Abstract
Background
There is growing concern about possible cognitive consequences of COVID-19, with reports of ‘Long COVID’ symptoms persisting into the chronic phase and case studies revealing neurological problems in severely affected patients. However, there is little information regarding the nature and broader prevalence of cognitive problems post-infection or across the full spread of disease severity.
Methods
We sought to confirm whether there was an association between cross-sectional cognitive performance data from 81,337 participants who between January and December 2020 undertook a clinically validated web-optimized assessment as part of the Great British Intelligence Test, and questionnaire items capturing self-report of suspected and confirmed COVID-19 infection and respiratory symptoms.
Findings
People who had recovered from COVID-19, including those no longer reporting symptoms, exhibited significant cognitive deficits versus controls when controlling for age, gender, education level, income, racial-ethnic group, pre-existing medical disorders, tiredness, depression and anxiety. The deficits were of substantial effect size for people who had been hospitalised (N = 192), but also for non-hospitalised cases who had biological confirmation of COVID-19 infection (N = 326). Analysing markers of premorbid intelligence did not support these differences being present prior to infection. Finer grained analysis of performance across sub-tests supported the hypothesis that COVID-19 has a multi-domain impact on human cognition.
Interpretation
Interpretation. These results accord with reports of ‘Long Covid’ cognitive symptoms that persist into the early-chronic phase. They should act as a clarion call for further research with longitudinal and neuroimaging cohorts to plot recovery trajectories and identify the biological basis of cognitive deficits in SARS-COV-2 survivors.
Funding
Funding. AH is supported by the UK Dementia Research Institute Care Research and Technology Centre and Biomedical Research Centre at Imperial College London. WT is supported by the EPSRC Centre for Doctoral Training in Neurotechnology. SRC is funded by a Wellcome Trust Clinical Fellowship 110,049/Z/15/Z. JMB is supported by Medical Research Council (MR/N013700/1). MAM, SCRW and PJH are, in part, supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London
Keywords: COVID-19, Long covid, Cognition, Deficits, Online assessment, Attention, Memory, Reasoning, Planning
Research in context.
Evidence before this study
Searching PubMed and Google scholar for the terms ‘COVID-19′, ‘long covid’, ‘SARS-CoV2’, ‘cognition’ and ‘brain fog’ highlights a growing body of studies reporting health changes that persist beyond the acute and sub-acute phases post COVID-19 infection, often termed ‘long COVID’. Much of this work depends on small scale studies and self-reported cognitive problems with little information regarding whether COVID-19 infection associates with objectively measured cognitive deficits or how this differs with respiratory symptom severity or hospitalisation status at a population level. Furthermore, many previous studies have been limited insofar as they lack sufficient scope and scale to account for key sociodemographic variables that associate with COVID-19 illness e.g., age, racial-ethnic group, pre-existing medical conditions and symptoms of depression, anxiety or insomnia.
Added value of this study
We report analyses of a large dataset comprising detailed cognitive assessment and questionnaire data pertaining to COVID-19 infection, from tens of thousands of individuals that span a large cross-section of the general public, around the time of the first wave within the UK. Importantly, as the data were collected in collaboration with BBC2 Horizon, COVID-19 was not mentioned in the promotional material, which mitigates sampling bias. We report that individuals who recovered from COVID-19, including biologically confirmed cases who remained at home and did not receive medical support, perform worse on a range of cognitive tests than would be expected given their detailed age and demographic profiles.
Implications of all the available evidence
This study confirms the hypothesis that individuals who have been infected with COVID-19 have persistent objectively measurable cognitive deficits after carefully controlling for pre-morbid IQ, pre-existing medical conditions, socio-demographic factors and mental health symptoms. Multiple studies are now using the online assessment technology reported here to investigate the neural correlates of cognitive deficits in people who have survived SARS-COV-2 infection, relate them to clinical outcomes and track at scale how they change over time.
Alt-text: Unlabelled box
Introduction
There is growing evidence that individuals with severe COVID-19 disease can have symptoms that persist beyond the initial illness, including through the sub-acute and into the early chronic phase. Often referred to as ‘Long COVID’ [1], [2], [3], there are colloquial reports of ‘brain fog’ with self-reported broad psychological symptoms including low energy, problems concentrating, disorientation and difficulty finding the right words. In parallel, case studies have provided evidence that COVID-19 patients can develop a range of neurological complications [4], [5], [6] including those arising from stroke [7,8], encephalopathies [9], inflammatory syndrome [7,10], microbleeds [7] and autoimmune responses [11]. There are concerns regarding potential neurological consequences due to sepsis, hypoxia and immune hyperstimulation [7,12,13], with reports of elevated cerebrospinal fluid autoantibodies in patients with neurological symptoms [14], white matter change in the brain [5,15,16], and psychological and psychiatric consequences at the point of discharge [17].
It is yet to be established whether COVID-19 infection is associated with cognitive deficits at the population level and how this differs with respiratory symptom severity [7,18]. Cognitive problems in those who have required a lengthy hospital stay or intubation are expected [19]. What is less clear is whether milder cases who have not been hospitalized also can suffer objectively measurable cognitive deficits. Measuring such associations is challenging. Longitudinal cognitive data from pre- to post-COVID-19 illness are scarce because infection is unpredictable. This issue is exacerbated by the cost of running standard face-to-face cognitive assessments in large enough populations to capture such change, or to account for potentially confounding population variables that correlate with cognitive performance. Furthermore, it is important to include key minority sub-populations, for example, older adults, racial-ethnic groups, and people with pre-existing medical conditions [20], [21], [22]. This motivated us to take a large-scale approach, whereby individuals who have recovered from COVID-19 infection were compared to concurrently obtained controls whilst accounting for the uneven sociodemographic distribution of virus prevalence and any associated population variability in cognition.
More specifically, at the time of writing, we had collected comprehensive cognitive test and questionnaire data from a very large cross-section of the general public, predominantly within the UK, as part of the Great British Intelligence Test - a collaborative project with BBC2 Horizon [23]. Notably, the online assessment platform had been optimized with NIHR support for remotely delivering cognitive assessments, including for older adults and patients with cognitive and motor deficits, with cross validation against commonly used neuropsychological scales. Due to the high visibility of the study, the cohort spanned a broad age and demographic range. During May, at the peak of the UK lockdown, we expanded the questionnaire (Table S1) to include questions pertaining to the impact of the pandemic, including suspected or confirmed COVID-19 illness, alongside details of symptom persistence and severity, relevant pre-existing medical conditions, and measures of depression, anxiety and post-traumatic stress [24,25].
Here, we analysed data from 81,337 individuals who completed the full extended questionnaire in order to test the hypothesis that those who had recovered from COVID-19 would show objective cognitive deficits when performing tests of attention, working memory, problem solving and emotional processing. We also determined whether the extent and/or nature of cognitive deficit related to severity of respiratory symptoms as gauged by level of medical assistance, positive verification of infection via a biological test, or time since illness onset.
Methods
Study promotion
We collected data from members of the general public, predominantly from the UK, who completed an extended questionnaire (inclusive of questions pertaining to COVID-19 infection) and series of cognitive tasks via The Great British Intelligence Test, a collaborative citizen science project with BBC2 Horizon that launched in late December 2019. At the beginning of January, articles promoting the study were placed on the Horizon homepage, BBC News homepage and main BBC homepage, and circulated via news meta-apps. They remained in prominent positions within the public eye throughout January. In May, aligned with report of initial results considered of interest to the general public via a BBC2 Horizon documentary, there was a further promotional push. This led to high recruitment in the months of January and May, with lower, but still substantial recruitment between and after these dates. The data analysed here includes responses from January until December 2020.
2.2. Data collection
The study was promoted as a free way for people to test themselves in order to find out what their greatest personal cognitive strengths were. It comprised a sequence of nine tests from the broader library that is available on our server system based on prior data showing that they can be used to measure distinct aspects of human cognition, spanning planning/reasoning, working memory, attention and emotion processing abilities, in a manner that is sensitive to population variables of interest whilst being robust against the type of device that a person is tested on. In this respect, the battery of tests should not be considered an IQ test in the classic sense, but instead, is intended to differentiate aspects of cognitive ability on a finer grain. The tests had been optimized for application with older adults and people with mild cognitive and motor impairments. This study was approved by the Imperial College Research Ethics Committee (17IC4009). Participants provided informed consent via the study website prior to starting the assessment.
All Cognitron tests were programmed in HTML5 with JavaScript by AH and WT. They were hosted on a custom server system (Cognitron) on the Amazon EC2 that can support diverse studies via custom websites. The server system was specifically developed to handle spikey acquisition profiles that are characteristic of main-stream media collaborative studies, fitting the number of server instances in an automated manner to rapid changes in demand. Here, maximum concurrent participants landing on the website information page was ~36,000, with this occurring at the point of the documentary airing on BBC2 in May.
After the nine cognitive tests, participants were presented with a detailed questionnaire with items capturing a broad range of socio-demographic, economic, vocational and lifestyle variables. During May, in response to the COVID-19 pandemic, the questionnaire was extended to include items pertaining to the direct and indirect impact of the virus, along with questions regarding common pre-existing medical conditions (Table S1) and 12 mood self-assessment items capturing aspects including depression, anxiety, insomnia, tiredness (Table S2). People who indicated that they had suspected having COVID-19 were presented further questions including whether they had breathing difficulties, what happened as a consequence of their breathing difficulties, and whether there had been positive confirmation via a biological test (Table S1). People under the age of 16 were not excluded. Instead, they were presented with an abbreviated questionnaire that did not include COVID-19 related items and were not analyzed here. This decision was made to help ensure accelerated approval via the ethics board.
On completing the questionnaire, participants were provided with a summary report of their performance relative to all other people who had undertaken each of the tests, which highlighted the cognitive tests that they performed relatively highest on. This report was used as a way to motivate people to take part in the study by finding out what their cognitive strengths were. The ordering of events as outlined above was designed to mitigate biases. Specifically, the study did not advertise as having a COVID-19 related questionnaire, eschewing biased sampling of people who were concerned that the illness had reduced their cognitive functions. Furthermore, when filling out the questionnaire, participants were yet to be shown how their performance compared to the normative population, thereby avoiding that feedback from biasing questionnaire responses.
2.3. Test designs
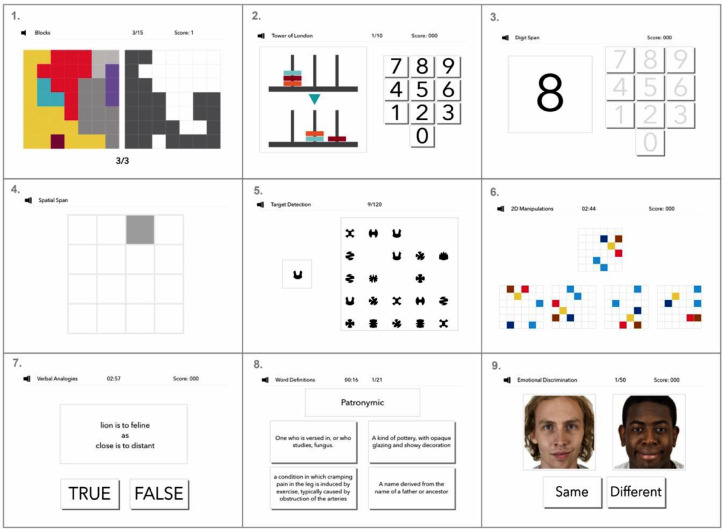
The cognitive tests included in this study (and three more recently added tests) can be viewed at https://gbit.cognitron.co.uk. In brief, the main study included nine tests that based on previous analyses were known to be robust across devices, sensitive to population variables of interest such as age, gender and education level, manageable for older adults and patients with mild cognitive or motor deficits, and not so strongly correlated as to measure just one overarching ability (Fig. 1). Further details regarding the test designs can be found in supplementary information.
Fig. 1.
Cognitive tests included within the great British intelligence test.
1. Block rearrange, a test designed to measure spatial problem solving. 2. Tower of London, designed to test measures of spatial planning. 3. Digit span, a test designed to measure working memory. 4. Spatial span, a test designed to measure spatial short-term memory capacity. 5. Target detection, designed to measure spatial visual attention 6. 2D mental rotations, a test designed to measure the ability to spatially manipulate objects in mind. 7. Analogical reasoning, measuring semantic reasoning abilities. 8. Rare word definitions, assessing the ability of individuals to identify the correct definitions of words. 9. Face emotional discrimination, designed to measure an individual's ability to identify and discern between emotions.
2.4. Statistical methods
All processing and analysis steps were conducted in MATLAB by AH with assistance from WT. Visualization was conducted in R (v4.0.2) by JMB and AJ. Pre-processing steps were as follows. Participants under 16 or who had not completed the extended questionnaire were removed from the analysis. Each test was designed to produce one primary accuracy-based performance measure (details of test designs are provided below). Values more than 5 standard deviations from the mean were winsorised. Nuisance variables were factored out by applying a generalized linear model and taking the standardized residuals forwards for analysis relative to the variables of interest. This two-step approach was chosen because it leverages the very large data when taking into account broadly applicable nuisance variables such as age whilst ensuring that the model applied to examine effects of interest had minimal possible complexity, thereby reducing any propensity for overfit when contrasting between smaller sub-groups. Nuisance variables were age, sex, racial-ethnicity, gender, handedness, first language (English vs other), country of residence (UK vs other), education level, vocational status and annual earning. Non-native English speakers who were resident outside of the UK were excluded during article revision based on reviewer feedback. Age in years was taken to the third order in the model to fit precisely the nonlinear age curves that are characteristic of the tests. Only complete datasets were included in the analyses and there was no imputation.
The overall summary score, component scores and nine individual test scores with nuisance variables factored out, were taken forwards for analysis with general linear modeling. The first analysis examined differences in scores relative to people who were not ill for those who reported that they believed they had recovered from the COVID-19 illness. These were subdivided along an approximate severity scale into (i) those who did not have trouble breathing, (ii) those who had breathing problems but received no medical assistance, (iii) those who had breathing problems and received medical assistance at home, (iv) those who were taken to hospital but were not put on a ventilator and (v) those who were fitted with a ventilator. Further models were then run focused on the summary score to examine if the observed deficits had a basis in other factors. These included as additional factors in the General Linear Model (GLM) (i) positive confirmation of COVID-19 infection through a biological test, (ii) people who reported residual COVID-19 symptoms, (iii) common pre-existing medical conditions that affect the respiratory system or immune system and that are associated with cognitive deficits, (iv) pre-existing psychiatric conditions, (v) 12 items of the NHS Mood Self-assessment pertaining to frequencies of depression, anxiety insomnia, tiredness and problems concentrating, and (vi) months from symptom onset to cognitive assessment.
2.5. Role of the funding source
The funder of the study had no role in the design of the study, data collection, data analysis, interpretation or writing of the report. All authors had full access to all data within the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
Out of 86,285 people who completed the extended questionnaire, 81,337 individuals fit the eligibility criteria and had complete data. These captured a broad demographic (mean age 46.75 years, 15.73 SD), including representation across sociodemographic and ethnic backgrounds (Table 1). Of these respondents, 93% indicated that their country of residence was in the UK. At the time of completing the extended survey and cognitive tests, a total of 12,689 individuals indicated that they suspected that they had experienced COVID-19, with varying degrees of respiratory severity (Table 2).
Table 1.
Groups within the Great British Intelligence Test cohort broken down by those who opted to complete the extended questionnaire (May-December 2020) and the remaining cohort (January-December 2020).
| COUNT |
PROPORTION |
|||
| Extended questionnaire | Pre-pandemic | Extended questionnaire | Pre-pandemic | |
| TOTAL COUNT | 81,337 | 269,264 | 81,337 | 269,264 |
| Age - mean | 46.7 | 42.8 | 46.7 | 42.8 |
| Age - SD | 15.7 | 15.5 | 15.7 | 15.5 |
| Sex | ||||
| Female | 44,826 | 117,757 | 0.551 | 0.437 |
| Male | 36,160 | 148,755 | 0.445 | 0.552 |
| Other | 351 | 2752 | 0.004 | 0.010 |
| Handedness | ||||
| Ambidextrous | 2078 | 7183 | 0.026 | 0.027 |
| Left | 8765 | 29,790 | 0.108 | 0.111 |
| Right | 70,494 | 232,291 | 0.867 | 0.863 |
| Ethnicity | ||||
| American Hispanic | 327 | 7820 | 0.004 | 0.029 |
| East Asian | 750 | 10,637 | 0.009 | 0.040 |
| Indian, South Asian or South-East Asian | 1995 | 10,275 | 0.025 | 0.038 |
| Mixed ethnicity | 1815 | 7155 | 0.022 | 0.027 |
| North African | 111 | 683 | 0.001 | 0.003 |
| Rom, Sinti or Bedouin | 50 | 403 | 0.001 | 0.001 |
| Sub-saharan African or Afro-american | 243 | 1595 | 0.003 | 0.006 |
| Unknown | 834 | 3514 | 0.010 | 0.013 |
| West-central Asian | 212 | 925 | 0.003 | 0.003 |
| White European or North American | 75,000 | 226,257 | 0.922 | 0.840 |
| Education level | ||||
| 01 No schooling | 94 | 673 | 0.001 | 0.002 |
| 02 Primary/Elementary school | 1553 | 6213 | 0.019 | 0.023 |
| 03 Secondary school/High school diploma | 28,827 | 84,860 | 0.354 | 0.315 |
| 04 University degree | 47,486 | 154,656 | 0.584 | 0.574 |
| 05 PhD | 3294 | 12,706 | 0.040 | 0.047 |
| Unknown | 83 | 10,156 | 0.001 | 0.038 |
| First Language | ||||
| English | 77,560 | 254,673 | 0.954 | 0.946 |
| Other | 3777 | 14,591 | 0.046 | 0.054 |
| Country of residence | ||||
| UK | 75,910 | 249,061 | 0.933 | 0.925 |
| Other | 5427 | 20,203 | 0.067 | 0.075 |
| Occupational status | ||||
| Disabled/Not applicable/Shielded employment | 845 | 2579 | 0.010 | 0.010 |
| Homemaker | 2575 | 8045 | 0.032 | 0.030 |
| Retired | 16,045 | 35,306 | 0.197 | 0.131 |
| Student | 6285 | 20,268 | 0.077 | 0.075 |
| Unemployed/Looking for work | 2500 | 6938 | 0.031 | 0.026 |
| Worker | 52,740 | 184,618 | 0.648 | 0.686 |
| Unknown | 347 | 11,510 | 0.004 | 0.043 |
| Yearly Earnings | ||||
| notworking | 28,597 | 84,646 | 0.352 | 0.314 |
| prefer not to say | 1859 | 5406 | 0.023 | 0.020 |
| £0–10K | 834 | 6211 | 0.010 | 0.023 |
| £10–20K | 8501 | 19,592 | 0.105 | 0.073 |
| £20–30K | 10,975 | 32,709 | 0.135 | 0.121 |
| £30–40K | 9885 | 33,556 | 0.122 | 0.125 |
| £40–50K | 6929 | 25,695 | 0.085 | 0.095 |
| £50–60K | 4145 | 17,228 | 0.051 | 0.064 |
| £60–70K | 2447 | 10,495 | 0.030 | 0.039 |
| £70–80K | 1782 | 7276 | 0.022 | 0.027 |
| £80–90K | 1110 | 5448 | 0.014 | 0.020 |
| £90–100K | 1078 | 5939 | 0.013 | 0.022 |
| >100K | 3195 | 15,063 | 0.039 | 0.056 |
Table 2.
Socio-demographics of the great British intelligence test cohort who completed the extended questionnaire broken down by respiratory severity. Values are proportions of overall count per severity unless otherwise stated.
| Group | Not ill | Ill without respiratory symptoms | No home assistance | Home assistance | Hospitalised No ventilator | Hospitalised +Ventilator |
|---|---|---|---|---|---|---|
| TOTAL COUNT | 68,648 | 8938 | 3386 | 173 | 148 | 44 |
| Age mean years | 47.3 | 43.7 | 43.4 | 43.7 | 45.0 | 41.0 |
| Age SD years | 15.9 | 14.9 | 13.9 | 12.2 | 13.9 | 14.9 |
| Sex | ||||||
| Female | 0.552 | 0.527 | 0.582 | 0.653 | 0.649 | 0.227 |
| Male | 0.443 | 0.468 | 0.411 | 0.335 | 0.351 | 0.705 |
| Other | 0.004 | 0.005 | 0.007 | 0.012 | 0.000 | 0.068 |
| Handedness | ||||||
| Ambidextrous | 0.024 | 0.029 | 0.042 | 0.040 | 0.061 | 0.068 |
| Left | 0.108 | 0.107 | 0.100 | 0.087 | 0.095 | 0.136 |
| Right | 0.867 | 0.865 | 0.859 | 0.873 | 0.845 | 0.795 |
| Ethnicity | ||||||
| American Hispanic | 0.004 | 0.005 | 0.002 | 0.012 | 0.000 | 0.000 |
| East Asian | 0.009 | 0.011 | 0.008 | 0.006 | 0.020 | 0.068 |
| Indian, South Asian/ South-East Asian | 0.024 | 0.032 | 0.022 | 0.058 | 0.020 | 0.114 |
| Mixed ethnicity | 0.021 | 0.032 | 0.031 | 0.035 | 0.020 | 0.023 |
| North African | 0.001 | 0.001 | 0.002 | 0.000 | 0.000 | 0.023 |
| Rom, Sinti or Bedouin | 0.001 | 0.000 | 0.001 | 0.006 | 0.007 | 0.000 |
| Sub-Saharan African or Afro American | 0.003 | 0.005 | 0.002 | 0.006 | 0.007 | 0.023 |
| Unknown | 0.010 | 0.012 | 0.013 | 0.023 | 0.027 | 0.045 |
| West-central Asian | 0.002 | 0.003 | 0.004 | 0.006 | 0.000 | 0.000 |
| White European or North American | 0.926 | 0.899 | 0.915 | 0.850 | 0.899 | 0.705 |
| Education level | ||||||
| No schooling | 0.001 | 0.001 | 0.001 | 0.006 | 0.000 | 0.023 |
| Primary/Elementary school | 0.020 | 0.013 | 0.020 | 0.012 | 0.027 | 0.068 |
| Secondary school/High school diploma | 0.360 | 0.318 | 0.343 | 0.312 | 0.399 | 0.182 |
| University degree | 0.578 | 0.624 | 0.593 | 0.618 | 0.541 | 0.636 |
| PhD | 0.040 | 0.043 | 0.043 | 0.052 | 0.034 | 0.045 |
| Other | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.045 |
| First Language | ||||||
| English | 0.956 | 0.935 | 0.954 | 0.890 | 0.966 | 0.909 |
| Other | 0.044 | 0.065 | 0.046 | 0.110 | 0.034 | 0.091 |
| Country of residence | ||||||
| UK | 0.932 | 0.938 | 0.945 | 0.925 | 0.912 | 0.841 |
| Other | 0.068 | 0.062 | 0.055 | 0.075 | 0.088 | 0.159 |
| Occupational status | ||||||
| Disabled/ Shielded | 0.010 | 0.009 | 0.020 | 0.058 | 0.020 | 0.000 |
| Homemaker | 0.031 | 0.031 | 0.039 | 0.046 | 0.061 | 0.000 |
| Retired | 0.211 | 0.130 | 0.103 | 0.092 | 0.128 | 0.091 |
| Student | 0.075 | 0.092 | 0.076 | 0.058 | 0.047 | 0.091 |
| Unemployed/Looking for work | 0.030 | 0.033 | 0.038 | 0.046 | 0.027 | 0.045 |
| Worker | 0.638 | 0.700 | 0.722 | 0.699 | 0.716 | 0.705 |
| Unknown | 0.004 | 0.005 | 0.002 | 0.000 | 0.000 | 0.068 |
| Yearly Earnings | ||||||
| notworking | 0.362 | 0.300 | 0.278 | 0.301 | 0.284 | 0.295 |
| prefer not to say | 0.023 | 0.021 | 0.019 | 0.029 | 0.054 | 0.023 |
| £0–10K | 0.010 | 0.009 | 0.012 | 0.017 | 0.020 | 0.000 |
| £10–20K | 0.105 | 0.096 | 0.115 | 0.121 | 0.169 | 0.023 |
| £20–30K | 0.133 | 0.139 | 0.157 | 0.127 | 0.142 | 0.114 |
| £30–40K | 0.119 | 0.133 | 0.139 | 0.116 | 0.122 | 0.227 |
| £40–50K | 0.083 | 0.097 | 0.095 | 0.075 | 0.068 | 0.000 |
| £50–60K | 0.049 | 0.060 | 0.056 | 0.098 | 0.027 | 0.136 |
| £60–70K | 0.030 | 0.033 | 0.027 | 0.040 | 0.020 | 0.023 |
| £70–80K | 0.021 | 0.025 | 0.024 | 0.029 | 0.020 | 0.045 |
| £80–90K | 0.013 | 0.018 | 0.012 | 0.000 | 0.034 | 0.000 |
| £90–100K | 0.013 | 0.016 | 0.017 | 0.023 | 0.014 | 0.023 |
| >100K | 0.037 | 0.052 | 0.051 | 0.023 | 0.027 | 0.091 |
| Positive COVID-19 biological test | ||||||
| No/awaiting results | 1.000 | 0.976 | 0.970 | 0.919 | 0.851 | 0.136 |
| Yes | 0.000 | 0.024 | 0.030 | 0.081 | 0.149 | 0.864 |
| Residual symptom rates | ||||||
| No | 1.000 | 0.962 | 0.942 | 0.908 | 0.878 | 0.159 |
| Yes | 0.000 | 0.038 | 0.058 | 0.092 | 0.122 | 0.841 |
| Pre-existing conditions affecting immune system | ||||||
| Weakened immune system (e.g., HIV/aids, medicines such as steroid tablets or chemotherapy). | 0.025 | 0.018 | 0.026 | 0.064 | 0.054 | 0.000 |
| Chronic kidney disease | 0.007 | 0.005 | 0.005 | 0.012 | 0.027 | 0.136 |
| Diabetes | 0.034 | 0.027 | 0.035 | 0.046 | 0.047 | 0.045 |
| Heart disease | 0.026 | 0.019 | 0.022 | 0.029 | 0.074 | 0.023 |
| High blood pressure | 0.011 | 0.012 | 0.013 | 0.035 | 0.027 | 0.000 |
| Irregular heartbeat atrial fibrillation | 0.003 | 0.003 | 0.006 | 0.006 | 0.000 | 0.000 |
| Liver disease e.g., hepatitis | 0.004 | 0.005 | 0.006 | 0.006 | 0.027 | 0.091 |
| Lung conditions e.g., asthma | 0.097 | 0.071 | 0.212 | 0.295 | 0.324 | 0.409 |
| Problems with your spleen e.g., sickle cell disease | 0.002 | 0.002 | 0.002 | 0.000 | 0.020 | 0.000 |
| Psychiatric conditions | ||||||
| Anxiety | 0.126 | 0.142 | 0.209 | 0.318 | 0.264 | 0.136 |
| Attentional deficit hyperactivity disorder | 0.005 | 0.007 | 0.012 | 0.000 | 0.034 | 0.068 |
| Bipolar | 0.004 | 0.006 | 0.011 | 0.006 | 0.007 | 0.023 |
| Depression | 0.126 | 0.140 | 0.216 | 0.382 | 0.243 | 0.341 |
| Obsessive compulsive disorder | 0.010 | 0.014 | 0.017 | 0.052 | 0.054 | 0.045 |
| Other | 0.013 | 0.018 | 0.035 | 0.064 | 0.054 | 0.000 |
Global cognitive scores were derived by taking the first unrotated principal component across all tests with the exception of Emotion Discrimination, which was excluded due to low communality with the cognitive tasks (Tables 3 & S3).
Table 3.
Average task summary scores for participants included within the study (n = 81,337) and task loadings for the global composite. Note the mean digit span and spatial span maximum scores achieved match previous reported national averages, according with the representative nature of the dataset. SD = standard deviation.
| Raw Population Task Scores |
Global component loading | ||||
|---|---|---|---|---|---|
| Task | Mean | SD | Max | Global cognitive score | |
| Digit Span | 7.04 | 1.51 | 15 | 0.38 | |
| Rare word definitions | 16.89 | 2.65 | 21 | 0.45 | |
| Analogical reasoning | 24.79 | 10.82 | 89 | 0.63 | |
| Target Detection | 56.89 | 11.68 | 90 | 0.24 | |
| 2D mental rotations | 26.87 | 8.13 | 70 | 0.35 | |
| Spatial span | 6.07 | 1.21 | 12 | 0.37 | |
| Block rearrange | 11.15 | 2.90 | 15 | 0.38 | |
| Tower of London | 6.72 | 2.44 | 10 | 0.39 | |
| Face emotional discrimination | 42.91 | 3.30 | 50 | NA | |
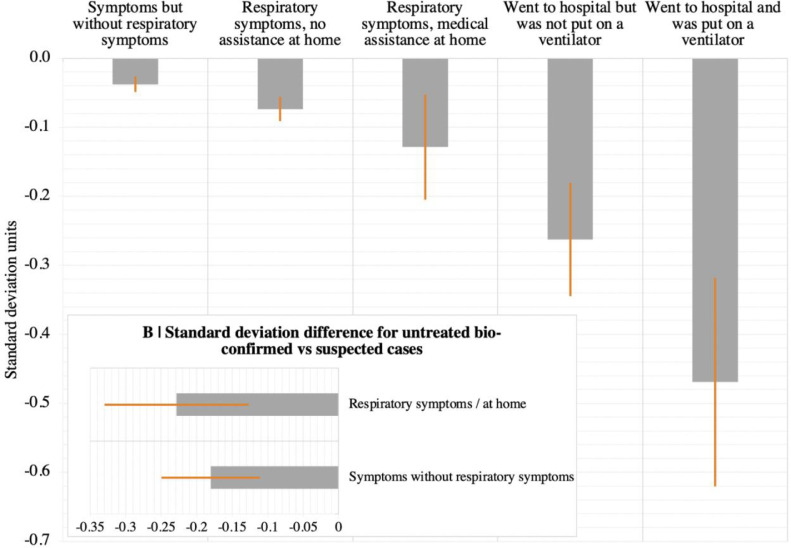
Generalised linear modelling (GLM) was applied to determine whether global cognitive scores covaried with respiratory COVID-19 symptom severity after factoring out age, sex, handedness, first language, education level, country of residence, occupational status and earnings. A one-sample Kolmogorov-Smirnov test failed to reject the null hypothesis that the global score that was the target variable was normally distributed (KS statistic = 0.0039, p = 0.1786) and a Bartlett test failed to reject the null hypothesis that global scores for groups with different respiratory symptoms came from normal distributions with the same variance (Bartlett's statistic 4.42, p = 0.49). There was a significant main effect (F(5,81,331) = 9.6867 p = 2.915e-09), with increasing degrees of cognitive underperformance relative to controls dependent on level of medical assistance received for COVID-19 respiratory symptoms (Fig. 2a-Table S4). People who had been hospitalised showed substantial scaled global performance deficits dependent on whether they were (−0.47 standard deviations (SDs) N = 44) vs. were not (−0.26 SDs N = 148) put onto a ventilator. Those who remained at home (i.e., without inpatient support) showed small statistically significant global performance deficits (assisted at home for respiratory difficulty −0.13 SD N = 173; no medical assistance but respiratory difficulty −0.07 SDs N = 3,386; ill without respiratory difficulty −0.04 SDs N = 8,938).
Fig. 2.
. Cognitive deficits in people with suspected and confirmed COVID-19 illness.
A | People who reported having recovered from COVID-19 performed worse in terms of global score. The scale of this deficit increased with the level of treatment received for respiratory difficulty. B | In individuals who did not receive medical assistance, the scale of this deficit was greater in biologically confirmed cases versus suspected cases of COVID-19. Error bars report the standard error.
The GLM was re-estimated including confirmation of COVID-19 by biological test as a main effect (Table S5a). In proportion with the number of UK confirmed cases when the bulk of data were collected, 386 people reported a positive biological test, including 86% of the hospitalised with ventilator sub-group. There were significant main effects of positive test (F(1,81,326) = 12.487 p = 0.0004 estimate = −0.19SDs) and respiratory severity (F(5,81,326) = 6.7 p = 3.165e-06). Intriguingly though, the interaction was non-significant (F(4,81,326) = 0.81 p = 0.51), indicating a possible deficit for mild cases who were bio-positive for COVID-19. A further GLM restricted to those who reported no breathing difficulties (bio-positive = 212 vs. suspected = 8,726) confirmed this (Fig. 2b, Table S5b), with a robustly greater global performance deficit for bio-positive cases (t=−2.592 p = 0.0048 (one tailed) estimate = −0.18SDs). Repeating the analysis for people who reported staying at home with breathing difficulty bio-positive = 100 suspected = 3,286) showed a similar scaled deficit (t = −2.25 p = 0.012 (one tailed) estimate = −0.23SDs). A larger relationship was evident amongst cases who went to hospital but were not put on a ventilator (bio-positive = 22 vs suspected = 126, t=−1.7923 p = 0.0375 (one tailed) estimate = −0.41SDs).
A common challenge in studies of COVID-19 is that differences between people who have vs. have not been ill could relate to premorbid differences. To address this issue, a linear model was trained on the broader independent GBIT dataset (N = 269,264) to predict general cognitive performance based on age (to the third order), sex, handedness, ethnicity, first language, country of residence, occupational status and earnings. Predicted and observed general performance correlated substantially r = 0.53), providing a proxy measure of premorbid intelligence of comparable performance to common explicit tests such as the National Adult Reading Test [26]. Regression of the same linear model with respiratory severity as the predictor indicated that people who were ill would on average be expected to have marginally higher as opposed to lower cognitive performance (Table S6). This relationship did not vary in a simple linear manner with symptom severity. Furthermore, when a follow up questionnaire was deployed in late December 2020, 275 respondents indicated that they had subsequently been ill with COVID-19 and received a positive biological test. Their baseline global cognitive scores did not differ significantly from the 7522 respondents who had not been ill (t = 0.7151, p = 0.4745 estimate = 0.0531SDs). Taken together, these findings indicate that the cognitive impairments detected in COVID-19 survivors were unlikely to reflect pre-morbid differences.
One possibility was that the observed cognitive deficits related to ongoing symptoms of COVID-19 infection, e.g., high temperature or respiratory problems. 4.8% of participants who were ill reported having residual symptoms, including 84.1% of the ventilator group, 12.2% hospitalised, 9.2% assisted at home, 5.8% unassisted and 3.8% without respiratory problems. Notably, 24.4% of participants who had positive biological tests reported persistent symptoms of illness compared with 4.2% who had not. When report of residual COVID-19 symptoms was included in the GLM (Table S7), the main effect of respiratory severity was undiminished (F(5, 81,287) = 8.2422 p = 8.54E-08). The main effect of residual symptoms was formally non-significant and of small effect size (F(1, 81,287) = 1.0633 p = 0.302 estimate = −0.0440 SDs).
We further examined whether there was a relationship between cognitive performance and time since symptom onset (Fig. S1) amongst bio-confirmed cases who did not report residual symptoms. In this sub-group, mean time from symptom onset was 1.96 months +/- 1.65SDs with an upper limit of 9 months. Analyzing this sub-group with time since symptom onset as the predictor showed no significant correlation (F(1,290) = 0.222 p = 0.638). Furthermore, expanding the analysis include those who were not bio-confirmed (mean time = 2.4610, SD=1.3481, max = 11) also showed no significant relationship between time and the magnitude of the observed deficit (F(1,12078) = 2.1196 p = 0.14545).
Another possibility was that the observed cognitive deficits had a basis in pre-existing conditions. When a GLM was estimated with additional predictors for common pre-existing conditions and 12 mood self-assessment items capturing aspects including depression, anxiety, insomnia, tiredness (Table S8), a number of them showed the expected association with reduced cognitive performance. However, the statistical significance and scale of the respiratory severity main effect remained approximately the same (F(5, 81,304) = 9.3355 p = 6.65E-09). Furthermore, the effect size for those who had been hospitalized was substantial relative to the other conditions examined.
Finally, the cognitive deficits were examined at a finer grain. Analysis of individual test summary scores (Tables 4 and S9a) highlighted a broad but variable profile of deficits across cognitive domains. A pattern was evident whereby the larger associations were for more complex tasks requiring reasoning, planning and problem solving such as verbal analogies, Blocks and Tower of London as opposed to more basic working memory functions such as Digit Span and Spatial Span or Emotional Discriminations. Analysis of individual task median response times also indicated significant slowing of responses (Table 4), particularly in the ventilated group (Table S9a). Further analysis of bio-confirmed cases revealed a broader set of tests sensitive to COVID-19 illness in the small-medium effect size range (Table S9b).
Table 4.
. Domain sensitivity of COVID-19 related cognitive deficits.
| Symptoms without respiratory symptoms | Respiratory symptoms / no home assistance | Respiratory symptoms / medical home assistance | Hospitalised / no ventilator | Hospitalised / ventilator | |
|---|---|---|---|---|---|
| ACCURACY | |||||
| Verbal Analogies | −0.017 | −0.047 | −0.050 | −0.358 | −0.433 |
| Tower of London | −0.023 | −0.040 | −0.049 | −0.145 | −0.363 |
| Target Detection | −0.010 | −0.024 | −0.133 | −0.158 | −0.318 |
| Word Definitions | 0.009 | −0.040 | −0.097 | −0.099 | −0.317 |
| Blocks | −0.037 | −0.006 | 0.043 | −0.087 | −0.310 |
| 2D Manipulation | −0.034 | −0.044 | −0.152 | −0.213 | −0.210 |
| Spatial Span | −0.037 | −0.056 | −0.144 | 0.009 | 0.076 |
| Emotion Discrimination | 0.043 | −0.019 | −0.053 | −0.134 | 0.113 |
| Digit Span | 0.013 | −0.013 | −0.036 | 0.077 | 0.136 |
| RESPONSE TIME | |||||
| Verbal Analogies | 0.051 | 0.032 | −0.044 | 0.104 | 0.526 |
| Blocks | 0.049 | 0.005 | 0.039 | 0.095 | 0.324 |
| Spatial Span | 0.006 | −0.019 | 0.012 | 0.021 | 0.277 |
| Digit Span | 0.026 | 0.031 | 0.078 | 0.050 | 0.219 |
| 2D Manipulation | 0.027 | 0.019 | 0.093 | 0.176 | 0.192 |
| Emotion Discrimination | 0.029 | 0.039 | 0.040 | −0.001 | 0.067 |
| Target Detection | −0.006 | −0.004 | 0.025 | 0.066 | −0.001 |
| Tower of London | 0.040 | 0.019 | 0.054 | −0.056 | −0.046 |
Upper | The effect size of cognitive deficits varied substantially across the nine test summary scores. Higher cognitive functions such as reasoning, planning and selective attention showed the greatest accuracy deficits. Lower | Slower response time latencies were also evident.
Discussion
Our analyses provide converging evidence to support the hypothesis that COVID-19 infection is associated with cognitive deficits that persist into the recovery phase. The observed deficits varied in scale with respiratory symptom severity, related to positive biological verification of having had the virus even amongst milder cases, could not be explained by differences in age, education or other demographic and socioeconomic variables, remained in those who had no other residual symptoms and was of greater scale than common pre-existing conditions that are associated with virus susceptibility and cognitive problems.
The scale of the observed deficit was not insubstantial; the 0.47 SD global composite score reduction for the hospitalized with ventilator sub-group was greater than the average 10-year decline in global performance between the ages of 20 to 70 within this dataset. It was larger than the mean deficit of 480 people who indicated they had previously suffered a stroke (−0.24SDs) and the 998 who reported learning disabilities (−0.38SDs). For comparison, in a classic intelligence test, 0.47 SDs equates to a 7-point difference in IQ.
In terms of cognitive profile, the assessment battery applied comprised tests that were designed to enable variance in different aspects of cognition to be examined at very large scale within the general population. The deficits affected multiple tests but to different degrees. When examining the entire population, the deficits were most pronounced for paradigms that tapped cognitive functions such as reasoning, problem solving, spatial planning and target detection whilst sparing tests of simpler functions such as working-memory span as well as emotional processing. These results accord with reports of long-COVID, where ‘brain fog’, trouble concentrating and difficulty finding the correct words are common. Notably, this profile cannot be explained by differences in the general sensitivity of our tests; e.g., Spatial Span and Digit Span scores show robust age-related differences. Instead, recovery from COVID-19 infection may be associated with particularly pronounced problems in aspects of higher cognitive or ‘executive’ function, an observation that accords with preliminary reports of executive dysfunction in some patients at hospital discharge [17], as well as previous studies of ventilated patients with acute respiratory distress syndrome pre-pandemic [19]. It should be noted though, that when the analysis of individual test scores was constrained to people who had positive biological tests, the profile in milder non-hospitalized cases extended to spatial span.
It is important to be cautious in inferring a neurobiological or psychological basis of the observed deficits without brain imaging data, although the assessment tasks used here have been shown to map to different networks within the human brain in terms of normal functional activity and connectivity as well as structural network damage [27], [28], [29]. Speculatively, we believe there are likely to be multiple contributing factors. For example, previous studies in hospitalised patients with respiratory disease not only demonstrate objective and subjective cognitive deficits but suggest these remain for some at 5-year follow-up [19]. Consequently, the observation of post-infection deficits in the subgroup who were put on a ventilator was not altogether surprising. Conversely, the scale of deficits in cases who were not put on a ventilator, particularly those who remained at home, was unexpected given the limited literature on other respiratory illnesses such as cold [30] Although these deficits were on average of small scale for those who remained at home, they were more substantial for people who had received positive confirmation of COVID-19 infection. A corollary of this is that cognitive deficits associated with other respiratory illnesses that are mistakenly self-diagnosed as COVID-19 are likely to be negligible. One possibility is that these deficits in milder bio-confirmed cases may reflect the lower grade consequences of less severe hypoxia. The observed correlation with severity of respiratory symptoms is in close concordance with this view; however, as noted in the introduction, there have been case reports of other forms of neurological damage in COVID-19 survivors, including some for whom such damage was the first detected symptom [7]. Accordingly, in the current study, bio-positive cases who reported being ill but remained at home showed a 0.23SD magnitude cognitive deficit. Based on this, we propose that a timely challenge is to cross-relate the multi-dimensional profile of cognitive deficits observed here to imaging markers that can confirm and differentiate the underlying psychological and neuropathological sequelae of COVID-19.
An important consideration for any cross-group study is biased sampling. Crucially, our study promotional material did not mention COVID-19. Instead, we raised the profile via a BBC2 Horizon documentary plus news features stating that people could undertake a free online assessment to identify their greatest cognitive strengths. This mitigated biased recruitment of people who suspected that COVID-19 had affected their cognitive faculties. Including the questionnaire post assessment also mitigated the potential for questionnaire items to bias expectations of poor self-performance due to COVID-19.
Normal limitations pertaining to inferences about cause and effect from cross-sectional studies also should be considered [6,31]. The large and socioeconomically diverse nature of the cohort enabled us to include many potentially confounding variables in our analysis, which goes some way to mitigating the possibility that observed differences were present prior to illness. Premorbid estimates also indicate that those who were ill were likely to have had somewhat higher as opposed to lower cognitive ability pre-illness. Nonetheless, longitudinal research, including follow-up of this cohort, should further confirm the cognitive impact of COVID-19 infection and determine deficit longevity as a function of respiratory symptom severity. A further consideration is that our results rely on self-report as we do not have access to participant clinical records. We note that this reliance will apply broadly for studies of the many Covid-19 patients who did not receive medical assistance during the acute phase.
Cross comparison to hospital recruited cohorts will provide further confirmation using the same cognitive tests reported here. This study did not set out to determine the biological basis of the COVID-19 cognitive deficit association in terms of neural systems or psychological mechanisms, just to confirm whether there is such an association. Further work is required to interrelate the deficits to underlying causes, e.g., neurological changes, fatigue and apathy. Relatedly, future studies should also examine the role of putatively protective population factors such as cognitive reserve. The observation of substantial associations reported here can guide assessment batteries applied in such studies. A fuller understanding of the marked deficits that our study shows will enable better preparedness in the post-pandemic recovery challenges.
Funding
AH is supported by the UK Dementia Research Institute Care Research and Technology Centre and Biomedical Research Centre at Imperial College London. WT is supported by the EPSRC Centre for Doctoral Training in Neurotechnology. SRC is funded by a Wellcome Trust Clinical Fellowship 110,049/Z/15/Z. JMB is supported by Medical Research Council (MR/N013700/1). MAM, SCRW and PJH are, in part, supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London
Declaration of Competing Interest
Dr. Hampshire reports grants from UK Dementia Research Institute, outside the submitted work and is Co-director and owner of H2CD Ltd, and owner and director of Future Cognition Ltd, which support online studies and develop custom cognitive assessment software respectively. Dr. Hellyer reports personal fees from H2CD Ltd, outside the submitted work. Dr. Chamberlain reports grants from Wellcome, personal fees from Elsevier, personal fees from Prometis (not current), outside the submitted work. Dr. Grant reports grants from Otsuka, grants from Biohaven, grants from Avanir, outside the submitted work. Dr. Patrick reports grants from H Lundbeck A/S, non-financial support from Astra Zeneca, non-financial support from Janssen, outside the submitted work. Dr. Mehta reports grants from H Lundbeck A/S, non-financial support from Astra Zeneca, non-financial support from Janssen, outside the submitted work. Dr. Williams has nothing to disclose. Dr. Mazibuko has nothing to disclose. Dr. Jolly has nothing to disclose. Mr. Trender has nothing to declare. Dr. Barnby has nothing to disclose.
Acknowledgments
Data sharing statement
Data sharing requests from researchers within academic institutions should be directed to the corresponding author.
Acknowledgments
We would like to thank the BBC2 Horizon team for their support in promoting this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101044.
Appendix. Supplementary materials
References
- 1.Baig A.M. Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2020 doi: 10.1002/jmv.26624. [DOI] [PubMed] [Google Scholar]
- 2.Perego E., Callard F., Stras L., Melville-Jóhannesson B., Pope R., Alwan N.A. Wellcome Open Research; 2020. Why the patient-made term 'long Covid' is needed. [Google Scholar]
- 3.Callard F., Perego E. How and why patients made Long Covid. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellul M.A., Benjamin L., Singh B. Neurological associations of COVID-19. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varatharaj A., Thomas N., Ellul M.A. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellul M., Varatharaj A., Nicholson T.R. Defining causality in COVID-19 and neurological disorders. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson R.W., Brown R.L., Benjamin L. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyrouti R., Adams M.E., Benjamin L. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toscano G., Palmerini F., Ravaglia S. Guillain-barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke C., Ferse C., Kreye J. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. 2021;93:415–419. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kremer S., Lersy F., de Seze J. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanin L., Saraceno G., Panciani P.P. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. (Wien) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers J.P., Chesney E., Oliver D. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown E., Gray R., Lo Monaco S. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79–87. doi: 10.1016/j.schres.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowkwanyun M., Reed A.L. Racial health disparities and COVID-19-caution and context. N Engl J Med. 2020 doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 21.P.H England.. Disparities in the risk and outcomes of COVID-19., 2020
- 22.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir Med. 2020;8(6):547–548. doi: 10.1016/S2213-2600(20)30228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampshire A. Research Square; 2020. Great british intelligence test protocol. [Google Scholar]
- 24.Chamberlain S.R., Grant J.E., Trender W., Hellyer P., Hampshire A. Post-traumatic stress disorder symptoms in COVID-19 survivors: online population survey. BJPsych Open. 2021;7(2):e47. doi: 10.1192/bjo.2021.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampshire A., Hellyer P.J., Soreq E. Associations between dimensions of behaviour, personality traits, and mental-health during the COVID-19 pandemic in the United Kingdom. Nat Commun. 2021;12(1):4111. doi: 10.1038/s41467-021-24365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGurn B., Starr J.M., Topfer J.A. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62(7):1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- 27.Hampshire A., Highfield R.R., Parkin B.L., Owen A.M. Fractionating human intelligence. Neuron. 2012;76(6):1225–1237. doi: 10.1016/j.neuron.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Jolly A.E., Scott G.T., Sharp D.J., Hampshire A. Distinct patterns of structural damage underlie working memory and reasoning deficits after traumatic brain injury. Brain. 2020;143(4):1158–1176. doi: 10.1093/brain/awaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soreq E., Violante I.R., Daws R.E., Hampshire A. Neuroimaging evidence for a network sampling theory of individual differences in human intelligence test performance. Nat Commun. 2021;12(1):2072. doi: 10.1038/s41467-021-22199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith A.P. Twenty-five years of research on the behavioural malaise associated with influenza and the common cold. Psychoneuroendocrinology. 2013;38(6):744–751. doi: 10.1016/j.psyneuen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes E.A., O'Connor R.C., Perry V.H. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.