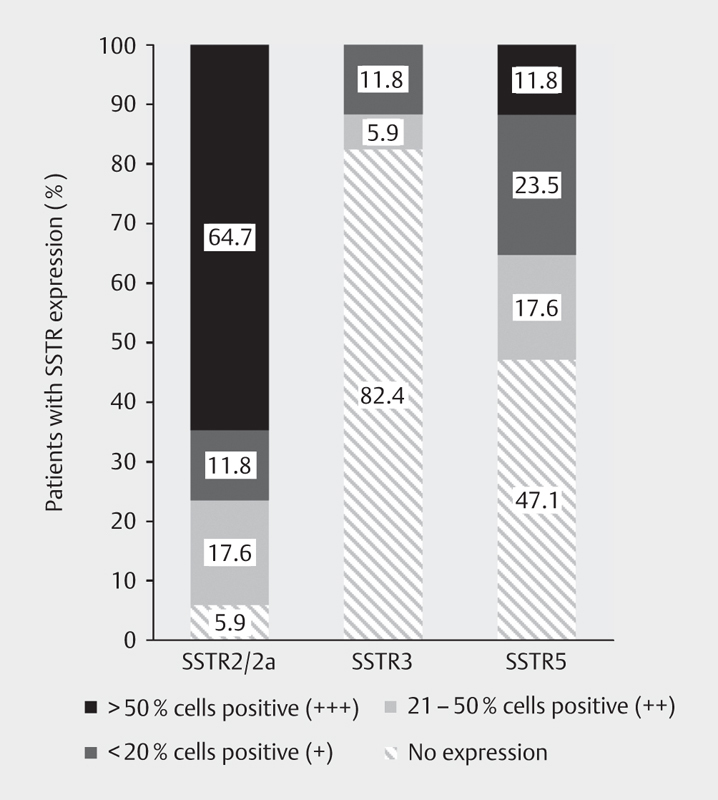
Expression of SSTR subtypes at baseline (n=17) (enrolled population); SSTR: somatostatin receptor.
80% of patients with NET and carcinoid syndrome with SSTR status available were SSTR-positive according to SSTR scintigraphy. This proportion of patients was similar to those with other NET subtypes (78.9%). Similar results were observed by location (intestinal: 71.4%, pancreatic: 81.8%, other: 71.4%) and grading (NET G1: 78.1%, NET G2: 77.8%, NET G3: 100%).
Lanreotide dose
At the baseline visit 45.6% of patients were administered LAN at a starting dose of 60 mg, 26.6% at a dose of 90 mg and 27.8% at a dose of 120 mg every 4 weeks ( Fig. 2 ). The proportion of enrolled patients receiving the 120 mg dose every 4 weeks was 53.3% at 12 months and 56.7% at 24 months.
Fig. 2.
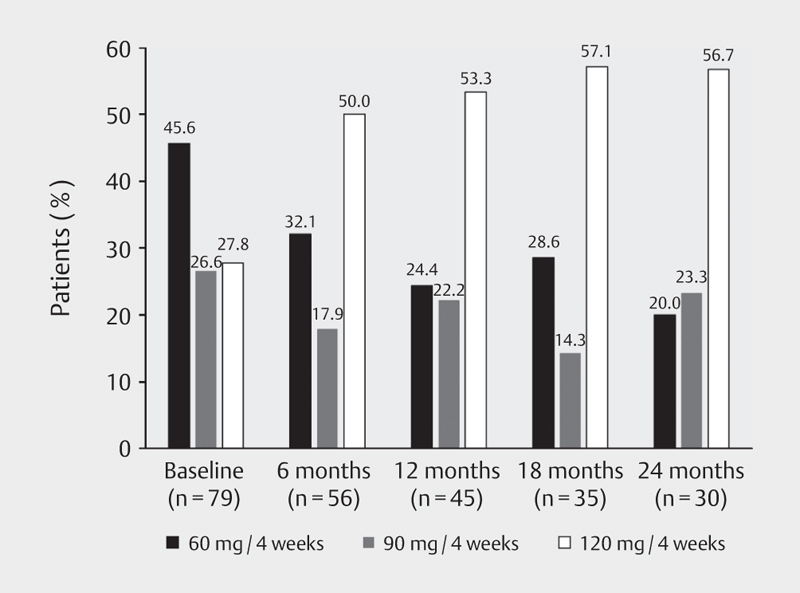
LAN doses during the course of the study (efficacy population); LAN: lanreotide autogel.
Of patients with carcinoid syndrome 49.0% were administered LAN at a starting dose of 60 mg at the baseline visit, 25.5% at a dose of 90 mg and 25.5% at a dose of 120 mg every 4 weeks. The proportion of patients with carcinoid syndrome receiving the 120-mg dose at month 12 was 59.4% and 68.0% at month 24. Throughout the study, the LAN injections were performed in more than 90% of the patients by a health care professional, in less than 10% by the patient or her/his partner.
During the study 16 (22.9%) patients received concomitant NET treatment. Of those, 43.8% received PRRT, 12.5% local ablative treatment, 18.8% chemotherapy and 43.8% targeted treatment with everolimus (n=6) or sunitinib (n=1) (multiple treatments possible).
Biochemical response
Effectiveness analyses are presented in the efficacy population. CgA levels were measured to assess long-term biochemical response to LAN treatment. The primary endpoint, disease control at 12 months, defined by a <50 % CgA increase compared with its lowest value between baseline and month 3, was achieved by 17 of 19 (89.5% [95% CI, 66.9–98.7]) patients with evaluable measurements. Since the effects of LAN are mediated by SSTR, biochemical response was evaluated post hoc in the subgroup of 12 patients with positive SSTR scintigraphy at baseline. In this subgroup, the primary endpoint was reached by 83.3% (95% CI, 51.6–97.9) patients.
Mean CgA (95% CI) levels were 13.2 x the upper limit of normal (ULN) (0.6–25.8) at baseline, 24.2 x ULN (0.0–67.7) at 6 months, 2.5 x ULN (0.7–4.4) at 12 months, and 2.4 x ULN (95% CI, 0.2–4.5) at 24 months. CgA levels >1 x ULN were observed in 27 (71.1%) patients and 9 (23.7%) patients had a CgA >10 x ULN at baseline. In a post hoc analysis in patients with carcinoid syndrome, mean (95 % CI) CgA levels were 21.1 x ULN (0.0–43.0) at baseline, 33.1 x ULN (0.0–94.8) at month 6, 3.0 x ULN (0.6–5.3) at month 12, and 2.7 x ULN (-0.3–5.7) at month 24. Due to low numbers, no data are presented for urine levels of 5-HIAA. Figure 3 shows the mean change of CgA values between baseline and 3, 6, 12, 18 and 24 months, and in the subgroup of patients with carcinoid syndrome.
Fig. 3.
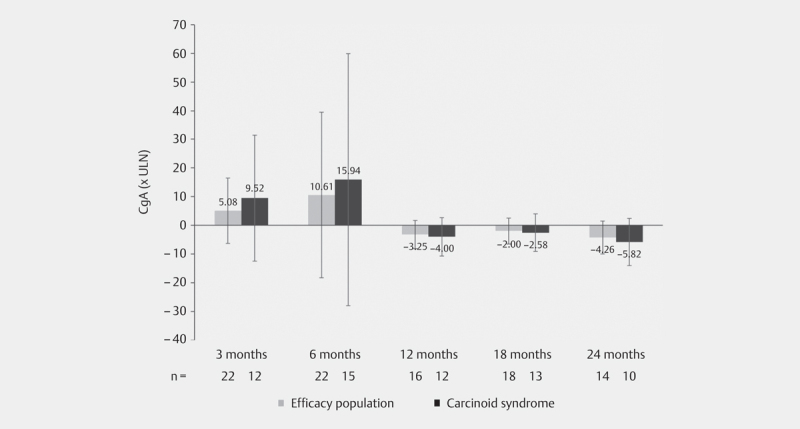
Mean change of CgA values (95% CI) from baseline during the course of the study in the patients of the efficacy population, and post hoc in patients with carcinoid syndrome; mean values at the baseline visit: efficacy population: 13.2 x ULN (95% CI, 0.61–25.81); patients with carcinoid syndrome: 21.13 × ULN (95% CI, 0.0–42.98); CgA: chromogranin A, ULN: upper limit of normal.
Symptoms and QoL, and glucose metabolism
38.5% of patients with carcinoid syndrome reported daily diarrhea episodes at baseline visit (70% of those with 4 or more daily bowel movements), 31.6% at month 12 and 21.4% at month 24 ( Fig. 4a ). At the baseline visit, 30.8% of patients with carcinoid syndrome experienced occasional or daily flushing episodes, 26.3% at 12 months and 14.2% patients at 24 months ( Fig. 4b ).
Fig. 4.

Patients with carcinoid syndrome reporting no, occasional or daily a . diarrhea or b . flushing episodes during the course of the study (efficacy population).
Table 2 shows mean (95% CI) raw scores and change from baseline to months 12 and months 24 for EORTC QLQ-C30 global health status and functional scales, symptom scales and single item scales as well as of the disease-specific GI-NET21 scales of the efficacy population. Improvements of mean scores of more than 10 points, i. e. changes deemed clinically significant, were observed for the QLQ-C30 single item scale appetite loss as well as for the GI-NET21 endocrine scale at month 24 ( Table 2 ). Worsening of more than 10 points was observed for the GI-NET21 treatment, muscle/bone pain symptom and body image scales at month 24 ( Table 2 ). No clinically significant differences with changes of more 10 points were observed either in the QLQ-C30 global health status/QoL and functional scales, the QLQ-C30 symptoms scales. However, confidence intervals were wide and the number of patients who completed the questionnaires was low.
Table 2 Mean baseline (95% CI) and changes from baseline to months 12 and 24 of EORTC QLQ-C30 and disease-specific EORTC GI-NET21 scale scores (efficacy population).
| Baseline | Change from baseline | |||||
|---|---|---|---|---|---|---|
| Month 12 | Month 24 | |||||
| n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | |
| QLQ-C30 global health status/QoL and functional scales a | ||||||
| Global health status | 36 | 54.4 (45.6–63.2) | 19 | 0.4 (−9.5–10.4) | 15 | −5.0 (−23.0–13.0) |
| Physical functioning | 36 | 72.8 (64.3–81.2) | 19 | −2.1 (−12.3–8.1) | 15 | −5.8 (−18.6–7.0) |
| Role functioning | 36 | 60.6 (48.9–72.4) | 19 | 0.0 (−15.6–15.6) | 15 | −3.3 (−25.7–19.1) |
| Emotional functioning | 7 | 63.2 (55.0–71.4) | 19 | −1.2 (−13.5–11.2) | 15 | −2.8 (−19.5–13.9) |
| Cognitive functioning | 36 | 79.2 (72.2–86.1) | 19 | −4.4 (−15.4–6.6) | 15 | 3.3 (−9.3–16.0) |
| Social functioning | 36 | 64.8 (54.0–75.6) | 19 | −6.1 (−22.0–9.7) | 15 | −8.9 (−33.5–15.7) |
| QLQ − C30 symptom scales b | ||||||
| Fatigue | 36 | 45.1 (35.1–55.1) | 19 | 4.1 (−9.9–18.1) | 15 | 7.4 (−4.1–19.0) |
| Nausea and vomiting | 36 | 14.4 (6.6–22.1) | 19 | 0.0 (−11.7–11.7) | 15 | −4.4 (−20.6–11.7) |
| Pain | 36 | 32.4 (21.5–43.3) | 19 | 7.9 (−11.3–27.1) | 15 | 7.8 (−12.8–28.4) |
| QLQ-C30 single item scales b | ||||||
| Dyspnea | 36 | 31.5 (20.7–42.2) | 19 | 1.8 (−10.8–14.3) | 15 | −2.2 (−20.0–15.5) |
| Insomnia | 36 | 41.7 (29.2–54.1) | 19 | 3.5 (−18.5–25.5) | 15 | 2.2 (−21.4–25.8) |
| Appetite loss | 35 | 22.9 (10.8–34.9) | 18 | −7.4 (−25.9–11.1) | 14 | −14.3 (−33.8–5.3) |
| Constipation | 36 | 2.8 (0.0–5.9) | 19 | 5.3 (−4.4–14.9) | 15 | 6.7 (−1.0–14.3) |
| Diarrhea | 36 | 51.9 (37.7–66.0) | 19 | −3.5 (−24.9–17.8) | 15 | −6.7 (−35.6–22.3) |
| Financial difficulties | 35 | 16.2 (6.4–26.0) | 18 | 7.4 (−6.0–20.8) | 14 | 7.1 (−11.6–25.9) |
| GI-NET21 scales b | ||||||
| Endocrine scale | 35 | 21.3 (12.2–30.3) | 18 | −7.4 (−17.8–3.0) | 14 | −11.9 (−25.1–1.3) |
| Gastrointestinal scale | 35 | 35.0 (28.2–41.7) | 18 | 5.6 (−7.1–18.2) | 14 | 0.5 (−10.5–11.4) |
| Treatment scale | 21 | 26.7 (14.4–39.0) | 11 | 1.0 (−12.2–14.2) | 9 | 11.1 (−5.3–27.5) |
| Social function scale | 35 | 60.3 (51.7–68.9) | 18 | 1.9 (−13.7–17.4) | 14 | 0.8 (−15.6–17.1) |
| Disease-related worries scale | 35 | 68.1 (59.6–76.6) | 17 | −8.5 (−23.3–6.3) | 14 | −7.5 (−27.6–12.5) |
| Muscle/bone pain symptom | 34 | 34.3 (22.0–46.6) | 18 | 9.3 (−10.3–28.8) | 14 | 21.4 (−2.0–44.8) |
| Sexual function | 19 | 50.9 (29.2–72.5) | 9 | 0.0 (−22.2–22.2) | 6 | 5.6 (−35.3–46.5) |
| Information/communication function | 34 | 19.6 (10.5–28.7) | 17 | 9.8 (−10.1–29.7) | 12 | 8.3 (−20.4–37.1) |
| Body image | 35 | 32.4 (19.8–45.0) | 18 | −1.9 (−22.7–19.0) | 14 | 16.7 (−6,9–40.2) |
Score ranges between a. 0 (worst functioning) and 100 (best functioning) – higher values indicate better quality of life, and b. between 0 (no problems) and 100 (highest level of problems/symptoms) – lower values indicate better quality of life; table cells highlighted in green indicate improvement vs. baseline. Green highlighting indicates improvement, red highlighting worsening of quality of life scores vs. baseline of more than 10 points; CI: confidence interval; EORTC: European Organisation for Research and Treatment of Cancer; QLQ: quality of life questionnaire; GI-NET: disease-specific scales for gastrointestinal neuroendocrine tumors.
Mean HbA 1c values were 5.8% (95% CI, 5.5–6.1) at baseline, 5.9% (95% CI, 5.7–6.1) at month 6, 5.9% (95% CI, 5.7–6.1) at month 12, and 7.0% (95% CI, 5.1–8.8) at month 24. The proportion of patients with HbA 1c values < 6.5% remained stable with ≥70% at all visits. Mean fasting glucose was essentially stable in the course of the study (range of mean values: 103.4–111.7 mg/dL). The proportion of patients with fasting glucose <140 mg/dL was ≥80% at all visits and the proportion of patients who received antidiabetic treatment remained <20% for all visits.
RECIST response and overall effectiveness
At baseline, 7.1% of patients with available imaging results (n=14) had complete response (CR), 7.1% partial response (PR), 42.9% stable disease (SD) and 42.9% progressive disease (PD). At month 12 (n=13) no patient had CR or PR, 76.9% had SD, and 23.1% had PD. At month 24 (n=8) 12.5% of patients had CR, no patient had PR, 75.0% had SD and 12.5% had PD.
Overall effectiveness of treatment with LAN was judged subjectively by the participating physicians as ‘very good’ or ‘good’ for 86.2% of patients at month 12 (n=29) and for 88.3% at month 24 (n=17) ( Fig. 5 ).
Fig. 5.
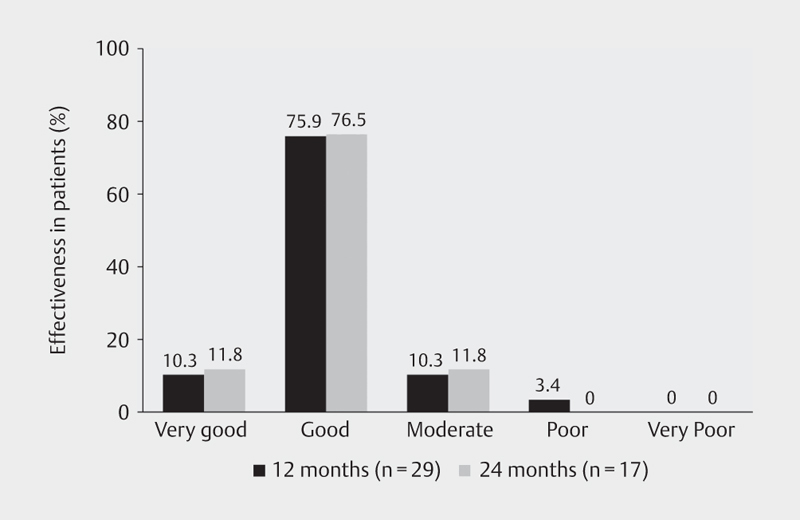
Global evaluation of LAN treatment effectiveness by physicians at months 12 and 24; LAN: lanreotide.
Safety and tolerability
No new safety concerns arose during this study and/or required further investigation. AEs were reported for 43 patients during the study following treatment with LAN. In 18 patients at least one AE was reported as serious, but no serious AEs were deemed ‘related’ or ‘possibly related’ to the study medication.
In the enrolled population, at baseline, mean heart rate was 74.4 (95% CI, 71.3−77.5) beats per minute (bpm), mean body weight was 73.7 (95% CI, 70.1−77.2) kg and mean systolic and diastolic blood pressures were 136.8 (95% CI, 132.2−141.4) mmHg and 78.8 (95% CI, 75.7−81.8) mmHg, respectively. During the study, heart rate, body weight and blood pressure remained unchanged. The proportion of patients who received antihypertensive treatment was 50.0% at baseline and ranged from 37.5% to 61.5% between month 3 and month 24.
Ultrasound examinations for gall bladder stones were performed in 49 patients at baseline, in 4 patients at month 1 and in 18 patients at months 6 to 18 during the follow-up. At baseline, gall bladder stones were detected in 4 patients and at month 24 in 7 patients.
Overall tolerability of treatment was judged as ‘very good’ or ‘good’ by 94.6% at month 12 and by 83.3% of patients at month 24.
Discussion
Our 24-month observational study of the treatment of NET with LAN in Germany and Austria provides insights into patient characteristics, treatment effectiveness, and disease control in various patient subgroups in a clinical ‘real-world’ setting. Nearly 90% of evaluable patients achieved the primary endpoint of a <50% increase of CgA levels at 12 months, which we defined as biochemically stable disease. In addition, imaging showed stable disease according to RECIST1.1 criteria in more than 75% of the patients at months 12 and 24. These observations are in line with the known antiproliferative activities of LAN in patients with GEP-NET 10 11 . Nevertheless, low sample sizes, missing data and variability of values due to local measurement of biochemical parameters limit the interpretation of our data: target enrolment was exceeded, but fewer patients than expected could be included in the efficacy population. Similarly, the proportion of patients with carcinoid syndrome reporting daily diarrhea at each visit in our post hoc analysis must be interpreted with caution, as different patients may be included at each visit.
Our study population represents patients from two European countries with similar health system characteristics. A median of 1.8 years between onset of symptoms and diagnosis indicates that the NET diagnostic delay was similar between our patient population and that reported in other studies 16 17 . With the exception of four patients, all tumors were classified as NET G1/G2 and the vast majority had metastasized. Almost all of the tumors studied immunohistochemically expressed SSTR2/5 and approximately 80% of patients with NET G1/2 and all patients with NET G3 had a positive SSTR scintigraphy. This is in agreement with literature data on SSTR expression in vitro 18 19 and on reported detection of carcinoid tumors reported from clinical studies (70–100%) 20 21 . This finding supports current clinical practice not to demand SSTR scintigraphy in order to predict response to somatostatin analogs. With respect to treatment, 90% of patients had been operated on previously. LAN was the first-line medical treatment being initiated for more than 80% of our patients. The use of first line somatostatin analogs is in accordance with current German and European guidelines 3 22 generally recommending treatment with somatostatin analogs in patients with advanced GEP-NET G1/G2 as initial antiproliferative or antisecretory therapy.
Within the study period no clinically relevant changes of mean values from baseline to month 24 were observed for 12 of 17 QoL scales assessed including scales for fatigue, pain and diarrhea, which are known to be negatively affected during the course of the disease in patients with NET, especially in those with carcinoid syndrome 23 . On the other hand, clinically relevant improvements of mean values were observed in two QLQ-C30 scales (appetite loss, endocrine scale) and worsening in three GI-NET21 scales (treatment, muscle/bone pain, body image). Still, evaluation of QoL and interpretation of results is difficult in the real-world setting due to the heterogeneity of the clinical presentation of NETs, which is also reflected by the wide confidence intervals.
Overall, participating physicians were satisfied with LAN treatment and rated the overall effectiveness of LAN in almost 90% of the patients as ‘good’ or ‘very good’. Nevertheless, only a little over half of the patients received the recommended antiproliferative standard LAN dose of 120 mg every 4 weeks at 24 months, suggesting that there may be potential to further optimize LAN treatment effectiveness in NET patients in a ‘real-world’ setting. In addition, patients with carcinoid syndrome might also have benefitted from using the full dose range of LAN in order to further reduce diarrhea or flushing episodes. We only can speculate about the reasons for not prescribing the recommended dose 120 mg LAN every 4 weeks in the patients. Some physicians may be worried about side effects, some may prefer individualized dosing and feel satisfied with treatment response to lower dosing. In fact, our data are in line with results of an observational study showing that 50% of GEP-NET patients treated in the community practice setting received octreotide at a relative dose intensity of less than 85%, 16.7% received above-label dose 24 .
No new safety signals for LAN were observed throughout our study. More than 90% of the participating patients rated tolerability of LAN as ‘good’ or ‘very good’, thus affirming the overall favorable safety profile obtained from controlled studies 5 6 7 8 9 10 11 .
Limitations of the study are essentially due to the ‘real-world’ nature of a non-interventional study. All decision-making concerning both diagnosis and treatment was in the hands of the treating physician. Patient population, treatment discontinuations and the number of patients who discontinued and the reduced numbers of valid measurements during the course of the study thus reflect routine clinical practice of the participating centers. Interpretation of results is clearly limited, in particular by a high dropout rate mainly due to decreasing efficacy of treatment and small sample sizes. We had planned to analyse whether early change in CgA was predictive of long-term treatment success, but the number of evaluable patients was too low. We were also unable to confirm the direct inhibitory action of LAN on excess serotonin production in the enrolled patients with carcinoid syndrome by analyzing urinary 5-HIAA excretion, due to low sample size. This heterogeneity of assessments reflects tailoring of care to individual patients. For example, only a few physicians performed ultrasound examinations during the follow-up visits, in line with guidelines that recommend ultrasound imaging in patients treated with somatostatin analogs at the start of therapy and for monitoring only if clinically indicated 25 26 .
In conclusion, this analysis shows favorable effectiveness with stabilization of disease-related symptoms and good tolerability of LAN in clinical practice.
Participating Centers and Principal Investigators
Department of Gastroenterology and Endocrinology, Philipps-University, Marburg, Germany (Anja Rinke), Hämatologisch-Onkologische Praxis, Würselen, Germany (Christoph Maintz), Praxis für Onkologie Leer-Emden-Papenburg, Leer, Germany (Lothar Müller), Department of Medicine 1, University Medical Center of the Johannes Gutenberg-University Mainz, Mainz, Germany (Matthias Weber), Department of Endocrinology and Metabolism, Division of Laboratory Research, University Hospital Essen, Germany (Harald Lahner), Department of Medicine 1, Division of Endocrinology, Universitätsklinikum Erlangen, Erlangen, Germany (Marianne Pavel), Department of Neuropathology, Pituitary Pathologist, University Hospital Eppendorf, Hamburg, Germany (Wolfgang Säger), ENDOC Center for Endocrine Tumors, Hamburg, Germany (Stephan Petersenn), Agaplesion Gesellschaft für Klinische Studien mbH, Frankfurt am Main, Germany (Claus Bolling), Klinikum der Johann Wolfgang Goethe-Universität - Medizinische Klinik I, Frankfurt am Main, Germany (Jörg Bojunga), Universitätsklinikum Greifswald - Klinik und Poliklinik für Innere, Greifswald, Germany (Markus Lerch), MVZ Endokrinologikum Berlin, Berlin, Germany (Henrik Biering), Städtische Kliniken Neuss - Chirurgische Klinik I, Neuss, Germany (Peter Goretzki), MVZ Balger Straße - Hämatologie, Internistische Onkologie, Baden-Baden, Germany (Kai Neben), Universitätsklinikum Tübingen - Innere Medizin I, Tübingen, Germany (Michael Bitzer), Onkologisches Versorgungszentrum Friedrichshain - Hämatologie, Onkologie und Palliativmedizin, Berlin, Germany (Herbert Lehahn), Universitätsklinikum Erlangen - Endokrinologie, Diabetes, Erlangen, Germany (Christof Schöfl), Praxis Dres. Etzrodt, Alexopoulos, Etzrodt-Walter, Ulm, Germany (Gwendolin Etzrodt-Walter), Onkologische Schwerpunktpraxis, Kronach, Germany (Martina Stauch), Charité Campus Mitte - Medizinische Poliklinik, Berlin, Germany (Yvonne Dörffel), MVZ Kloster Paradiese, Soest, Germany (Eckhard Böcher), Universitätsklinikum der PMU - Klinik für Innere Medizin III, Salzburg, Austria (Richard Greil), Universitätsklinikum des Saarlandes, Homburg, Germany (Frank Lammert), Medizinische Universität Wien - Universitätsklinik für Innere, Wien, Austria (Markus Raderer), Universitätsklinikum Heidelberg - Innere Medizin I, Heidelberg, Germany (Christian Kasperk), Krankenhaus der Elisabethinen Linz GmbH, Linz, Austria (Karl Aichberger), ODEM Gbr, Lohne, Germany (Matthias Penke), Klinikum Wels-Grieskirchen GmbH, Wels, Austria (Thomas Kühr), Clinical Research Stolberg GmbH, Stolberg, Germany (Matthias Groschek), phase drei - Hämato-Onkologischer Studienkreis am Klinikum, Aschaffenburg, Germany (Manfred Welslau)
Where patient data can be anonymised, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to datasharing@ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
Acknowledgements
This study was sponsored by Ipsen. The authors thank all patients involved in the study, as well as their caregivers, care team, investigators and research staff in participating institutions. The authors thank Dr. Philipp Hoffmanns, of Dr. Hoffmanns Gesundheitsberatung GmbH, (formerly an employee of Ipsen) for constructive contributions to the manuscript content, and Dr. Joachim Sauer of DP-Medsystems AG, Germany, for providing medical writing support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
Footnotes
Conflict of Interest A.R. has received honoraria for presentations by AAA, Falk, IPSEN and Novartis. She also received honoraria for attendance at advisory board meetings (Ipsen, Novartis) and travel/congress reimbursements (Ipsen, Novartis). H.L. has received honoraria for presentations by Pfizer, Ipsen and Novartis. He also received honoraria for attendance at advisory board meetings (Pfizer, Ipsen, Novartis) and travel/congress reimbursements (Pfizer, Ipsen, Novartis). C.M. has no COI. S.P. has served as an advisory board member for Ipsen, Crinetics, Takeda, and Novartis, and has received honoraria for speaking at symposia for Ipsen, Novartis, Takeda, and Pfizer. A.H., H.U. are Ipsen employee. L.M. has served as an advisory board member for Ipsen. M.P. received honoraria for presentations by IPSEN, AAA, Novartis, Falk, and Boehringer-Ingelheim, and for advisory role from AAA, IPSEN, Riemser, Lilly, Amgen. W.S. has no COI. M.M.W. has received honoraria as a speaker and consultant from Ipsen and Novartis.
References
- 1.Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberg K E.Gastrointestinal neuroendocrine tumors Ann Oncol 201021Suppl 7vii72–vii80. [DOI] [PubMed] [Google Scholar]
- 3.Pavel M, O'Toole D, Costa F et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 4.Grozinsky-Glasberg S, Grossman A B, Gross D J. Carcinoid Heart Disease: From Pathophysiology to Treatment–'Something in the Way It Moves’. Neuroendocrinology. 2015;101:263–273. doi: 10.1159/000381930. [DOI] [PubMed] [Google Scholar]
- 5.Vinik A I, Wolin E M, Liyanage N et al. Evaluation of lanreotide depot/Autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): A randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22:1068–1080. doi: 10.4158/EP151172.OR. [DOI] [PubMed] [Google Scholar]
- 6.O'Toole D, Ducreux M, Bommelaer G et al. Treatment of carcinoid syndrome: A prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer. 2000;88:770–776. doi: 10.1002/(sici)1097-0142(20000215)88:4<770::aid-cncr6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Fisher G A, Wolin E M, Liyanage N et al. Lanreotide therapy in carcinoid syndrome: prospective analysis of patient-reported symptoms in patients responsive to prior octreotide therapy and patients naive to somatostatin analogue therapy in the ELECT phase 3 study. Endocr Pract. 2018;24:243–255. doi: 10.4158/EP172000.OR. [DOI] [PubMed] [Google Scholar]
- 8.Ruszniewski P, Valle J W, Lombard-Bohas C et al. Patient-reported outcomes with lanreotide Autogel/Depot for carcinoid syndrome: An international observational study. Dig Liver Dis. 2016;48:552–558. doi: 10.1016/j.dld.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Ruszniewski P, Ish-Shalom S, Wymenga M et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: Results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80:244–251. doi: 10.1159/000082875. [DOI] [PubMed] [Google Scholar]
- 10.Caplin M E, Pavel M, Cwikla J B et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 11.Caplin M E, Pavel M, Cwikla J B et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somatuline Autogel EMA Summary of product characteristics Ipsen Pharma GmbH, October 2018, https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/21/2019/12/11153003/Fachinformation_Somatuline-60_90_120-mg-Filmtabletten_Stand-Okt-2019.pdf
- 13.Somatuline Autogel US Prescribing Information Ipsen Biopharmaceuticals, Inc, June 2019, https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/2019/08/30162316/Somatuline_Depot_Full_Prescribing_Information_7.22.19.pdf
- 14.Pavel M, Unger N, Borbath I et al. Safety and QOL in patients with Advanced NET in a phase 3b expanded access study of everolimus. Target Oncol. 2016;11:667–675. doi: 10.1007/s11523-016-0440-y. [DOI] [PubMed] [Google Scholar]
- 15.Osoba D, Rodrigues G, Myles J et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Granberg D, Wolin E et al. Patient-Reported Burden of a Neuroendocrine Tumor (NET) Diagnosis: Results From the First Global Survey of Patients With NETs. J Glob Oncol. 2017;3:43–53. doi: 10.1200/JGO.2015.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basuroy R, Bouvier C, Ramage J K et al. Presenting symptoms and delay in diagnosis of gastrointestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 2018;107:42–49. doi: 10.1159/000488510. [DOI] [PubMed] [Google Scholar]
- 18.de Herder W W, Hofland L J, van der Lely A J et al. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10:451–458. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- 19.Reubi J C, Laissue J, Waser B et al. Expression of somatostatin receptors in normal, inflamed, and neoplastic human gastrointestinal tissues. Ann NY Acad Sci. 1994;733:122–137. doi: 10.1111/j.1749-6632.1994.tb17262.x. [DOI] [PubMed] [Google Scholar]
- 20.Dimitroulopoulos D, Xynopoulos D, Tsamakidis K et al. Scintigraphic detection of carcinoid tumors with a cost effectiveness analysis. World J Gastroenterol. 2004;10:3628–3633. doi: 10.3748/wjg.v10.i24.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberg K, Eriksson B. Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:265–276. doi: 10.1016/j.beem.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Rinke A, Wiedenmann B, Auernhammer C et al. S2k-Leitlinie Neuroendokrine Tumore, AWMF Register Nr. 021-26. Z Gastroenterol. 2018;56:583–681. doi: 10.1055/a-0604-2924. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Beaumont J L, Hudgens S et al. Relationship between symptoms and health-related quality-of-life benefits in patients with carcinoid syndrome: Post Hoc Analyses From TELESTAR. Clin Ther. 2018;40:2006–INF. doi: 10.1016/j.clinthera.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Jiao X, Pulgar S, Boyd M et al. Treatment patterns and clinical outcomes in patients with metastatic gastroenteropancreatic neuroendocrine tumors treated in the community practice setting in the United States. Pancreas. 2018;47:173–182. doi: 10.1097/MPA.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 25.Katznelson L, Laws E R, Melmed S et al. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 26.Pavel M, Valle J W, Eriksson B et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms: Systemic therapy - biotherapy and novel targeted agents. Neuroendocrinology. 2017;105:266–280. doi: 10.1159/000471880. [DOI] [PubMed] [Google Scholar]


