Abstract
Introduction.
Symptom improvement was assessed as changes in the Chronic Respiratory Infection Symptom Score (CRISS) during intravenous antimicrobial exacerbation treatments among subjects from study NCT02109822.
Methods.
Median daily CRISS reduction (i.e., improvement) and covariates associated with CRISS reduction by Day 14 were assessed by logistic regression.
Results.
Among 173 subjects, median baseline CRISS was 49 [IQR 41, 56]; 93.6% had a CRISS reduction of ≥11 (minimal clinically important difference); median time to −11 reduction was 2 days [95% CI 2, 3]. The greatest median CRISS difference from baseline, on Day 17, was −26 [−29, −23]. Odds of −26 CRISS change by Day 14 were greater in subjects with higher baseline CRISS (P=.006) and younger ages (P=.041).
Conclusions.
CRISS response has good dynamic range and may be a useful efficacy endpoint for PEx interventional trials. The optimal use of CRISS change as an endpoint remains uncharacterized.
Keywords: cystic fibrosis, exacerbations, symptoms, clinical trials
Introduction.
People with cystic fibrosis (CF) experience recurrent pulmonary exacerbations (PEx), typically described as a worsening of respiratory signs and symptoms [1]. PEx management often includes antimicrobial treatment and aggressive chest physiotherapy with a goal of lung function improvement, but patients have expressed a greater interest in relief of PEx respiratory symptoms than lung function recovery [2], and treatment-associated lung function and respiratory symptom changes do not strongly correlate [3]. The Standardized Treatment of Pulmonary Exacerbations Observational study (STOP-OB; NCT02109822) was a prospective observational study of intravenous (IV) antimicrobial response to CF PEx treatment intended to provide information to design future PEx intervention studies [4]. In the current analysis, we assess daily change of respiratory symptoms during PEx treatment as an efficacy endpoint.
Methods.
STOP-OB was an observational study conducted at eleven US CF centers between January 2014 and January 2015 approved by each participating center’s Institutional Review Board. People with CF ages ≥12 years old admitted to the hospital for IV antimicrobial treatment of PEx were studied. Subject symptoms were captured using the Cystic Fibrosis Respiratory Symptom Diary (CFRSD) Chronic Respiratory Infection Symptom Score (CRISS) [5–7] daily for up to 28 days (lower score = fewer symptoms). The CRISS has been used as a key secondary outcome in previous CF studies [2,8,9] and a completed validation dossier is currently under review by the Food and Drug Administration Division of Clinical Outcome Assessment (DDT COA #000007). To be included in the current analysis, subjects had to have had at least one CRISS measure recorded by Day 2 (the first measure being defined as “baseline”) and >9 and >15 measures recorded by Days 14 and Day 28, respectively. Mean, median, and interquartile ranges of CRISS values were plotted by study day using notched box-and-whickers, and values at Days 1, 14, and 28 were plotted for age subgroups (<18, 18 to <25, 25 to <35, and ≥35 years) and baseline percent predicted forced expiratory volume in 1 second (ppFEV1) quartiles by box-and-whisker. Hodges-Lehman median differences from baseline CRISS at Days 14 and 28 and 95% confidence intervals (CI) were determined among all subjects, for younger versus older subjects, and for subjects in the lowest versus highest ppFEV1 quartiles. Median difference significance was assessed by Mann-Whitney test for independent samples. Medians and 95% CI for times to an 11-point CRISS reduction and to the greatest observed median CRISS reduction from baseline were assessed by Kaplan-Meier method. Odds ratios of CRISS reductions at Day 14 were assessed by logistic regression using baseline CRISS, age, sex, baseline ppFEV1, body mass index (BMI), and age*ppFEV1 interaction as covariates. A sensitivity analysis excluded subjects with lower baseline CRISS values. Analyses were conducted with MedCalc Statistical Software version 19.2 (Ostend, Belgium).
Results.
Of 220 STOP-OB subjects, 173 were included in this analysis. Mean baseline age was 26.3 (SD 9.5) years; mean ppFEV1 (N=159) was 51.0 (21.6). Median baseline CRISS was 49 [IQR 41, 56; range 0, 73]. Mean ppFEV1, median CRISS, and sex distributions were similar at baseline for STOP-OB subjects excluded from analyses; excluded subjects were on average younger than those included: 22.0 (SD 6.4) years versus 27.2 (10.1) years, P<.001. Median CRISS at Day 14 was 23 [IQR 14, 34] (N=166; Figure 1). Median CRISS difference from baseline at Day 14 was −24 [95% CI −28, −22], P<.001. The greatest median CRISS difference from baseline, observed at Day 17, was −26 [95% CI −29, −23]. The difference between Day 28 median CRISS (N=120) and baseline was −21 [95% CI −24, −28], P<.001 (Figure 1). Most subjects (N=162; 93.6%) experienced a CRISS reduction of ≥11 (the minimal clinical important difference [6]); 78.0% experienced a reduction of ≥26. Median times to 11- and 26-point CRISS reductions were 2 [95% CI 2, 3] and 11 [10, 13] days, respectively (Figure 2).
Figure 1. Notch-and-whisker plot of CRISS measure distributions by study day.
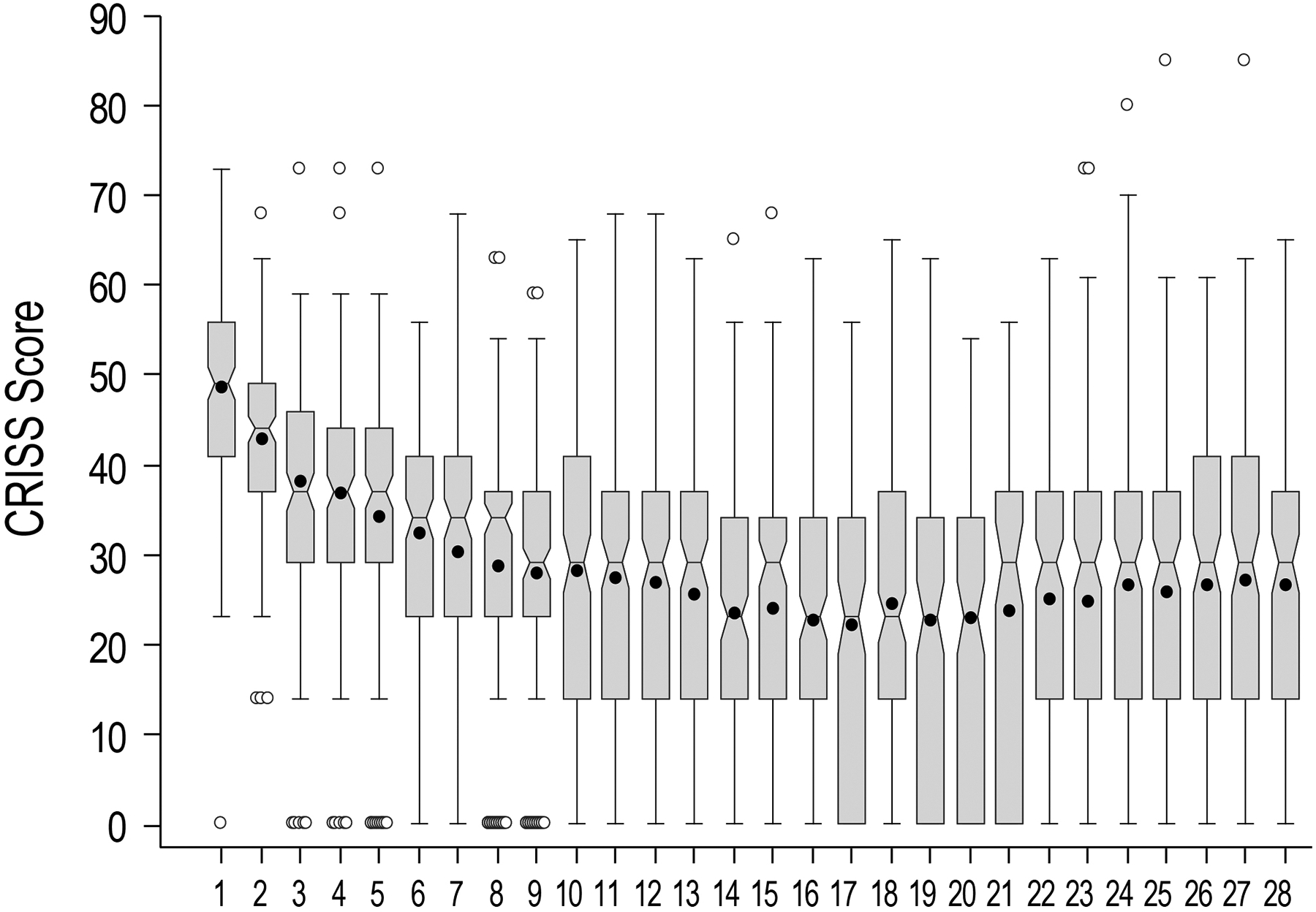
Horizontal lines within boxes are median values, boxes identify interquartile ranges, notches display median confidence intervals, and whiskers display ranges. Means are shown as solid circles and outliers as open circles.
Figure 2. Kaplan Meier curves of time to achieving 11-point and 26-point CRISS reductions from baseline.
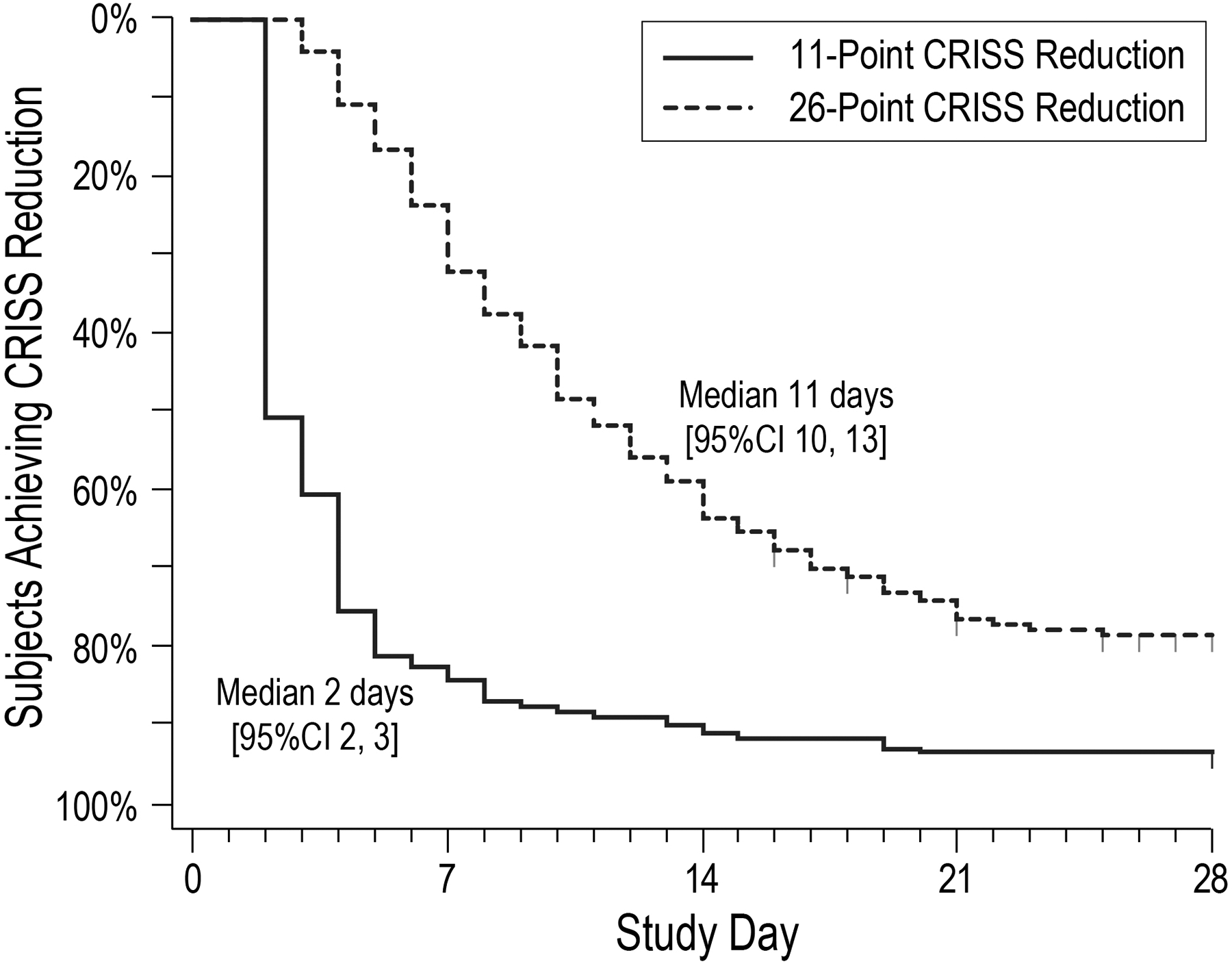
Time to an 11-point CRISS reduction from baseline is shown as a solid line; time to a 26-point reduction is shown as a hashed line.
Higher baseline CRISS score was associated with probability of reaching both an 11-point and a 26-point CRISS reduction by Day 14 in adjusted logistic regressions, with odds ratios of 1.12 [95% CI 1.05, 1.19], P<.001, and 1.05 [1.02, 1.09], P=.006 respectively. Exclusion of subjects with lower baseline CRISS did not result in greater proportions of subjects reaching treatment goals (data not shown). Greater baseline ppFEV1 was also associated with greater probability of reaching an 11-point CRISS reduction by Day 14 (odds ratio 1.13 [1.01, 1.27]), P=.036, but not with that of reaching a 26-point reduction by Day 14. In contrast, younger subject age was also associated with greater probability of reaching a 26-point CRISS reduction by Day 14 (odds ratio 1.13 [1.01, 1.27], P=.041), but not with probability of reaching an 11-point reduction by Day 14. BMI, sex, and age*ppFEV1 interaction were not significant covariates in CRISS reduction models.
CRISS values tended to be greater in older subjects at baseline, Day 14, and Day 28 (Figure 3A). Median CRISS for 29 subjects <18 years old was less than that of 29 subjects ≥35 years old (44 vs. 55, P=.020) at baseline. Median CRISS differences were more pronounced between youngest and oldest age groups at Day 14 (14 vs. 34, P=.007) and Day 28 (14 vs. 34, P=.005). CRISS values did not significantly differ between subjects from the highest (≥54.4, N=40) and lowest (<32.8, N=40) ppFEV1 quartiles at baseline (medians of 45 vs. 50.5, respectively, P=.061) (Figure 3B). However, medians for these groups did differ at Day 14 (14 for highest ppFEV1 quartile subjects vs 29 for the lowest quartile, P=.013), but not at Day 28 (medians of 29 and 34, respectively, P=.32).
Figure 3. CRISS values at baseline, Day 14, and Day 28 stratified by age and lung function.
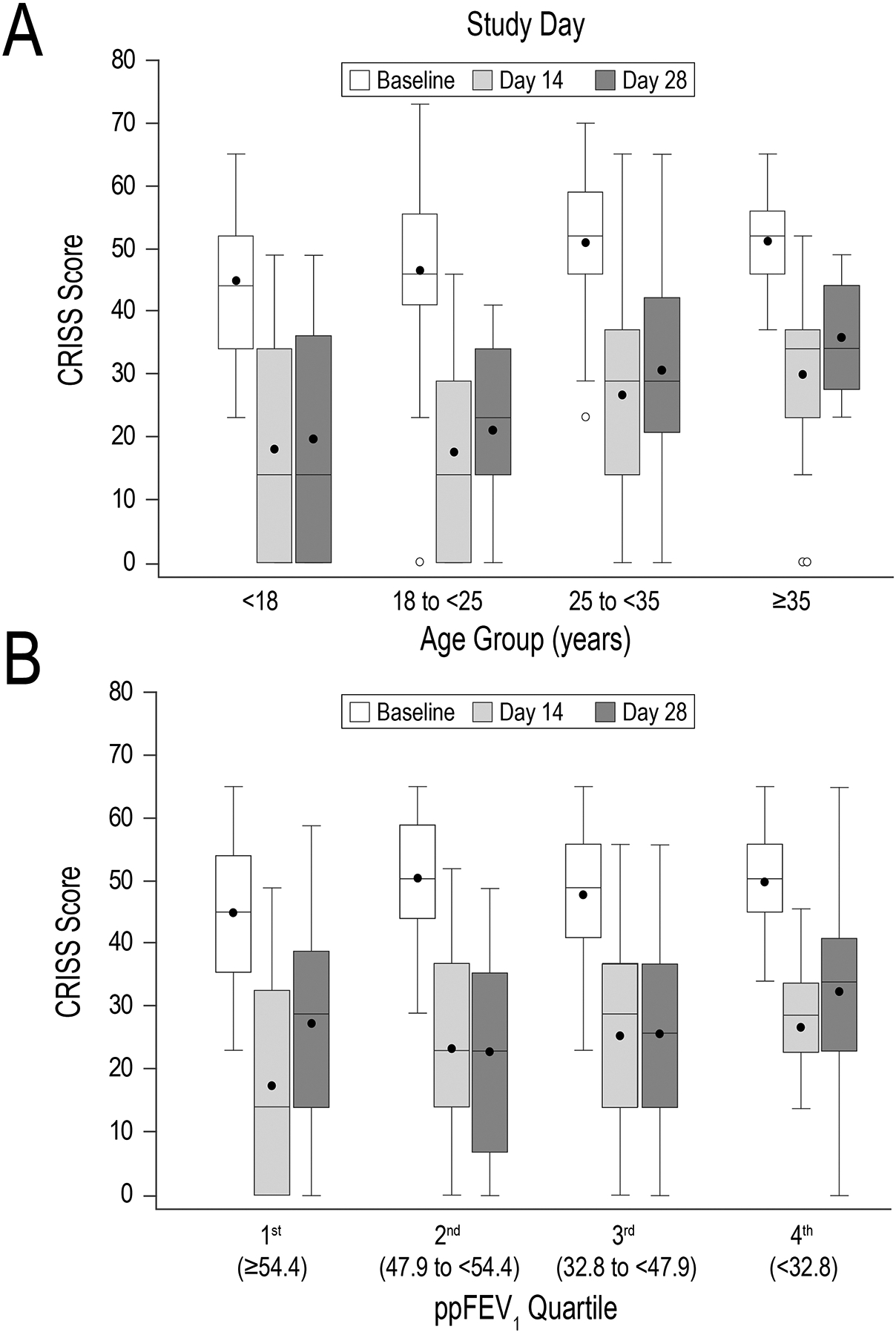
Panel A, box-and-whisker CRISS plots stratified by age group. Panel B, plots stratified by ppFEV1 quartiles. Boxes display medians and interquartile ranges, whiskers display ranges, solid circles display means, and open circles display outliers. White bars represent baseline values; light and dark gray boxes represent Day 14 and Day 28 values, respectively.
Conclusions.
People with CF have reported that symptom reduction is their greatest priority with respect to assessing recovery during PEx treatment [2]. The CRISS is a validated patient-reported outcome tool that is simple to administer, with only 8 questions to measure PEx-associated symptom changes. Our analyses indicate that treatment-associated CRISS change from baseline is a viable efficacy endpoint for clinical studies of PEx interventions with attractive characteristics, including rapid change, good dynamic range, and sensitivity across broad age and lung function groups. Presumably, active comparator studies would assess mean differences between treatment groups in CRISS change at a fixed time point. Our results indicate a strong association between greater CRISS values at PEx treatment initiation and the probability of substantial CRISS reduction by 14 days (a typical PEx treatment duration), which might suggest that protocols using the CRISS as an endpoint might benefit from setting a minimum CRISS score as an inclusion criterion. However, sensitivity analyses in which subjects with lower CRISS scores at baseline were excluded from logistic regressions did not suggest an advantage to this approach. Trends towards older subjects and those with more advanced lung disease having higher CRISS values both prior to and after PEx treatment when compared to younger subjects suggest that studies might benefit from including stratification by subject age and/or ppFEV1 during treatment group allocation.
Our analysis has several limitations. Adherence to daily diary entry was challenging for subjects, and some subjects were excluded from analyses due to missing data. However, daily CRISS collection may not be necessary for effective assessment of treatment response. STOP-OB was not a randomized comparison of PEx treatments, providing no basis to conclude that CRISS changes during the study would have been affected by, for instance, different antimicrobial treatments. In fact, the only successful placebo-controlled study of antimicrobial PEx treatment was able to identify a treatment-associated difference in ppFEV1 change from baseline but not of respiratory symptoms [10]. It is not clear whether the established CRISS minimal clinically important difference of 11 points is an appropriate endpoint when used in this context, as it was observed to occur very quickly. Our choice to study a 26-point CRISS reduction (the greatest daily median CRISS reduction) as a response endpoint was arbitrary. Finally, it is not clear whether difference in magnitude of CRISS reduction or difference in time to a given reduction would perform better as an efficacy endpoint (it is possible to imagine different scenarios where one but not both endpoints could be positive). Answers to these and other questions will likely require the addition of CRISS collection to future randomized studies employing other efficacy endpoints.
Acknowledgements
The STOP-OB study was supported by grants from the Cystic Fibrosis Foundation (SANDERS14A0, HELTSH13A1, GOSS13A0, FLUME13A1, CLANCY09Y0, SORSCH15RO, ORENST14Y0, NICKR0, DAINES14Y0). We thank Bruce Marshall for his continued support of the STOP program and goal to improve the health of our patients. We would like to thank the patients, families, care providers, and clinic coordinators at CF Centers in STOP and throughout the CFF Therapeutic Development Network for their contributions to the study. Finally, we would like to thank all the participating sites, investigators, and research coordinators.
References
- [1].Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148(2):259–264. [DOI] [PubMed] [Google Scholar]
- [2].Heltshe SL, West NE, VanDevanter DR, Sanders DB, Beckett VV, Flume PA, Goss CH; STOP Study Group. Study design considerations for the Standardized Treatment of Pulmonary Exacerbations 2 (STOP2): A trial to compare intravenous antibiotic treatment durations in CF. Contemp Clin Trials. 2017. November 21;64:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].VanDevanter DR, Heltshe SL, Spahr J, Beckett VV, Daines CL, Dasenbrook EC, Gibson RL, Jain R, Sanders DB, Goss CH, Flume PA; STOP Study Group. Rationalizing endpoints for prospective studies of pulmonary exacerbation treatment response in cystic fibrosis. J Cyst Fibros. 2017. September;16(5):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, VanDevanter DR, Spahr JE, Gibson RL, Nick JA, Marshall BC, Flume PA, Goss CH; STOP Study Group. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017. September;16(5):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. July 2009;8(4):245–52. [DOI] [PubMed] [Google Scholar]
- [6].Goss CH, Caldwell E, Gries K, Leidy N, Edwards T, Flume PA, et al. Validation of a novel patient-reported respiratory symptoms instrument in cystic fibrosis: CFRSD-CRISS. Pediatr Pulmonol. 2013:A251. [Google Scholar]
- [7].Schmid-Mohler G, Caress AL, Spirig R, Benden C, Yorke J. Patient-Reported Outcome Measures for Symptom Perception During a Cystic Fibrosis Exacerbation. Respir Care. 2018;63(3):353–366. [DOI] [PubMed] [Google Scholar]
- [8].Lechtzin N, Mayer-Hamblett N, West NE, Allgood S, Wilhelm E, Khan U, Aitken ML, Ramsey BW, Boyle MP, Mogayzel PJ Jr, Gibson RL, Orenstein D, Milla C, Clancy JP, Antony V, Goss CH; eICE Study Team. Home Monitoring of Patients with Cystic Fibrosis to Identify and Treat Acute Pulmonary Exacerbations. eICE Study Results. Am J Respir Crit Care Med. 2017;196(9):1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gold LS, Patrick DL, Hansen RN, Beckett V, Goss CH, Kessler L. Correspondence between symptoms and preference-based health status measures in the STOP study. J Cyst Fibros. 2019;18(2):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis. 1990;141(4 Pt 1):914–921. [DOI] [PubMed] [Google Scholar]


